Abstract
Electrophysiological recordings in the dorsal cochlear nucleus (DCN) were conducted to determine the nature of changes in single unit activity following intense sound exposure and how they relate to changes in multiunit activity. Single and multiunit spontaneous discharge rates and auditory response properties were recorded from the left DCN of tone exposed and control hamsters. The exposure condition consisted of a 10 kHz tone presented in the free-field at a level of 115 dB for 4 hrs. Recordings conducted at 5–6 days postexposure revealed several important changes. Increases in multiunit spontaneous neural activity were observed at surface and subsurface levels of the DCN of exposed animals, reaching a peak at intermediate depths corresponding to the fusiform cell layer and upper level of the deep layer. Extracellular spikes from single units in the DCN of both control and exposed animals characteristically displayed either M- or W-shaped waveforms, although the proportion of units with M-shaped spikes was higher in exposed animals than in controls. W-shaped spikes showed significant increases in the duration of their major peaks after exposure, suggestive of changes in the intrinsic membrane properties of neurons. Spike amplitudes were not found to be significantly increased in exposed animals. Spontaneous discharge rates of single units increased significantly from 8.7 spikes/s in controls to 15.9 spikes/s after exposure. Units with the highest activity in exposed animals displayed type III electrophysiological responses patterns, properties usually attributed to fusiform cells. Increases in spontaneous discharge rate were significantly larger when the comparison was limited to a subset of units having type III frequency response patterns. There was an increase in the incidence of simple spiking activity as well as in the incidence of spontaneous bursting activity, although the incidence of spikes occurring in bursts was low in both animal groups (i.e., <30%). Despite this low incidence, approximately half of the increase in spontaneous activity in exposed animals was accounted for by an increase in bursting activity. Finally, we found no evidence of an increase in the mean number of spontaneously active units in electrode penetrations of exposed animals compared to those in controls. Overall our results indicate that the increase in multiunit activity observed at the DCN surface reflects primarily an increase in the spontaneous discharge rates of single units below the DCN surface, of which approximately half was contributed by spikes in bursts. The highest level of hyperactivity was observed among units having the response properties most commonly attributed to fusiform cells.
Keywords: Burst, Tinnitus, Spike waveform, Couplet, Fusiform cells, dorsal cochlear nucleus
INTRODUCTION
Increased spontaneous neural activity is a major consequence of intense sound exposure that has been observed at several levels of the mammalian auditory system, including the cochlear nucleus, inferior colliculus and auditory cortex (Kaltenbach and Afman, 2000; Kaltenbach et al., 1998, 2000; Zhang and Kaltenbach, 1998; Ma et al., 2006; Dong et al., 2009; Brozoski et al., 2002; Komiya and Eggermont, 2000; Seki and Eggermont, 2003; Norena and Eggermont, 2003; Wallhauser-Frank et al., 2003). This alteration has been implicated as a possible factor contributing to the induction of tinnitus (Kaltenbach et al., 2004, 2005; Brozoski et al., 2002; Shore et al., 2008; Imig and Durham, 2005; Eggermont and Roberts, 2004; Ma et al., 2006; Wallhauser-Franke et al., 2003) and could play an important role in other auditory deficits. For example, excessive levels of activity may induce plastic changes in or cause excitotoxic injury to post-synaptic neurons (Kim et al., 1997). Such changes are potentially disruptive to normal signal detection and information processing in neurons further downstream from hyperactive cell populations. Excitotoxic damage to postsynaptic neurons could result in loss of inhibitory interneurons, which could enhance responses to suprathreshold stimuli, leading to recruitment, hyper-responsiveness and hyperacusis. Knowledge of the mechanisms underlying the induction of hyperactivity would thus seem likely to have important implications for the understanding and treatment of tinnitus and other related hearing disorders.
Much work in our laboratory has focused on hyperactivity in the dorsal cochlear nucleus (DCN) as a possible factor contributing to tinnitus (Kaltenbach and McCaslin, 1996; Kaltenbach et al., 1998; Zhang and Kaltenbach, 1998; Kaltenbach et al., 2000). These studies have been performed primarily at the multiunit level in order to provide details concerning the distribution pattern of hyperactivity in the different layers of the DCN (Kaltenbach and Falzarano, 2002), the profile of this activity along the tonotopic axis (Kaltenbach and Afman, 2000; Zhang and Kaltenbach, 1998), the relationship of activity changes to the type and distribution of hair cell injury along the cochlear partition (Kaltenbach et al., 2002), and the time course of its progression (Kaltenbach et al., 2000). However, the multiunit data is limited in what it can reveal about the nature of the changes in neuronal activity leading to hyperactivity. Increases in multiunit activity might be assumed to reflect increases in single cell spontaneous discharge rates, but in fact, other factors such as increases in the number of active neurons, average spike amplitude, or the incidence of bursting discharges, could also play important roles. Which of these changes occurs after intense sound exposure is critical for an understanding of underlying mechanisms. For example, increases in the discharge rates of individual neurons or in the density of spontaneously active units could indicate perturbations in neuronal transmission or network connections due to loss/weakening of inhibition or a net gain of excitation. In contrast, increases in spike amplitude or duration could signify changes in excitability due to changes in intrinsic membrane properties or loss of the intercellular insulation (e.g., demyelination). Finally, increases in bursting activity could point to changes in the spike generation mechanism.
The present study was conducted to determine which of the above changes contribute to the large increases in activity observed at the multiunit level. We performed multiunit and single unit recordings in the DCNs of animals previously exposed to intense sound as well as unexposed control animals. Multiunit activity was first mapped in a region of the tonotopic range where hyperactivity has previously been shown to be robust. Single unit recordings were then conducted at various depths within this same region. In these areas, we recorded the activity of well-isolated single units in the DCN. From these recordings we analyzed the mean discharge rates, mean spike amplitudes, the incidence of bursting activity and the numbers of units/penetration. Finally, we recorded the frequency-dependent and temporal responses of neurons in an effort to obtain clues concerning the categories of cells displaying abnormal activity.
MATERIALS and METHODS
Animal Preparation and sound exposures
The Wayne State University Animal Investigation Committee approved the care and use of all animals in this study. Syrian golden hamsters (male) ranging in age from 2 to 3 months, were divided into 2 groups. One group was exposed to an intense sound, while the other served as unexposed controls.
Sound exposure
Acoustic exposures were performed inside a sound attenuation booth (Industrial Acoustics Corporation). A cage was placed on a table inside the booth, and a loudspeaker (JBL 2404H) was suspended above the cage and used to deliver a continuous 10 kHz tone. Before placing the animals in the cage, the sound output was adjusted so that the level of the tone at the center of the cage was 115 dB SPL and usually varied by no more than ± 6 dB at other locations inside the cage. Acoustic measures were performed with an Etymotic ER-7C probe tube microphone. The sound was then switched off, and the animals were placed awake and freely moving inside the cage in groups of 2–3. The sound was switched back on at a level of 80 dB then turned up 5 dB every 2–3 minutes until the desired exposure level of 115 dB SPL was reached. Using this method, the animals readily accommodated to the loud sound without displaying any behavioral signs of acoustic stress. The sound was maintained at this level for 4 hours. At the end of the exposure period, the sound was turned off, and the animals returned to the animal holding facility where they were allowed a postexposure recovery period of 5–6 days before commencement of electrophysiological studies. This recovery period was chosen to maximize the area over which hyperactivity is induced, as a previous study showed multiunit activity increases over a broader area of the DCN between 5 and 14 days than at later time points (Kaltenbach, et al., 2000). Control animals were similarly placed in a cage inside an acoustic booth but were not exposed to sound. Researchers were blinded to the group to which each animal belonged. This was accomplished by sub-cutaneously injecting a tracking chip (Microchip I.D., Avid) in each animal, after documenting the exposure history. The tracking chip was removed and read at the end of each electrophysiological recording session, but the animal’s identity with respect to group was not determined until all experiments were completed.
Surgical procedures
Following the post-exposure recovery period, each animal was prepared surgically for electrophysiological studies in a sound attenuation booth. Animals were anesthetized by i.m. injection of ketamine (85 mg/kg) and xylazine (15 mg/kg). Areflexia was maintained with supplementary injections of anesthetic. Core body temperature was maintained at 37°C using a rectal probe and heating pad. The animal’s head was held firmly in a brace, and the left DCN exposed by a parieto-occipital craniotomy followed by aspiration of the overlying portion of the cerebellum.
Recordings of multiunit activity
Multiunit activity at the DCN surface was recorded with 0.4 MΩ electrodes filled with 0.3 M NaCl. Contact of the electrode with the surface of the DCN was indicated by a sudden increase in the loudness of the signal from the audio monitor and the emergence of neural signals which appeared as continuous trains of multiunit potentials. Electrode positions on the DCN surface were digitally captured with Image-Pro Plus software (MediaCybernetics) using a CCD camera mounted on a Nikon stereomicroscope. The level discriminator was adjusted to trigger on amplified (1000X) neural potentials at a threshold voltage of −100 mV, as described previously (Kaltenbach and Afman, 2000). The output from the level discriminator was fed to a universal counter. Spontaneous activity was then measured at each of the recording sites by counting the number of output events from the level discriminator over a period of 90 s. Upon completion of each count, the electrode was moved to another site, where a new measure of spontaneous activity was performed. Such measures were repeated at various locations on the DCN surface until recordings of spontaneous activity were completed in a grid consisting of 3 rows of 7 sites (21 sites total) spaced 100 μm apart from 0.5 to 1.1 mm from the lateral edge of the DCN. This range corresponds to the region in which multiunit activity has previously been found to be very high in exposed animals at 5 days after exposure (Kaltenbach et al., 2000). In each animal, measures of multiunit activity were first averaged across the 3 rows and were used to obtain an activity profile for each animal, as described previously (Kaltenbach et al., 2000). The mean activity profiles were then averaged across animals within each group. After all experiments were completed, the average multiunit activity profiles in exposed and control animal groups were compared to establish whether the sound exposure had induced hyperactivity in the DCN.
In a separate experiment, multiunit recordings were performed at each of a series of depths spaced at 20 micron intervals, based on readings from the micrometer scale on the micromanipulator. At each depth, activity was measured for 90 seconds, as in the surface recordings, then the electrode was moved to the next deepest location. This procedure was followed until recordings were performed from up to 19 depths in each penetration. The mean activity was then plotted vs. depth to yield an activity profile spanning the radial axis of the DCN.
