Introduction
There has been significant progress over the past two decades in the surgical treatment of spinal disorders, including advances in spinal instrumentation, minimally invasive spine surgery, biomaterials, and artificial disc replacement. Although improvements in surgical treatments are on track, basic science research on the pathogenesis of intervertebral disc (IVD) degeneration and the mechanism of pain is nascent. A better understanding of the pathogenesis of IVD degeneration will culminate in greater advances in treatment and improve patient selection and indications for emerging techniques and procedures. In this article, we review the relevant literature on biological and biomechanical research, including our own recent publications.
Biological factors
Although the exact pathogenesis is unknown, the mechanically- induced and biologically-mediated degenerative disc is conceptualized to be a consequence of pathological or aging changes associated with genetic background. The IVD consists of an outer anulus fibrosus (AF), which is rich in collagens that account for its tensile strength, and an inner nucleus pulposus (NP), which contains large proteoglycans (PGs) that retain water for resisting loading by compression. Biologically, disc cells residing in both the AF and NP actively regulate the homeostasis of IVD tissues by maintaining a balance between anabolism and catabolism. Disc cells modulate their activity by a variety of substances, including cytokines, growth factors, enzymes, and enzyme inhibitors, in a paracrine or/and autocrine fashion.1,2
Anabolic regulators include polypeptide growth factors, such as insulin-like growth factor (IGF), transforming growth factor-β (TGF-β), and the bone morphogenetic proteins (BMPs).1–4 Other small molecules, such as the synthetic peptide of link protein, have also been reported to be regulators of matrix synthesis.5 The catabolic process is mediated by various enzymes, such as the matrix metalloproteinases (MMPs),6–16 members of a disintegrin-like and metalloprotease with thrombospondin motifs (ADAMTS) family (aggrecanases),17–19 and cytokines.13,20–26 Disc degeneration can result from an imbalance between the anabolic and catabolic processes or the loss of steady-state metabolism that is maintained in the normal disc. With IVD degeneration, the gradual loss of large PGs, such as aggrecan and versican, from the NP has been reported; however, in the AF, there is an initial upregulation of these proteins in the early stages of disc degeneration, followed by a downregulation in the late stage of disc degeneration.27 Therefore, therapeutic strategies might be different depending on the stage of disc degeneration and for the NP versus the AF. Therapeutic strategies for disc degeneration include an upregulation of important matrix proteins, such as aggrecan, or downregulation of proinflammatory cytokines and matrix-degrading enzymes, such as the MMPs and ADAMTSs, possibly by applying both strategies.
The importance of nutrition to the IVD, which is the largest avascular tissue in the body, in the pathogenesis of disc disease is well recognized.28–36 To maintain the steady-state metabolism of the cells, the IVD requires proper nutrition, mainly by diffusion from the vertebral bodies and endplates.29 Trauma, cigarette smoking, deposition of calcium crystal, and other factors that disrupt the integrity of the endplates may affect diffusion and disturb the nutrition of the disc cells.28 It is important to assess the nutritional status of the degenerated disc before contemplating biological treatment because such treatment will be ineffective if there is a complete loss of diffusion or nutrition to the disc.
For delivering therapeutic biologic agents, several methods of administration can be considered. Protein injection is relatively simple and practical; however, efficacy, duration of action, and the possibility of adverse effects should be thoroughly tested. In gene therapy, DNAs that encode specific proteins are delivered into the cells by viral or nonviral transfection; the transfected cells eventually produce proteins to, theoretically, prolong the duration of action. Both viral and nonviral transfection methods have pros and cons. Safety and immunological reactions as well as the control of expression in viral-mediated gene therapy are potential problems. On the other hand, the transfection efficiency of nonviral methods is generally inferior.37 Cell transplantation has the potential to regenerate the disc matrix,38–40 but it involves cell procurement and its efficacy has not been fully established.41,42 Depending on the severity of disc degeneration, a combined approach may be needed. It is assumed that matrix repair will bring about the biomechanical restoration of the disc and the functional motion segment, and, optimistically, relieve the patient’s discogenic pain. These assumptions are numerous; much needs to be done to test these hypotheses and assumptions.
Growth factors and BMPs
When the clinical application of growth factors, including BMPs, is contemplated, several factors, such as indication, mode of delivery, dose, duration, and side effects, should be taken into consideration to achieve the greatest beneficial therapeutic effect. Each growth factor may have a different effect on the NP and AF, and the action at different stages of degeneration may be different. An in vitro study using a validated method is essential to explore the therapeutic use of growth factors.
In vitro evidence for the feasibility of growth factor application
Thompson et al. first reported the positive effects of various growth factors, including TGF-β, epidermal growth factor (EGF), and basic fibroblast growth factor (bFGF),3 on PG synthesis; these effects were more pronounced in the NP. Osada et al. have shown that IGF-1 stimulated PG synthesis by bovine NP cells in serum-free conditions in a dose-dependent manner.4 Recently, Tim Yoon et al. showed that recombinant human BMP-2 (10–1000 ng/ml) increased cell proliferation, PG synthesis, mRNA expression of type II collagen, aggrecan, SOX9, and osteocalcin in the monolayer culture of rat AF cells.43 Furthermore, these authors showed that the stimulatory effect of BMP-2 (at 1–100 ng/ml) was inhibited by the addition of nicotine (10–100¼g/ml) in the culture media in rat NP cell culture.44 These results suggested that nicotine may contribute to the process of disc degeneration by a direct effect on NP cells, possibly by antagonizing the effect of BMP-2.
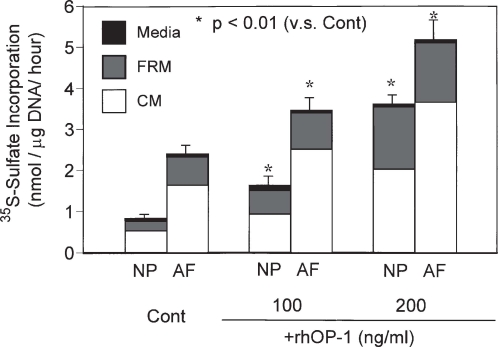
We have recently reported the in vitro stimulative effect of osteogenic protein 1 (OP-1, otherwise known as BMP-7) on PG and collagen metabolism in rabbit NP and AF cells cultured in alginate beads.45 In both cell types, recombinant human OP-1 (rhOP-1) stimulated the synthesis of PG and collagens in a dose-dependent manner (50–200 ng/ml) in the presence of 10% fetal bovine serum (FBS) (Fig. 1). Although the responsiveness of IVD cells to IGF-1 and TGF-β has been shown to decrease with increasing age in rabbit IVD cells,46 OP-1 effectively stimulated PG synthesis of fetal, adult, and old bovine NP and AF cells.47 In fact, the IVD cells of the older animals in this study were very responsive to OP-1. The age-related and degeneration stage-related change in the response to growth factors is of special interest for future studies because of the emerging trend to treat disc degeneration in adults or older individuals by the local injection of growth factors.48
Fig. 1.
Effect of recombinant human osteogenic protein 1 (rhOP-1) treatment on proteoglycan (PG) synthesis by rabbit nucleus pulposus (NP) and anulus fibrosus (AF) cells cultured in alginate beads. In both rabbit NP and AF cultures, significant dose-dependent increases in the rate of PG synthesis per mg DNA were observed on the addition of rhOP-1 to the medium [Dulbecco’s modified essential medium (DMEM)/F12 + 10% fetal bovine serum (FBS)]. (From ref. 45, with permission.) FRM, Further removed matrix; CM, cell-associated matrix
Several in vitro feasibility studies were performed to test if growth factors can overcome a negative balance of matrix homeostasis. To model the repair process after matrix depletion in IVD cells, degeneration of the matrix of rabbit IVD cells was first induced by exposure to the proinflammatory cytokine interleukin 1 (IL-1), using the alginate culture system; subsequently, rhOP-1 was applied in culture.49 OP-1 (100 ng/ml) was effective in promoting, in vitro, the repair of the damaged matrix surrounding the NP and AF cells after exposure to IL-1. A similar study was performed using a brief exposure to the glycosidase chondroitinase-ABC (C-ABC) to deplete PGs and hyaluronan in the matrix around NP and AF cells.50 After the matrices around the cells were depleted by C-ABC digestion, the cultures were then maintained in media containing 10% FBS and OP-1 (100 ng/ml). As seen in the case of IL-1 exposure, OP-1 stimulated the repair of the C-ABC-treated matrix around NP and AF cells, actually resulting in an increase above the normal level of matrix molecules. These two studies became the fundamental basis for our strategy that growth factor injection therapy can be applied to repair the matrix of degenerated human IVDs or to promote matrix repair in conjunction with chemonucleolysis.
Because the cellular phenotypes of young animals, especially rat and rabbit cells, are different from those of adult human cells, studies using human disc cells are essential for the clinical development of growth factor therapy. There is considerable evidence in the recent literature that human disc cells, healthy or degenerated, will respond to growth factors and BMPs. Gruber et al. reported that human AF cells in three-dimensional cultures (agarose gel and alginate beads) produced and accumulated more abundant extracellular matrix molecules, such as PGs, than those cultured in monolayer; under those conditions; they also showed that cell proliferation was stimulated by TGF-β.51
Imai et al. reported that OP-1 (100–200 ng/ml) enhanced the production and accumulation of PGs by human NP and AF cells cultured in alginate beads in the presence of 10% FBS.52 Interestingly, AF cells, which are more fibrochondrocytic, strongly responded to OP-1, suggesting that OP-1 might be beneficial not only for nucleus repair but for anulus repair as well.
