Abstract
Background
ADAMTS13 is a secreted metalloprotease that cleaves von Willebrand Factor multimers and maintains proper homeostasis. A severe deficiency in ADAMTS13 triggers a disorder known as thrombotic thrombocytopenic purpura (TTP). At present, ADAMTS13 expression levels are determined by immunoblotting.
Methods
We established a flow cytometry methodology to detect intracellular ADAMTS13 in liver and kidney cells using a polyclonal antibody, BL154G, and several monoclonal antibodies previously used to detect ADAMTS13 by immunoblotting. Results were validated using confocal microscopy, immunoblotting and an activity assay (FRETS-VWF73).
Results
We show that labeling ADAMTS13 with specific antibodies and detection by flow cytometry yields results that are comparable to previously established methods for ADAMTS13 detection. Specifically, we compared the endogenous expression levels of ADAMTS13 in various liver cell lines using flow cytometry and obtained results that parallel immunoblot analysis. Knock-down of ADAMTS13 expression via targeted siRNA resulted in significantly reduced median signal, displaying the sensitivity of this detection method. A further analysis of reliability and specificity was achieved through plasmid DNA and transfection reagent dose response studies.
Conclusions
The flow cytometry method described here is useful in determining the expression of both endogenous and recombinant forms of intracellular ADAMTS13. Flow cytometry is a convenient, efficient and cost effective way to measure the expression levels of ADAMTS13.
Key terms: ADAMTS13, flow cytometry, intracellular protein expression
INTRODUCTION
ADAMTS13, the von Willebrand Factor (VWF) cleavage protease, prevents intravascular thrombosis leading to the TTP (Thrombotic Thrombocytopenic Purpura) disorder (1–3). This therapeutically important protein is 150 kDa in size and expressed in a variety of cell types, but mainly by hepatic stellate cells (4,5), platelets (6), venous and arterial endothelial cells (7), and podocytes in the kidney. ADAMTS13 is a member of the ADAMTS (a disintegrin-like and metalloprotease with thrombospondin motifs) family of secreted metalloproteases. It differs from the other members of its family by bearing two C-terminal CUB (Complement C1r/C1s, Uegf (EGF-related sea urchin protein) and BMP-1 (bone morphogenic protein-1) domains (8). Unlike other secreted metalloproteases, it is fully active before secretion from the cell.
Protein expression levels in cell culture can be measured by either immunoblot analysis or flow cytometry. Various studies demonstrate that flow cytometry and immunoblots provide comparable results when measuring intracellular quantities of secreted proteins (9–11), including clotting and anti-clotting factors (12,13). Presently, immunoblotting is the technique of choice in quantifying secreted ADAMST13 levels although it requires more cells and is more time consuming than cytometry (7,14–18). Moreover, detecting endogenous levels of intracellular ADAMTS13 through Western blot is exceedingly difficult without further purification and/or concentration of cell lysates—an additional time intensive task. Various research groups have studied ADAMTS13 by using specific antibodies to detect and measure ADAMTS13 expression by means of immunoblots. Establishing flow cytometry as tool to measure intracellular expression of ADAMTS13 would provide researchers with a swift yet reliable means by which to monitor ADAMTS13.
Here, we describe the reliability and utility of flow cytometry as a tool to measure the intracellular level of fully active, wild-type ADAMTS13. Our cytometric measurements were found to be very similar, if not identical, to those obtained by immunoblotting. For example, human stellate cells (LX2), tested by both methods, were found to express the highest known level of ADAMTS13. The antibodies employed in this study were also useful in the confocal imaging of ADAMTS13. Their specificity to ADAMTS13 in flow cytometry experiments were confirmed by reduced fluorescence intensity readings when testing ADAMTS13 siRNA-knockdown cells. Flow cytometry was found to be just as accurate as the traditionally employed immunoblot method for ADAMTS13 quantification and is certainly superior to immunoblotting, given its rapid and simple nature.
MATERIALS AND METHODS
Cell Lines and Cell Culture
Human embryonic kidney (HEK293) cells (ATCC, Manassas, VA) were used in all transfection experiments. A panel of liver cells was tested in the flow cytometry and immunoblotting experiments of ADAMTS13: Hep3B (ATCC), Huh7, Alexander (a gift from Sara Ladu, National Cancer Institute (NCI), NIH), 7404 cells (a gift from Michael M. Gottesman, NCI, NIH), and highly expressing ADAMTS13 hepatic stellate cells (LX2) (19). All cells were grown in Dulbecco’s Modified Eagle Medium with 1% glutamine, 1% penicillin- streptomycin, and 10% fetal bovine serum (Invitrogen, Carlsbad, CA) at 37°C under humid conditions in 5% CO2.
Transfection
In preparation for confocal microscopy, flow cytometry or for immunoblotting, 5 × 105 cells were plated in MatTeK dishes (MatTeK, Ashland, MA), or 6-well plates, or T-75 flasks 24 hours before transfection. Cells were transfected with either 2 or 20 µg pcDNA4-ADAMTS13 (respective, to the container size; a gift from Evan Sadler, St. Louis, MO), or the control pcDNA4 empty vector, using Lipofectamine Plus or Lipofectamine 2000 (Invitrogen) or Fugene6 (Roche, Pleasanton, CA) according to the manufacturer’s recommended protocol. We used several different transfection reagents to assure that the increase in fluorescence was not determined by any individual reagent. Several dose response experiments were performed with modifications to the amount of the transfected DNA or the transfection reagent.
