Abstract
Purpose:
NKG2D binds to cellular ligands of the MIC and ULBP/RAET family. These ligands have restricted expression in normal tissue, but are frequently expressed on primary tumors. The role of NKG2D ligands is thought to be important in carcinogenesis but prognostic impact has not been investigated in such a large cohort.
Experimental Design:
In our study 462 primary colorectal tumors were screened for expression of all MIC / ULBP / RAET proteins and NK cell infiltration. Tumor microarray technology was used for the purpose of this investigation.
Results:
NKG2D ligands were expressed by the majority of colorectal tumors; however the level of expression varied considerably. High expression of MIC (68 versus 56 months) or RAET1G (74 versus 62 months) demonstrated improved patient survival. Tumors expressing high levels of MIC and RAET1G showed improved survival of 77 months over tumors that expressed high levels of one ligand or low levels of both. High-level expression of all ligands was frequent in TNM stage 1 tumors, but became progressively less frequent in stage 2, 3 and 4 tumors. Expression of MIC was correlated with NK cellular infiltration.
Conclusion:
The observations presented are consistent with an immunoediting mechanism that selects tumor cells that have lost or reduced their expression of NKG2D ligands. Combination of MIC and TNM stage was found to be the strongest predictor of survival splitting patients into 8 groups and suggesting prognostic value in clinical assessment. Of particular interest were stage 1 patients with low expression of MIC who had a similar survival to stage 3 patients, and may be candidates for adjuvant therapy.
Keywords: NKG2D, colorectal cancer, tissue microarray
Introduction
NKG2D (Natural Killer Group 2, member D) is a stimulatory receptor expressed on the surface of NK cells and subsets of T cells (1). It is unusual amongst activating receptors in binding to a diverse array of cellular ligands (2). Human NKG2D ligands comprize two members of the MIC (MHC class I-related chain) family and six members of the ULBP/RAET (UL16 binding protein, or retinoic acid early transcript) family (3-7). In mice they include five members of the Rae1 (retinoic acid early inducible) family, the minor histocompatibility antigen H60, and Mult1 (Murine ULBP-like transcript) (8-10).
NKG2D ligand expression is generally absent from healthy tissues but can be induced on infection, and by cell stress stimuli. NKG2D ligands are also widely expressed on a variety of cancer cell lines, as well as primary solid tumors and leukemia (4, 11-14). The mechanisms regulating NKG2D ligand expression in cancer are not well understood, although activation of DNA damage response pathways have been implicated, as has the expression of the BCR/ABL oncogene (15-17).
In mouse models it has been shown that tumor cell lines transfected with Rae1 are rejected in vivo via NKG2D mediated immunity (18, 19). The recent generation of an NKG2D knockout mouse has provided the most convincing evidence to date for NKG2D involvement in anti-tumor immune responses (20). Using the knockout mice in conjunction with several different cancer models it became clear NKG2D interactions are variable between different types of cancer. For example there was no increase in the incidence of methylcolanthrene-induced tumors in the knockout compared to wild type, however NKG2D deficiency was associated with increased incidence of prostate adenocarcinomas and accelerated progression of Eμ-myc induced lymphomas (20).
It is now widely accepted that tumors develop ways to evade anti-cancer immunity through a process termed immunoediting (21). A number of mechanisms have been proposed by which cancers could evade NKG2D mediated immune responses. In some systems persistent expression of NKG2D ligands can result in downregulation of NKG2D expression (22-24). It is also proposed that tumors may shed soluble NKG2D ligands, or secrete immunosuppressive cytokines such as TGF-beta, in order to downregulate NKG2D expression (25-27).
It is known that NKG2D ligands can be expressed independently of each other in human cell lines and primary tumors (4, 12, 28). It is also clear that different ligands can be expressed in response to different cancer specific pathways, for example in the cell line K562 the BCR/ABL oncogene induced expression of MICA but not ULBP1 and ULBP2 (16). NKG2D ligand expression was also heterogeneous between different tumors that arose in the knockout mouse. Prostate tumors arising in these mice had higher levels of NKG2D ligand expression than in wild type mice. This suggests that tumor cells under selection switch off NKG2D ligand expression as part of an immunoediting process (20). A similar observation has been made in perforin deficient mice (29).