Recombinant human BMP-2 (rhBMP-2) has been shown to stimulate PG synthesis at relatively high doses (67% at 300 ng/ml and 200% at 1500 ng/ml rhBMP-2).53 Ahn et al. recently reported that both BMP-2 and BMP-12 stimulated PG and collagen synthesis by human NP cells from degenerated discs cultured in monolayer in the absence of serum.54
A decrease in cell number is one characteristic of aging IVD tissue.55 Growth factor therapy may be beneficial to maintain functional cells in IVD tissues. Gruber et al. reported a high incidence of apoptosis in AF tissues.56 Furthermore, they found that the surviving AF cells were not synthetically inactive but were, rather, producing inappropriate matrix molecules during aging and degeneration. Based on these initial findings, they studied the effects of IGF-1 and platelet-derived growth factor (PDGF) on apoptosis in human AF cells grown in monolayer culture.57 In monolayer cultures of human degenerative IVD cells, Wehling recently reported that the combination of autologous IL- 1 receptor antagonist (IL-1ra)/IGF-1/PDGF proteins, which was produced in vitro by stimulating monocytes and thrombocytes, reduced the percentage of apoptosis and the production of biochemical markers of disc degeneration, such as IL-1 and IL-6.58
In vivo evidence of the feasibility of growth factor application
Walsh et al. reported the in vivo effects of a single injection of other growth factors, such as bFGF, growth differentiation factor 5 (GDF-5), IGF-1, or TGF-β, in the mouse caudal disc with degeneration induced by static compression.59 An injection of GDF-5 was effective in promoting disc regeneration. Multiple injections (four injections, once per week) of TGF-β showed a stimulatory effect, although the other growth factors did not show a significant enhancement of their original effect.59
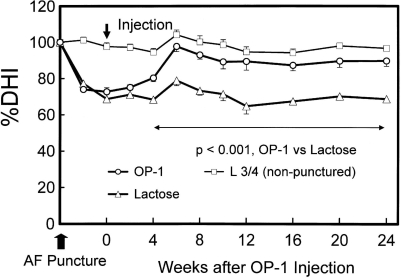
As the first feasibility in vivo study on the effect of OP-1, we reported that the in vivo intradiscal administration of the growth factor OP-1 resulted in an increase in disc height and in the PG content of the NP in normal rabbits.60 At 2 weeks after the injections of OP-1 (2¼g in saline), the mean disc height index of the OP-1-injected discs was 15% greater than that of the saline group, and the increase was sustained for up to 8 weeks. Biochemically, a significant increase in the PG content of the NP was observed in OP-1-injected discs when compared to the saline-injected group at the 2-week time point. Using a defined-gauge needle puncture of the AF,61 we conducted a study of the radiographic and magnetic resonance imaging (MRI) changes in the rabbit IVD after injection of OP-1 into the NP in the anular-puncture disc degeneration model. Six weeks after the OP-1 injection, a restoration of disc height and MRI changes were observed and sustained for the entire experimental period, up to 24 weeks (Fig. 2).62 In another degeneration model using C-ABC, 4 weeks following the injection of C-ABC, OP-1 (100¼g/disc) injection restored the disc height up to 12 weeks after the OP-1 injection.63 These data suggest that OP-1 may be utilized in patients with disc degeneration who had received chemonucleolysis in the past that resulted in some loss of disc height.
Fig. 2.
Changes in intervertebral disc height Index (DHI) after anular fibrosus (AF) puncture and osteogenic protein 1 (OP-1) injection. The percent DHI (%DHI = postoperative DHI/preoperative DHI × 100) was measured at each time point to quantify changes in disc height. By 4 weeks after the OP-1 injection, the mean %DHI of injected discs in the OP-1 group was significantly higher than that in the lactose control group [P < 0.001, repeated analysis of variance (ANOVA)]. This significant difference in mean %DHI was maintained during the follow-up period (P < 0.001). (From ref. 62, with permission)62
Gene therapy
Nishida et al. injected an adenovirus construct encoding the TGF-β gene (Ad/CMV-hTGF-β1) into lumbar discs of skeletally mature rabbits and showed the feasibility of using the gene therapy approach to induce biological changes in IVD tissues in vivo. The NP tissues from the discs injected with Ad/CMV-hTGF-β1 showed a significant increase in production of active and latent TGF-β1. They found that these tissues exhibited an increase of 100% in PG synthesis when compared to intact control tissues.64 However, the effect of TGF-β on PG synthesis remains controversial. Wallach et al., from the same group, recently reported the inhibition of PG synthesis by the transduction of the TGF-β1 gene.65 The authors suggested that the inhibitory response to TGF-β1 may have resulted from increased cell-to-cell interactions within the three-dimensional pellet culture, which in turn may have produced higher, and potentially toxic, TGF-β1 concentrations in the microcellular environment. More biologically relevant in vivo studies, such as those using a large animal model, might be required to reach a conclusion on the effect of TGF-β1. Ahn et al. recently reported that the introduction of the BMP-12 gene into human IVD cells in pellet culture by an adenoviral vector significantly increased cell proliferation and matrix production (PG and collagen).66 The increase in mRNA for collagen types I, II, and VI in the AF cells was more pronounced than that in NP cells.
Matsumoto et al. have also shown that the nonviral gene transfer of the human OP-1 gene into bovine NP and AF cells, using the gene gun (particle-mediated gene transfer method), can stimulate PG synthesis.67 On day 3 after transfection of the human OP-1 gene, transfected cells synthesized PGs at a faster rate in both the AF (+24%) and NP (+44%). In an in vivo experiment, the injection of OP-1-transfected cells (allogenic cells from a donor rabbit) into the NP induced a significant preservation of disc height and retention of PG content when compared to the control vector-transfected group.68
Wang et al. showed that transfection of the GDF-5 gene into rabbit and human IVD cells with adenovirus encoding the GDF-5 gene or the TGF-β gene induced a significant increase of GDF-5 and TGF-β protein production,69 respectively.
Paul et al. reported the successful adenovirus-mediated gene transfer of the SOX9 gene and the BMP-2 gene, both with green fluorescent protein (GFP), into IVD cells obtained from human degenerated IVD tissues70 with a steady expression over the 80% level for 7 days in culture and also with an increased level of type II collagen synthesis. The in vivo transfection of the SOX9 gene by adenoviral vector using the rabbit stab model resulted in maintenance of a chondrocytic appearance over 5 weeks.70
Wallach et al. also reported that gene transfer of the tissue inhibitor of metalloproteinase 1 (TIMP-1), an inhibitor of catabolic enzymes, can increase PG accumulation in pellet cultures of human IVD cells.71 This study introduced a new approach to modulate a course of disc degeneration by inhibiting the catabolic pathway rather than stimulating the anabolic pathway. The combined use of anabolic factors with an inhibitor of a catabolic enzyme is one way to induce regeneration of IVD disc undergoing degenerative changes.
Yoon et al. reported that overexpression of LIM mineralization protein 1 (LMP-1),72 an intercellular protein, using an adenoviral vector (AdLMP-1) in rat IVD cells resulted in an increase of BMP-2 and BMP-7 gene expression and PG synthesis.73 BMP-2 and BMP-7 protein levels increased by two- to threefold. The mRNA levels of BMP-2 and BMP-7 were also upregulated. In addition, the in vivo injection of AdLMP-1 resulted in a significant elevation of mRNA levels of LMP-1, BMP-2, and BMP-7. Based on these findings, the authors suggested that LMP-1 upregulates the gene expression of BMP-2 and BMP-7 and that the increased level of BMP production resulted in an increased PG synthesis.
Based on these studies, gene therapy has the potential to upregulate matrix synthesis and downregulate catabolic processes by introducing cDNAs that encode specific proteins. With any gene therapy, safety issues should be resolved, as well as transfection efficiency and duration of action. In addition, further research using degenerated human IVD cells may be necessary before taking this approach to a clinical setting.
Summary of the biological factors
In the treatment of degenerative disc diseases, no clinical option currently exists between conservative therapies and more aggressive therapies, such as fusion or disc replacement. The prevention of disc degeneration and the stimulation of the biological disc repair process by the injection of growth factors, cytokine inhibitors, enzyme inhibitors, and cells transfected with therapeutic genes will create a new category of therapy for degenerative disc diseases. In addition, two or more different approaches might be applied, resulting in the prevention of disc degeneration and/or the active stimulation of the repair process of the degenerated disc. These therapies could produce an amelioration of pain and restructuring of the degenerated disc.
Biomechanical factors
Kinematic and load-bearing characteristics are key factors describing the biomechanical behavior of the spine. Spinal instability, which has been thought to be one of the most important biomechanical factors in the degenerative IVD, is poorly defined and understood.74–82 It has also been suggested that degenerative changes of the IVD precede facet joint degeneration through an altered load-bearing in the spine. The following sections describe biomechanical changes in segmental movement and load-bearing through the facet joint in the degenerated lumbar spine.