Flow Cytometry
Fixation and permeabilization of the trypsinized cells were performed according to the manufacturer’s instructions (IntraPrep™ Beckman Coulter, Marseille, France). Similar results were also obtained when the permeabilization and fixation were performed with BD Cytofix/Cytoperm (BD biosciences, San Jose, CA) following the company’s instruction manual. Unpermeablized cells were used as a control. Dilutions from stock of 1 mg/ml of Wh2-11-1 Wh2-22-1A, Wh10, W688X6-1, W688X3-69, BL154G (Bethyl Laboratories, Montgomery, TX) and anti-V5 antibody (Invitrogen) were used for the labeling. The isotopes, anti-Mouse IgG2aκ and anti-mouse IgG1κ antibodies served as controls. Alexa Fluor 488 goat anti-mouse IgG secondary antibody (Invitrogen) was used to detect all the monoclonal antibodies while Rabbit anti-Goat IgG FITC (Bethyl) was used to identify the primary polyclonal ADAMTS13 antibody BL154G. Washes after antibody labeling were with PBS (Invitrogen) 0.1% bovine serum albumin (BSA; Sigma) three times. Antibody labeling was performed for 30 minutes at 37°C. The cells were then analyzed using the Becton Dickinson FACS Calibur. Median values were calculated using CellQuest software by Becton Dickinson.
Quantification of ADAMTS13 using Western Blotting
The cell lysates were prepared by washing the harvested cells with chilled PBS and lysed by suspension in cell lysis buffer (20mM Tris-HCl, 150mM NaCl, 1% Triton X-100, Protease Inhibitor Cocktail Tablet (Roche, Florence, SC) and 1mM PMSF) and then stored at −20°C. The total protein in the lysates was quantified by Bradford protein quantification assay (Bio-Rad, New York, NY).
For electrophoresis, 30–320 µg of total protein was mixed with loading buffer, heated at 95°C for 5 minutes, sonicated at room temperature for 5 minutes, and separated by electrophoresis on a 3–8% Tris-Acetate SDS gel (Invitrogen) immersed in 1x NuPAGE Tris-Acetate running buffer (Invitrogen). The protein was then transferred to a nitrocellulose membrane (Invitrogen) immersed in 1x NuPAGE Tris-Acetate transfer buffer (Invitrogen) containing 20% MeOH, 0.02% SDS, and 0.1% NuPAGE Antioxidant (Invitrogen). After transfer, the membrane was blocked in 5% non-fat milk at room temperature. Immunostaining was performed using the primary polyclonal antibody ADAMTS13 BL154G followed by secondary antibody staining with anti-Rabbit IgG HRP (1.0 mg/ml; Invitrogen). Detection was carried out using West Pico Chemiluminescent Substrate (Pierce, Rockford, IL, USA) and quantified using ImageQuaNT software (GE Healthcare, Piscataway, NJ).
Measurement of Protease Activity using FRETS-VWF73 Assay
Fluorescence Resonance Energy Transfer Substrate-von Willebrand Factor 73 (FRETS-VWF73) (Peptide International, Osaka, Japan) is a fluorogenic substrate that was developed to test the activity of ADAMTS13. Samples were prepared according to the manufacturer. The fluorescence was read by a GeminiMax plate reader (Molecular Devices, Sunnyvale, CA) every 5 minutes for one hour with mixing at an excitation of 340 nm and emission of 450 nm.
Confocal Microscopy
Transfected cells were washed twice with Phosphate-Buffered Saline (PBS) with 0.1% Bovine Serum Albumin (BSA), and then fixed and permeablized with 4% paraformaldehyde (Sigma) for 30 minutes with an optional step of 70% ethanol for 15 minutes. Labeling was done with anti-V5 and Wh2-11-1 monoclonal antibodies for one hour at room temperature and Alexa Fluor 488 goat anti-mouse secondary antibody. Immediately before scanning the dish, DAPI (4',6-diamidino-2-phenylindole) (Invitrogen, Molecular Probes) was added to a final concentration of 10 µg/ml to stain the nucleus. Labeling with the secondary antibody only served as a control.
Confocal images were sequentially acquired with Zeiss AIM software on a Zeiss LSM 510 Confocal system (Carl Zeiss Inc, Thornwood, NY) with a Zeiss Axiovert 100M inverted microscope and 50 mW argon UV laser tuned to 364 nm, a 25 mW Argon visible laser tuned to 488 nm and a 1 mW HeNe laser tuned to 543 nm. A 63x Plan-Neofluar 1.4 NA oil immersion objective was used at various digital zoom settings. Emission signals after sequential excitation of DAPI and Alexa Fluor 488 goat anti-mouse by the 364nm or 488 nm laser lines were collected with a BP 435–485 or BP 505–550 filter respectively, using individual photomultipliers.
Knock down of ADAMTS13 using siRNA
Transient expression of ADAMTS13 in HEK293 cells was knocked down using pooled ADAMTS13-targeted siRNA (Invitrogen) introduced into cells at a final concentration of 200 nM using Lipofectamine 2000 (Invitrogen) as per manufacturer’s protocol. The control cells were transfected with AllStars scrambled siRNA (Qiagen, Valencia, CA) at a final concentration of 200 nM. Cells were harvested 24 hours post-transfection and either immunostained for flow cytometry or lyzed for Western blot as described above.