Recordings of single unit activity
Immediately upon completion of multiunit recordings, single unit activity was recorded with electrodes filled with 0.3 M NaCl and having tip impedances of 13 to 24 MΩ. Electrode signals were preamplified and bandpass filtered (200 Hz to 4 kHz) (WPI DAM-80). Single units were recorded in penetrations that were perpendicular to the DCN surface over the same topographic range where multiunit activity was recorded. Electrodes were advanced remotely using a Narashige XYZ micromanipulator. The depth of each recording was measured by taking the difference between readings on the micrometer scale of the micromanipulator at the surface and at the recording site. The number of cells recorded in each penetration was determined and used to compute the average number of cells/penetration in each animal group.
Single unit spikes were recorded during periods of spontaneous activity (SA) and detected using a level discriminator. The spontaneous discharge rate (spikes/s) of each unit was quantified by counting the number of spikes measured over periods of 90 s. Oscilloscopic traces of activity were digitized at a 20 kHz sampling rate (Apple Quadra 650, Hardware: National Instruments NB-MIO-16 ADC; software: A/Dvance, McKellar design).
Acoustic response properties
Frequency response areas were obtained by testing the responses of units to pure-tones (50 ms including 5 ms rise-fall ramps) over a range of frequencies and intensities. Pure tones were generated by a computer controlled HP 3325A function generator. The level was controlled by computer using a Coulbourn programmable attenuator. Stimuli were presented monaurally at a rate of 10/s to the left ear through a Beyer Dynamic DT-48 headphone coupled to the external auditory meatus using a plastic conical insert. Stimulus frequencies were increased in 50 logarithmic increments from 3 to 32 kHz, and the intensities were increased in 6 dB steps from 6 to 96 dB SPL, yielding a total of 800 frequency/intensity combinations. Frequency response areas, based on presentations of each tone only once, were displayed on-line by plotting response bars whose heights were proportioned to the number of spikes collected during each stimulus.
When possible, peri-stimulus time histograms (PSTH) were also collected. These were calculated from spike time occurrences in relation to tone onset with a time resolution of 0.1 ms. The PSTH was determined from responses to 250 presentations of 50 ms best frequency tones at 20 dB above threshold, presented at 4/s.
Data analysis
Levels of spontaneous activity can be influenced by a number of external factors beyond those caused by our controlled noise exposure, such as quality of surgical preparation, depth of anesthesia, tissue dehydration, etc. Due to these other factors, animals occasionally yielded spontaneous rates far outside their normal ranges. Although such occurrences were rare, when they were encountered, their inclusion in the analysis greatly biased the mean spontaneous rate values of their respective animal groups. To avoid the biasing effects of these outliers, we rejected data from animals yielding mean multiunit spontaneous rates greater than or less than 1.5 standard deviations of the mean values obtained in a previous study (Kaltenbach and Afman, 2000).
For each unit, we obtained digitized records of spontaneous activity for periods of up to 90 seconds. From these records, a series of 10 traces, each trace representing 100–200 ms of activity, were subjected to a detailed analysis to obtain clear measures of spike amplitude and waveform, and the incidence of bursting activity. Note, however, that the measures of spike amplitude and waveform were performed only on simple spikes and excluded spikes occurring in bursts, which comprised less than 30% of all spikes in both animal groups. Because this analysis required a high degree of consistency across the population of spikes from each unit, records displaying signs of instability in the recording were not included in the analysis.
The analysis of spike trains was conducted in several steps. The unit was first classified as having either a W-shaped or M-shaped waveform, based on the polarity and configuration of the first three major peaks in the spike. W-shaped spikes displayed waveforms characterized by an upward peak before and after large downward peaks, while M-shaped spikes exhibited a downward peak before and after upward peaks. The criteria for this classification are described in more detail in Finlayson and Kaltenbach (2006). Mean spike amplitude was then calculated from a sample of at least 8 successive spikes recorded from each unit by measuring the peak-to-peak voltage. Spike waveforms were averaged primarily to ensure that for comparisons of spikes, accurate representative waveforms from each unit were used. Spikes were carefully aligned (manually) so that all peaks were in register before averaging. This is possible, but very cumbersome with hundreds of spikes. The averages of 8 spikes were good representations of individual spikes (based on comparisons of average waveforms to individual spikes (Finlayson and Kaltenbach, 2006). The increase in the signal-to-noise ratio was an added benefit of averaging. Consistency in the spike waveform and amplitude confirmed that a given spike train represented only one neuron. The mean peak-to-peak voltage of the entire population of neurons within each treatment group (exposed and control) was then computed. In a similar manner, using the same traces used to measure spike amplitude, the mean duration of each of the three major peaks in the spike waveform were measured for each unit. The measures were made at the zero crossings for each peak. The mean duration of each peak was then calculated for all units in each of the treatment groups. Lastly, the incidence of burst events over the entire period spanned by the 10 traces was determined by counting the number of clusters of two or more spikes in which the interspike intervals were separated by intervals of 10 ms or less. From these counts, the number of bursts/s for each unit was determined and the mean number of bursts for all units within each of the treatment groups was calculated.
All summary data are presented as means ± standard error (S.E.). Multiunit activity in control and exposed animals was compared using a 2-way ANOVA for averaged activity as a function of the distance along the medial-lateral axis of the DCN. Comparisons of spike amplitudes, duration of spike waveform peaks, spontaneous discharge rates of all cells and those with type III frequency responses, percentage of units firing in bursts and thresholds at the characteristic frequency (CF) based on frequency responses between control and exposed animals were made with the Student t-test and Bartlett’s test for unequal variances. The t-test was also used to compare waveform shapes and bursting activity between control and exposed animals. The level of significance was set at 0.05.
RESULTS
Multiunit recordings, sufficient to map spontaneous activity in 3 rows along the medial-lateral (tonotopic) axis of the DCN (activity profiles), were successfully obtained from 25 animals, including 15 exposed and 10 controls. Of these, 23 animals yielded data that met the criteria for inclusion in the final data analysis. The two animals that were excluded (one exposed and one control) displayed very abnormal multiunit activity levels that fell outside 1.5 standard deviations of the mean values that are typical of exposed and control animals, as published previously (see methods). Inclusion of these animals in the analysis resulted in an unacceptably high level of skewness in the data distributions, which increased variances and greatly reduced the statistical differences between levels of activity in exposed and control animals. Excluding these outliers, we were able to obtain a sample of 91 well isolated single units on which to compare spontaneous discharge rates in control (36 units) and exposed (55 units) animals. Acoustic response properties (PST histogram and/or frequency response patterns) were obtained in 39 units in exposed animals and 18 units in controls.
Multiunit recordings
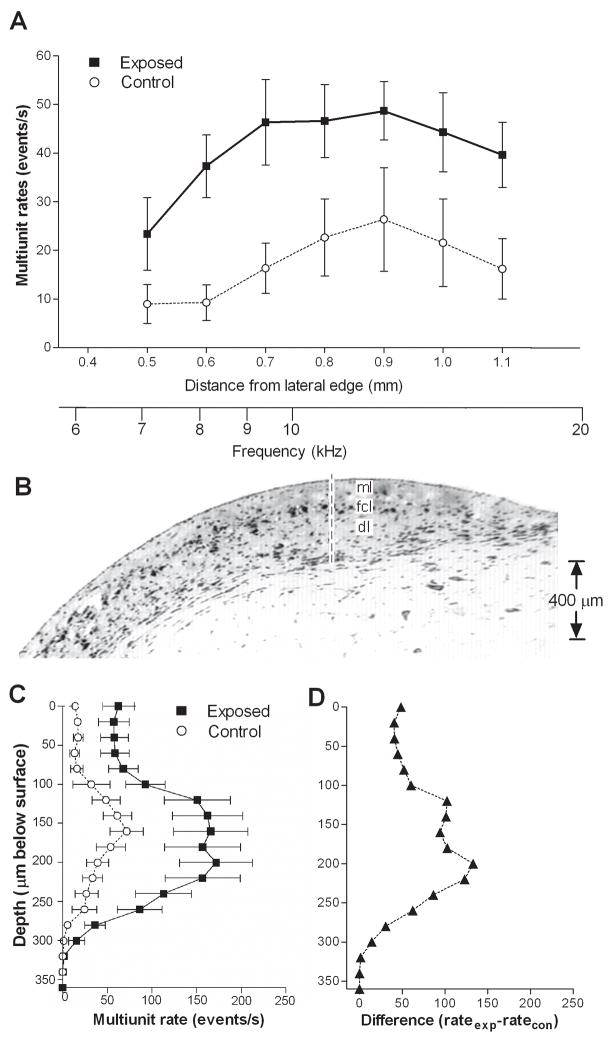
An increase in multiunit spontaneous activity in exposed animals is shown in Fig. 1A and Table I, comparing mean rates (averaged across the 3 rows of recording sites) vs. distance at loci between 0.5 to 1.1 mm from the lateral edge of the DCN. Mean activity in exposed animals was higher than that measured in control animals at all locations between 0.6 and 1.1 mm. Whereas control rates in this range consistently averaged less than 27 events/s, the mean rates measured in exposed animals over this range were consistently above 35 events/s. When data from all sites along the medial-lateral axis were pooled, activity over the full range of loci averaged 17.4 events/s in control animals and 40.9 events/s in exposed animals. This difference represents an increase of activity of 134 % and was highly significant (p < 0.0001).
Figure 1.
Comparison of multiunit spontaneous activity profiles in exposed and control animals. A. Activity profile for the DCN surface. Each point represents the group mean (±S.E.) of rates measured in the three rows of recordings sites. Rates in exposed animals were significantly higher than control values. Distances specified on the abscissa are relative to the lateral edge of the DCN. The activity profile along a vertical penetration through the DCN (B) demonstrates that multiunit spontaneous activity was highest at depths corresponding to the fusiform cell layer (FCL), as shown in panel C. When the data were plotted as a difference, relative to controls, (D), it can be seen that the largest increase in spontaneous activity was also observed at these depths. The exposure sound was a 10 kHz tone at a level of 115 dB SPL for 4 hrs. Spontaneous activity was measured 5–6 days following the exposure.
Table I.