Relationship between disc degeneration and segmental instability of the lumbar spine
The basic concept of spinal instability is that excessive motion beyond normal constraints causes either compression or stretching of the neural elements or causes abnormal deformations of ligaments, joint capsules, annular fibers, or endplates, which are known to have a significant number of nociceptors.80 Even though several studies have indicated that excessive motion on flexion-extension radiographs is associated with degenerative disc disease,83 other studies cite decreased motion in patients with degenerative changes.84,85 Lumbar segmental instability may be associated with a spectrum of clinical manifestations of degenerative changes in the IVD.77,86,87 Intervertebral disc degeneration has been studied using magnetic resonance (MR) imaging, and grades of degeneration have been reported.87–89 There also have been numerous biomechanical studies on disc degeneration and instability.85,90–101 The relationship between the types (or grades) of disc degeneration and kinematic characteristics of the motion segment has been studied using cadaveric spinal motion segments. 102–107 Despite variations in results, likely because of different loading conditions and methods of grading degenerative disc changes, the overall results of these studies indicate that the biomechanical characteristics of the motion segment can become significantly altered when degenerative changes develop in the IVD. In vitro biomechanical studies by Fujiwara et al. determined the changes in the rotational mobility of the lumbar motion segment in relation to the degenerative changes in the disc and facet joints.102 The authors found that segmental motion increased with increasing severity of disc degeneration to grade 4 but decreased when the disc degeneration advanced up to grade 5. Such segmental motion changes were much greater in axial rotation when compared to those in lateral bending, flexion, and extension, demonstrating the importance of torsional instability in diagnosing spinal instability. The results of these studies are important for understanding the kinematic changes in relation to the types or grades of disc degeneration. However, these results were obtained in vitro from cadaveric specimens. Therefore, we developed a noninvasive in vivo segmental lumbar motion measurement system and clarified the relationship between the lumbar segmental motion and disc degeneration, as indicated in the following sections.
In vivo measurement of lumbar segmental movement
Many methods have been used in an attempt to quantify segmental instability in patients, but the most typical method has been flexion-extension lateral radiographs. The use of radiographs has been shown to be limited by poor accuracy, and radiographic studies are impractical to measure out-of-plane rotation.108,109 In addition, the range of motion measured in many radiographic studies is affected by the variability in voluntary efforts that the subject applies at the time of examination and can also be limited because of pain. Other two-dimensional (2D) methods for measuring axial rotation, as opposed to flexion/extension, have involved MR imaging scanning of subjects in various rotated positions.109,110 Although these studies were noninvasive and controlled for voluntary motions, they could only determine changes in vertebral motion around one axis. It has been suggested that coupled motions could play an important role in determining spinal instability. To measure these coupled motions, studies have been conducted to measure 3D motions in vivo. More invasive techniques involve inserting wires into the spinous process of subjects to determine 3D motion.94 Although this method has proven more accurate than radiographs, its invasive nature limits its widespread clinical use. Other researchers have used biplanar radiography, in which subjects are filmed from two directions simultaneously and 3D motions are determined from anatomical landmarks in corresponding images.111,112 There has been some concern about the accuracy of determining anatomical landmarks for biplanar radiography, as well as a lack of equipment for this method in typical clinical settings. To combat some of these limitations to 3D motion measurement, Lim et al. developed a 3D imaging technique using parallel computed tomography (CT) scans to determine rotations and translations in individual cadaveric cervical vertebrae.108 The authors illustrated that accurate measurements (±1mm and ±1°) can be made using CT in vitro. We have expanded on this technique to measure vertebral segmental rotations and translations, the motion between adjacent vertebral bodies, in human lumbar spines in vivo.
Relationship between instability and disc degeneration
Most patients with segmental instability have disc degeneration, but the relationship between instability and degeneration has not been clarified. Disc degeneration has been extensively studied, particularly with MR imaging.113–116 Yu et al.116 described the MR imaging appearance of degenerating discs as a change in signal intensity associated with a radial tear of the AF. Schiebler et al.115 showed that before the obvious MR imaging signal changes, such changes as infolding of the central fibers of the outer anulus and the central dot may be early signs of disc degeneration. Recently, using MR imaging, Hedman and Fernie113 reported that certain patterns such as a dark nucleus with annular tears were highly associated with positive symptoms on discography. These studies indicate the ability of MR imaging to identify anatomical changes in the IVD and possibly identify dynamic changes of the disc with different loadings, and even, potentially, to identify symptomatic discs. Takekuchi et al.117 presented a study using MR images in which T1 relaxation time was decreased in degenerative discs and the energy dissipated to axial loading was linearly correlated with T1 relaxation time. The authors attempted to correlate the intrinsic biomechanical properties of the disc with MR imaging findings, but no information could be derived about the segmental motion characteristics from this study.
Results of the in vitro studies of segmental motion characteristics and disc degeneration done by Fujiwara et al. demonstrated that torsional motion was most significantly affected by the degenerative changes in disc and facet joints. In addition, some investigators advocate the importance of torsional loads and stability on the injuries and degeneration of the motion segments.118,119 However, torsional instability in relation to the degenerative changes in the disc had not been well investigated in vivo, mostly because of difficulties in measurements.
To resolve the accuracy problems in spinal motion measurements and to establish a meaningful relationship between segmental instability and degenerative changes in the disc and facet joints, we have established a method to measure 3D vertebral motion noninvasively using CT images of the vertebrae.108 We have developed an in vivo measurement system of lumbar segmental motion with an accuracy of less than 0.2mm in translation and 0.2° in rotation (Fig. 3). We also developed a fixture to apply axial rotation to the lumbar spine in a controlled manner with minimal voluntary effort of the subjects (Fig. 4). Using the new techniques, we found that a relationship exists between the severity of IVD degeneration and increases in torsional and flexion-extension movements in vivo, which was previously demonstrated only in cadaveric studies.120
Fig. 3.
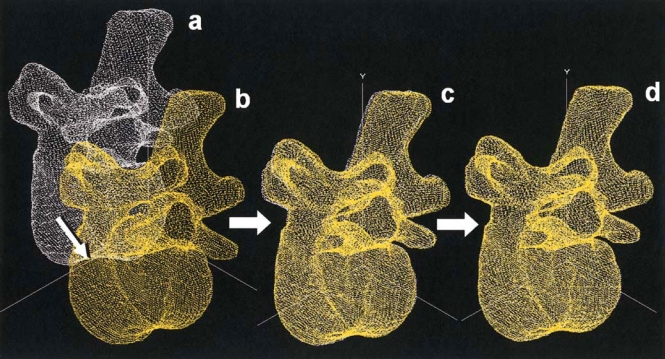
Lumbar segmental motion in vivo measurement system. The vertebral body in the neutral position (a) was virtually rotated and translated toward the real rotated position (b). The roughly merged position (c) was further rotated and translated with 0.1° and 0.1-mm increments, respectively, until the highest value of volume merge was calculated (d)
Fig. 4.
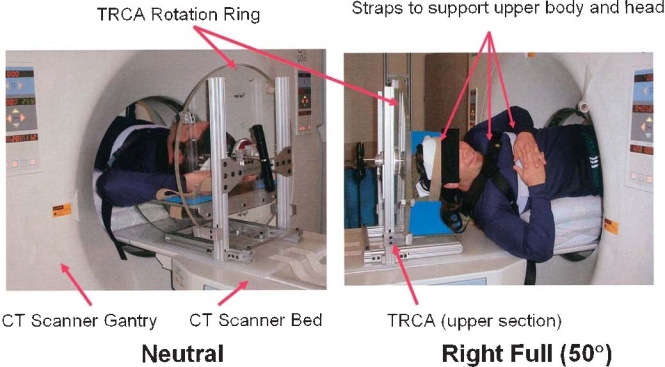
A torsion control apparatus (TCRA) to apply axial rotation to the lumbar spine. A TCRA with a subject shown in the neutral position (left) and with a right rotation of 50° (right)
Facet joint degeneration associated with disc degeneration
Facet load transmission
Facet joints are described as motion limiters having a role in injury prevention.101 The facet joints also contribute to load transmission in the motion segment. Many studies have evaluated the loads carried by the facets during physiological motion using indirect methodologies. 121–128 These methods have used proportions of loads in cadaver studies or predicted loads in mathematical models. Yang and King measured facet loads indirectly by calculating the difference between disc load and total applied loads in cadaver lumbar motion segments.129 The authors determined that facet loads in two body motion segments increased with extension posture (30% of applied load) from neutral (25% of applied load) or flexion postures (22% of applied load). In addition, the authors determined that facet loads increased with the presence of facet osteoarthritis in two specimens (49% of applied load) in extension postures. Similarly, Adams and Hutton130 determined that the facets supported 16% of the applied load through the lower facet joints in a “standing” posture by comparing load-deformation curves of specimens with and without facets. In another paper, Adams and Hutton determined that the facets are major contributors to the resistance to torsion because of bony interactions.131 Using strain gauges applied to the exterior facet surface, Schendel et al.132 determined locations of peak forces on the facets for various motions. They calculated that flexion resulted in no measurable facet forces whereas extension produced the greatest forces. The facet load versus applied extension moment was biphasic in that measured facet stiffness changed slope after 4.5–6.0Nm of applied load. This pattern was not seen with torsional or lateral bending moments. The authors explained that this change in stiffness in extension loading could be caused by impingement of one facet on the other. Peak forces were located on lateral and inferior margins of the superior facet.
Other researchers have used more direct methods for measuring facet loads. In an early study, Lorenz et al.121 used pressure-sensitive film to determine the proportion of load carried by the facets during axial compression and extension loading. The authors calculated that a greater proportion of load was carried in the upper (L2–L3) versus the lower (L4–L5) facets for similarly applied external loads. Furthermore, a decrease in total percent loads supported by the facets at higher axial compressive loads was observed with greater peak pressures at the facets, illustrating the phenomenon of compressive preload affecting measured facet loads. More recently, Hedman and Fernie measured facet loads with an inserted load cell in combination with Fuji film for determination of facet load area.133 The authors measured relative mean facet loads of 50 N for extension postures compared to 5.6 N in flexed postures at L4–L5 in lumbar spinal segments. The authors noted that the combined transducers placed in the facet joint resulted in joint distraction and that only 56% of the facet loads passed through the sensitive area of the load cell, creating a “relative” versus total load measurement.122
Mathematical models have also confirmed that the loss of disc height anteriorly results in a dorsal load transfer to the posterior elements.95,104,134 Shirazi-Adl135 predicted that vertebral body motions under torsion were decreased with larger facet gap distances, indicating the presence of a healthy facet. This model predicted a linear increase in facet contact force with increasing axial torque.