Tissue microarray technology allows simultaneous immunohistochemical analysis of hundreds of tumor specimens for target protein expression (30). Data derived from these analyses can then be linked to clinicopathological data so as to evaluate potential prognostic markers. In this article we describe analysis of the expression of all human NKG2D ligands using a large series of formalin-fixed paraffin-embedded colorectal cancer tissue arrays, demonstrating that several findings from the NKG2D knockout mouse, such as heterogeneous NKG2D ligand expression and evidence for immunoediting, are also a feature of human disease. Our analysis identified the two strongest prognostic factors in colorectal cancer that retain independent significance as TNM stage and MIC expression. Using a mathematical prognostic model these two factors indicated the presence of an at-risk group of TNM 1 patients who would potentially benefit from additional adjuvant therapy.
Methods
Patients and study design
The study population comprised a series of 462 consecutive patients undergoing elective surgical resection of a histologically proven sporadic primary colorectal cancer at the University Hospital, Nottingham, UK and has been reported on previously (31-35). Follow-up was calculated from time of resection of the original tumor with all surviving cases being censored for data analysis at 31st December 2003, this produced a median follow up of 37 months (range 0–116) for all patients and 75 months (range 36–116) for survivors.
Adjuvant chemotherapy consisting of 5-FU and folinic acid was reserved for those patients with positive lymph nodes, although, surgical and adjuvant treatment was at the discretion of the supervising physician. Prior ethical review of the study was conducted by the Nottingham Local Research and Ethics Committee, who granted approval for the study.
Construction of the array blocks incorporated a wide spectrum of electively resected colorectal tumors and was found to be broadly representative of the colorectal cancer population in the UK. 266 (58%) patients were male and 196 (42%) female. The median age at the time of surgery was 72 years, consistent with a median age at diagnosis of colorectal cancer of 70–74 years in the UK (36). 69 (15%) tumors arrayed were TNM stage 1, 174 (38%) stage 2, 155 (34%) stage 3 and 54 (11%) stage 4; there were 3 cases of in-situ disease. These figures are comparable with national figures for distribution of stage 1–4 at diagnosis of 11, 35, 26 and 29% respectively (37). The majority of tumors were adenocarcinomas (392, 85%), and were most frequently of a moderate histological grade (353, 77%).
At the time of censoring for data analysis 228 (49%) patients had died from their disease, 64 (14%) were deceased from all other causes, and 169 (37%) were alive. The median five-year disease-specific survival for the cohort was 58 months, comparable with a national average of approximately 45% five-year survival for colorectal cancer in the UK (37). The reporting of this study adheres to the REMARK guidelines (38). Investigators conducting statistical analysis were blinded to the clinicopathological data prior to analysis.
Specimen characteristics
All tumors received following resection in the operating theatre were incised, fixed immediately in 10% neutral buffered formalin followed by standard processing through to embedding in paraffin wax, ensuring optimal tissue fixation and preservation for histological examination. The construction of the colorectal tissue micro-array has been described previously (33).
Antibody sources
Anti-MIC monoclonal antibody SR99 (a kind gift of Dr Sophie Caillat-Zucman); anti-ULBP1 goat polyclonal antibody (R&D Systems Inc., MN); anti-ULBP2 monoclonal antibody M311 (a kind gift of Dr David Cosman); anti-ULBP3 goat polyclonal antibody (R&D Systems); anti-RAET1E rabbit polyclonal (this study); anti-RAET1G rabbit polyclonal (39); anti-CD16 mouse monoclonal antibody, clone DJ130c (AbD Serotec).
Production of polyclonal ULBP4/RAET1E antisera
Recombinant ULBP4/RAET1E protein was produced by cloning the extracellular domain of ULBP4/RAET1E into an N-terminal six histidine vector (a kind gift of David Owen, Cambridge, UK) and transduction into bacterial pLysis cells. Cultures were induced with 1mM IPTG and incubated overnight at 20°C. Inclusion bodies were dissolved in 8M urea and purified using nickel beads. Refolding was performed by dilution in refolding buffer (100mM Tris pH8, 400mM arginine, 2mM EDTA, 5mM reduced glutathione, 0.5mM oxidized glutathione and 0.1 mM PMSF). Concentrated protein was run on a 12% SDS page gel and mass spectrometry confirmed that the band was ULPBP4/RAET1E. The protein was used to immunize rabbits by CovalAB (Lyon, France) to generate serum specific to ULBP4/RAET1E.