Changes in the properties of single and multiunit recordings in the DCN following intense sound exposure
| Spike Amplitude | MU Events/s | All SU Spikes/s | Type III SU Spikes/s | |
|---|---|---|---|---|
| Control | 331 ± 29 (54) | 17.4 | 8.9 ± 1.6 (36) | 6.8 ± 1.7 (12) |
| Exposed | 422 ± 46 (71) | 40.9 | 15.9 ± 2.5 (55) | 18.8 ± 3.2 (17) |
| % increase Exposed/Control | 28% | 134% | 83% | 176% |
| Statistics | t-test; ns, p = 0.12 | p< 0.0001 | t-test; **, p = 0.038 | t-test; **, p = 0.007 |
MU: mean multiunit spontaneous activity; SU: Single unit spontaneous discharge rates. All SU refers to the entire sample of single units. Type III SUs refers to units displaying type III response patterns.
Multiunit spontaneous activity is examined as a function of depth in Fig. 1B–D. As can be seen, mean activity is increased at all depths, but especially in the intermediate range between 100 and 250 μm below the DCN surface (Fig. 1C–D). In this range, the increase in activity in exposed animals was much stronger than at shallower and deeper levels. Post-hoc analysis of the data revealed that the intermediate depth was focused on the fusiform cell layer and shallow level of the deep layer (Fig. 1B).
Single Unit recordings
Spike waveforms
Since deafferentation of sensory systems has been shown to trigger plasticity in voltage-gated channels (Devor, 2006), it was of interest to examine how the proportions of units with different waveforms might be affected in exposed animals. We focused first on the properties of simple spikes in single unit recordings, since a previous study indicated that simple spikes in the hamster DCN fall into two distinct categories. As detailed in the methods section of this paper, these include M-shaped and W-shaped spikes based on the configuration of the first three peaks in their waveforms (Finlayson and Kaltenbach, 2006). This differentiation is important because W-shaped waveforms were found in a previous study to be distributed almost exclusively in the fusiform cell layer (Finlayson and Kaltenbach, 2006). We also examined the incidences of units that exhibited complex spikes, characterized by bursts of spikes occurring close together. These will be described in a separate section (see section titled ‘Bursting activity’ below).
Simple spikes in both groups of animals displayed either M- or W-shaped waveforms (see methods). No units were observed with mixtures of both waveforms, and waveforms were found to be constant with changes in electrode position, despite the fact that changes in the peak-to-peak amplitude of the waveforms were usually apparent as the electrode was moved to deeper levels. In control animals there was a slightly higher occurrence of M-shaped spikes (51.4%) than W-shaped spikes (48.6%). Exposed animals showed a much higher percentage of M-shaped (72.7%) than W-shaped (27.3%) spikes. This change was statistically significant (Fisher’s exact test, 2-tailed, p=0.0058).
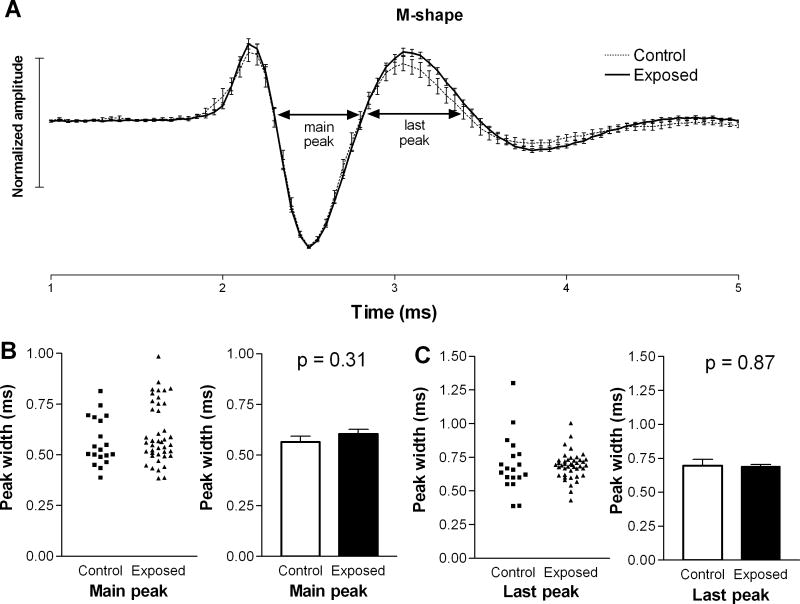
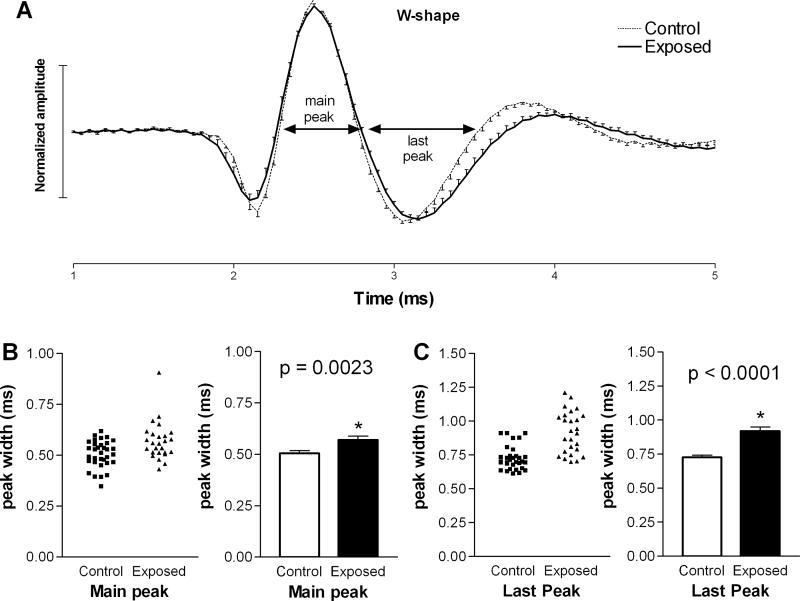
We also compared the microstructures of each of the two waveform types in the two animal groups. The average waveforms of units with M-shaped spikes were almost indistinguishable in control and exposed animals (Fig. 2A), and the durations of each peak at zero crossings were not statistically longer in units from exposed animals compared to those in controls (Fig. 2B, C). A larger difference was observed in the average waveform of units with W-shaped spikes in exposed animals compared to those in controls (Fig. 3A). This difference was apparent as a prolongation of the second (main) and last (third) peaks in the spike waveform. Specifically, the average duration of the second peak increased from 0.51 ± 0.01 ms in controls to 0.57 ± 0.02 ms in exposed animals (Fig. 3B), and the last peak increased from 0.73 ± 0.02 ms in control animals to 0.92 ± 0.03 ms in exposed animals (Fig. 3C). These increases were statistically significant (p=0.003; p< 0.0001, respectively) and suggest that intense sound exposure may have caused changes in the biophysical properties of neurons in the DCN fusiform cell layer.
Figure 2.
Comparison of M-shaped spike waveforms in the DCNs of exposed and control animals. The mean spike waveform (± S.E.), normalized by dividing all amplitude values by the absolute values of the maximum peaks, of M-shaped spikes in exposed animals were not found to be significantly different from those in controls. Although there was a small increase in the average amplitude of the last peak following sound exposure (A), this increase was not statistically significant. The durations of individual peaks in the M-shaped spike waveforms were also not statistically different in control and exposed animals (B, C).
Figure 3.
Examination of W-shaped spike waveforms in exposed and control animals. Panel A compares the mean waveforms from the two groups, normalized as in Fig. 2. Note that the durations of the main and last major peaks in the waveform (B, C) were significantly increased in units from exposed animals compared to those from controls.
Spike amplitudes
Amplitudes of spikes in single unit recordings were examined to determine if an increase in spike amplitude from cells in exposed animals could account for all or part of the increased rates from exposed animals observed in multiunit recordings. The average spike amplitude from exposed animals was 422 ± 46 μV, which was larger than 330 ± 29 μV for cells in control animals. However, this increase was not statistically significant (p=0.12; Table I). Recording electrodes had similar properties in both groups, with a mean impedance of 21 MΩ in each group.
Spontaneous discharge rates
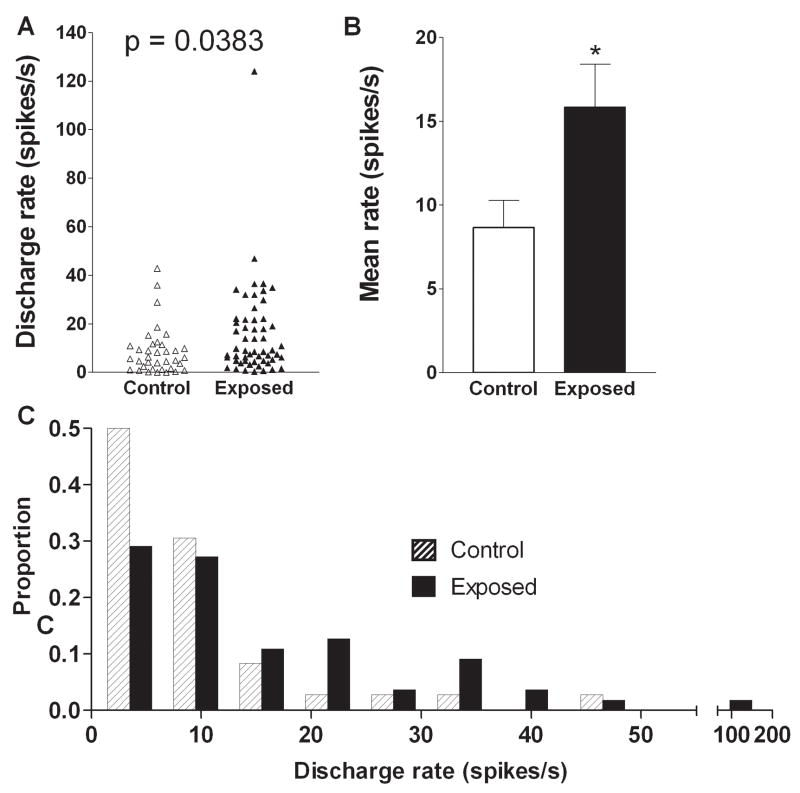
The elevated level of multiunit activity in exposed animals was paralleled by elevated spontaneous activity of single units. The mean single unit spontaneous rate in control animals was 8.7 ± 1.6 spikes/s, while that in exposed animals was 15.9 ± 2.5 spikes/s (Fig. 4A, B, Table I). Thus, previous sound exposure raised the mean spontaneous rate by 83% above control levels, and this increase was statistically significant (p = 0.038).
Figure 4.