Facet joint degeneration
Facet joints are synovial articulations and undergo degenerative changes similar to those of other weight-bearing joints.96,104,136–138 In healthy facets, the articular surfaces allow smooth motion between vertebrae. These surfaces consist of a thin layer of hyaline cartilage with synovial fluid allowing for a low-friction articulation. Alterations in contact pressures as a result of disc degeneration may create areas of focally increased contact, hastening facet cartilage catabolism.104,105,121,124,125,139–143 Clinically, it has been observed that disc degeneration precedes facet degeneration. Butler et al. demonstrated the occurrence of facet degeneration following IVD degeneration using MR imaging analysis.144
Relationship between disc degeneration and facet degeneration
Biomechanical alterations in motion resulting from IVD degeneration and their ensuing effects on facet kinematics have been largely ignored. The facet joints are one of the primary structures for stability in the spinal motion segment.90,99,100,121,123–125,130,136,140,145–156 Load-displacement relationships in the intervertebral motion segments are a complex interaction involving at least six degrees of freedom and structural and material nonlinearity.126,157 As such, alteration in the load-bearing role of the facet joint most likely occurs with IVD degeneration because of the alterations in the structural properties of the disc.97,135,141,158
However, it was not until the work of Fujiwara et al. that the association between segmental motion characteristics of the IVD and the facet joints was analyzed.102,159–161 The most pronounced association was found between segmental motion and the severity of subchondral sclerosis. The authors believed that the severity of subchondral sclerosis reflected the magnitude of applied stresses on the involved facet joints. However, a mechanistic relationship between disc degeneration and facet joint degeneration still remains unclear. Further studies are needed to understand facet joint degeneration associated with disc degeneration.
Summary of the biomechanical factors
The newly developed noninvasive 3D analytical method to determine segmental motion of the lumbar spine permitted the demonstration of a relationship that exists between the severity of IVD degeneration and increases in segmental lumbar motion in vivo. Abnormal motions could accelerate the facet degeneration processes and may lead to facet osteoarthritis. A better understanding of the effects of IVD degeneration on alternations in facet kinematics and load-bearing would provide a clear understanding of the pathogenesis of the facet degeneration derivative of IVD degeneration. A clarification of the relationship between structure and the mechanical properties of the degeneration of the disc and facet joints would also provide a strategy for treatment modality selection at different IVD degeneration levels.
Acknowledgments
This study was supported by NIH grant P01-AR 48152. The authors acknowledge Mary Ellen Lenz, M.S., for her assistance in the preparation of the manuscript.
Footnotes
This Instructional Lecture was presentd at the 20th Annual Research Meeting of the Japanese Orthopaedic Association, Mie, Japan, October 2005.
References
- 1.Masuda K, Oegema TR, Jr, An HS. Growth factors and treatment of intervertebral disc degeneration. Spine. 2004;29:2757–69. doi: 10.1097/01.brs.0000146048.14946.af. [DOI] [PubMed] [Google Scholar]
- 2.Masuda K, An HS. Growth factors and the intervertebral disc. Spine J. 2004;4:330S–40S. doi: 10.1016/j.spinee.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 3.Thompson JP, Oegema TJ, Bradford DS. Stimulation of mature canine intervertebral disc by growth factors. Spine. 1991;16:253–60. doi: 10.1097/00007632-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Osada R, Ohshima H, Ishihara H, Yudoh K, Sakai K, Matsui H, et al. Autocrine/paracrine mechanism of insulin-like growth factor-1 secretion, and the effect of insulin-like growth factor-1 on proteoglycan synthesis in bovine intervertebral discs. J Orthop Res. 1996;14:690–9. doi: 10.1002/jor.1100140503. [DOI] [PubMed] [Google Scholar]
- 5.Mwale F, Demers CN, Petit A, Roughley P, Poole AR, Steffen T, et al. A synthetic peptide of link protein stimulates the biosynthesis of collagens II, IX and proteoglycan by cells of the intervertebral disc. J Cell Biochem. 2003;88:1202–13. doi: 10.1002/jcb.10479. [DOI] [PubMed] [Google Scholar]
- 6.Liu J, Roughley PJ, Mort JS. Identification of human intervertebral disc stromelysin and its involvement in matrix degradation. J Orthop Res. 1991;9:568–75. doi: 10.1002/jor.1100090413. [DOI] [PubMed] [Google Scholar]
- 7.Nemoto O, Yamagishi M, Yamada H, Kikuchi T, Takaishi H. Matrix metalloproteinase-3 production by human degenerated intervertebral disc. J Spinal Disord. 1997;10:493–8. [PubMed] [Google Scholar]
- 8.Matsui Y, Maeda M, Nakagami W, Iwata H. The involvement of matrix metalloproteinases and inflammation in lumbar disc herniation. Spine. 1998;23:863–8. doi: 10.1097/00007632-199804150-00005. [DOI] [PubMed] [Google Scholar]
- 9.Nishida T. Kinetics of tissue and serum matrix metalloproteinase-3 and tissue inhibitor of metalloproteinases-1 in intervertebral disc degeneration and disc herniation. Kurume Med J. 1999;46:39–50. doi: 10.2739/kurumemedj.46.39. [DOI] [PubMed] [Google Scholar]
- 10.Goupille P, Jayson MI, Valat JP, Freemont AJ. Matrix metalloproteinases: the clue to intervertebral disc degeneration? Spine. 1998;23:1612–26. doi: 10.1097/00007632-199807150-00021. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi H, Suguro T, Okazima Y, Motegi M, Okada Y, Kakiuchi T. Inflammatory cytokines in the herniated disc of the lumbar spine. Spine. 1996;21:218–24. doi: 10.1097/00007632-199601150-00011. [DOI] [PubMed] [Google Scholar]
- 12.Fujita K, Nakagawa T, Hirabayashi K, Nagai Y. Neutral proteinases in human intervertebral disc. Role in degeneration and probable origin. Spine. 1993;18:1766–73. doi: 10.1097/00007632-199310000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Kang JD, Georgescu HI, McIntyre-Larkin L, Stefanovic-Racic M, Donaldson WF, 3rd, Evans CH. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine. 1996;21:271–7. doi: 10.1097/00007632-199602010-00003. [DOI] [PubMed] [Google Scholar]
- 14.Kang JD, Georgescu HI, McIntyre-Larkin L, Stefanovic-Racic M, Evans CH. Herniated cervical intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine. 1995;20:2373–8. doi: 10.1097/00007632-199511001-00001. [DOI] [PubMed] [Google Scholar]
- 15.Furusawa N, Baba H, Miyoshi N, Maezawa Y, Uchida K, Kokubo Y, et al. Herniation of cervical intervertebral disc: immunohistochemical examination and measurement of nitric oxide production. Spine. 2001;26:1110–16. doi: 10.1097/00007632-200105150-00004. [DOI] [PubMed] [Google Scholar]
- 16.Weiler C, Nerlich AG, Zipperer J, Bachmeier BE, Boos N. 2002 SSE Award Competition in Basic Science: expression of major matrix metalloproteinases is associated with intervertebral disc degradation and resorption. Eur Spine J. 2002;11:308–20. doi: 10.1007/s00586-002-0472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Maitre CL, Freemont AJ, Hoyland JA. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol. 2004;204:47–54. doi: 10.1002/path.1608. [DOI] [PubMed] [Google Scholar]
- 18.Roberts S, Caterson B, Menage J, Evans EH, Jaffray DC, Eisenstein SM. Matrix metalloproteinases and aggrecanase: their role in disorders of the human intervertebral disc. Spine. 2000;25:3005–13. doi: 10.1097/00007632-200012010-00007. [DOI] [PubMed] [Google Scholar]
- 19.Sztrolovics R, Alini M, Roughley PJ, Mort JS. Aggrecan degradation in human intervertebral disc and articular cartilage. Biochem J. 1997;326:235–41. doi: 10.1042/bj3260235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahn SH, Cho YW, Ahn MW, Jang SH, Sohn YK, Kim HS. mRNA expression of cytokines and chemokines in herniated lumbar intervertebral discs. Spine. 2002;27:911–17. doi: 10.1097/00007632-200205010-00005. [DOI] [PubMed] [Google Scholar]
- 21.Burke JG, RW GW, Conhyea D, McCormack D, Dowling FE, Walsh MG, et al. Human nucleus pulposus can respond to a pro-inflammatory stimulus. Spine. 2003;28:2685–93. doi: 10.1097/01.BRS.0000103341.45133.F3. [DOI] [PubMed] [Google Scholar]