RESULTS AND DISCUSSION
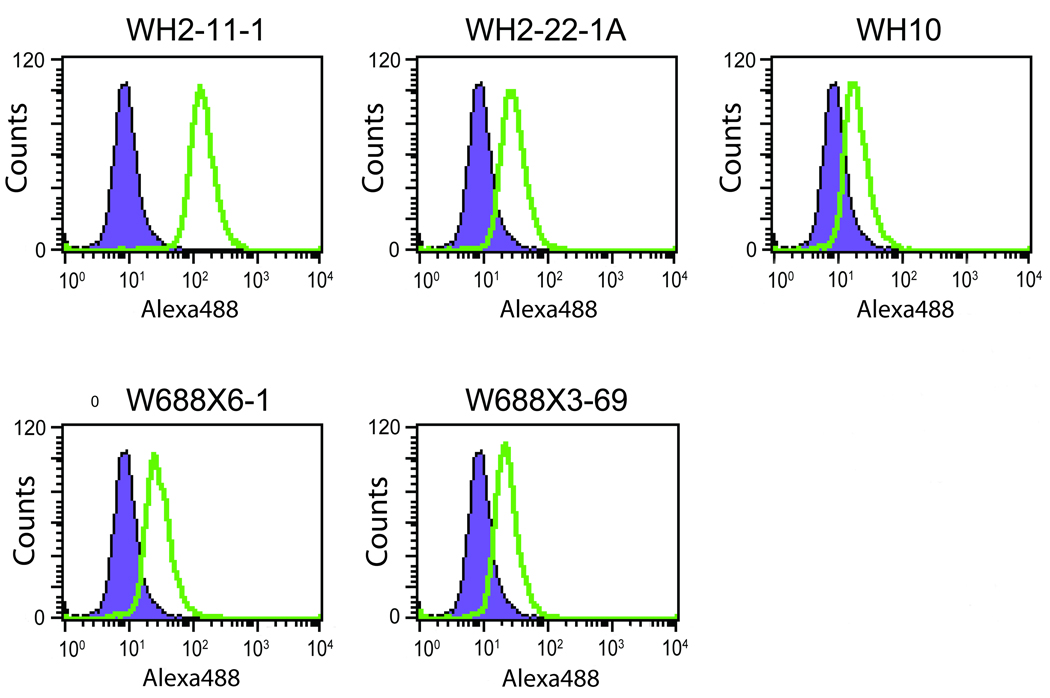
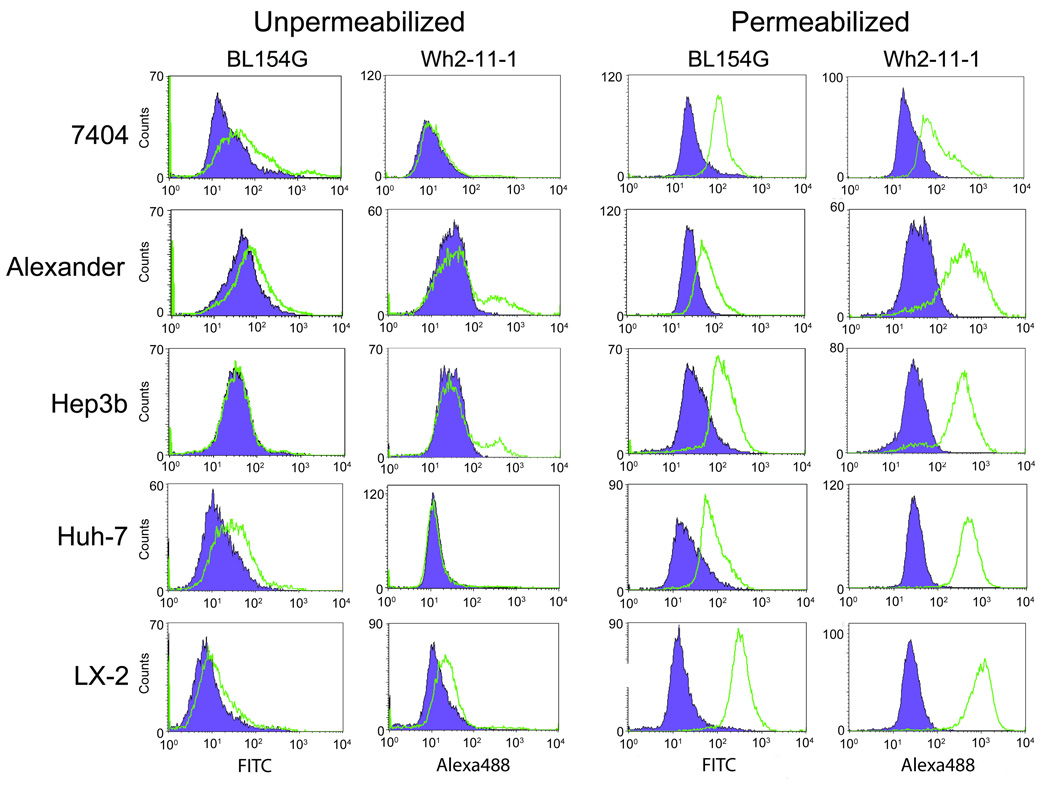
The various ADAMTS13-specific monoclonal antibodies described by Soejima and coworkers (15) (Wh2-11-1, Wh2-22-1A, Wh-10, W688X6-1 and W688X3-69) and the commercially available ADAMTS13 polyclonal antibody BL154G were previously tested to detect ADAMTS13 on Western blots (http://www.natutec.de/pdf_bethyl/A300-391A.pdf). Here we used flow cytometry to test these antibodies for their efficacy in detecting the endogenous ADAMTS13 in HEK293 cells (Figure 1). A preliminary test of both isotype controls alongside a secondary-only control was conducted (Figure S1) revealing comparable histogram curves and median signal measurements. All figures contain histograms of secondary-only data to ensure clarity, although all three controls were conducted for each experimental setup. Significantly high fluorescence intensities were measured for each antibody relative to control staining with secondary antibody only or anti-mouse IgG2aκ or IgG1κ isotypic antibodies (Figure 1). Of the antibodies that we tested, Wh2-11-1 had the highest reactivity to ADAMTS13. Therefore, we used this antibody alongside the polyclonal antibody BL154G to study the endogenous expression of ADAMTS13 in multiple liver cell lines: 7404, Alexander, Hep3b, Huh-7 and LX2 (human hepatic stellate cells). In addition, the cells were fixed and permeabilized with two alternative methods as discussed in the Materials and Methods section, or left untreated (unfixed and unpermeabilized) prior to incubating with the ADAMTS13 antibodies. Following treatment with the primary antibody, the cells were stained with the secondary antibody and analyzed by flow cytometry. These results are depicted in Figure 2A and clearly demonstrate that histogram shifts occur using both ADAMTS13 antibodies; these shifts are dramatically pronounced when the cells are permeabilized. Finally, among all the cell lines tested, LX2, the liver stellate cell line (19), showed the highest median fluorescence (Figure 2A). This observation is consistent with the report of Fujimura and coworkers that liver stellate cells show the highest expression of ADAMTS13 and are the major contributors of ADAMTS13 found in blood (4). The median fluorescence of the permeabilized cells in this assay is a measure of relative ADAMTS13 protein. Endogenous levels of intracellular ADAMTS13 were not detected using conventional cell lysate preparation and Western blotting procedures (data not shown). This finding adds to the significance of detecting endogenous levels of intracellular ADAMTS13 via flow cytometry.