Western blot
ULBP/RAET extracellular domains were cloned into pDisplay vector (Invitrogen, Paisley, UK). Approximately 106 Cos7 (a kind gift of Dr A Barrow) were transiently transfected with 1μg plasmid using Lipofectamine reagent (Invitrogen). After 48 hours, cells were harvested into reducing SDS-PAGE buffer and boiled. Lysates were subject to SDS-PAGE and blotted onto Immobilon P membrane (Millipore, MA). As a loading control western blots were also probed with anti-β-actin monoclonal antibody (Sigma-Aldrich, Dorset, UK) and anti-HA tag monoclonal antibody 6E2 (Cell Signaling Technology, MA) followed by goat anti-mouse HRP (Dako, Ely, UK). Blots were also probed with the anti-ULBP/RAET antisera followed by goat anti-rabbit HRP or rabbit anti-goat HRP (Dako).
Flow cytometry
Approximately 106 CHO cells (a kind gift of Dr A Barrow) were transiently transfected with 1μg of the pDisplay plasmids using Lipofectamine reagent. After 48 hours cells were detached in PBS 1mM EDTA and analyzed by flow cytometry. Transfection efficiency was assessed by staining with the anti-HA tag monoclonal antibody followed by goat anti-mouse FITC (Dako). Cells were also stained with the M311 anti-ULBP2 monoclonal antibody and an IgG2a isotype control (Dako). Flow cytometry was carried out on a BD FACSCalibur.
Immunohistochemistry
Immunohistochemical analysis of MIC, ULBP1, ULBP2, ULBP3, RAET1E and RAET1G expression and CD16+ cell distribution was performed using a routine streptavidin-biotin peroxidase method. Tissue array sections were first deparaffinized with xylene and then rehydrated through graded alcohol. In order to retrieve antigenicity, sections were immersed in 500mls of pH 6.0 Citrate buffer and heated for 10 min in an 800W microwave at high power, followed by 10 min at low power. Sections were then immersed in PBS containing 0.3% hydrogen peroxide for 20 minutes to block endogenous peroxidase activity. In order to block non-specific binding of the primary antibody sections were then treated with 100μl of 1/50 normal blocking serum (Vector Labs, CA) in PBS for 20 min, with the exception of sections to be stained with anti-ULBP1 and anti-ULBP3, which were treated with 100μl of 1/50 rabbit blocking serum (Vector Labs) in PBS for 20 min. Endogenous avidin/biotin binding was blocked using an avidin/biotin blocking kit (Vector Labs).
Test sections were incubated with 100μl of monoclonal antibody (SR99) recognizing MICA, with partial cross-reactivity against MICB, (40) which was found to show optimal staining at 20μg/ml in PBS, or polyclonal antibody recognizing ULBP1 (figure 1a) at 2.5μg/ml, or polyclonal antibody (M311) recognizing ULBP2 (figure 1b) at 2.5μg/ml, or polyclonal antibody recognizing ULBP3 (figure 1a) at 2.5μg/ml, or polyclonal antibody recognizing RAET1E (figure 1a) at 2.5μg/ml in PBS or polyclonal antibody recognizing RAET1G (39) at 5μg/ml in PBS or monoclonal antibody recognizing CD16 at 1:100 dilution in PBS for 60 min at room temperature. Positive control tissue comprised whole sections of colorectal cancer tissue. The primary antibody was omitted from the negative control, which was left incubating in blocking serum.
Figure 1. Specificity of α-ULBP1, α-ULBP2, α-ULBP3 and α-RAET1E antibodies.
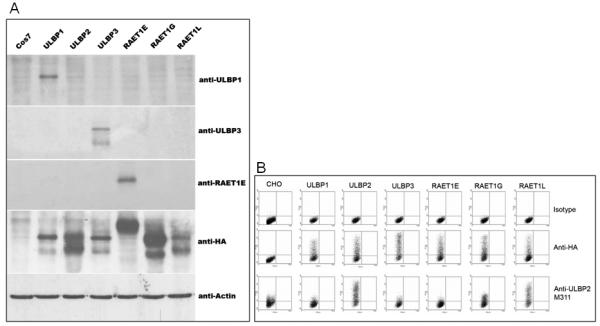
(A) Western blot demonstrates that the antibodies to ULBP1, ULBP3 and RAET1E are specific to extracts from cells expressing the appropriate ligand. Cos7 non-transfected cells were included as a negative control. Anti-actin antibody positive control shows bands in each lane. (B) Flow cytometry confirmed the specificity of anti-ULBP2.