- 22.Weiler C, Nerlich AG, Bachmeier BE, Boos N. Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: a study in surgical specimen and autopsy controls. Spine. 2005;30:44–53. doi: 10.1097/01.brs.0000149186.63457.20. [DOI] [PubMed] [Google Scholar]
- 23.Igarashi T, Kikuchi S, Shubayev V, Myers RR. 2000 Volvo Award winner in basic science studies: exogenous tumor necrosis factor-alpha mimics nucleus pulposus-induced neuropathology. Molecular, histologic, and behavioral comparisons in rats. Spine. 2000;25:2975–80. doi: 10.1097/00007632-200012010-00003. [DOI] [PubMed] [Google Scholar]
- 24.Olmarker K, Larsson K. Tumor necrosis factor alpha and nucleus-pulposus-induced nerve root injury. Spine. 1998;23:2538–44. doi: 10.1097/00007632-199812010-00008. [DOI] [PubMed] [Google Scholar]
- 25.Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7:R732–45. doi: 10.1186/ar1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seguin CA, Pilliar RM, Roughley PJ, Kandel RA. Tumor necrosis factor-alpha modulates matrix production and catabolism in nucleus pulposus tissue. Spine. 2005;30:1940–8. doi: 10.1097/01.brs.0000176188.40263.f9. [DOI] [PubMed] [Google Scholar]
- 27.Cs-Szabo G, Ragasa-San Juan D, Turumella V, Masuda K, Thonar EJ, An HS. Changes in mRNA and protein levels of proteoglycans of the anulus fibrosus and nucleus pulposus during intervertebral disc degeneration. Spine. 2002;27:2212–19. doi: 10.1097/00007632-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 28.Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine. 2004;29:2700–9. doi: 10.1097/01.brs.0000146499.97948.52. [DOI] [PubMed] [Google Scholar]
- 29.Urban JP, Holm S, Maroudas A, Nachemson A. Nutrition of the intervertebral disk. An in vivo study of solute transport. Clin Orthop. 1977;129:101–14. [PubMed] [Google Scholar]
- 30.Bartels EM, Fairbank JC, Winlove CP, Urban JP. Oxygen and lactate concentrations measured in vivo in the intervertebral discs of patients with scoliosis and back pain. Spine. 1998;23:1–7. doi: 10.1097/00007632-199801010-00001. [DOI] [PubMed] [Google Scholar]
- 31.Selard E, Shirazi-Adl A, Urban JP. Finite element study of nutrient diffusion in the human intervertebral disc. Spine. 2003;28:1945–53. doi: 10.1097/01.BRS.0000087210.93541.23. [DOI] [PubMed] [Google Scholar]
- 32.Urban MR, Fairbank JC, Etherington PJ, Loh FL, Winlove CP, Urban JP. Electrochemical measurement of transport into scoliotic intervertebral discs in vivo using nitrous oxide as a tracer. Spine. 2001;26:984–90. doi: 10.1097/00007632-200104150-00028. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen-minh C, Riley L, III, Ho KC, Xu R, An H, Haughton VM. Effect of degeneration of the intervertebral disk on the process of diffusion. AJNR Am J Neuroradiol. 1997;18:435–42. [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen-minh C, Haughton VM, Papke RA, An H, Censky SC. Measuring diffusion of solutes into intervertebral disks with MR imaging and paramagnetic contrast medium. AJNR Am J Neuroradiol. 1998;19:1781–4. [PMC free article] [PubMed] [Google Scholar]
- 35.Horner HA, Urban JP. 2001 Volvo Award Winner in Basic Science Studies: effect of nutrient supply on the viability of cells from the nucleus pulposus of the intervertebral disc. Spine. 2001;26:2543–9. doi: 10.1097/00007632-200112010-00006. [DOI] [PubMed] [Google Scholar]
- 36.Kurunlahti M, Kerttula L, Jauhiainen J, Karppinen J, Tervonen O. Correlation of diffusion in lumbar intervertebral disks with occlusion of lumbar arteries: a study in adult volunteers. Radiology. 2001;221:779–86. doi: 10.1148/radiol.2213010134. [DOI] [PubMed] [Google Scholar]
- 37.Yoon ST. Molecular therapy of the intervertebral disc. Spine J. 2005;5:280S–6S. doi: 10.1016/j.spinee.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 38.Mochida J. New strategies for disc repair: novel preclinical trials. J Orthop Sci. 2005;10:112–18. doi: 10.1007/s00776-004-0864-6. [DOI] [PubMed] [Google Scholar]
- 39.Okuma M, Mochida J, Nishimura K, Sakabe K, Seiki K. Reinsertion of stimulated nucleus pulposus cells retards intervertebral disc degeneration: an in vitro and in vivo experimental study. J Orthop Res. 2000;18:988–97. doi: 10.1002/jor.1100180620. [DOI] [PubMed] [Google Scholar]
- 40.Nishimura K, Mochida J. Percutaneous reinsertion of the nucleus pulposus. An experimental study. Spine. 1998;23:1531–8. doi: 10.1097/00007632-199807150-00006. [DOI] [PubMed] [Google Scholar]
- 41.Anderson DG, Albert TJ, Fraser JK, Risbud M, Wuisman P, Meisel HJ, et al. Cellular therapy for disc degeneration. Spine. 2005;30:S14–19. doi: 10.1097/01.brs.0000175174.50235.ba. [DOI] [PubMed] [Google Scholar]
- 42.Ganey T, Libera J, Moos V, Alasevic O, Fritsch KG, Meisel HJ, et al. Disc chondrocyte transplantation in a canine model: a treatment for degenerated or damaged intervertebral disc. Spine. 2003;28:2609–20. doi: 10.1097/01.BRS.0000097891.63063.78. [DOI] [PubMed] [Google Scholar]
- 43.Tim Yoon S, Su Kim K, Li J, Soo Park J, Akamaru T, Elmer WA, et al. The effect of bone morphogenetic protein-2 on rat intervertebral disc cells in vitro. Spine. 2003;28:1773–80. doi: 10.1097/01.BRS.0000083204.44190.34. [DOI] [PubMed] [Google Scholar]
- 44.Kim KS, Yoon ST, Park JS, Li J, Park MS, Hutton WC. Inhibition of proteoglycan and type II collagen synthesis of disc nucleus cells by nicotine. J Neurosurg. 2003;99:291–7. doi: 10.3171/spi.2003.99.3.0291. [DOI] [PubMed] [Google Scholar]
- 45.Masuda K, Takegami K, An H, Kumano F, Chiba K, Andersson GB, et al. Recombinant osteogenic protein-1 upregulates extracellular matrix metabolism by rabbit annulus fibrosus and nucleus pulposus cells cultured in alginate beads. J Orthop Res. 2003;21:922–30. doi: 10.1016/S0736-0266(03)00037-8. [DOI] [PubMed] [Google Scholar]
- 46.Okuda S, Myoui A, Ariga K, Nakase T, Yonenobu K, Yoshikawa H. Mechanisms of age-related decline in insulin-like growth factor-I dependent proteoglycan synthesis in rat intervertebral disc cells. Spine. 2001;26:2421–6. doi: 10.1097/00007632-200111150-00005. [DOI] [PubMed] [Google Scholar]
- 47.Matsumoto T, An H, Thonar E, Andersson G, Masuda K. Effect of osteogenic protein-1 on the metabolism of proteoglycan of intervertebral disc cells in aging. Trans Orthop Res Soc. 2002;27:826. [Google Scholar]
- 48.An HS, Thonar EJ, Masuda K. Biological repair of intervertebral disc. Spine. 2003;28:S86–92. doi: 10.1097/01.BRS.0000076904.99434.40. [DOI] [PubMed] [Google Scholar]
- 49.Takegami K, Thonar EJ, An HS, Kamada H, Masuda K. Osteogenic protein-1 enhances matrix replenishment by intervertebral disc cells previously exposed to interleukin-1. Spine. 2002;27:1318–25. doi: 10.1097/00007632-200206150-00014. [DOI] [PubMed] [Google Scholar]
- 50.Takegami K, An HS, Kumano F, Chiba K, Thonar EJ, Singh K, et al. Osteogenic protein-1 is most effective in stimulating nucleus pulposus and annulus fibrosus cells to repair their matrix after chondroitinase ABC-induced in vitro chemonucleolysis. Spine J. 2005;5:231–8. doi: 10.1016/j.spinee.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 51.Gruber HE, Fisher EC, Jr, Desai B, Stasky AA, Hoelscher G, Hanley EN., Jr Human intervertebral disc cells from the annulus: three-dimensional culture in agarose or alginate and responsiveness to TGF-beta1. Exp Cell Res. 1997;235:13–21. doi: 10.1006/excr.1997.3647. [DOI] [PubMed] [Google Scholar]
- 52.Imai Y, An H, Pichika R, Thonar E, Otten L, Andersson G, et al. Recombinant human osteogenic protein-1 upregulates extracellular matrix metabolism by human annulus fibrosus and nucleus pulposus cells. Trans Orthop Res Soc. 2003;28:1140. doi: 10.1016/S0736-0266(03)00037-8. [DOI] [PubMed] [Google Scholar]
- 53.Kim DJ, Moon SH, Kim H, Kwon UH, Park MS, Han KJ, et al. Bone morphogenetic protein-2 facilitates expression of chondrogenic, not osteogenic, phenotype of human intervertebral disc cells. Spine. 2003;28:2679–84. doi: 10.1097/01.BRS.0000101445.46487.16. [DOI] [PubMed] [Google Scholar]
- 54.Ahn S-H, Teng P-N, Niyibizi C, Gilbertson L, Kang J. The effects of BMP-12 and BMP-2 on proteoglycan and collagen synthesis in nucleus pulposus cells from human degenerated discs. In: Proceedings, The International Society for the Study of the Lumbar Spine, 29th Annual Meeting, May 14–18, Cleveland, OH, 2002:49