Figure 1. Flow cytometry detection of intracellular ADAMTS13 in HEK293 cells using various specific anti-ADAMTS13 monoclonal antibodies.
HEK293 cells from a two-day old culture were harvested after trypsinization. Cells were fixed and permeablized. The specific monoclonal primary antibodies tested were Wh2-11-1, Wh2-22-1A, Wh-10, W688X6-1 and W688X3-69 (all green histograms) followed by secondary antibody labeling with Alexa Fluor 488 goat anti-mouse; various isotypes and secondary antibody only served as controls (purple histograms, secondary antibody only).
Figure 2. Detection of ADAMTS13 intracellular expression in various liver ADAMTS13-expressing cell lines.
Flow cytometry: The cells were harvested from a two-day-old culture. One-half of the cells were fixed and permeablized using IntraPrep reagent according to the product manual (right two columns). Both the unpermeablized cells (left two columns) and permeablized/fixed cells were labeled using primary polyclonal antibody BL154G (left column for each treatment) or monoclonal Wh2-11-1 (right column for each treatment); green curves) followed by a secondary antibody FITC anti-goat for the former and Alexa Fluor 488 goat anti-mouse IgG for the later. Corresponding cell samples were labeled side by side with fluorescent-tagged secondary antibody only (purple curves), which served as controls. Fluorescence readings were taken in the FACSCalibur flow cytometer.
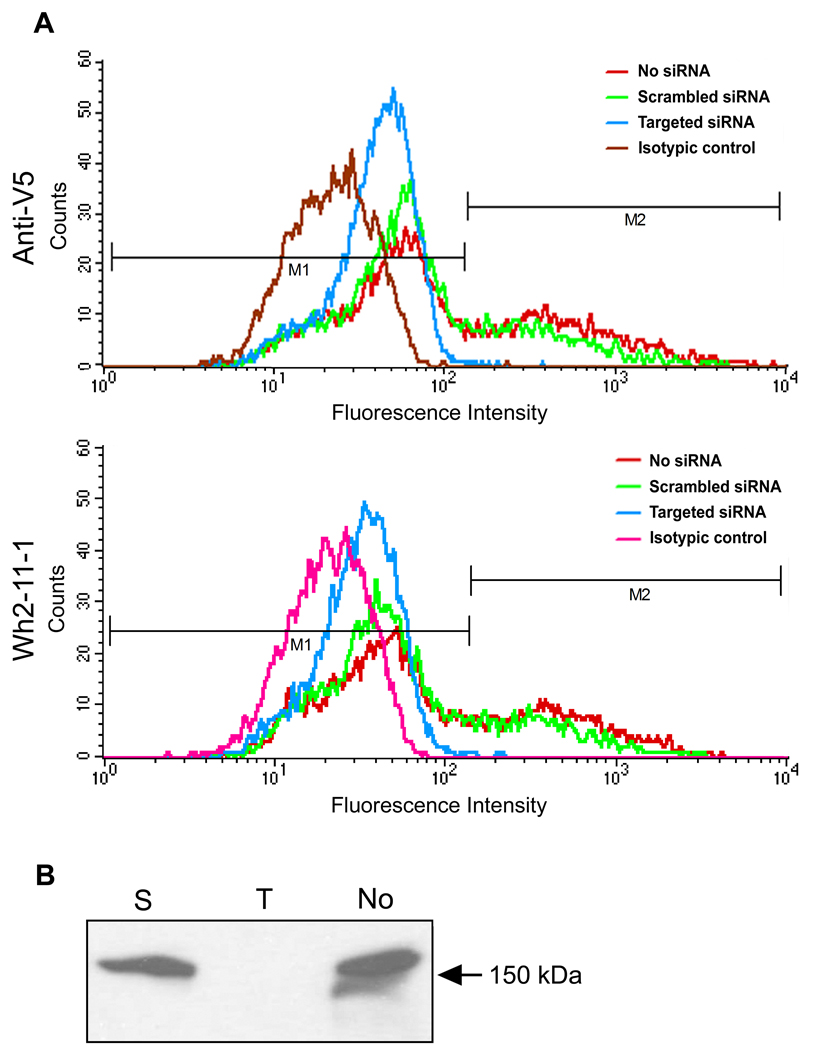
As a further demonstration of this method’s sensitivity, transient expression of ADAMTS13 was knocked down in HEK293 cells using siRNA as described in Materials and Methods. Upon targeted siRNA transfection, median fluorescence fell to 31.91/41.05 a.u. from 55.73/69.78 a.u for Wh2-11-1 and anti-V5 respectively (Figure 3A). With isotypic readings subtracted, cells probed with Wh2-11-1 showed a 68% reduction in expression, while anti-V5 probed cells showed a 60% decline. One reason for this slight difference may be that the use of Wh2-11-2 estimates the reduction in both endogenous and transfected ADAMTS13 while the use of the anti-V5 antibody only