Abstract
Metabolism of oxygen, while central to life, also produces reactive oxygen species (ROS) that have been implicated in processes as diverse as cancer, cardiovascular disease, and aging. It has recently been shown that central nervous system stem cells1, 2 and hematopoietic stem cells and early progenitors3-6 contain lower levels of ROS than their more mature progeny and that these differences appear to be critical for maintaining stem cell function. We hypothesized that epithelial tissue stem cells and their cancer stem cell (CSC) counterparts may also share this property. Here we show that normal mammary epithelial stem cells contain lower concentrations of ROS than their more mature progeny cells. Congruently, subsets of CSCs in some human and murine breast tumors contain lower ROS levels than corresponding non-tumorigenic cells (NTCs). Consistent with ROS being critical mediators of ionizing radiation-induced cell killing7, 8, CSCs in these tumors develop less DNA damage and are preferentially spared after irradiation compared to NTCs. Lower ROS levels in CSCs are associated with increased expression of free radical scavenging systems. Pharmacologic depletion of ROS scavengers in CSCs significantly decreases their clonogenicity and results in radiosensitization. These results indicate that, similar to normal tissue stem cells, subsets of CSCs in some tumors contain lower ROS levels and enhanced ROS defenses compared to their non-tumorigenic progeny, which may contribute to tumor radioresistance.
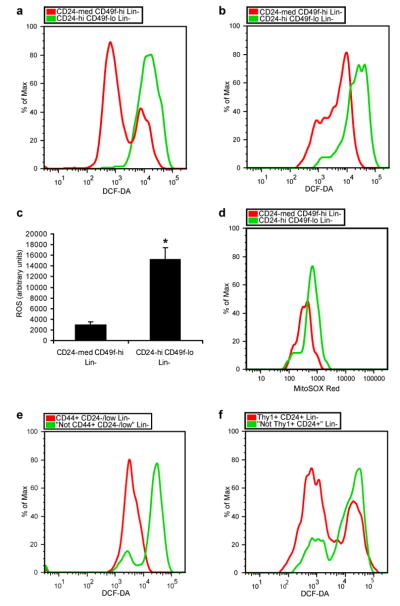
We began by asking whether low ROS concentrations that appear to be critical to self renewal of hematopoietic stem cells (HSCs)3, 5 are also a property of mammary epithelial stem cells9, 10 by isolating CD24medCD49fhighLin- mammary cells, a population enriched for mammary repopulating units (MRUs), and CD24highCD49flowLin- progenitor cells by flow cytometry (Supplementary Fig. S1) and measuring intracellular concentrations of prooxidants using 2′-7′-dichlorofluorescein diacetate (DCF-DA) staining3. Cells in the MRU-enriched population contained significantly lower concentrations of ROS than the progenitor-enriched cells in two different strains of mice (Fig. 1a-c). Specifically, the MRU-enriched populations displayed low to intermediate ROS levels, while the progenitor-enriched populations contained more uniformly high levels of ROS. Similarly, analysis of the two populations with MitoSOX Red, a highly selective detection method for mitochondrial superoxide, revealed lower superoxide levels in the MRU-enriched population (Fig. 1d). In order to assess if mammary repopulating activity was related to intracellular concentrations of ROS, we transplanted CD24medCD49fhighLin- cells based on their levels of DCF-DA staining. Mammary stem cells with both low and intermediate ROS levels gave rise to epithelial outgrowths when transplanted into cleared fat pads (Supplementary Table 1). Similar heterogeneity of ROS concentrations was recently demonstrated in HSC-enriched populations5, 11, where it may have functional significance in modulating the HSC-niche interaction12.