- 55.Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine. 1995;20:1307–14. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- 56.Gruber HE, Hanley EN., Jr Analysis of aging and degeneration of the human intervertebral disc. Comparison of surgical specimens with normal controls. Spine. 1998;23:751–7. doi: 10.1097/00007632-199804010-00001. [DOI] [PubMed] [Google Scholar]
- 57.Gruber HE, Norton HJ, Hanley EN., Jr Anti-apoptotic effects of IGF-1 and PDGF on human intervertebral disc cells in vitro. Spine. 2000;25:2153–7. doi: 10.1097/00007632-200009010-00002. [DOI] [PubMed] [Google Scholar]
- 58.Wehling P. Antiapoptotic and antidegenerative effect of an autologous IL-1ra/IGF-1/PDGF combination on human intervertebral disc cells in vivo. In: Proceedings, The International Society for the Study of the Lumbar Spine, 29th Annual Meeting, May 14–8, Cleveland, OH, 2002:24
- 59.Walsh AJ, Bradford DS, Lotz JC. In vivo growth factor treatment of degenerated intervertebral discs. Spine. 2004;29:156–63. doi: 10.1097/01.BRS.0000107231.67854.9F. [DOI] [PubMed] [Google Scholar]
- 60.An HS, Takegami K, Kamada H, Nguyen CM, Thonar EJ, Singh K, et al. Intradiscal administration of osteogenic protein-1 increases intervertebral disc height and proteoglycan content in the nucleus pulposus in normal adolescent rabbits. Spine. 2005;30:25–31. doi: 10.1097/01.brs.0000148002.68656.4d. [DOI] [PubMed] [Google Scholar]
- 61.Masuda K, Aota Y, Muehleman C, Imai Y, Okuma M, Thonar EJ, et al. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine. 2005;30:5–14. doi: 10.1097/01.brs.0000148152.04401.20. [DOI] [PubMed] [Google Scholar]
- 62.Masuda K, Imai Y, Okuma M, Muehleman C, Nakagawa K, Akeda K, et al. Osteogenic protein-1 (OP-1) injection into a degenerated disc induces the restoration of disc height and structural changes in the rabbit annular puncture model. Spine. 2006;31:742–54. doi: 10.1097/01.brs.0000206358.66412.7b. [DOI] [PubMed] [Google Scholar]
- 63.Imai Y, An H, Matsumoto T, Nguyen C, Andersson G, Thonar E, et al. Intervertebral disc regeneration with rhOP-1 following C-ABC chemonucleolysis: An in vivo study using the rabbit model. In: Proceedings, The International Society for the Study of the Lumbar Spine. Proceedings, 29th annual meeting, May 14–18, Cleveland, OH, 2002:71
- 64.Nishida K, Kang JD, Gilbertson LG, Moon SH, Suh JK, Vogt MT, et al. Modulation of the biologic activity of the rabbit intervertebral disc by gene therapy: an in vivo study of adenovirus-mediated transfer of the human transforming growth factor beta 1 encoding gene. Spine. 1999;24:2419–25. doi: 10.1097/00007632-199912010-00002. [DOI] [PubMed] [Google Scholar]
- 65.Wallach CJ, Latterman C, Gilbertson L, Kang J. Transduction of TGF-β1 inhibits proteoglycan synthesis in intervertebral disc cells in a three dimensional culture. North American Spine Society. Spine J. 2002;2:67S. [Google Scholar]
- 66.Ahn S-H, Sobajima S, Teng P-N, Niyibizi C, Robins P, Gilbertson L, et al. The effects of adenovirus mediated delivery of BMP-12 cDNA on matrix synthesis in annulus fibrosus and nucleus pulposus cells from degenerated human discs. Spine J. 2003;3:70S. [Google Scholar]
- 67.Matsumoto T, Masuda K, Chen S, An H, Andersson GBJ, Aota Y, et al. Transfer of osteogenic protein-1 gene by gene gun system promotes matrix synthesis in bovine intervertebral disc and articular cartilage cells. Orthop Res Soc Trans. 2001;26:30. [Google Scholar]
- 68.Matsumoto T, An H, Imai Y, Pietryla D, Andersson G, Thonar E, et al. Intervertebral disc regeneration with cells transfected with the OP-1 gene: an ex vivo gene transfer using the rabbit model. Trans Orthop Res Soc 2003;349
- 69.Wang H, Kroeber M, Hanke M, Ries R, Schmid C, Poller W, et al. Release of active and depot GDF-5 after adenovirus-mediated overexpression stimulates rabbit and human intervertebral disc cells. J Mol Med. 2004;82:126–34. doi: 10.1007/s00109-003-0507-y. [DOI] [PubMed] [Google Scholar]
- 70.Paul R, Haydon RC, Cheng H, Ishikawa A, Nenadovich N, Jiang W, et al. Potential use of sox9 gene therapy for intervertebral degenerative disc disease. Spine. 2003;28:755–63. [PMC free article] [PubMed] [Google Scholar]
- 71.Wallach CJ, Sobajima S, Watanabe Y, Kim JS, Georgescu HI, Robbins P, et al. Gene transfer of the catabolic inhibitor TIMP-1 increases measured proteoglycans in cells from degenerated human intervertebral discs. Spine. 2003;28:2331–7. doi: 10.1097/01.BRS.0000085303.67942.94. [DOI] [PubMed] [Google Scholar]
- 72.Boden SD. Biology of lumbar spine fusion and use of bone graft substitutes: present, future, and next generation. Tissue Eng. 2000;6:383–99. doi: 10.1089/107632700418092. [DOI] [PubMed] [Google Scholar]
- 73.Yoon ST, Park JS, Kim KS, Li J, Attallah-Wasif ES, Hutton WC, et al. ISSLS prize winner: LMP-1 upregulates intervertebral disc cell production of proteoglycans and BMPs in vitro and in vivo. Spine. 2004;29:2603–11. doi: 10.1097/01.brs.0000146103.94600.85. [DOI] [PubMed] [Google Scholar]
- 74.Farfan HF, Gracovetsky S. The nature of instability. Spine. 1984;9:714–19. doi: 10.1097/00007632-198410000-00011. [DOI] [PubMed] [Google Scholar]
- 75.Frymoyer JW, Newberg A, Pope MH, Wilder DG, Clements J, MacPherson B. Spine radiographs in patients with low-back pain. An epidemiological study in men. J Bone Joint Surg [Am] 1984;66:1048–55. [PubMed] [Google Scholar]
- 76.Gertzbein SD, Seligman J, Holtby R, Chan KH, Kapasouri A, Tile M, et al. Centrode patterns and segmental instability in degenerative disc disease. Spine. 1985;10:257–61. doi: 10.1097/00007632-198504000-00014. [DOI] [PubMed] [Google Scholar]
- 77.Kirkaldy-Willis WH, Farfan HF. Instability of the lumbar spine. Clin Orthop Relat Res. 1982;165:110–23. [PubMed] [Google Scholar]
- 78.Morgan FP, King T. Primary instability of lumbar vertebrae as a common cause of low back pain. J Bone Joint Surg [Br] 1957;39B:6–22. doi: 10.1302/0301-620X.39B1.6. [DOI] [PubMed] [Google Scholar]
- 79.Nachemson A. Lumbar spine instability. A critical update and symposium summary. Spine. 1985;10:290–1. [PubMed] [Google Scholar]
- 80.Panjabi M. Low back pain and spinal instability. In: Weinstein J, Gordon SL, editors. Low back pain: a scientific and clinical overview. Rosemont: American Academy of Orthopedic Surgeons; 1996. pp. 367–84. [Google Scholar]
- 81.Pope MH, Panjabi M. Biomechanical definitions of spinal instability. Spine. 1985;10:255–6. doi: 10.1097/00007632-198504000-00013. [DOI] [PubMed] [Google Scholar]
- 82.Stokes IA, Frymoyer JW. Segmental motion and instability. Spine. 1987;12:688–91. doi: 10.1097/00007632-198709000-00009. [DOI] [PubMed] [Google Scholar]
- 83.Hayes MA, Howard TC, Gruel CR, Kopta JA. Roentgenographic evaluation of lumbar spine flexion-extension in asymptomatic individuals. Spine. 1989;14:327–31. doi: 10.1097/00007632-198903000-00014. [DOI] [PubMed] [Google Scholar]
- 84.Gracovetsky S, Newman N, Pawlowsky M, Lanzo V, Davey B, Robinson L. A database for estimating normal spinal motion derived from noninvasive measurements. Spine. 1995;20:1036–46. doi: 10.1097/00007632-199505000-00010. [DOI] [PubMed] [Google Scholar]
- 85.Dvorak J, Panjabi MM, Grob D, Novotny JE, Antinnes JA. Clinical validation of functional flexion/extension radiographs of the cervical spine. Spine. 1993;18:120–7. doi: 10.1097/00007632-199301000-00018. [DOI] [PubMed] [Google Scholar]
- 86.Modic MT, Masaryk TJ, Ross JS, Carter JR. Imaging of degenerative disk disease. Radiology. 1988;168:177–86. doi: 10.1148/radiology.168.1.3289089. [DOI] [PubMed] [Google Scholar]
- 87.Berns DH, Blaser SI, Modic MT. Magnetic resonance imaging of the spine. Clin Orthop Relat Res 1989:78–100 [PubMed]
- 88.Nowicki BH, Haughton VM. Neural foraminal ligaments of the lumbar spine: appearance at CT and MR imaging. Radiology. 1992;183:257–64. doi: 10.1148/radiology.183.1.1549683. [DOI] [PubMed] [Google Scholar]
- 89.Thompson JP, Pearce RH, Schechter MT, Adams ME, Tsang IK, Bishop PB. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine. 1990;15:411–15. doi: 10.1097/00007632-199005000-00012. [DOI] [PubMed] [Google Scholar]