estimates the reduction in the transfected ADAMTS13. Conversely, cells transfected with control scrambled siRNA retained 73% and 80% of their expression, when probing with Wh2-11-1 and anti-V5 respectively. A similar reduction in ADAMTS13 expression was observed through Western blotting. Relative to cells transfected with plasmid ADAMTS13 alone, cells administered scrambled siRNA retained 79% of ADAMTS13 expression, while targeted siRNA transfection obliterated detectable levels of ADAMTS13 (Figure 3B).
Figure 3. Measurement of transient ADAMTS13 expression via FACS after siRNA knock-down.
(A) HEK293 cells were co-transfected with pADAMTS13 in addition to either targeted (blue), scrambled (green) or no siRNA (red). Cells were harvested 24 hours post-transfection and immunolabeled with either Wh2-11-1, anti-V5 or a respective isotypic control antibody (brown/pink). Fluorescence readings were measured in FACSCalibur flow cytometer. The proportion of the transfected (under M2) vs. untransfected cells (under M1) was calculated using the Statistics program of CellQuest software (Beckton Dickinson). (B) A proportion of the cells from (A) were lysed and analyzed by SDS-PAGE followed by immunoblotting using the anti-V5 antibody. Lanes: S (scrambled siRNA); T (targeted siRNA); No (pADAMTS13 only).
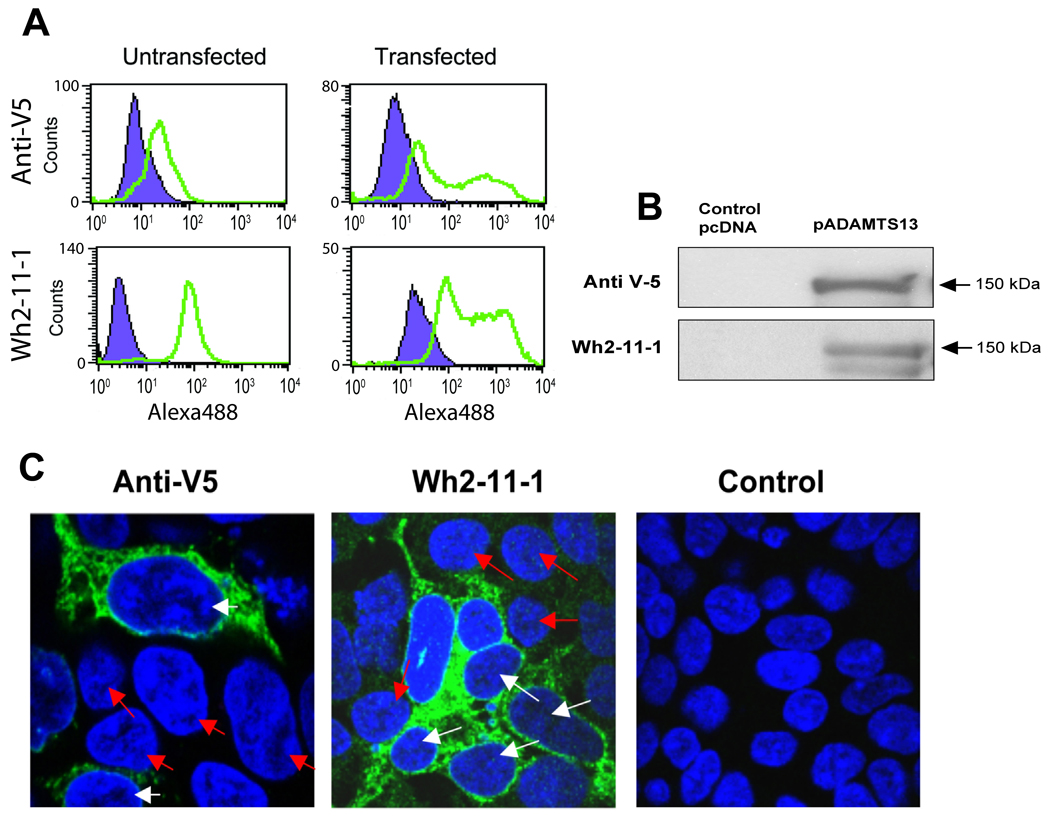
Analogously, we used Wh2-11-1 and an antibody against the C-terminal V5 tag of recombinant ADAMTS13 to detect gains in ADAMTS13-specific antibody reactivity which is reflected by gains in median fluorescence upon transfection with plasmid ADAMTS13 (pADAMTS13) in HEK293 cells. The anti-V5 antibody detects the recombinant protein only, while Wh2-11-1 measures total ADAMTS13 within the cell. Both antibodies yielded a significant gain in detection following transfection with pADAMTS13 (Figure 3A). The median fluorescence observed was always higher in pADAMTS13-transfected cells than in cells transfected with the empty vector control, which was verified by an immunoblot of cell lysates using the same two antibodies (Figure 3B). To accompany this flow cytometry study, confocal imaging of ADAMTS13 following pADAMTS13 transfection was performed. The increase in intracellular expression of ADAMTS13 following transfection is clearly evident in images of immunolabeled HEK293 cells using the same antibodies—anti-V5 and Wh2-11-1 (Figure 3C).