Figure 1. Analysis of ROS levels in normal mammary and breast cancer stem cells and their progeny.
a, CD24medCD49fhighLin- mammary cells (mammary stem cell-containing population) and CD24highCD49flowLin- mammary cells (progenitor cell-containing population) were isolated from C57Bl/6J female mice using flow cytometry and intracellular ROS concentrations were measured by DCF-DA staining. b, as in a but using 29S1/SvImJ mice. c, Mean ± s.e.m. for replicates of a and b (n=6; p=0.001). d, as in b but using MitoSOX Red instead of DCF-DA. Data shown are representative of two independent experiments. e, CD44+CD24-/lowLin- breast cancer cells (CSC-containing population) and “Not CD44+CD24-/low” Lin- cells (non-tumorigenic population) were isolated from a primary human breast tumor by flow cytometry and ROS levels were analyzed using DCF-DA. f, as in e but using murine Thy1+CD24+Lin- breast cancer cells (CSC-containing population) and “Not Thy1+CD24+” Lin- cells (non-tumorigenic population) isolated from an MMTV-Wnt-1 breast tumor.
Given the conservation of low ROS levels in several types of normal tissue stem cells, we hypothesized that CSCs in some tumors may also contain lower concentrations of ROS than their non-tumorigenic progeny. In order to investigate ROS biology in human CSCs, we began by examining the expression of genes involved in ROS metabolism in primary human breast CSCs and NTCs. Using microarray data from human breast CSC-enriched populations and NTCs13 and a curated list of genes involved in ROS metabolism5 (see methods), Gene Set Enrichment Analysis (GSEA)14 revealed that the expression of ROS genes was highly overrepresented in the CD44+CD24-/lowLin- breast CSC-enriched population compared to NTCs (p<0.001; Supplementary Fig. S2). The ROS genes identified as the core enriched genes by GSEA included a number of important antioxidant genes (Supplementary Table 2). Thus, gene expression profiles of human breast CSC-containing populations suggest that they contain higher levels of antioxidant defense systems than NTCs.
Next, we directly assessed ROS levels in human tumor subpopulations. To do this the CD44+CD24-/lowLin- breast CSC-enriched population and the corresponding “Not CD44+CD24-/low” Lin- NTC population were purified from surgically resected breast tumors (Supplementary Fig. S3). DCF-DA staining revealed that the CSC-enriched population in the human breast tumors we examined contained significantly lower levels of prooxidants than the NTC population. In some breast tumors, the vast majority of cells in the CSC-containing fraction displayed a low ROS phenotype compared to NTCs (Fig. 1e) while in others it was restricted to a significant subset of CSCs (Supplementary Fig. S4). We found a similar enrichment of cells with low ROS concentrations in a head and neck tumor (Supplementary Fig. S4). Thus, CSC-enriched populations from some human tumors contain lower average intracellular ROS levels than corresponding NTC populations.
Recently, we have demonstrated that Thy1+CD24+Lin- cells in the majority of spontaneously developing breast tumors from MMTV-Wnt-1 mice are highly enriched for tumorigenic activity15 (Supplementary Fig. S5a). We therefore asked whether the CSC-enriched population in this model system also displays a low ROS phenotype. ROS analysis using DCF-DA revealed that in tumors in which the Thy1+CD24+Lin- population was enriched for CSCs, this population contained a significantly higher fraction of cells with low prooxidant levels than the “Not Thy1+CD24+” Lin- non-tumorigenic population (Fig. 1f). Thy1+CD24+Lin- cells contained two main sub-populations of cells based on ROS concentration, with the low ROS subpopulation being significantly overrepresented compared to NTCs (Supplementary Fig. S5b). In order to confirm the presence of CSCs within the low ROS subpopulation, we transplanted CSCs based on their DCF-DA staining and found that both the low and high ROS subsets of Thy1+CD24+Lin- cells gave rise to tumors in recipient animals (Supplementary Table 3). Thus, a subset of CSCs from these MMTV-Wnt-1 tumors displayed low baseline levels of ROS compared to NTCs.