Effects of intense tone exposure on single unit spontaneous discharge rate. A. Mean spontaneous rates were significantly higher in exposed animals relative to those in controls. The scatter plot in panel A and the bar graph in panel B show the distributions of units with different rates and the difference in mean rates in the two animal groups, respectively. C. Change in percent of the total number of units as a function of discharge rate. The data show an increase in the proportion of units with high discharge rates (≥ 20 spikes/s) and a reduction in the percentage of units with low discharge rates (≤ 12 spikes/s) in exposed animals.
In order to determine whether this hyperactivity in exposed animals reflected a general increase in activity among neurons spanning the full range of spontaneous rates, or instead, resulted from an increase in the proportion of cells having only high rates of activity, we plotted the proportion of cells from exposed and control animals grouped in different rate categories as shown in Fig. 4C. As can be seen, following sound exposure, more cells had firing rates greater than 20 spikes/s and fewer had rates between 0 and 10 spikes/s. Thus, the increase in mean discharge rates for single units in exposed animals was associated with an increase in the proportion of units with high rates and a decrease in the proportion of units with low rates.
Bursting activity
Bursting activity refers to the firing of a single unit in groups of 2 or more spikes in close temporal proximity. We define close proximity as interspike intervals of 10 ms or less. Examples of non-bursting and bursting activity are shown in Fig. 5. In our analysis, we differentiated three categories of spikes, including simple spikes (Fig. 5A), which occurred singly, and two forms of bursting activity, including spike couplets (Fig. 5B), consisting of spikes occurring in pairs, and spike runs (Fig. 5C), consisting of close successions of three or more spikes. Note that no units displayed exclusively bursting activity; spikes occurring in bursts always alternated with simple spikes. Cells with W-shaped spikes were more likely to exhibit bursting activity (75% of) than M-shaped units (43% of) and this was significant (chi-square =9.73, DF=2, p = 0.0077). However, exposed and control animals did not differ in the proportions of units with W and M shaped spikes showing bursting activity.
Figure 5.

Examples of spike trains of DCN neurons from exposed animals. A. Simple spikes (non-bursting activity). These occurred in regular (upper trace) and irregular (lower trace) discharge patterns. B. Spike bursts in the form of couplets, characterized by spike pairs. Each couplet is marked by a horizontal bar above the trace. C. Spike bursts in the form of runs. Most runs consisted of clusters of 3–5 spikes, but in some animals barrages of more than 10 spikes were sometimes observed (bottom trace). Interspike intervals in couplets and runs were ≤ 10 ms. Horizontal scale bars = 10 ms; vertical scale bar = 100 μV in A, and 50 μV in B and C.
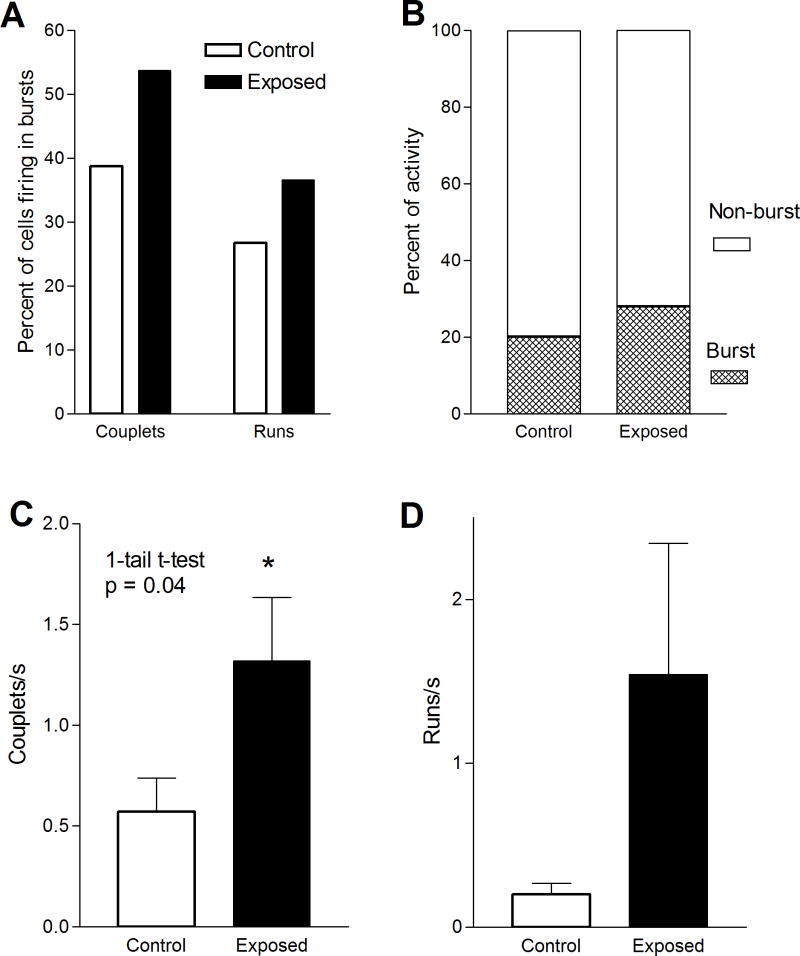
We found several lines of evidence for an increase in bursting activity in tone-exposed animals. First, exposed animals showed an increase in the proportion of units displaying spike bursts. In exposed animals 56% (31/55) of units displayed bursting activity, compared with 47% (17/36) in controls (Fig. 6A). This reflected increases in both couplets and bursts. The proportion of units with couplets increased from 38% in controls to 53% in exposed animals, while those with runs increased from 25% to 37%. Second, the percent of spikes occurring in bursts of activity increased. That is, 28.2% of spikes occurred in the form of couplets or runs in exposed animals, compared to only 20.3% in controls (Fig. 6B). And third, there was an increase in the rate of bursts in exposed animals (Fig. 6C–D). Most of this increase was due to an increase in the incidence of spike couplets, which averaged 0.57 couplets/s in controls and 1.32 couplets/s in exposed animals (Fig. 6C). This increase was statistically significant (p = 0.04). Spike runs also increased slightly, from 0.20 runs/s in controls to 1.54 runs/s in exposed animals (Fig. 6D), although this difference was not statistically significant. This difference was due to units that had rates of over 2 runs/s, which were only observed in exposed animals.
Figure 6.
Intense tone exposure-induced changes in bursting activity of DCN units. The figure compares the percentage of units with couplets and runs (A), percent of spikes in bursts B), couplet rate (C) and run rate (D) in exposed and control animals. Note that the increase in units with high rate activity and the burst rate observed in exposed animals was paralleled by increases in the rates of couplets and runs, although only the increase in couplet rate was statistically significant. Overall, the results are indicative of an increase in bursting activity in the DCN of exposed animals.
To determine the relative weight of the contributions of increased bursting vs. non bursting activity to the overall increase in spontaneous discharge rate in exposed animals reported above (see previous section ‘Spontaneous discharge rates’) we computed the proportion of the increase in mean discharge rate that could be accounted for by the increase in the mean discharge rates of spikes occurring in couplets and runs, based on the analysis of spike trains in the digitized traces. This proportion was obtained using the following ratio:
where βe and βc are the mean number of spikes in bursts per second in exposed and control animals, respectively, and σe and σc are the mean spontaneous discharge rates of all spikes combined (those in bursts and those not in bursts) in exposed and control animals, respectively. Based on this ratio, it was determined that 51.3% of the increase in mean spontaneous discharge rate in exposed animals was accounted for by an increase in the discharge rates of spikes present in bursts. The remainder of the increase (48.7%) occurred as simple spikes. Thus, spikes in bursts and simple spikes contributed approximately equally to the overall increase in mean discharge rate. Note, however, that 20.3% of spikes in control animals occurred in bursts, while in exposed animals 28.2% of spikes occurred in bursts. Thus, although spikes in bursts were a relatively minor share of the total spike population in exposed animals, their contribution to the overall increase in mean discharge rate was about equal to that of simple spikes.
Number of units/penetration
In an effort to determine whether intense sound exposure may have caused an increase in the numbers of spontaneously active neurons, we compared the mean numbers of spontaneously active units per electrode penetration through the DCN in the two animal groups. This comparison was only marginally supportive of a change in the numbers of spontaneously active neurons. The average number of cells recorded per penetration was 1.03 ± 0.12 in controls and 1.21 ± 0.12 in exposed. This difference, however, was not significant (p=0.26). The mean number of recorded units/animal was 9 in the control group and 8.3 in the exposed group.
Frequency responses, thresholds and PSTH response patterns
Unit thresholds and frequency response patterns were obtained from 18 units in control animals and 39 units in exposed animals. Frequency responses of single units recorded from control animals were predominantly (12/18) type III (Fig. 7A), one gave a type V response, and 5 showed poor or no determinate responses. Although many units (17/39) recorded in exposed animals exhibited type III frequency responses, one unit had a type V response, and a larger proportion (21/39) of units exhibited poor or no responses to tones. An example of a unit with a type III response is shown in Fig. 7B.
Figure 7.