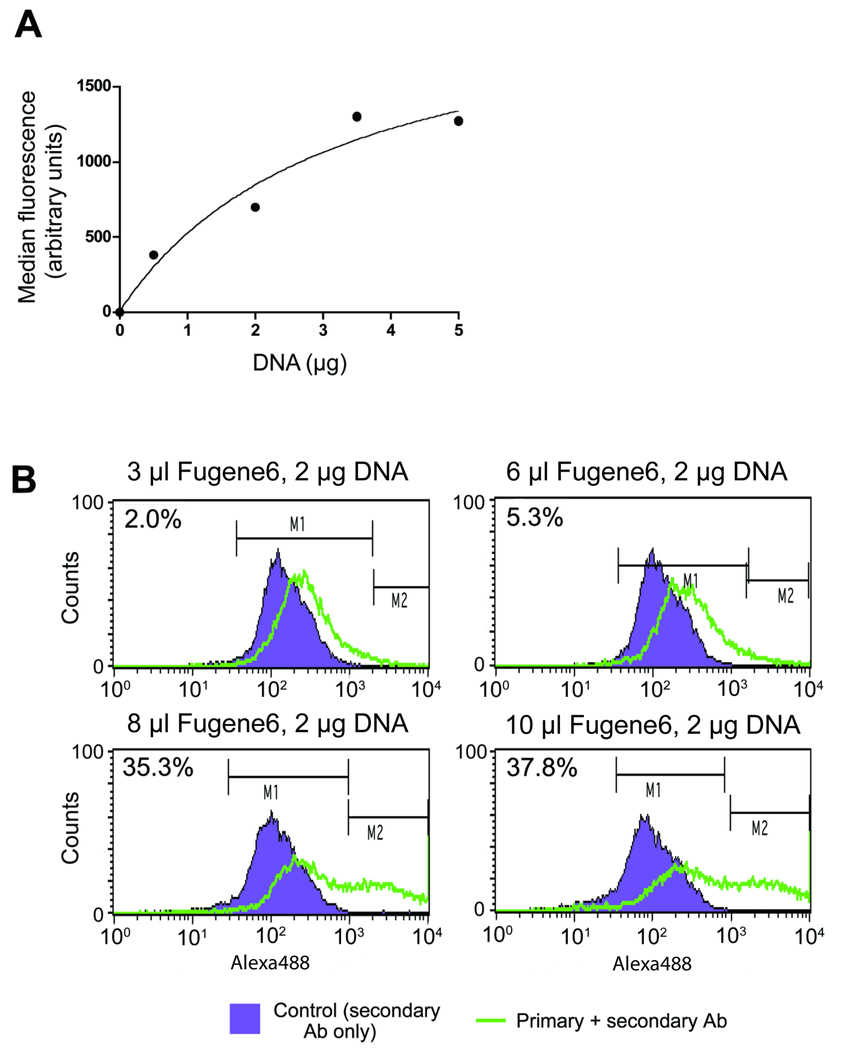
Finally, dose response experiments were conducted to determine the sensitivity of flow cytometry to various amounts of transfected DNA and transfection regent used during the process of transfecting pADAMTS13 into HEK293 cells. The response to increasing concentrations of DNA is clearly reflected in the increasing median fluorescence intensity measured by flow cytometry using Wh2-11-1 (Figure 4A). The saturation point was reached when using 5 µg DNA per 5 × 105 cells in the transfection procedure. In another experiment, we varied the amount of Fugene6 transfection reagent but retained a constant amount of DNA (2 µg) (Figure 4B). In this experiment, we found a progressive increase in the percentage of transfected cells as we increased the concentration of the transfection reagent. With the use of 3, 6, 8 and 12 µL of Fugene6 transfection reagent, the percentage of transfected cells increased to 2.0, 5.3, 35.3 and 37.8, respectively. A further increase in the quantity of transfection reagent yielded no further increase in the transfected cells, and the percent of stained cells reached a plateau. The nature of this incremental increase in fluorescence signal obtained by flow cytometry clearly shows a dose response curve vis-à-vis the amount of DNA used to transfect the cells as well as the transfection reagent itself.
Figure 4. Detection of intracellular ADAMTS13 in transfected HEK293 via flow cytometry, Western blotting and confocal microscopy.