After washing with PBS, sections were incubated with 100μl of biotinylated goat anti-mouse/rabbit immunoglobulin (Vector Labs) diluted 1/50 in normal blocking serum:PBS for 30 min, excluding sections incubated with anti-ULBP1 and anti-ULBP2, which were incubated with biotinylated rabbit anti-goat immunoglobulin (Vector Labs) diluted 1/50 in rabbit blocking serum:PBS for 30 min. Sections were washed in PBS and next incubated with 100μl of streptavidin-biotin/HRP complex (Vector Labs) for 30 min. Subsequently, visualization was achieved using DAB Peroxidase Substrate Kit (Vector Labs). Finally, sections were lightly counterstained with haematoxylin, dehydrated in alcohol, cleared in xylene and mounted with DPX.
Evaluation of staining
Immunostaining of our TMA sections was evaluated using a Chromavision Automated Cellular Imaging System (ACIS) with TMA analysis-specific software (ChromaVision Medical Systems, San Juan Capistrano, CA). In this system, images of each slide are captured and stored digitally on a computer, and the intensity is calculated automatically, without any reference to clinicopathological variables. Color threshold settings for the optimal discrimination between brown and blue staining in this system were set prior to analysis and left unchanged throughout. In addition, one investigator who was blinded to the clinicopathology (RWM) reviewed the images of each individual core to confirm both its presence on the slide, and the presence of tumor tissue within the core.
Expression of MIC, RAET1E, and RAET1G was uniform with no negative cores present in the sample. The intensity of staining was used as the discriminator with tumors categorized as low and high intensity of expression. This was used as the discriminator for ULBP1/2/3 to maintain the same assessment criteria, although there were a small number of negative cores, which were classed within the low expression group. For analysis of CD16, a cut-off system of positive versus negative was adopted, in which a core was considered positive when any number of positive cells were observed and negative when no cells showed staining. 254 of the cores (61%) were completely negative for CD16 cells, with 164 cores positive (39%).
Statistical analysis
Statistical analysis of the study data was performed by RWM using the ‘SPSS’ package (SPSS Inc., Chicago, IL), and confirmed by GB using the ‘Statistica’ package (Statsoft Inc., Tulsa, OK). Pearson χ2 (chi-square) tests were used to determine the significance of associations between categorical variables. Disease-specific survival calculations included all patients whose death related to colorectal cancer. Patients whose deaths resulted from non-colorectal cancer related causes and without evidence of cancer recurrence were censored at the time of death. Kaplan-Meier curves were used to assess factors influencing survival. The statistical significance of differences in disease-specific survival between groups with differing expression was estimated using the log-rank test and univariate Cox regression. Significance was established where the p-value was found to be 0.05 or less. Combined factorial analysis was considered significant with p-values of 0.025 or less, applying Bonferroni correction.
Significant prognostic factors were incorporated into a formula that would represent the prognostic outcome for a given individual. This was conducted by iteratively adding to the variable combination starting at TNM category. Only factors of independent prognostic significance were retained. Factors having prognostic significance were incorporated into a formula based on the beta value derived from the Cox proportional hazard model. This function is not being used to prove or disprove a hypothesis but to show a relationship between a prognostic index and probability of survival at a given time. As we are not testing significance between populations but showing a correlation between factors this does not require an assumption of a normal distribution. This approach is largely based on the methods used for breast cancer by Blamey et al (41). This formula was applied to the study population and the distribution of scores determined. The distribution of scores was then used to derive cut-off values for prognostic groups. These prognostic groups were assessed by plotting Kaplan-Meier curves. From these curves the percentage ten year survival was determined. Median prognostic score within each group was then related to survival by production of a scatter plot in Microsoft Excel and fitting an appropriate curve, determined by regression analysis. Where possible a balance between simplicity and performance was sought in order to prevent the risk of overfitting.
Results
Comparison of patient/tumor characteristics and prognosis
Relationships between patient/tumor characteristics and disease specific survival have been published previously (33). We are aware that there may be potential selection bias due to the nature of a single-hospital study but distribution of patient sex, age, stage, histological type and grade were found to be representative of the UK colorectal cancer population (33). Highly significant relationships were demonstrated between disease specific survival and TNM stage (log rank 211.37, p<0.001), and between disease specific survival and the presence of extramural vascular invasion (log rank 44.30, p<0.001). There were no other significant correlations found in the analysis.