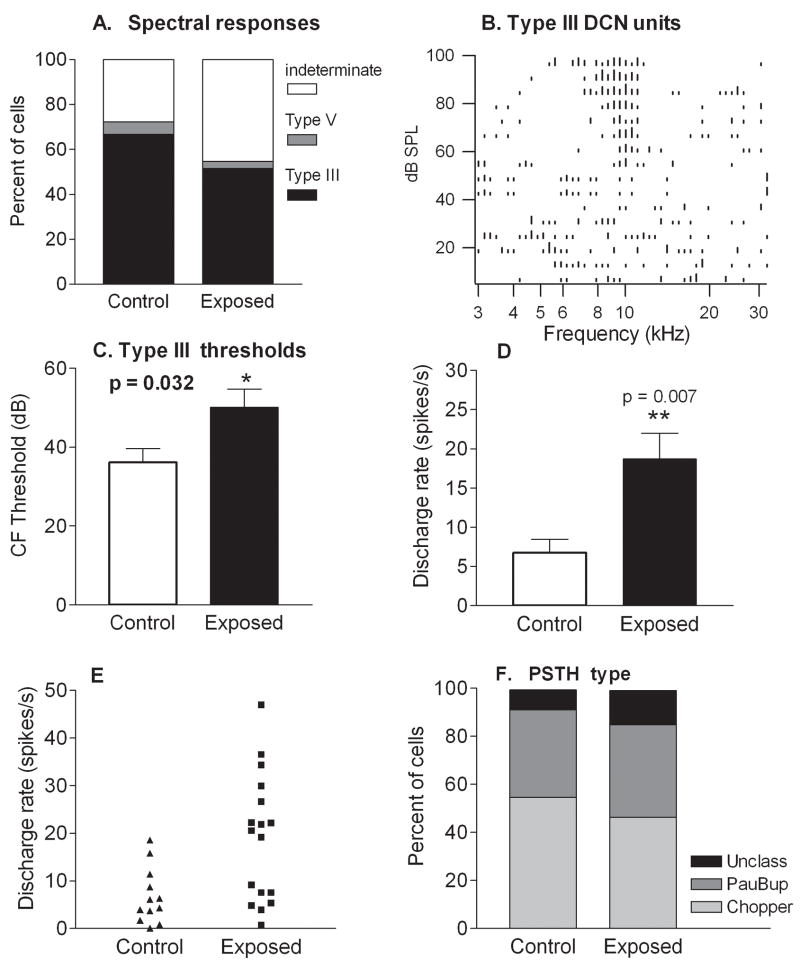
Comparison of response properties and spontaneous rates in control and tone-exposed animals. A. Proportions of unit populations in control and exposed animals with different frequency response patterns. The predominant frequency pattern was type III in both control and exposed animals. A decrease in the percentage of units showing type III frequency responses in exposed animals was accounted for by an increase in the percentage of units in which the frequency response type was indeterminate, due to weak or absent responses. B. An example of a type III frequency (spectral) response area from an exposed animal. This response type is characterized by a sharply tuned excitatory area flanked by side band (lateral) inhibition. C. Type III units in exposed animals displayed higher thresholds than those in controls. Mean thresholds were 14 dB higher in exposed animals than in controls, although the true magnitude of the threshold difference is underestimated due to the increased number of units in which thresholds were indeterminate. D. Mean spontaneous discharge rates of type III units were distinctly elevated in exposed animals relative to controls. E. This increase was due to an elevation in the number of type III units with high activity (rates above 20 spikes/s). F. Proportions of unit populations in control and exposed animals yielding different PST histogram patterns. Similar proportions of DCN cells exhibited chopper and buildup temporal firing patterns to sound (PSTH response type) in control and exposed animals.
Thresholds measured from type III frequency responses were elevated in the exposed animals relative to controls. The average characteristic frequency (CF) threshold was 14 dB greater in exposed animals than in control animals (p=0.03; Fig. 7C). However, this is very likely an underestimate of the threshold changes, as measures of thresholds could not be obtained from units whose responses to tones were very weak or absent.
Examination of the data in Fig. 7D revealed an increase in mean rates of units with type III response patterns in exposed animals (Fig. 7D). The average rate for this unit type increased from 6.8 ± 1.7 spikes/s (n=12) in controls to 18.8 ± 3.2 spikes/s (n=17) in exposed animals. No type III cells in controls had spontaneous discharge rates greater than 20 spikes/s, whereas, after exposure, over half of type III cells exhibited spontaneous rates greater than 20 spikes/s (Fig. 7E), and were the majority of units (9/17) with these higher rates. Bursting activity was observed in many units with type III frequency responses (Control, 6/12; Exposed 11/17; Fig. 8). These findings suggest that type III units were major contributors to the increases in mean firing rate observed across the general population of units in exposed animals.
Figure 8.
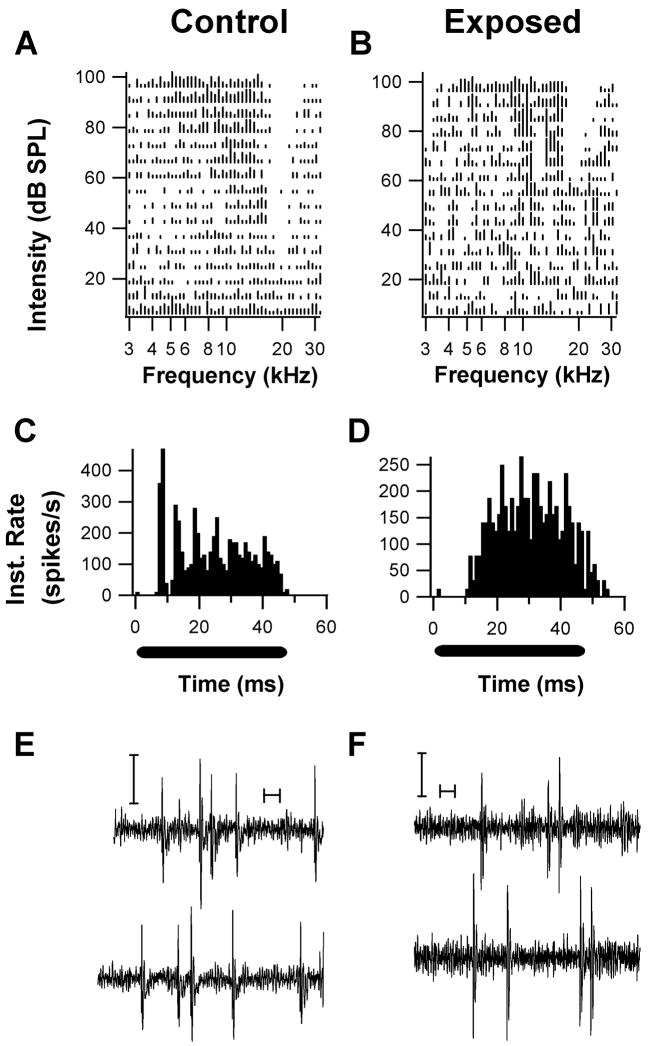
Examples of units with type III spectral response patterns and their associated temporal response patterns. (A. Unit from a control animal; B, Unit from an exposed animal). The response areas both show a V-shaped excitatory area with a lateral inhibitory side band in which spontaneous activity was inhibited. One unit exhibited a chopper response pattern (C) and W-shaped spikes (E), while the other unit exhibited a buildup pattern (D) and W-shaped spikes. Both PST patterns were obtained in response to a CF tone burst 20 –30 dB above threshold. (Y-axis represents Instantaneous rate; black bars under PSTHs indicate tonal stimulus period). These response patterns are typical of DCN fusiform cells. Both units exhibited couplet (pairs of spikes with interspike intervals of less than 10 ms) firing activity during recordings of spontaneous activity (E, F, calibration bars are 10 ms, and 50 μV).
Peristimulus time histograms (PSTH) were collected from 11 type III units from control animals and 14 type III units from exposed animals. Units displayed similar temporal response patterns, typically “Pauser-buildup” (controls: 6/11; exposed: 6/14) and “Wide Chopper” (control: 4/11; exposed: 6/14), and in similar proportions in control and exposed animals (Fig. 7F). Figure 8 shows two examples of units with type III response patterns (Fig. 8A, B) along with their associated temporal response patterns. One of these units, which was obtained from a control animal, yielded a chopper PSTH pattern (Fig. 8C), while the other, which was obtained from an exposed animal, yielded a buildup pattern (Fig. 8D). Both units showed simple spiking activity (W-shaped waveforms) alternating with bursts consisting of spike couplets.
DISCUSSION
These results demonstrate that increased multiunit activity induced by intense sound exposure reflects increases in spontaneous discharge rate of single units. Included in this general increase in activity was an increase in non-bursting as well as bursting activity. Both types of increases in activity have been reported to occur in the inferior colliculus and auditory cortex following noise exposure (Norena and Eggermont, 2003; Bauer et al., 2008) and/or salicylate treatment (Chen and Jastreboff, 1995). In the present study, although we found no change in the interspike intervals in bursts, approximately half of the increase in discharge rate was accounted for by spikes present in bursts. This is all the more remarkable, given that spikes in bursts were a relatively small percentage of the total number of spikes in both exposed (28%) and control (20%) animals. Chen and Jastreboff (1995) found increases in spontaneous rate of rat inferior colliculus neurons after salicylate treatment. Later data from their group suggested that behavioral evidence for tinnitus correlated better with bursting activity than with the general increase in spontaneous activity (Kwon et al., 1999). Bauer et al. (2008) reported that the 1000 Hz tinnitus in chinchillas following sound exposure correlated with the firing rate of spikes in bursts. Although we have previously reported that behavioral evidence for tinnitus correlates moderately well with increases in spontaneous activity (Kaltenbach et al., 2004), it is possible that an even stronger correlation would be found if the relationship between tinnitus and the increase in activity were limited to spikes in bursts. Confirmation of this prediction awaits further study.
Interestingly, the magnitude of the increase in single unit discharge rate (83%) was smaller than the magnitude of the increase in multiunit activity observed in the present study (134%). This indicates that one or more other factors must contribute to the increase in multiunit activity. One possible other factor that would not be mirrored in single unit firing rates is an increase in the number of spontaneously active units by recruitment of activity from units that are normally silent. Although there was a slight increase in the number of units/penetration in exposed animals, the difference was not statistically significant. Thus, the data seem only marginally supportive of an increase in the size of the spontaneously active neural population. Another possibility is that the higher multiunit activity after exposure reflects not only an increase in mean discharge rate of neurons but also an increase in the amplitude of single unit spikes. There was little evidence to support this explanation, as mean single unit spike amplitudes were only slightly higher in exposed animals than in controls, but again, the difference was not statistically significant.
Other explanations of the higher level of multiunit activity compared to the increase in single unit discharge rates include an increase in glial activation and/or and increase in neural synchrony. Although we cannot yet comment on the role of glial activity in our measures of multiunit activity, increases in neural synchrony have been reported to occur in the auditory cortex following noise exposure (Norena and Eggermont, 2006; Seki and Eggermont, 2003). Elevations in neural discharge rates and in the incidence of spike bursts would be expected to increase the number of component spikes contributing to a multiunit waveform and the number of spikes from different neurons falling into the same time bin, increasing coincidence of spike events. Constructive interference among coincident spikes produces an enhancement of the summated multiunit waveform amplitudes. Because the summations of spikes from different units occur more or less in random phase, the summated waveforms are likely to be more complex and to display higher amplitudes than are characteristic of the spikes of the contributing units. The resulting higher amplitudes of the multiunit waveform may therefore have a multiplicative effect on rate, increasing the number of multiunit potentials reaching the threshold voltage of our measurements.