(A) HEK293 untransfected (left column) vs. ADAMTS13- HEK293 transfected cells (right column) were harvested twenty four hours post-transfection and labeled with the anti-V5 (top) and Wh2-11-1 (bottom) antibodies followed by the secondary antibody Alexa Fluor 488 goat anti-mouse. Untransfected cells were labeled similarly. The increased expression level of ADAMTS13 as a result of transfection is reflected in the heightened fluorescence readings relative to untransfected cells (green histograms). Staining with the secondary antibody only (purple histogram) served as a control (Alexa Fluor 488 goat anti-mouse). (B) Immunoblot: The same cells were lysed and analyzed by SDS-PAGE using the same two antibodies anti-V5 (top) and Wh2-11-1 (bottom). (C) ADAMTS13-transfected HEK293 cells were labeled with anti-V5 (left) to detect transfected cells only or Wh2-11-1 (middle) to detect both endogenous and recombinant ADAMTS13. Primary antibody incubation was followed by secondary antibody labeling with Alexa Fluor 488 goat anti-mouse and the background fluorescence for both antibodies was detected by labeling with secondary antibody only (right). Nuclear staining was done using DAPI (4',6-diamidino-2-phenylindole). Confocal images were sequentially acquired and overlaid to identify the transfected and untransfected cells. The figures are the merge of the 488 nm channel and DAPI fluorescence signal. White arrows point to transfected cells and the red arrows to the untransfected cells. Images were collected with a 60X planapochromat lens.
ADAMTS13 is an unusual secreted protein, as it has both intracellular and extracellular functionality (8). We measured the activity of the secreted protein using the fluorescent substrate FRETS-VWF73, as described previously by Kokame and coworkers (20). As expected, an increase in intracellular ADAMTS13 measured by flow cytometry accurately predicts increased proteolytic activity of extracellular ADAMTS13 (data not shown), due to higher secretion levels of ADAMTS13 into the culture media following transfection.
Here, we have introduced flow cytometry as a novel method to detect and quantitatively measure expression levels of intracellular ADAMTS13. We have shown that the expression level of intracellular ADAMTS13 as determined by flow cytometry correlates well with previously established assays for ADAMTS13 detection: Western blotting, confocal imaging and FRETS-VWF activity assay.
The method described here, unlike immunoblotting of cell lysates, easily lends itself to high throughput assays. Thus, the technique could be useful in the standardization of protocols for the production of recombinant ADAMTS13. In addition, during large-scale production of ADAMTS13 this method would be useful in monitoring the expression levels at frequent intervals to ensure the quality of the protein, which is the most crucial issue for the production of any recombinant therapeutic protein.
Supplementary Material
Figure 5. Detection of recombinant ADAMTS13 in transfected HEK293 cells.
(A) Transfected DNA dose response. Increasing amounts of DNA (0, 0.2, 2.0, 3.5 and 5.0 µg) were transfected into HEK293 cells. Twenty-four hours post transfection the cells were labeled with the ADAMTS13 monoclonal Wh2-11-1 antibody and Alexa Fluor 488 goat anti-mouse secondary antibody. Fluorescence intensity was measured by flow cytometry and median fluorescence levels were plotted on the graph. (B) Transfection efficiency monitored by flow cytometry. Varying amounts of Fugene6 transfection reagent (3, 6, 8 and 10 µL) with a constant amount of DNA (2 µg) were used for transfection. Antibody labeling (24 hours post transfection) for flow cytometry was achieved using Wh2-11-1 antibody followed by a secondary Alexa Fluor 488 goat anti-mouse (green histogram). Transfection efficiency was determined using flow cytometry. The fluorescence intensity was monitored and compared with secondary only staining, Alexa Fluor 488 goat anti-mouse (purple histogram). The proportion of the transfected (under M2) vs. untransfected cells (under M1) was calculated using the Statistics program of CellQuest software (Beckton Dickinson) and indicated as a percentage for each panel.
ACKNOWLEDGMENTS
We thank Dr. Evan Sadler, Washington University School of Medicine, St Louis, MO for the ADAMTS13-expressing plasmid. We thank Dr. Michael M. Gottesman and Dr. Sara Ladu, NCI, NIH for the 7404 and Alexander cell lines, respectively. In addition, our special thanks is expressed to Mr. George Leiman, NCI, NIH for insightful editorial assistance and to Dr. Robert Fisher, CBER, FDA for fruitful discussions.
Footnotes
COMPETING INTERESTS STATEMENT
The authors declare no competing interests.