Expression of individual NKG2D ligands in colorectal cancer and associations with prognosis
A total of 6 antibodies were used in this analysis to determine the expression of NKG2D ligands on our tissue microarray containing 462 colorectal tumors. Western Blot analysis was used to demonstrate the specificity of the antibodies to ULBP1, ULBP3, and RAET1E, shown in figure 1. The specificity of the anti-ULBP2 reagent was determined by flow cytometry, where there was good recognition of ULBP2 but an element of cross-reactivity with the highly related molecule RAET1L, and to a lesser extent with RAET1G (figure 1). We are not aware of any antibodies that can specifically discriminate between ULBP2, RAET1L and RAET1G extracellular domains (R.A. Eagle, unpublished data). All antibodies are reactive against extracellular domains with the exception of the anti-RAET1G antiserum that was raised against the cytoplasmic tail. The specificity of the anti-MIC monoclonal (40) and anti-RAET1G anti-serum (39) have been described elsewhere.
The majority of ligands examined in this study were expressed in colorectal cancer with few cores staining negatively. MIC, RAET1E and RAET1G (figure 2) stained in a similar pattern with a broad distribution of intensity from low to high. ULBP1, ULBP2 and ULBP3 (figure 2) stained the majority of tissues but showed a skewed distribution where the staining of tumor tissue was mostly at the lower scale of intensity. In each of the six cases the median intensity of staining was used to separate the two populations corresponding to high and low expression. There was no visible staining of nucleus or stromal elements in any of the analysis carried out.
Figure 2. Photographs showing typical staining for MIC, RAET1E, RAET1G, ULBP1, ULBP2, and ULBP3.
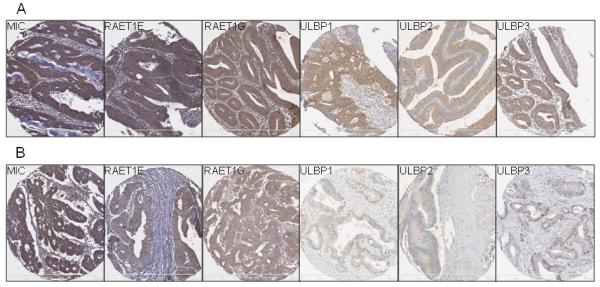
The photographs presented are from representative tissue micro array cores showing high (A) and low (B) expression at x100 magnification. Scale bar represents 0-500μm in 100μm increments.
When analyzed individually for impact on survival, summarized in table I, MIC and RAET1G expression showed significant survival advantages. Kaplan-Meier survival analysis of MIC (figure 3) and RAET1G (figure 3) showed a significant association between disease specific survival and high levels of expression (Log-rank p=0.035, Cox regression p=0.018; Log-rank p=0.01, Cox regression p=0.03 respectively). The mean survival at 10 years for MIC expression was 56 months in the low group, compared with 68 months for high expression levels. For RAET1G expression the mean survival at ten years was 62 months in the low group and 74 months in the high expressing patients. Median survival for high expressing patients could not be calculated in either case as greater than 50% of these patients were still alive at the data endpoint.
Table I. Disease specific survival analysis of the NKG2D ligands MIC, RAET1E, RAET1G, ULBP1, ULBP2 and ULBP3.
Data presented for each ligand expression case. Combinations of ligand expression are only presented in cases where statistical significance was found.
| Variable | Mean DSS (months) |
Survival range (months) |
Log-rank p-value |
Cox regression p-value |
HR | Lower CI |
Upper CI |
|---|---|---|---|---|---|---|---|
| MICA expression |
|||||||
| High | 68 | 62-74 | 0.035 | 0.04 | 0.76 | 0.59 | 0.99 |
| Low | 56 | 47-65 | |||||
|
| |||||||
| ULBP1 expression |
|||||||
| High | 67 | 61-73 | 0.055 | 0.045 | 0.81 | 0.64 | 1.05 |
| Low | 58 | 50-66 | |||||
|
| |||||||
| ULBP2 expression |
|||||||
| High | 60 | 54-66 | 0.466 | 0.39 | 1.13 | ||
| Low | 67 | 60-74 | |||||
|
| |||||||
| ULBP3 expression |
|||||||
| High | 66 | 58-74 | 0.53 | 0.492 | 0.91 | ||
| Low | 63 | 57-70 | |||||
|
| |||||||
| RAET1E expression |
|||||||