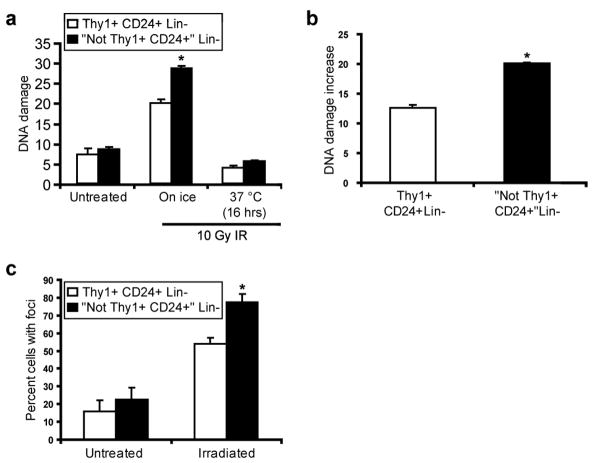
It is well established that cell killing after exposure to ionizing radiation (IR) and a subset of cytotoxic chemotherapeutics is partially mediated by free radicals16. Given our observations of increased expression of ROS defense genes in CSCs, we were therefore interested in testing whether CSC-enriched populations develop less DNA damage after IR than NTCs. In order to examine DNA damage immediately after irradiation, we purified Thy1+CD24+Lin- cells and NTCs from MMTV-Wnt-1 tumors by flow cytometry and irradiated them on ice. Cells were then either left on ice or incubated at 37°C, before being analyzed using the alkaline comet assay17. While untreated cells did not display significantly different levels of DNA damage, there were fewer DNA-strand breaks in the Thy1+CD24+Lin- cells than NTCs immediately after exposure to IR (Fig. 2a-b). These findings are consistent with the hypothesis that enhanced expression of ROS defenses in CSCs contributes to reduced levels of DNA damage after irradiation.
Figure 2. Thy1+CD24+Lin- CSC-enriched cells develop less DNA damage after irradiation than non-tumorigenic cells.
a, Thy1+CD24+Lin- cells (CSC-enriched population) and “Not Thy1+CD24+” Lin- non-tumorigenic cells were isolated from MMTV-Wnt-1 breast tumors by flow cytometry and irradiated with 10 Gy of IR. DNA damage was measured before irradiation, immediately after irradiation, and 16 hrs later using the alkaline comet assay. Mean of median tail moments ± s.e.m. (n=3; p=0.05). b, Using the data from a, the difference in median tail moments between the untreated and “on ice” time points was calculated. Mean ± s.e.m. (n=3; p=0.004). c, Thy1+CD24+Lin- cells and “Not Thy1+CD24+” Lin- non-tumorigenic cells from MMTV-Wnt-1 tumors that were processed and collected 15 minutes after being irradiated in vivo with 1 Gy of IR were immunostained for γ-H2AX, a marker of DNA double strand breaks. Mean ± s.e.m. (n=2; p=0.04).
Since the alkaline comet assay mainly measures single strand breaks and since double strand breaks are important for IR-induced lethality18, we also analyzed levels of double strand breaks as reflected by phosphorylated histone 2AX (H2AX) nuclear foci after in vitro irradiation. As with the comet assay, we again observed significantly lower levels of DNA damage in Thy1+CD24+Lin- CSC-enriched cells than in NTCs (Supplementary Fig. S6). We also measured phosphorylated H2AX foci after in vivo irradiation of MMTV-Wnt-1 tumors, and again found that Thy1+CD24+Lin- cells contained fewer foci than NTCs (Fig. 2c). Thus, consistent with their lower baseline levels of ROS, Thy1+CD24+Lin- CSC-enriched cells isolated from these tumors developed less DNA strand breaks than NTCs after exposure to IR.