- 90.Adams MA, Hutton WC, Stott JR. The resistance to flexion of the lumbar intervertebral joint. Spine. 1980;5:245–53. doi: 10.1097/00007632-198005000-00007. [DOI] [PubMed] [Google Scholar]
- 91.Autilio-Gambetti L, Crane R, Gambetti P. Binding of Bodian’s silver and monoclonal antibodies to defined regions of human neurofilament subunits: Bodian’s silver reacts with a highly charged unique domain of neurofilaments. J Neurochem. 1986;46:366–70. doi: 10.1111/j.1471-4159.1986.tb12977.x. [DOI] [PubMed] [Google Scholar]
- 92.Crane B, An H, Ochia R, Conrin S, Chen K, Andersson G, et al. In vivo measurement of changes in lumbar intervertebral disc height distribution during torsion. Trans Orthop Res Soc 2006:1217
- 93.Crisco JJ, Pike S, Hulsizer-Galvin DL, Akelman E, Weiss AP, Wolfe SW. Carpal bone postures and motions are abnormal in both wrists of patients with unilateral scapholunate interosseous ligament tears. J Hand Surg [Am] 2003;28:926–37. doi: 10.1016/s0363-5023(03)00422-2. [DOI] [PubMed] [Google Scholar]
- 94.Dickey JP, Pierrynowski MR, Bednar DA, Yang SX. Relationship between pain and vertebral motion in chronic low-back pain subjects. Clin Biomech. 2002;17:345–52. doi: 10.1016/s0268-0033(02)00032-3. [DOI] [PubMed] [Google Scholar]
- 95.Dooris AP, Goel VK, Grosland NM, Gilbertson LG, Wilder DG. Load-sharing between anterior and posterior elements in a lumbar motion segment implanted with an artificial disc. Spine. 2001;26:E122–9. doi: 10.1097/00007632-200103150-00004. [DOI] [PubMed] [Google Scholar]
- 96.Dreyer SJ, Dreyfuss PH. Low back pain and the zygapophysial (facet) joints. Arch Phys Med Rehabil. 1996;77:290–300. doi: 10.1016/s0003-9993(96)90115-x. [DOI] [PubMed] [Google Scholar]
- 97.Dunlop RB, Adams MA, Hutton WC. Disc space narrowing and the lumbar facet joints. J Bone Joint Surg [Br] 1984;66:706–10. doi: 10.1302/0301-620X.66B5.6501365. [DOI] [PubMed] [Google Scholar]
- 98.Dupuis PR, Yong-Hing K, Cassidy JD, Kirkaldy-Willis WH. Radiologic diagnosis of degenerative lumbar spinal instability. Spine. 1985;10:262–76. doi: 10.1097/00007632-198504000-00015. [DOI] [PubMed] [Google Scholar]
- 99.Ebraheim NA, Xu R, Challgren E, Yeasting RA. Quantitative anatomy of the cervical facet and the posterior projection of its inferior facet. J Spinal Disord. 1997;10:308–16. [PubMed] [Google Scholar]
- 100.Ebraheim NA, Xu R, Ahmad M, Yeasting RA. The quantitative anatomy of the thoracic facet and the posterior projection of its inferior facet. Spine. 1997;22:1811–17. doi: 10.1097/00007632-199708150-00002. [DOI] [PubMed] [Google Scholar]
- 101.Ewing CL, King AI, Prasad P. Structural consideration of the human vertebral column under +Gz impact acceleration. J Aircraft. 1972;9:84–90. [Google Scholar]
- 102.Fujiwara A, Lim TH, An HS, Tanaka N, Jeon CH, Andersson GB, et al. The effect of disc degeneration and facet joint osteoarthritis on the segmental flexibility of the lumbar spine. Spine. 2000;25:3036–44. doi: 10.1097/00007632-200012010-00011. [DOI] [PubMed] [Google Scholar]
- 103.Haughton VM, Schmidt TA, Keele K, An HS, Lim TH. Flexibility of lumbar spinal motion segments correlated to type of tears in the annulus fibrosus. J Neurosurg. 2000;92:81–6. doi: 10.3171/spi.2000.92.1.0081. [DOI] [PubMed] [Google Scholar]
- 104.Kurowski P, Kubo A. The relationship of degeneration of the intervertebral disc to mechanical loading conditions on lumbar vertebrae. Spine. 1986;11:726–31. doi: 10.1097/00007632-198609000-00012. [DOI] [PubMed] [Google Scholar]
- 105.Mimura M, Panjabi MM, Oxland TR, Crisco JJ, Yamamoto I, Vasavada A. Disc degeneration affects the multidirectional flexibility of the lumbar spine. Spine. 1994;19:1371–80. doi: 10.1097/00007632-199406000-00011. [DOI] [PubMed] [Google Scholar]
- 106.Nowicki BH, Yu S, Reinartz J, Pintar F, Yoganandan N, Haughton VM. Effect of axial loading on neural foramina and nerve roots in the lumbar spine. Radiology. 1990;176:433–7. doi: 10.1148/radiology.176.2.2367657. [DOI] [PubMed] [Google Scholar]
- 107.Schmidt TA, An HS, Lim TH, Nowicki BH, Haughton VM. The stiffness of lumbar spinal motion segments with a high-intensity zone in the anulus fibrosus. Spine. 1998;23:2167–73. doi: 10.1097/00007632-199810150-00005. [DOI] [PubMed] [Google Scholar]
- 108.Lim TH, Eck JC, An HS, McGrady LM, Harris GF, Haughton VM. A noninvasive, three-dimensional spinal motion analysis method. Spine. 1997;22:1996–2000. doi: 10.1097/00007632-199709010-00011. [DOI] [PubMed] [Google Scholar]
- 109.Rogers BP, Haughton VM, Arfanakis K, Meyerand ME. Application of image registration to measurement of intervertebral rotation in the lumbar spine. Magn Reson Med. 2002;48:1072–5. doi: 10.1002/mrm.10319. [DOI] [PubMed] [Google Scholar]
- 110.Haughton VM, Rogers B, Meyerand ME, Resnick DK. Measuring the axial rotation of lumbar vertebrae in vivo with MR imaging. AJNR Am J Neuroradiol. 2002;23:1110–16. [PMC free article] [PubMed] [Google Scholar]
- 111.Pearcy MJ. Stereo radiography of lumbar spine motion. Acta Orthop Scand Suppl. 1985;212:1–45. doi: 10.3109/17453678509154154. [DOI] [PubMed] [Google Scholar]
- 112.Pearcy M, Portek I, Shepherd J. Three-dimensional x-ray analysis of normal movement in the lumbar spine. Spine. 1984;9:294–7. doi: 10.1097/00007632-198404000-00013. [DOI] [PubMed] [Google Scholar]
- 113.Hedman TP, Fernie GR. Mechanical response of the lumbar spine to seated postural loads. Spine. 1997;22:734–43. doi: 10.1097/00007632-199704010-00004. [DOI] [PubMed] [Google Scholar]
- 114.Horton WC, Daftari TK. Which disc as visualized by magnetic resonance imaging is actually a source of pain? A correlation between magnetic resonance imaging and discography. Spine. 1992;17:S164–71. doi: 10.1097/00007632-199206001-00018. [DOI] [PubMed] [Google Scholar]
- 115.Schiebler ML, Camerino VJ, Fallon MD, Zlatkin MB, Grenier N, Kressel HY. In vivo and ex vivo magnetic resonance imaging evaluation of early disc degeneration with histopathologic correlation. Spine. 1991;16:635–40. doi: 10.1097/00007632-199106000-00007. [DOI] [PubMed] [Google Scholar]
- 116.Yu SW, Sether LA, Ho PS, Wagner M, Haughton VM. Tears of the anulus fibrosus: correlation between MR and pathologic findings in cadavers. AJNR Am J Neuroradiol. 1988;9:367–70. [PMC free article] [PubMed] [Google Scholar]
- 117.Takeuchi T, Shea M, White AA. Correlation of magnetic resonance relaxation times with degeneration and biomechanical properties in human lumbar intervertebral disks. Trans Orthop Res Soc 1992:191
- 118.Farfan HF. Mechanical disorders of the low back pain. Philadelphia: Lea and Febiger; 1973. [Google Scholar]
- 119.Farfan HF, Cossette JW, Robertson GH, Wells RV, Kraus H. The effects of torsion on the lumbar intervertebral joints: the role of torsion in the production of disc degeneration. J Bone Joint Surg [Am] 1970;52:468–97. [PubMed] [Google Scholar]
- 120.Ochia R, inoue N, Renner S, Lorenz E, Lim T, Andersson G, et al. Three-dimensional in vivo measurement of lumbar segmental motion. Spine 31(18):2073–8 [DOI] [PubMed]
- 121.Lorenz M, Patwardhan A, Vanderby R., Jr Load-bearing characteristics of lumbar facets in normal and surgically altered spinal segments. Spine. 1983;8:122–30. doi: 10.1097/00007632-198303000-00002. [DOI] [PubMed] [Google Scholar]
- 122.Hedman TP. A new transducer for facet force measurement in the lumbar spine: benchmark and in vitro test results. J Biomech. 1992;25:69–80. doi: 10.1016/0021-9290(92)90246-w. [DOI] [PubMed] [Google Scholar]
- 123.Haher TR, O’Brien M, Dryer JW, Nucci R, Zipnick R, Leone DJ. The role of the lumbar facet joints in spinal stability. Identification of alternative paths of loading. Spine. 1994;19:2667–70. [PubMed] [Google Scholar]
- 124.Little JS, Ianuzzi A, Chiu JB, Baitner A, Khalsa PS. Human lumbar facet joint capsule strains: II. Alteration of strains subsequent to anterior interbody fixation. Spine J. 2004;4:153–62. doi: 10.1016/j.spinee.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 125.Natarajan RN, Andersson GB, Patwardhan AG, Andriacchi TP. Study on effect of graded facetectomy on change in lumbar motion segment torsional flexibility using three-dimensional continuum contact representation for facet joints. J Biomech Eng. 1999;121:215–21. doi: 10.1115/1.2835106. [DOI] [PubMed] [Google Scholar]