Comparison with other studies
A few previous studies in other laboratories have examined the effects of intense noise exposure on single unit spontaneous activity in the DCN. Brozoski et al. (2002) compared the mean spontaneous discharge rates of DCN neurons in exposed and control chinchillas. The animals had been exposed to a 4 kHz monaural tone for 1 hour at 80 dB SPL. While ABR thresholds were within normal limits 5 months after exposure, mean spontaneous rates in the ipsilateral DCN were found to be 35% higher than control levels. Shore et al. (2008) compared spontaneous rates of single units in the DCN of control and noise-exposed (broadband noise at 120 dB SPL for 4 hrs) guinea pigs. Neural response threshold shifts in the DCN were between 30 and 40 dB in exposed animals. A 35% increase in single unit spontaneous activity was observed in the DCN one week after exposure. Overall, there seems to be a reasonable degree of correspondence between the results obtained in these studies and those reported here, except that the 35% increases observed in the chinchilla and guinea pig are considerably lower than the 83% increase observed in the present study. Variations in morphology or physiology in the DCN among these species or in effects of sound exposure may be important factors in this difference.
Two other studies which have demonstrated increases in spontaneous activity in the DCN have involved the use of non-electrophysiological measures. Imig and Durham (2005) obtained evidence using the 2-deoxyglucose method for an increase in metabolic activity of the DCN and inferior colliculus (IC) of Long-Evans rats exposed monaurally to intense (114–116 dB SPL) narrow band (15–20 kHz) noise for a period of one hour. The increase was apparent one week after exposure as an elevation of optical density in the low frequency region of the ipsilateral DCN and contralateral IC. The high frequency region showed a decrease of optical density. The increase in the low frequency region was augmented by decortication, suggesting that corticofugal pathways have a suppressive effect on hyperactivity. A study by Wallhauser-Franke et al. (2003) found an increase in c-fos expression in the DCN and in other auditory nuclei (IC and auditory cortex) after intense impulse noise (136–142 dB SPL). The increase was observed 1 and 7 hours after exposure. Unfortunately, comparison with the present study is difficult as data for later postexposure times were not reported. However, electrophysiologically demonstrated increases in spontaneous activity have been observed in the DCN within hours following moderate levels of sound exposure (Kaltenbach et al., 2005). The onset of hyperactivity following more intense exposures requires several days (Kaltenbach et al., 2000). Thus the onset of hyperactivity may be affected by the type of cochlear injury, which would likely be different for impulse noise than for continuous tone exposure.
One other study, published by Ma and Young (2006), compared in vivo spontaneous activity in cats binaurally exposed to an intense narrow band of noise centered at 10 kHz at a level of 105–120 dB SPL for 4 hrs. Single unit spontaneous activity was recorded in the ipsilateral DCN beginning at 28 days after exposure. Although spontaneous activity was higher among units with high CFs than in those with low CFs, the difference was not significant in any of several unit types in the DCN of exposed animals relative to controls. It is not clear why this study yielded such different results from those reported here and by the other investigators referred to above. Ma and Young acknowledged that species differences or differences in methodology may play a role. It is perhaps worthy of note that the study by Ma and Young was conducted in decerebrate animals, whereas all other electrophysiological studies cited above were conducted in anesthetized animals. The DCN in decerebrate animals may adjust to loss of normal peripheral input in a different manner from that of anesthetized animals, in which the inputs to the DCN from higher order auditory and non-auditory structures remain intact (Weedman and Ryugo, 1996; Shore et al., 2000; Itoh et al., 1987; Weinberg and Rustioni, 1987).
Identity of the hyperactive cells
Identification of the cell populations giving rise to hyperactivity is a critical step towards unraveling the mechanisms by which tinnitus-related hyperactivity is induced. This is a difficult task, as our results indicate that the increase in mean rate only partially reflects the emergence of high rate units. Much of the increase is due to a shift in rate distribution within the normal range, with high rate units becoming more common and low rate units becoming a smaller proportion of the rate distribution after tone exposure. Although units with spontaneous rates that lie outside the range of rates observed in control animals were present in our sample of units from exposed animals, their numbers were very limited when the sample as a whole was examined (Fig. 4A). The limited number of high rate units is a challenge to any effort to identify hyperactive neurons by intracellular and juxtacellular labeling methods since the latter methods inherently yield small numbers of successfully labeled cells. For this reason, we sought to obtain clues concerning the identity of hyperactive cell populations by inspection of unit response properties and by examining the laminar distribution of hyperactivity as a function of depth below the DCN surface.
We found that the degree of multiunit hyperactivity in the DCN of exposed animals was greater at intermediate depths than at deeper and shallower levels (Fig. 1C, D). This observation points to neurons in the fusiform cell layer and/or shallow levels of the deep layer as sources of hyperactivity. Among units with the highest rates of spontaneous activity in exposed animals, when a response area was collected, it was of the type III pattern, characterized by an excitatory peak flanked by side bands of inhibition (Fig. 7C and Fig. 8). Also, as shown in Fig. 7C (middle panel), the proportion of units with elevated rates of spontaneous activity was larger when a subpopulation of units having type III response areas was examined. All type III units in control animals had rates below 20 spikes/s, while in exposed animals most had rates above 20 spikes/s. This suggests that type III units may be a major source of hyperactivity in the DCN. The available evidence suggests that type III response patterns are derived mainly from fusiform cells, although the possibility that type III patterns could arise from cell types in exposed animals that normally have other response properties cannot be ruled out. The inference that fusiform cells are a major source of DCN hyperactivity after noise exposure is also supported by two other works. The studies of Shore et al. (2008) in guinea pigs and by Brozoski et al. (2002) in chinchillas, showed that units displaying increased spontaneous discharge rates after intense noise exposure, exhibited pauser/buildup PST histogram patterns and/or non-monotonic rate/level functions. These characteristics are generally considered to be typical of fusiform cells (Godfrey et al., 1975a, b; Rhode & Smith, 1986; Stabler et al., 1996; Young, 1980).
The increase in bursting activity in our exposed animal group might be regarded as evidence for a non-fusiform cell origin of hyperactivity. Indeed, 47% of our units in controls and 55% of units in exposed animals, including units with elevated spontaneous activity, displayed bursting activity. Some in vitro studies have suggested an association of bursting activity in the DCN with cartwheel cells rather than fusiform cells, which tend to exhibit more regular firing (Manis, 1990; Oertel and Wu, 1989; Manis et al., 1993; Zhang and Oertel, 1993). This is consistent with the results of Chang et al. (2002) based on their in vitro recordings of rat DCN following intense noise exposure. However, the association of bursting activity with cartwheel cells based on in vivo recordings, is less well established. While some have asserted that cartwheel cells may fire in bursts in vivo, bursting activity has also been observed occasionally in cells at depths outside the range in which cartwheel cells are normally distributed in the DCN (Parham and Kim, 1995). Bursting activity has also been observed occasionally on cells that have the physiological characteristics of fusiform cells (Ding et al., 1999; Pal et al., 2003; Rhode and Smith, 1986). In our sample, bursting activity was often observed in units displaying type III frequency response areas (Fig. 8), a property that is not known to be generated by cartwheel cells (Portfors and Roberts, 2007; Parham and Kim, 1995; Parham et al., 2000). Finally, in a preliminary study using juxtacellular labeling, we observed bursting activity originating from a neuron that was found to have the morphological characteristics of a fusiform cell (Finlayson and Kaltenbach, 2005). Based on these considerations, it seems likely that the increase in bursting activity may be indicative of an increase in bursting of fusiform cells. However, the possibility that cartwheel cells might also contribute to the increases in activity on week after exposure cannot be ruled out.
Mechanisms underlying induction of hyperactivity
Previously, discussions of mechanisms of tinnitus-related hyperactivity in the DCN have focused on the synaptic elements underlying the perturbations in the balance of excitatory and inhibitory inputs. A loss of inhibition in the DCN has been observed following unilateral cochlear ablation (Potashner et al., 2000; Suneja et al., 1998; Asako et al., 2005). There is evidence for a down-regulation of the inhibitory neurotransmitter, glycine, in the DCN after cochlear ablation (Suneja et al., 1998; Potashner et al., 2000) and a decrease in glycine receptor expression in the DCN after noise exposure (Asako et al., 2005). At the same time, complex time-dependent up-regulation of aspartate release has been observed in the DCN after intense sound exposure (Muly, et al., 2004), suggestive of an increase in excitation. Certain other excitatory neurotransmitters (acetylcholine and serotonin) or their receptors (glutamate), have also been shown to be up-regulated following noise exposure (Cransac, et al., 1998; Chang et al., 2002; Kaltenbach and Zhang, 2007) or cochlear ablation (Jin, et al., 2006; Suneja et al., 2000; Illing et al., 2005). Overall, the changes suggest that there may be a net increase in synaptic excitation compared to inhibition in the DCN after noise exposure and other related damage to the auditory periphery (see review by Kaltenbach, 2007).
One or more ion channel pathologies may also be involved in the emergence of DCN hyperactivity. Numerous studies in other sensory systems have shown that peripheral injury causes changes in ion conductances of first and second order neurons (Waxman, 1999; Abdulla and Smith, 2001a, 2001b; Liu et al., 2000; Kim et al., 2002; Hains et al., 2003; Chaplan et al., 2003). Francis and Manis (2000) invoked alterations in ion conductances in cochlear nucleus neurons to explain changes in input resistance and spike waveforms of ventral cochlear nucleus neurons after cochlear ablation. In the present study, the increase in the proportion of cells with M-shaped spike waveforms, and the increase in the duration of W-shaped spike waveforms in exposed animals are consistent with changes in ionic channels. Increases in spike duration have been shown to result when potassium channels are blocked (Zhang, et al., 2004). Down-regulation of the A-type potassium (IA) conductance, a prominent conductance in fusiform cells (Manis, 1990; Serodio and Rudy, 1998; Kanold and Manis, 1999; Pal, et al, 2003) is associated with hypersensitivity in pain (Hu et al., 2006) and increased bursting in pyramidal neurons in the electrosensory lateral line lobe (Ellis, et al., 2007), which are likely homologous to DCN fusiform cells (Mugnaini and Maler, 1993). Persistent sodium channels (INaP) are exhibited by DCN fusiform cells (Manis et al., 2003) and cause subthreshold membrane potential oscillations that could increase generation of burst firing. Fusiform cells also exhibit hyperpolarization-activated mixed cationic conductances (Ih; Mouse: Pal et al., 2003; Guinea Pig: Manis, 1990). An up-regulation of either Ih or INaP would increase excitability and spontaneous activity. DCN cells also exhibit many other ion channels whose regulation could increase excitability (Kim and Trussel, 2007; Molitor and Manis, 1999; Manis and Molitor, 2003; Ruszsnak et al., 2000). Although, we do not exactly know how excitatory and inhibitory transmission affects the pattern of spiking activity in postsynaptic neurons following noise exposure, it is possible that some of the alterations in synaptic release discussed above, coupled with changes in ionic conductances, could underlie the observed increases in spontaneous activity in the DCN after intense sound exposure.