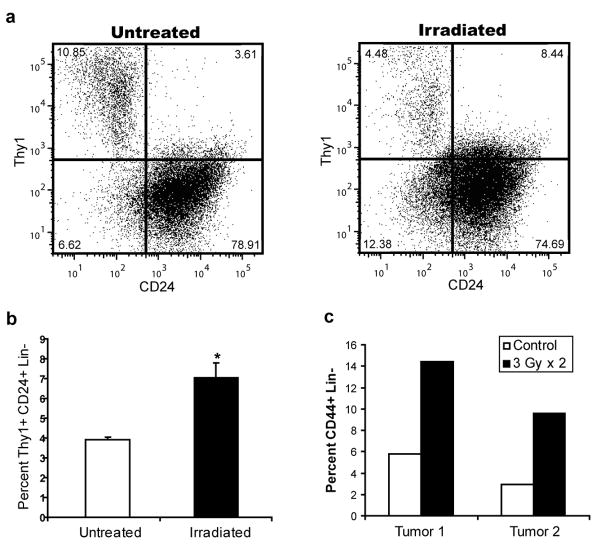
Given these findings, CSCs would be expected to preferentially survive exposure to IR in intact tumors. Mice bearing MMTV-Wnt-1 tumors were therefore treated with short, fractionated courses of IR and the percentage of the Thy1+CD24+Lin- CSC-enriched population before and after irradiation was analyzed using flow cytometry. On average, we found an approximately 2 fold increase in the percentage of the Thy1+CD24+Lin- CSC-enriched population compared with “Not Thy1+CD24+” Lin- NTCs in the irradiated tumors, suggesting that CSCs are relatively radioresistant compared with NTCs (Fig. 3a-b). We found a similar increase in the fraction of CD44+Lin- CSC-enriched population when we irradiated human head and neck cancer xenografts grown in immunodeficient mice (Fig. 3c). Other investigators have documented similar radioresistance of CSCs in brain tumors19 and a breast cancer cell line20. Thus, CSCs in some murine and human tumors are relatively radioresistant compared to their NTC counterparts.
Figure 3. Enrichment of CSCs after in vivo irradiation.
a, Breast tumors from MMTV-Wnt-1 mice were irradiated in vivo with 3 × 5 Gy or 5 × 2 Gy. Percentage of Thy1+CD24+Lin- cells in untreated and irradiated tumors were quantified by flow cytometry 72 hours after the last fraction was delivered. b, Mean ± s.e.m. for replicates of a (n=6; p=0.008). c, First generation xenografts established from two different primary human head and neck cancers were irradiated in vivo with 2 × 3 Gy. Percentage of CD44+Lin- cells was quantified as above.
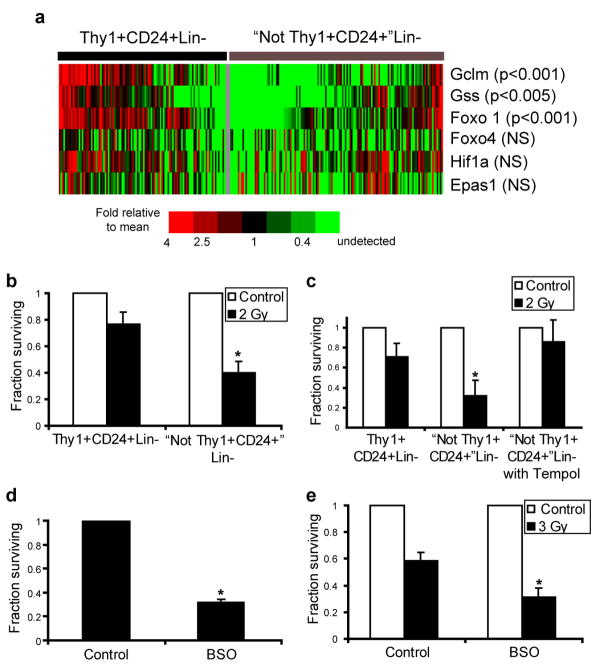
Since a significant fraction of murine breast CSCs contained relatively low levels of ROS, we hypothesized that these cells may express enhanced levels of ROS defenses compared to their NTC counterparts. We were particularly interested in glutathione (GSH), a critical cellular reducing agent and antioxidant which has been implicated in chemotherapy and radiotherapy resistance of cancer cells21. Since our prior analyses revealed heterogeneity within CSC-enriched populations and since single cell gene expression studies have revealed significant variation in gene expression in other stem cell populations22, we investigated expression of critical GSH biosynthesis genes in MMTV-Wnt-1 CSCs and NTCs using single cell qRT-PCR. This analysis revealed significant overexpression of Gclm (p<0.001) and Gss (p<0.005) in a large fraction of cells within the CSC-enriched population, the former of which encodes the regulatory subunit of the enzyme (glutamate-cysteine ligase) that catalyzes the rate limiting step of GSH synthesis (Fig4a)23. Furthermore, Foxo1, a transcription factor implicated in the regulation of an anti-ROS gene expression program in HSCs5, was also overexpressed in CSCs compared to NTCs (p<0.001; Fig.4a). Other genes, including Hif1a, Epas1, and Foxo4 were not differentially expressed. Thus, genes controlling GSH biosynthesis were overexpressed by many cells within the CSC-enriched population isolated from this tumor.