REFERENCES
- 1.Furlan M, Robles R, Solenthaler M, Wassmer M, Sandoz P, Lammle B. Deficient activity of von Willebrand factor-cleaving protease in chronic relapsing thrombotic thrombocytopenic purpura. Blood. 1997;89(9):3097–3103. [PubMed] [Google Scholar]
- 2.Furlan M, Robles R, Solenthaler M, Lammle B. Acquired deficiency of von Willebrand factor-cleaving protease in a patient with thrombotic thrombocytopenic purpura. Blood. 1998;91(8):2839–2846. [PubMed] [Google Scholar]
- 3.Zheng X, Chung D, Takayama TK, Majerus EM, Sadler JE, Fujikawa K. Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J Biol Chem. 2001;276(44):41059–41063. doi: 10.1074/jbc.C100515200. [DOI] [PubMed] [Google Scholar]
- 4.Uemura M, Tatsumi K, Matsumoto M, Fujimoto M, Matsuyama T, Ishikawa M, Iwamoto TA, Mori T, Wanaka A, Fukui H. Localization of ADAMTS13 to the stellate cells of human liver. Blood. 2005;106(3):922–924. doi: 10.1182/blood-2005-01-0152. and others. [DOI] [PubMed] [Google Scholar]
- 5.Zhou W, Inada M, Lee TP, Benten D, Lyubsky S, Bouhassira EE, Gupta S, Tsai HM. ADAMTS13 is expressed in hepatic stellate cells. Lab Invest. 2005;85(6):780–788. doi: 10.1038/labinvest.3700275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki M, Murata M, Matsubara Y, Uchida T, Ishihara H, Shibano T, Ashida S, Soejima K, Okada Y, Ikeda Y. Detection of von Willebrand factor-cleaving protease (ADAMTS-13) in human platelets. Biochem Biophys Res Commun. 2004;313(1):212–216. doi: 10.1016/j.bbrc.2003.11.111. [DOI] [PubMed] [Google Scholar]
- 7.Turner N, Nolasco L, Tao Z, Dong JF, Moake J. Human endothelial cells synthesize and release ADAMTS-13. J Thromb Haemost. 2006;4(6):1396–1404. doi: 10.1111/j.1538-7836.2006.01959.x. [DOI] [PubMed] [Google Scholar]
- 8.Majerus EM, Zheng X, Tuley EA, Sadler JE. Cleavage of the ADAMTS13 propeptide is not required for protease activity. J Biol Chem. 2003;278(47):46643–46648. doi: 10.1074/jbc.M309872200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He L, Fox MH. Comparison of flow cytometry and western blotting to measure Hsp70. Cytometry. 1996;25(3):280–286. doi: 10.1002/(SICI)1097-0320(19961101)25:3<280::AID-CYTO9>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 10.Dragowska WH, Lopes de Menezes DE, Sartor J, Mayer LD. Quantitative fluorescence cytometric analysis of Bcl-2 levels in tumor cells exhibiting a wide range of inherent Bcl-2 protein expression: correlation with Western blot analysis. Cytometry. 2000;40(4):346–352. doi: 10.1002/1097-0320(20000801)40:4<346::aid-cyto10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 11.Kannan M, Ahmad F, Yadav BK, Kumar P, Jain P, Kumar R, Saxena R. Carrier detection in Glanzmann thrombasthenia: comparison of flow cytometry and Western blot with respect to DNA mutation. Am J Clin Pathol. 2008;130(1):93–98. doi: 10.1309/HYE4AP9961CEP0C0. [DOI] [PubMed] [Google Scholar]
- 12.Mei B, Chen Y, Chen J, Pan CQ, Murphy JE. Expression of human coagulation factor VIII in a human hybrid cell line, HKB11. Mol Biotechnol. 2006;34(2):165–178. doi: 10.1385/MB:34:2:165. [DOI] [PubMed] [Google Scholar]
- 13.Tomer A. Human marrow megakaryocyte differentiation: multiparameter correlative analysis identifies von Willebrand factor as a sensitive and distinctive marker for early (2N and 4N) megakaryocytes. Blood. 2004;104(9):2722–2727. doi: 10.1182/blood-2004-02-0769. [DOI] [PubMed] [Google Scholar]
- 14.Tao Z, Wang Y, Choi H, Bernardo A, Nishio K, Sadler JE, Lopez JA, Dong JF. Cleavage of ultralarge multimers of von Willebrand factor by C-terminal-truncated mutants of ADAMTS-13 under flow. Blood. 2005;106(1):141–143. doi: 10.1182/blood-2004-11-4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soejima K, Nakamura H, Hirashima M, Morikawa W, Nozaki C, Nakagaki T. Analysis on the molecular species and concentration of circulating ADAMTS13 in Blood. J Biochem. 2006;139(1):147–154. doi: 10.1093/jb/mvj013. [DOI] [PubMed] [Google Scholar]
- 16.Ricketts LM, Dlugosz M, Luther KB, Haltiwanger RS, Majerus EM. O-fucosylation is required for ADAMTS13 secretion. J Biol Chem. 2007;282(23):17014–17023. doi: 10.1074/jbc.M700317200. [DOI] [PubMed] [Google Scholar]
- 17.Plaimauer B, Zimmermann K, Volkel D, Antoine G, Kerschbaumer R, Jenab P, Furlan M, Gerritsen H, Lammle B, Schwarz HP. Cloning, expression, and functional characterization of the von Willebrand factor-cleaving protease (ADAMTS13) Blood. 2002;100(10):3626–3632. doi: 10.1182/blood-2002-05-1397. and others. [DOI] [PubMed] [Google Scholar]
- 18.Kokame K, Matsumoto M, Soejima K, Yagi H, Ishizashi H, Funato M, Tamai H, Konno M, Kamide K, Kawano Y. Mutations and common polymorphisms in ADAMTS13 gene responsible for von Willebrand factor-cleaving protease activity. Proc Natl Acad Sci U S A. 2002;99(18):11902–11907. doi: 10.1073/pnas.172277399. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu L, Hui AY, Albanis E, Arthur MJ, O'Byrne SM, Blaner WS, Mukherjee P, Friedman SL, Eng FJ. Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut. 2005;54(1):142–151. doi: 10.1136/gut.2004.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kokame K, Nobe Y, Kokubo Y, Okayama A, Miyata T. FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br J Haematol. 2005;129(1):93–100. doi: 10.1111/j.1365-2141.2005.05420.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.