Acknowledgments
This project was supported by a grant from NIDCD (R01 DC03258).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdulla FA, Smith PA. Axotomy- and autotomy-induced changes in Ca2+ and K+ channel currents of rat dorsal root ganglion neurons. J Neurophysiol. 2001;85:644–58. doi: 10.1152/jn.2001.85.2.644. [DOI] [PubMed] [Google Scholar]
- Abdulla FA, Smith PA. Axotomy- and autotomy-induced changes in the excitability of rat dorsal root ganglion neurons. J Neurophysiol. 2001;85:630–43. doi: 10.1152/jn.2001.85.2.630. [DOI] [PubMed] [Google Scholar]
- Asako M, Holt AG, Griffith RD, Buras ED, Altschuler RA. Deafness-related decreases in glycine-immunoreactive labeling in the rat cochlear nucleus. J Neurosci Res. 2005;81:102–9. doi: 10.1002/jnr.20542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CA, Turner JG, Caspary DM, Myers KS, Brozoski TJ. Tinnitus and inferior colliculus activity in chinchillas related to three distinct patterns of cochlear trauma. J Neurosci Res. 2008;86:2564–78. doi: 10.1002/jnr.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Bauer CA, Caspary DM. Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psychophysical evidence of tinnitus. J Neurosci. 2002;22:2383–90. doi: 10.1523/JNEUROSCI.22-06-02383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Chen K, Kaltenbach JA, Zhang J, Godfrey DA. Effects of acoustic trauma on dorsal cochlear nucleus neuron activity in slices. Hear Res. 2002;164:59–68. doi: 10.1016/s0378-5955(01)00410-5. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Guo HQ, Lee DH, Luo L, Liu C, Kuei C, Velumian AA, Butler MP, Brown SM, Dubin AE. Neuronal hyperpolarization-activated pacemaker channels drive neuropathic pain. J Neurosci. 2003;23:1169–78. doi: 10.1523/JNEUROSCI.23-04-01169.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GD, Jastreboff PJ. Salicylate-induced abnormal activity in the inferior colliculus of rats. Hear Res. 1995;82:158–78. doi: 10.1016/0378-5955(94)00174-o. [DOI] [PubMed] [Google Scholar]
- Cransac H, Cottet-Emard JM, Hellstrom S, Peyrin L. Specific sound-induced noradrenergic and serotonergic activation in central auditory structures. Hear Res. 1998;118:151–6. doi: 10.1016/s0378-5955(98)00031-8. [DOI] [PubMed] [Google Scholar]
- Devor M. Sodium channels and mechanisms of neuropathic pain. J Pain. 2006;7:S3–S12. doi: 10.1016/j.jpain.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Ding J, Benson TE, Voigt HF. Acoustic and current-pulse responses of identified neurons in the dorsal cochlear nucleus of unanesthetized, decerebrate gerbils. J Neurophysiol. 1999;82:3434–57. doi: 10.1152/jn.1999.82.6.3434. [DOI] [PubMed] [Google Scholar]
- Dong S, Mulders WH, Rodger J, Robertson D. Changes in neuronal activity and gene expression in guinea-pig auditory brainstem after unilateral partial hearing loss. Neuroscience. 2009;159:1164–74. doi: 10.1016/j.neuroscience.2009.01.043. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends Neurosci. 2004;27:676–82. doi: 10.1016/j.tins.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Ellis LD, Mehaffey WH, Harvey-Girard E, Turner RW, Maler L, Dunn RJ. SK channels provide a novel mechanism for the control of frequency tuning in electrosensory neurons. J Neurosci. 2007;27:9491–502. doi: 10.1523/JNEUROSCI.1106-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson PG, Kaltenbach JA. Differentiation of simple spike waveforms in the hamster dorsal cochlear nucleus. Brain research. 2006;1069:63–74. doi: 10.1016/j.brainres.2005.10.097. [DOI] [PubMed] [Google Scholar]
- Finlayson P, Kaltenbach J. Identification of Hyperactive Cells in the DCN of a Noise-Induced Tinnitus Model, Using Spike Classification and Juxtacellular Labeling. Assoc Res Otolaryngol. 2005 Abstract 654. [Google Scholar]
- Francis HW, Manis PB. Effects of deafferentation on the electrophysiology of ventral cochlear nucleus neurons. Hear Res. 2000;149:91–105. doi: 10.1016/s0378-5955(00)00165-9. [DOI] [PubMed] [Google Scholar]
- Godfrey DA, Kiang NY, Norris BE. Single unit activity in the dorsal cochlear nucleus of the cat. J Comp Neurol. 1975;162:269–84. doi: 10.1002/cne.901620207. [DOI] [PubMed] [Google Scholar]
- Godfrey DA, Kiang NY, Norris BE. Single unit activity in the posteroventral cochlear nucleus of the cat. J Comp Neurol. 1975;162:247–68. doi: 10.1002/cne.901620206. [DOI] [PubMed] [Google Scholar]
- Hains BC, Klein JP, Saab CY, Craner MJ, Black JA, Waxman SG. Upregulation of sodium channel Nav1.3 and functional involvement in neuronal hyperexcitability associated with central neuropathic pain after spinal cord injury. J Neurosci. 2003;23:8881–92. doi: 10.1523/JNEUROSCI.23-26-08881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HJ, Carrasquillo Y, Karim F, Jung WE, Nerbonne JM, Schwarz TL, Gereau RWT. The kv4.2 potassium channel subunit is required for pain plasticity. Neuron. 2006;50:89–100. doi: 10.1016/j.neuron.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Illing RB, Kraus KS, Meidinger MA. Reconnecting neuronal networks in the auditory brainstem following unilateral deafening. Hear Res. 2005;206:185–99. doi: 10.1016/j.heares.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Imig TJ, Durham D. Effect of unilateral noise exposure on the tonotopic distribution of spontaneous activity in the cochlear nucleus and inferior colliculus in the cortically intact and decorticate rat. J Comp Neurol. 2005;490:391–413. doi: 10.1002/cne.20674. [DOI] [PubMed] [Google Scholar]
- Itoh K, Kamiya H, Mitani A, Yasui Y, Takada M, Mizuno N. Direct projectsions from the dorsal column nuclei and the spinal trigeminal nuclei to the cochlear nuclei in the cat. Brain Res. 1987;400:145–150. doi: 10.1016/0006-8993(87)90662-7. [DOI] [PubMed] [Google Scholar]
- Jin YM, Godfrey DA. Effects of cochlear ablation on muscarinic acetylcholine receptor binding in the rat cochlear nucleus. J Neurosci Res. 2006;83:157–66. doi: 10.1002/jnr.20706. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, McCaslin DL. Increases in spontaneous activity of dorsal cochlear nucleus neurons following intense sound exposure. Auditory Neuroscience. 1996;3:57–78. [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach JA, Godfrey DA, Neumann JB, McCaslin DL, Afman CE, Zhang J. Changes in spontaneous neural activity in the dorsal cochlear nucleus following exposure to intense sound: relation to threshold shift. Hear Res. 1998;124:78–84. doi: 10.1016/s0378-5955(98)00119-1. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Afman CE. Hyperactivity in the dorsal cochlear nucleus after intense sound exposure and its resemblance to tone-evoked activity: a physiological model for tinnitus. Hear Res. 2000;140:165–72. doi: 10.1016/s0378-5955(99)00197-5. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Zhang J, Afman CE. Plasticity of spontaneous neural activity in the dorsal cochlear nucleus after intense sound exposure. Hear Res. 2000;147:282–92. doi: 10.1016/s0378-5955(00)00138-6. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Falzarno PR. DCN hyperactivity induced by previous intense sound exposure: origin with respect to cell layer. Abstracts of the Association for Research in Otolaryngology. 2002;25:208. [Google Scholar]
- Kaltenbach JA, Rachel JD, Mathog TA, Zhang J, Falzarano PR, Lewandowski M. Cisplatin-induced hyperactivity in the dorsal cochlear nucleus and its relation to outer hair cell loss: relevance to tinnitus. J Neurophysiol. 2002;88:699–714. doi: 10.1152/jn.2002.88.2.699. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Zacharek MA, Zhang J, Frederick S. Activity in the dorsal cochlear nucleus of hamsters previously tested for tinnitus following intense tone exposure. Neurosci Lett. 2004;355:121–5. doi: 10.1016/j.neulet.2003.10.038. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Zhang J, Finlayson P. Tinnitus as a plastic phenomenon and its possible neural underpinnings in the dorsal cochlear nucleus. Hear Res. 2005;206:200–26. doi: 10.1016/j.heares.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Zhang J. Intense sound-induced plasticity in the dorsal cochlear nucleus of rats: Evidence for cholinergic receptor upregulation. Hear Res. 2007;226:232–43. doi: 10.1016/j.heares.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA. The dorsal cochlear nucleus as a contributor to tinnitus: mechanisms underlying the induction of hyperactivity. Prog Brain Res. 2007;166:89–106. doi: 10.1016/S0079-6123(07)66009-9. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Manis PB. Transient potassium currents regulate the discharge patterns of dorsal cochlear nucleus pyramidal cells. J Neurosci. 1999;19:2195–208. doi: 10.1523/JNEUROSCI.19-06-02195.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Morest DK, Bohne BA. Degeneration of axons in the brainstem of the chinchilla after auditory overstimulation. Hear Res. 1997;103:169–91. doi: 10.1016/s0378-5955(96)00173-6. [DOI] [PubMed] [Google Scholar]
- Kim DS, Choi JO, Rim HD, Cho HJ. Downregulation of voltage-gated potassium channel alpha gene expression in dorsal root ganglia following chronic constriction injury of the rat sciatic nerve. Brain Res Mol Brain Res. 2002;105:146–52. doi: 10.1016/s0169-328x(02)00388-1. [DOI] [PubMed] [Google Scholar]