Figure 4. Thy1+CD24+Lin- cells overexpress genes involved in ROS scavenging and pharmacologic modulation of ROS levels affects the radiosensitivity of Thy1+CD24+Lin- and “Not Thy1+CD24+” Lin-cells.
a, Single cell qRT-PCR analysis of gene expression in Thy1+CD24+Lin- CSC-enriched cells and “Not Thy1+CD24+” Lin- non-tumorigenic cells. The heatmap displays mean centered CT values. b, Clonogenic survival of Thy1+CD24+Lin- CSC-enriched cells and “Not Thy1+CD24+” Lin- non-tumorigenic cells before and after 2 Gy of ionizing radiation. Mean ± s.e.m. (n=3; p=0.001). c, Clonogenic survival of “Not Thy1+CD24+” Lin- non-tumorigenic cells in the presence or absence of the ROS scavenger tempol (10 mM). Mean ± s.e.m. (n=2; p=0.03). d, Clonogenic survival of Thy1+CD24+Lin- CSC-enriched cells in the presence or absence of 24 hour pre-treatment with the glutathione synthesis inhibitor L-S,R-Buthionine Sulfoximine (BSO, 1 mM). Mean ± s.e.m. (n=3; p=0.002). e, Clonogenic survival of Thy1+CD24+Lin- CSC-enriched cells after 3 Gy of ionizing radiation with or without BSO pretreatment. Mean ± s.e.m. (n=3; p=0.03).
In order to pharmacologically manipulate ROS levels separately in CSCs and NTCs, we employed in vitro culture conditions that allowed both cell populations to produce colonies upon co-culture with irradiated feeder cells. We found that Thy1+CD24+Lin- CSC-enriched cells were relatively radioresistant compared with NTCs (Fig. 4b and Supplementary Fig. S7). When exposed to 2 Gy, a dose commonly administered clinically during daily treatments of breast cancer patients, 2.0-fold +/- 0.2 more CSC-enriched colonies survived than NTC colonies. Next, we attempted to radioprotect NTCs by exposing them to the nitroxide antioxidant tempol24. Pretreatment with tempol radioprotected NTCs, and resulted in survival levels similar to those seen in the CSC-enriched population (Fig. 4c).
Given the overexpression of genes involved in GSH synthesis by CSCs, we wished to assess the sensitivity of these cells to ROS elevation via pharmacologic depletion of GSH. Exposure of Thy1+CD24+Lin- CSC-enriched cells to Buthionine Sulfoximine (BSO), which inhibits glutamate-cysteine ligase25, decreased their colony forming ability by approximately three fold (Fig. 4d). Finally, we asked if GSH depletion would radiosensitize CSCs. As shown in Fig. 4e, BSO pretreatment of Thy1+CD24+Lin- CSC-enriched cells led to significant radiosensitization. These data demonstrate the importance of low ROS levels and antioxidant defenses to CSC survival and radiosensitivity in these tumors.
Our data indicate that normal breast stem cells and a subset of CSCs in some tumors arising in both mice and humans contain lower levels of ROS than their cellular descendants. Taken together with previous reports of low ROS concentrations in other normal tissue stem cells, these findings suggest that stem cells in diverse systems have conserved this attribute, which likely helps to protect their genomes from endogenous and exogenous ROS-mediated damage. The mechanism leading to low ROS levels in some CSCs appears to be at least partially due to the increased production of free radical scavengers. Notably, there appears to be significant heterogeneity of ROS levels in both normal stem cell and CSC-enriched populations, which could reflect that the enriched populations contain both stem and non-stem cells and/or that ROS levels within stem cells can differ based on environmental factors that alter the balance of endogenous production and the expression of scavenging pathways. The low ROS subset found in the various stem cell populations may also represent a quiescent subpopulation. Heterogeneity of ROS levels may influence the extent to which CSC-enriched populations are resistant to therapies such as ionizing radiation.