- 126.Panjabi MM, Krag MH, Goel VK. A technique for measurement and description of three-dimensional six degree-of-freedom motion of a body joint with an application to the human spine. J Biomech. 1981;14:447–60. doi: 10.1016/0021-9290(81)90095-6. [DOI] [PubMed] [Google Scholar]
- 127.Pal GP, Routal RV, Saggu SK. The orientation of the articular facets of the zygapophyseal joints at the cervical and upper thoracic region. J Anat. 2001;198:431–41. doi: 10.1046/j.1469-7580.2001.19840431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Olsewski JM, Garvey TA, Schendel MJ. Biomechanical analysis of facet and graft loading in a Smith-Robinson type cervical spine model. Spine. 1994;19:2540–4. doi: 10.1097/00007632-199411001-00008. [DOI] [PubMed] [Google Scholar]
- 129.Yang KH, King AI. Mechanism of facet load transmission as a hypothesis for low-back pain. Spine. 1984;9:557–65. doi: 10.1097/00007632-198409000-00005. [DOI] [PubMed] [Google Scholar]
- 130.Adams MA, Hutton WC. The effect of posture on the role of the apophysial joints in resisting intervertebral compressive forces. J Bone Joint Surg [Br] 1980;62:358–62. doi: 10.1302/0301-620X.62B3.6447702. [DOI] [PubMed] [Google Scholar]
- 131.Adams MA, Hutton WC. The relevance of torsion to the mechanical derangement of the lumbar spine. Spine. 1981;6:241–8. doi: 10.1097/00007632-198105000-00006. [DOI] [PubMed] [Google Scholar]
- 132.Schendel MJ, Wood KB, Buttermann GR, Lewis JL, Ogilvie JW. Experimental measurement of ligament force, facet force, and segment motion in the human lumbar spine. J Biomech. 1993;26:427–38. doi: 10.1016/0021-9290(93)90006-z. [DOI] [PubMed] [Google Scholar]
- 133.Hedman TP, Fernie GR. Mechanical response of the lumbar spine to seated postural loads. Spine. 1997;22:734–43. doi: 10.1097/00007632-199704010-00004. [DOI] [PubMed] [Google Scholar]
- 134.Shirazi-Adl A, Ahmed AM, Shrivastava SC. Mechanical response of a lumbar motion segment in axial torque alone and combined with compression. Spine. 1986;11:914–27. doi: 10.1097/00007632-198611000-00012. [DOI] [PubMed] [Google Scholar]
- 135.Shirazi-Adl A. Biomechanics of the lumbar spine in sagittal/lateral moments. Spine. 1994;19:2407–14. doi: 10.1097/00007632-199411000-00007. [DOI] [PubMed] [Google Scholar]
- 136.Adams MA, Hutton WC. Cadaver lumbar intervertebral joints. Spine. 1980;5:483–4. [PubMed] [Google Scholar]
- 137.Barry M, Livesley P. Facet joint hypertrophy: the cross-sectional area of the superior articular process of L4 and L5. Eur Spine J. 1997;6:121–4. doi: 10.1007/BF01358744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Berlemann U, Jeszenszky DJ, Buhler DW, Harms J. Facet joint remodeling in degenerative spondylolisthesis: an investigation of joint orientation and tropism. Eur Spine J. 1998;7:376–80. doi: 10.1007/s005860050093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Niosi CA, Oxland TR. Degenerative mechanics of the lumbar spine. Spine J. 2004;4:202S–8S. doi: 10.1016/j.spinee.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 140.Masharawi Y, Rothschild B, Dar G, Peleg S, Robinson D, Been E, et al. Facet orientation in the thoracolumbar spine: three-dimensional anatomic and biomechanical analysis. Spine. 2004;29:1755–63. doi: 10.1097/01.brs.0000134575.04084.ef. [DOI] [PubMed] [Google Scholar]
- 141.Panjabi MM, Krag MH, Chung TQ. Effects of disc injury on mechanical behavior of the human spine. Spine. 1984;9:707–13. doi: 10.1097/00007632-198410000-00010. [DOI] [PubMed] [Google Scholar]
- 142.An HS, Singh K, Vaccaro AR, Wang G, Yoshida H, Eck J, et al. Biomechanical evaluation of contemporary posterior spinal internal fixation configurations in an unstable burst-fracture calf spine model: special references of hook configurations and pedicle screws. Spine. 2004;29:257–62. doi: 10.1097/01.brs.0000106979.54651.d6. [DOI] [PubMed] [Google Scholar]
- 143.Moore RJ, Crotti TN, Osti OL, Fraser RD, Vernon-Roberts B. Osteoarthrosis of the facet joints resulting from anular rim lesions in sheep lumbar discs. Spine. 1999;24:519–25. doi: 10.1097/00007632-199903150-00003. [DOI] [PubMed] [Google Scholar]
- 144.Butler D, Trafimow JH, Andersson GB, McNeill TW, Huckman MS. Discs degenerate before facets. Spine. 1990;15:111–13. doi: 10.1097/00007632-199002000-00012. [DOI] [PubMed] [Google Scholar]
- 145.Adams MA, Hutton WC. The mechanical function of the lumbar apophyseal joints. Spine. 1983;8:327–30. doi: 10.1097/00007632-198304000-00017. [DOI] [PubMed] [Google Scholar]
- 146.Abumi K, Panjabi MM, Kramer KM, Duranceau J, Oxland T, Crisco JJ. Biomechanical evaluation of lumbar spinal stability after graded facetectomies. Spine. 1990;15:1142–7. doi: 10.1097/00007632-199011010-00011. [DOI] [PubMed] [Google Scholar]
- 147.Ianuzzi A, Little JS, Chiu JB, Baitner A, Kawchuk G, Khalsa PS. Human lumbar facet joint capsule strains: I. During physiological motions. Spine J. 2004;4:141–52. doi: 10.1016/j.spinee.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 148.Pearson AM, Ivancic PC, Ito S, Panjabi MM. Facet joint kinematics and injury mechanisms during simulated whiplash. Spine. 2004;29:390–7. doi: 10.1097/01.brs.0000090836.50508.f7. [DOI] [PubMed] [Google Scholar]
- 149.Panjabi MM, Takata K, Goel V, Federico D, Oxland T, Duranceau J, et al. Thoracic human vertebrae. Quantitative three-dimensional anatomy. Spine. 1991;16:888–901. doi: 10.1097/00007632-199108000-00006. [DOI] [PubMed] [Google Scholar]
- 150.Panjabi MM, Oxland T, Takata K, Goel V, Duranceau J, Krag M. Articular facets of the human spine. Quantitative three-dimensional anatomy. Spine. 1993;18:1298–310. doi: 10.1097/00007632-199308000-00009. [DOI] [PubMed] [Google Scholar]
- 151.Panjabi MM, Goel V, Oxland T, Takata K, Duranceau J, Krag M, et al. Human lumbar vertebrae. Quantitative three-dimensional anatomy. Spine. 1992;17:299–306. doi: 10.1097/00007632-199203000-00010. [DOI] [PubMed] [Google Scholar]
- 152.Pellengahr C, Pfahler M, Kuhr M, Hohmann D. Influence of facet joint angles and asymmetric disk collapse on degenerative olisthesis of the cervical spine. Orthopedics. 2000;23:697–701. doi: 10.3928/0147-7447-20000701-17. [DOI] [PubMed] [Google Scholar]
- 153.Sharma M, Langrana NA, Rodriguez J. Modeling of facet articulation as a nonlinear moving contact problem: sensitivity study on lumbar facet response. J Biomech Eng. 1998;120:118–25. doi: 10.1115/1.2834291. [DOI] [PubMed] [Google Scholar]
- 154.van Schaik JP. Lumbar facet joint morphology. J Spinal Disord. 2000;13:88–9. doi: 10.1097/00002517-200002000-00017. [DOI] [PubMed] [Google Scholar]
- 155.White AA, Panjabi M. Clinical biomechanics of the spine. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 1990. [Google Scholar]
- 156.Zander T, Rohlmann A, Klockner C, Bergmann G. Influence of graded facetectomy and laminectomy on spinal biomechanics. Eur Spine J. 2003;12:427–34. doi: 10.1007/s00586-003-0540-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Panjabi MM, Oxland TR, Yamamoto I, Crisco JJ. Mechanical behavior of the human lumbar and lumbosacral spine as shown by three-dimensional load-displacement curves. J Bone Joint Surg [Am] 1994;76:413–24. doi: 10.2106/00004623-199403000-00012. [DOI] [PubMed] [Google Scholar]
- 158.Sato K, Kikuchi S, Yonezawa T. In vivo intradiscal pressure measurement in healthy individuals and in patients with ongoing back problems. Spine. 1999;24:2468–74. doi: 10.1097/00007632-199912010-00008. [DOI] [PubMed] [Google Scholar]
- 159.Fujiwara A, Tamai K, An HS, Kurihashi T, Lim TH, Yoshida H, et al. The relationship between disc degeneration, facet joint osteoarthritis, and stability of the degenerative lumbar spine. J Spinal Disord. 2000;13:444–50. doi: 10.1097/00002517-200010000-00013. [DOI] [PubMed] [Google Scholar]
- 160.Fujiwara A, Tamai K, Yamato M, An HS, Yoshida H, Saotome K, et al. The relationship between facet joint osteoarthritis and disc degeneration of the lumbar spine: an MRI study. Eur Spine J. 1999;8:396–401. doi: 10.1007/s005860050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fujiwara A, Tamai K, An HS, Lim TH, Yoshida H, Kurihashi A, et al. Orientation and osteoarthritis of the lumbar facet joint. Clin Orthop Relat Res 2001:88–94 [DOI] [PubMed]