- Kim Y, Trussell LO. Ion channels generating complex spikes in cartwheel cells of the dorsal cochlear nucleus. J Neurophysiol. 2007;97:1705–25. doi: 10.1152/jn.00536.2006. [DOI] [PubMed] [Google Scholar]
- Komiya H, Eggermont JJ. Spontaneous firing activity of cortical neurons in adult cats with reorganized tonotopic map following pure-tone trauma. Acta Otolaryngol. 2000;120:750–6. doi: 10.1080/000164800750000298. [DOI] [PubMed] [Google Scholar]
- Kwon O, Jastreboff MM, Hu S, Shi J, Jastreboff PJ. Modification of single unit atcivity related to noise-induced tinnitus in rats. Proc. of the 6th Internat. Tinn. Sem; 1999. pp. 459–462. [Google Scholar]
- Liu CN, Michaelis M, Amir R, Devor M. Spinal nerve injury enhances subthreshold membrane potential oscillations in DRG neurons: relation to neuropathic pain. J Neurophysiol. 2000;84:205–15. doi: 10.1152/jn.2000.84.1.205. [DOI] [PubMed] [Google Scholar]
- Ma WL, Hidaka H, May BJ. Spontaneous activity in the inferior colliculus of CBA/J mice after manipulations that induce tinnitus. Hear Res. 2006;212:9–21. doi: 10.1016/j.heares.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Ma WL, Young ED. Dorsal cochlear nucleus response properties following acoustic trauma: response maps and spontaneous activity. Hear Res. 2006:216–217. 176–88. doi: 10.1016/j.heares.2006.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manis PB. Membrane properties and discharge characteristics of guinea pig dorsal cochlear nucleus neurons studied in vitro. J Neurosci. 1990;10:2338–51. doi: 10.1523/JNEUROSCI.10-07-02338.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manis PB, Spirou GA, Wright DD, Paydar S, Ryugo DK. Physiology and morphology of complex spiking neurons in the guinea pig dorsal cochlear nucleus. J Comp Neurol. 1994;348:261–76. doi: 10.1002/cne.903480208. [DOI] [PubMed] [Google Scholar]
- Manis PB, Molitor SC, Wu H. Subthreshold oscillations generated by TTX-sensitive sodium currents in dorsal cochlear nucleus pyramidal cells. Exp Brain Res. 2003;153:443–51. doi: 10.1007/s00221-003-1639-6. [DOI] [PubMed] [Google Scholar]
- Molitor SC, Manis PB. Voltage-gated Ca2+ conductances in acutely isolated guinea pig dorsal cochlear nucleus neurons. J Neurophysiol. 1999;81:985–98. doi: 10.1152/jn.1999.81.3.985. [DOI] [PubMed] [Google Scholar]
- Molitor SC, Manis PB. Dendritic Ca2+ transients evoked by action potentials in rat dorsal cochlear nucleus pyramidal and cartwheel neurons. J Neurophysiol. 2003;89:2225–37. doi: 10.1152/jn.00709.2002. [DOI] [PubMed] [Google Scholar]
- Mugnaini E, Maler L. Comparison between the fish electrosensory lateral line lobe and the mammalian dorsal cochlear nucleus. J Comp Physiol [A] 1993;173:683– 685. [Google Scholar]
- Muly SM, Gross JS, Potashner SJ. Noise trauma alters D-[3H]aspartate release and AMPA binding in chinchilla cochlear nucleus. J Neurosci Res. 2004;75:585–96. doi: 10.1002/jnr.20011. [DOI] [PubMed] [Google Scholar]
- Norena AJ, Eggermont JJ. Changes in spontaneous neural activity immediately after an acoustic trauma: implications for neural correlates of tinnitus. Hear Res. 2003;183:137–53. doi: 10.1016/s0378-5955(03)00225-9. [DOI] [PubMed] [Google Scholar]
- Norena AJ, Eggermont JJ. Enriched acoustic environment after noise trauma abolishes neural signs of tinnitus. Neuroreport. 2006;17:559–63. doi: 10.1097/00001756-200604240-00001. [DOI] [PubMed] [Google Scholar]
- Oertel D, Wu SH. Morphology and physiology of cells in slice preparations of the dorsal cochlear nucleus of mice. J Comp Neurol. 1989;283:228–47. doi: 10.1002/cne.902830206. [DOI] [PubMed] [Google Scholar]
- Pal B, Por A, Szucs G, Kovacs I, Rusznak Z. HCN channels contribute to the intrinsic activity of cochlear pyramidal cells. Cell Mol Life Sci. 2003;60:2189–99. doi: 10.1007/s00018-003-3187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham K, Kim DO. Spontaneous and sound-evoked discharge characteristics of complex-spiking neurons in the dorsal cochlear nucleus of the unanesthetized decerebrate cat. J Neurophysiol. 1995;73:550–61. doi: 10.1152/jn.1995.73.2.550. [DOI] [PubMed] [Google Scholar]
- Parham K, Bonaiuto G, Carlson S, Turner JG, D’Angelo WR, Bross LS, Fox A, Willott JF, Kim DO. Purkinje cell degeneration and control mice: responses of single units in the dorsal cochlear nucleus and the acoustic startle response. Hear Res. 2000;148:137–52. doi: 10.1016/s0378-5955(00)00147-7. [DOI] [PubMed] [Google Scholar]
- Portfors CV, Roberts PD. Temporal and frequency characteristics of cartwheel cells in the dorsal cochlear nucleus of the awake mouse. J Neurophysiol. 2007;98:744–56. doi: 10.1152/jn.01356.2006. [DOI] [PubMed] [Google Scholar]
- Potashner SJ, Suneja SK, Benson CG. Altered glycinergic synaptic activities in guinea pig brain stem auditory nuclei after unilateral cochlear ablation. Hear Res. 2000;147:125–36. doi: 10.1016/s0378-5955(00)00126-x. [DOI] [PubMed] [Google Scholar]
- Rhode WS, Smith PH. Physiological studies on neurons in the dorsal cochlear nucleus of cat. J Neurophysiol. 1986;56:287–307. doi: 10.1152/jn.1986.56.2.287. [DOI] [PubMed] [Google Scholar]
- Rusznak Z, Harasztosi C, Stanfield PR, Kovacs L, Szucs G. Potassium-depolarization-induced cytoplasmic [Ca2+] transient in freshly dissociated pyramidal neurones of the rat dorsal cochlear nucleus. Pflugers Arch. 2000;440:462–6. doi: 10.1007/s004240000314. [DOI] [PubMed] [Google Scholar]
- Seki S, Eggermont JJ. Changes in spontaneous firing rate and neural synchrony in cat primary auditory cortex after localized tone-induced hearing loss. Hear Res. 2003;180:28–38. doi: 10.1016/s0378-5955(03)00074-1. [DOI] [PubMed] [Google Scholar]
- Serodio P, Rudy B. Differential expression of Kv4 K+ channel subunits mediating subthreshold transient K+ (A-type) currents in rat brain. J Neurophysiol. 1998;79:1081–91. doi: 10.1152/jn.1998.79.2.1081. [DOI] [PubMed] [Google Scholar]
- Shore SE, Vass Z, Wys NL, Altschuler RA. Trigeminal ganglion innervates the auditory brainstem. J Comp Neurol. 2000;419:271–85. doi: 10.1002/(sici)1096-9861(20000410)419:3<271::aid-cne1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Shore SE, Koehler S, Oldakowski M, Hughes LF, Syed S. Dorsal cochlear nucleus responses to somatosensory stimulation are enhanced after noise-induced hearing loss. Eur J Neurosci. 2008;27:155–68. doi: 10.1111/j.1460-9568.2007.05983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabler SE, Palmer AR, Winter IM. Temporal and mean rate discharge patterns of single units in the dorsal cochlear nucleus of the anesthetized guinea pig. J Neurophysiol. 1996;76:1667–88. doi: 10.1152/jn.1996.76.3.1667. [DOI] [PubMed] [Google Scholar]
- Suneja SK, Potashner SJ, Benson CG. Plastic changes in glycine and GABA release and uptake in adult brain stem auditory nuclei after unilateral middle ear ossicle removal and cochlear ablation. Exp Neurol. 1998;151:273–88. doi: 10.1006/exnr.1998.6812. [DOI] [PubMed] [Google Scholar]
- Suneja SK, Potashner SJ, Benson CG. AMPA receptor binding in adult guinea pig brain stem auditory nuclei after unilateral cochlear ablation. Exp Neurol. 2000;165:355–69. doi: 10.1006/exnr.2000.7471. [DOI] [PubMed] [Google Scholar]
- Wallhausser-Franke E, Mahlke C, Oliva R, Braun S, Wenz G, Langner G. Expression of c-fos in auditory and non-auditory brain regions of the gerbil after manipulations that induce tinnitus. Exp Brain Res. 2003;153:649–54. doi: 10.1007/s00221-003-1614-2. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Dib-Hajj S, Cummins TR, Black JA. Sodium channels and pain. Proc Natl Acad Sci U S A. 1999;96:7635–9. doi: 10.1073/pnas.96.14.7635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weedman DL, Ryugo DK. Projections from auditory cortex to the cochlear nucleus in rats: synapses on granule cell dendrites. J Comp Neurol. 1996;371:311–24. doi: 10.1002/(SICI)1096-9861(19960722)371:2<311::AID-CNE10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Weinberg RJ, Rustioni A. A cuneocochlear pathway in the rat. Neurosci. 1987;20:209–219. doi: 10.1016/0306-4522(87)90013-3. [DOI] [PubMed] [Google Scholar]
- Young ED. Identification of response properties of ascending axons from dorsal cochlear nucleus. Brain research. 1980;200:23–37. doi: 10.1016/0006-8993(80)91091-4. [DOI] [PubMed] [Google Scholar]
- Zhang JS, Kaltenbach JA. Increases in spontaneous activity in the dorsal cochlear nucleus of the rat following exposure to high-intensity sound. Neurosci Lett. 1998;250:197–200. doi: 10.1016/s0304-3940(98)00482-0. [DOI] [PubMed] [Google Scholar]
- Zhang S, Oertel D. Tuberculoventral cells of the dorsal cochlear nucleus of mice: intracellular recordings in slices. J Neurophysiol. 1993;69:1409–21. doi: 10.1152/jn.1993.69.5.1409. [DOI] [PubMed] [Google Scholar]
- Zhang H, Wu SH, Kelly JB. Regulation of auditory responses in the central nucleus of the inferior colliculus by tetraethylammonium-sensitive potassium channels. J Neurophysiol. 2004;91:2194–204. doi: 10.1152/jn.00730.2003. [DOI] [PubMed] [Google Scholar]