| High | 65 | 58-72 | 0.642 | 0.299 | 0.84 | ||
| Low | 64 | 57-71 | |||||
|
| |||||||
| RAET1G expression |
|||||||
| High | 74 | 65-83 | 0.01 | 0.03 | 0.74 | 0.551 | 1 |
| Low | 62 | 56-67 | |||||
|
| |||||||
| MICA and ULBP1 |
|||||||
| Both high | 67 | 60-73 | 0.018 | 0.006 | 0.83 | 0.7 | 0.97 |
| Single high | 66 | 57-74 | |||||
| Both low | 50 | 38-62 | |||||
|
| |||||||
| MICA and RAET1E |
|||||||
| Both high | 68 | 62-74 | 0.02 | 0.014 | 0.86 | 0.77 | 0.97 |
| Single high | 61 | 51-72 | |||||
| Both low | 44 | 30-59 | |||||
|
| |||||||
| MICA and RAET1G |
|||||||
| Both high | 77 | 68-87 | 0.003 | 0.005 | 0.84 | 0.74 | 0.95 |
| Single high | 64 | 56-71 | |||||
| Both low | 54 | 44-64 | |||||
|
| |||||||
| ULBP1 and RAET1E |
|||||||
| Both high | 68 | 59-72 | 0.041 | 0.039 | 0.81 | ||
| Single high | 62 | 55-70 | |||||
| Both low | 45 | 27-63 | |||||
|
| |||||||
| ULBP1 and RAET1G |
|||||||
| Both high | 73 | 63-84 | 0.027 | 0.032 | 0.83 | ||
| Single high | 65 | 58-72 | |||||
| Both low | 57 | 48-65 | |||||
|
| |||||||
| RAET1E and RAET1G |
|||||||
| Both high | 76 | 67-86 | 0.004 | 0.002 | 0.84 | 0.77 | 0.99 |
| Single low/ Both low |
61 | 55-67 | |||||
Figure 3.
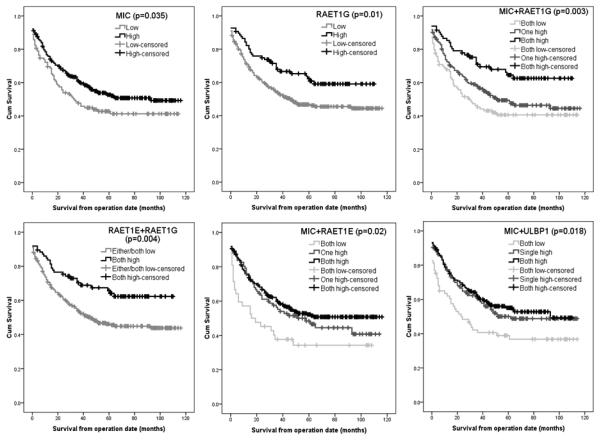
Kaplan-Meier plots showing disease specific survival in statistically significant cases.
The ligands RAET1E and the ULBPs did not demonstrate any statistically significant association with disease specific survival. When considered in univariate analysis against the clinico-pathological factors the expression of MIC correlates with tumor grade (p=0.037), RAET1E with TNM stage (p=0.017), ULBP1 with tumor grade (p=0.006), and ULBP3 with Duke's stage (p=0.022). There was no association with MSI which does not confer a survival benefit in this cohort of patients..
NKG2D ligand expression is heterogenous and combinations of ligands are highly associated with prognosis
Ligands for NKG2D are not expressed independently on the cell surface (2) and we were interested to find out what the prognostic impact of combined analysis would be. Each ligand was combined with each of the five other ligands and then subjected to statistical analysis as discussed previously. Although it would be of major interest to analyse expression of more than two ligands at any one time, this is not feasible due to statistical limitations. The statistical power of the cohort is lost with the large number of groups generated. The significance of each ligand expression in multi-factorial analysis, tested by the Cox regression model, is discussed later.
MIC expression considered on its own had a p-value of 0.035, so when MICA and RAET1G expression patterns were combined the relationship between both factors and disease specific survival was shown to be highly significant (p=0.003) (figure 3). Certain ligands that on their own had no impact on significance were found to have an association with survival when considered in combination with another ligand. RAET1E had no significant association with survival unless combined with RAET1G expression, which indicates an improved prognosis (p=0.004) (figure 3). In the same manner, the combination between RAET1E and MIC expression produced a p-value of 0.02 (figure 3) in relation to disease specific survival. This appears to indicate a close association between ligands that is not directly related to patient survival. The combination of ULBP1 and MIC gave a p-value of 0.018 (figure 3), indicating a similar relationship. These data provide an indication that NKG2D ligands are co-operative in a manner that, can either directly or indirectly, impact on patient survival.
All combinations were analyzed but no other combinations than those discussed here demonstrated any statistically significant association with disease specific survival.