In light of recent findings that CSCs in glioblastoma multiforme display enhanced DNA repair capabilities19, it appears that CSCs may resist standard cytotoxic therapies through a combination of mechanisms, and that these may be unique to a given tumor. In the case of human CSCs, the frequency with which these cells display low ROS levels or enhanced DNA repair remains to be determined, particularly since the normal transformation precursor may be either stem or progenitor cells26, 27 and since ROS concentration (Fig. 1 and 5, 28) and increased DNA repair capabilities appear to partially reflect differentiation state. Clinical therapies could likely be optimized by patient- and tumor-specific identification of CSC-resistance mechanisms and overcoming low ROS levels within CSCs may be a useful method for improving local and systemic oncologic therapies.
Methods Summary
Cells were analyzed, collected by FACS, and injected into recipient mice as described with minor modifications from mouse mammary glands10, human breast29 cancers, human head and neck30 cancers, and MMTV-Wnt-1 mouse tumors15. For human samples, informed consent was obtained after approval of protocols by the Stanford University and City of Hope Institutional Review Boards. For intracellular ROS analysis, cells were loaded with 10 μM DCF-DA (Invitrogen), incubated at 37°C for 30 min, and immediately analyzed by flow cytometry. Cells were re-sorted based on their level of DCF-DA staining for transplant experiments. For MitoSOX Red experiments, cells were loaded with 5 uM MitoSOX Red at 37°C for 20 min. DNA damage was evaluated using the single-cell gel electrophoresis assay under alkaline conditions17. For γ-H2AX immunostaining, purified cells were cytospun onto poly-L-lysine coated slides, fixed, permeabilized, and stained with a phospho-specific (Ser 139) histone H2AX antibody (Cell Signaling Technology) followed by a secondary Alexa Fluor 488-conjugated antibody (Invitrogen). For single cell gene expression analysis, cells were sorted into 96 well plates containing CellsDirect qRT-PCR mix (Invitrogen). After reverse transcription, genes were pre-amplified (22 cycles) using the same Taqman primers (Applied Biosystems) used for quantification. Products were analyzed using qPCR DynamicArray microfluidic chips (Fluidigm). For in vitro colony assays, cells were cultured in Epicult B medium (StemCell Technologies) with 5% serum in the presence of ∼13,000 cm-2 irradiated NIH-3T3 cells. After 24-48 hrs, the media was replaced with serum-free Epicult B, and colonies counted ∼7 days later. GSEA14 was performed using previously published microarray data13 of CSCs and NTCs from primary breast tumor samples and a curated list of ROS genes (Supplementary Table 4). Levels of significance were determined by Student's t-tests using α=0.05.
Supplementary Material
Acknowledgments
We thank D. Spitz for helpful discussions and D. Menke, D. Rossi, and J. Seita for technical assistance. This work was supported by grants from the National Institutes of Health (M.F.C. and I.L.W.), the Virginia and D.K. Ludwig Foundation (M.F.C. and I.L.W.), the Breast Cancer Research Foundation (M.F.C.), the Machiah foundation (T.K.), the American Society for Therapeutic Radiology and Oncology (M.D.), and the Radiological Society of North America (M.D.). M.D. is a recipient of the Leonard B. Holman Research Pathway fellowship.
Footnotes
Author Contributions M.D. and R.W.C. contributed equally to this work. M.D, R.W.C., N.L., T.K., M.J.D., A.K., D.Q., J.S.L., L.A., and M.W. performed the experiments. B.J., M.J.K, I.W., F.W., G.S., C.G., B.P., J.S., and S.K.L. aided in human tumor tissue acquisition. G.S. designed a pre-operative protocol allowing for tissue acquisition. M.D., R.W.C., and M.F.C. designed the experiments and wrote the manuscript. S.R.Q., J.M.B., and I.L.W. provided intellectual input and aided in experimental design.
Author Information Reprints and permissions information is available at npg.nature.com/reprintsandpermissions.