High level NKG2D ligand expression reduces with increasing tumor stage
The patient data for expression of each ligand was divided according to their TNM classification, as 1, 2, 3, and 4, where 1 included patients with stage 0 and 1 tumors. Each stage was subdivided into high and low expression as a percentage of the total, as shown in figure 4. When considering all the expression data it was noted that a trend occurred in all but one case. As tumor stage progresses the frequency of high level expression decreases through to stage 4, which has the worst prognosis. This data trend shows a direct relationship between the patients worsening prognosis and loss of expression, indicating that late stage tumours have been driven towards lower expression levels.
Figure 4. Tumor Expression of NKG2D Ligands Decreases as Tumor Grade Increases.
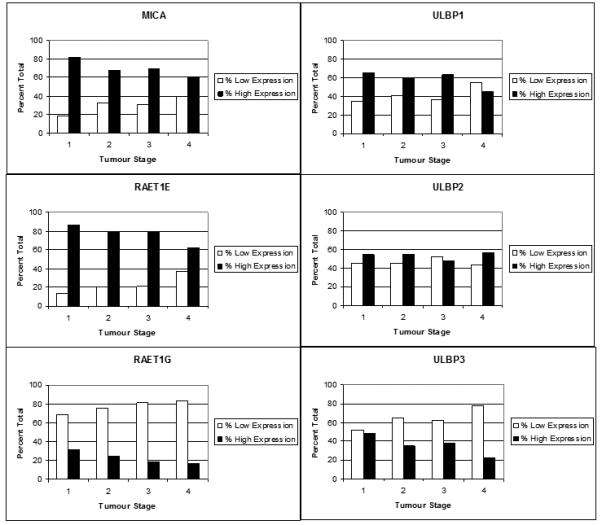
The graphs above show the number of patients classified as low or high expression represented as a percentage of the total for each TNM stage where stages 0 and 1 were classified together as 1. Stages 2, 3, and 4 were left as individual groups.
Multivariate analysis uncovers potential relationships between NKG2D ligands in overall survival of colorectal cancer patients
Multivariate analysis highlighted the factors with a link between the clinicopathological data and the ligands tested, were TNM stage (p<0.001) and MIC expression (p=0.012). Although Cox regression analysis of RAET1G (p=0.029) demonstrated significance, it appears to be co-correlated with TNM stage as this significance was lost in multi-factorial Cox regression analysis (p=0.244). Other combinations did not demonstrate independent prognostic value. This is likely to be due to the partial co-correlation and interdependence of parameters.
Beta values were used to derive a prognostic scoring formula for the two prognostic factors. TNM stage had a beta value of 0.717 and MIC had a beta value of −0.381. This resulted in the following formula.
Prognostic scores were calculated for the experimental population and the distribution of scores determined (results not shown). Based on this distribution prognostic categories were defined. Kaplan Meier survival curves were plotted for these categories and the median 10 year survival determined for each category. Regression analysis was used to define the relationship between median category and percentage 10 year survival. A good relationship was seen between score and median 10 year survival having an r-squared value of 0.912. This initial prototype prognostic index has been developed on a single centre sample set. As such it should be validated in other centres for prognostic performance.
The cumulative survival plot for patients sorted according to their prognostic index score (figure 5) indentified unusually poor survival in group 2 patients. This group contains patients who have been classified as TNM stage 1 and low MIC expression level, and according to the prognostic model have a median 10 year survival rate of 50%. When we consider group 1 patients who have the same TNM classification but high MIC expression, their median 10 year survival is 83.8%. The expected survival rate of TNM stage 1 colorectal cancer patients is 80-95%, so those patients with low levels of MIC expression should be selected for adjuvant therapy following surgical resection of their tumor.
Figure 5. 10 Year Survival Plot of Patient Prognostic Groups According to Their Prognostic Index Score.
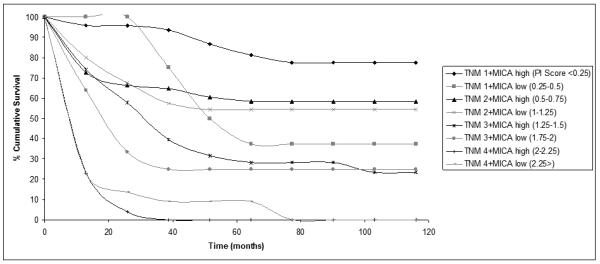
The graph shows cumulative survival for each of the prognostic groups as sub-divided according to our prognostic model generated from the Cox Regression analysis. The prognostic model was generated based on TNM category and MIC expression level. Each prognostic group is indicated in the legend with the corresponding prognostic index score.