References
- 1.Smith J, Ladi E, Mayer-Proschel M, Noble M. Redox state is a central modulator of the balance between self-renewal and differentiation in a dividing glial precursor cell. Proc Natl Acad Sci U S A. 2000;97:10032–7. doi: 10.1073/pnas.170209797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsatmali M, Walcott EC, Crossin KL. Newborn neurons acquire high levels of reactive oxygen species and increased mitochondrial proteins upon differentiation from progenitors. Brain Res. 2005;1040:137–50. doi: 10.1016/j.brainres.2005.01.087. [DOI] [PubMed] [Google Scholar]
- 3.Ito K, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 4.Ito K, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–51. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 5.Tothova Z, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–39. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Miyamoto K, et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–12. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Powell S, McMillan TJ. DNA damage and repair following treatment with ionizing radiation. Radiother Oncol. 1990;19:95–108. doi: 10.1016/0167-8140(90)90123-e. [DOI] [PubMed] [Google Scholar]
- 8.Ward JF. Biochemistry of DNA lesions. Radiat Res. 1985 8:S103–11. [PubMed] [Google Scholar]
- 9.Shackleton M, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–8. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 10.Stingl J, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–7. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 11.Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110:3056–63. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosokawa K, et al. Function of oxidative stress in the regulation of hematopoietic stem cell-niche interaction. Biochem Biophys Res Commun. 2007;363:578–83. doi: 10.1016/j.bbrc.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Liu R, et al. The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Engl J Med. 2007;356:217–26. doi: 10.1056/NEJMoa063994. [DOI] [PubMed] [Google Scholar]
- 14.Subramanian A, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–50. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho R, et al. Isolation and molecular characterization of cancer stem cells in MMTV-Wnt-1 murine breast tumors. Stem Cells. 2008;26:364–71. doi: 10.1634/stemcells.2007-0440. [DOI] [PubMed] [Google Scholar]
- 16.Riley PA. Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int J Radiat Biol. 1994;65:27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- 17.Dorie MJ, et al. DNA damage measured by the comet assay in head and neck cancer patients treated with tirapazamine. Neoplasia. 1999;1:461–7. doi: 10.1038/sj.neo.7900060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jonathan EC, Bernhard EJ, McKenna WG. How does radiation kill cells? Curr Opin Chem Biol. 1999;3:77–83. doi: 10.1016/s1367-5931(99)80014-3. [DOI] [PubMed] [Google Scholar]
- 19.Bao S, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 20.Phillips TM, McBride WH, Pajonk F. The response of CD24(-/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98:1777–85. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 21.Estrela JM, Ortega A, Obrador E. Glutathione in cancer biology and therapy. Crit Rev Clin Lab Sci. 2006;43:143–81. doi: 10.1080/10408360500523878. [DOI] [PubMed] [Google Scholar]
- 22.Warren L, Bryder D, Weissman IL, Quake SR. Transcription factor profiling in individual hematopoietic progenitors by digital RT-PCR. Proc Natl Acad Sci U S A. 2006;103:17807–12. doi: 10.1073/pnas.0608512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffith OW. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic Biol Med. 1999;27:922–35. doi: 10.1016/s0891-5849(99)00176-8. [DOI] [PubMed] [Google Scholar]
- 24.Hahn SM, et al. Potential use of nitroxides in radiation oncology. Cancer Res. 1994;54:2006s–2010s. [PubMed] [Google Scholar]
- 25.Bailey HH. L-S,R-buthionine sulfoximine: historical development and clinical issues. Chem Biol Interact. 1998;111-112:239–54. doi: 10.1016/s0009-2797(97)00164-6. [DOI] [PubMed] [Google Scholar]
- 26.Jamieson CH, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351:657–67. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 27.Akala OO, et al. Long-term haematopoietic reconstitution by Trp53-/-p16Ink4a-/-p19Arf-/- multipotent progenitors. Nature. 2008;453:228–32. doi: 10.1038/nature06869. [DOI] [PubMed] [Google Scholar]
- 28.Saretzki G, et al. Downregulation of multiple stress defense mechanisms during differentiation of human embryonic stem cells. Stem Cells. 2008;26:455–64. doi: 10.1634/stemcells.2007-0628. [DOI] [PubMed] [Google Scholar]
- 29.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prince ME, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007 doi: 10.1073/pnas.0610117104. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.