As MIC was the only ligand shown to be an independent marker of good prognosis in colorectal cancer, its expression was correlated with innate cell infiltration. CD16 is expressed by innate mononuclear cells including NK cells and monocytes which both express high levels of NKG2D. There was a strong positive correlation (p=0.004) between MIC expression and cellular infiltrate.
Discussion
After remaining an unproven theory for many years, cancer immunosurveillance has undergone a renaissance (21). Diverse lines of evidence from in vivo models suggest that the immune system attacks early stage tumors. Cancer cells that survive must adapted to avoid the immune system, a process variously described as immunoediting, immune sculpting, or cancer immune evasion (21). Studies with in vivo cancer models strongly suggest that the activating immune receptor NKG2D is involved in anti-cancer immune responses (18-20, 29, 42). In humans both primary tumors and tumor-derived cell lines frequently express NKG2D ligands (11, 12, 14). In this study we show that there is a good correlation between MICA expression and infiltration of colorectal tumours with innate mononuclear cells.
Tissue microarray technology allows protein expression to be assessed and compared in a large number of samples simultaneously. This allows the identification of broad trends in expression patterns, and correlations to be made between expression, clinicopathological features and patient prognosis. We have previously shown that MICA has independent prognostic value in colorectal carcinoma using polyclonal anti-sera (33). In this study we confirmed this result with a monoclonal antibody that recognises MICA and MICB, showing that high expression is associated with improved patient survival. The study was extended using antibodies that recognize all members of the ULBP/RAET family of NKG2D ligands. The first observation from this analysis was that NKG2D ligand expression was heterogeneous in primary cancers, as not all ligands were expressed highly in the same tumor. Individually MIC and RAET1G showed an association with prognosis, but other NKG2D ligands did not. Significantly the combinations of these two ligands were as good as TNM stage at predicting patient prognosis. These results make a strong case for NKG2D mediated immunity involvement in human colorectal cancer development.
There are two explanations for NKG2D ligand heterogeneity on tumors that are not mutually exclusive. Firstly, the heterogeneity may reflect that fact that NKG2D ligands have different promoters and can be expressed independently in response to different stress response pathways. Also there is now evidence for post-transcriptional regulation of NKG2D ligand expression that also may allow differential regulation of the expression of different NKG2D ligands, an example being a potential role of microRNAs (43). Some stimuli have been reported to result in the expression of all NKG2D ligands tested, such as DNA damage responses in mice (15). Others have been shown to be specific to some NKG2D ligands; for example BCR/ABL regulates MICA but not ULBP1-2 in K562 cells and treatment of hepatoma cells with histone deacetylase inhibitors induced MICA, but not ULBP1-3 (16, 44).
If this explanation was exclusively true it would imply that heterogeneous NKG2D ligand expression on tumors was simply reflecting the activation stage of various cancer related pathways, and was not as a result of selective pressure placed on the cancer by the immune system to develop evasion strategies. In perforin knockout mice that are deficient in immune cell cytotoxicity chemically induced tumors are seen to have much higher expression of Rae-1 than wild type mice. In NKG2D knockout mice early arising prostate tumors have higher levels of NKG2D ligand expression than tumors arising in wild type mice. This indicates that cytotoxic immune responses, and NKG2D mediated immunity, can place selection on developing tumors to switch off NKG2D ligand expression as part of a cancer immune evasion strategy or immunoediting. Our study indicated that for all six NKG2D ligands, expression was highest in the early stage I tumors, but then decreased in later stages II, III and IV, with highly aggressive stage IV tumors having the lowest expression. The data are consistent with a model where developing cancers lose expression of NKG2D ligands to avoid the immune system. This does not preclude other NKG2D specific cancer immune evasion mechanisms being important, such as release of soluble NKG2D ligands or immunosuppressive cytokines such as TGF-beta (25-27).
It has been suggested that NKG2D ligands are important in the immune response against tumors and we have shown this both through our prognostic model and the significant correlation between MIC expression and NK cellular infiltration. The poor prognosis of early stage colorectal cancer patients with low MIC expression is evident and has not previously been described. Currently the generally accepted regimen for TNM stage I patients is surgery alone, although our data suggests that the group of patients with low level MIC would benefit from additional adjuvant therapy. Patients having colonoscopy could easily have a biopsy sample histologically stained for NKG2D ligand expression in order to further classify their tumor in line with our results.
In conclusion tissue microarrays provide a further line of evidence for NKG2D involvement in cancer immunosurveillance and combinatorial analysis of NKG2D ligand expression is an attractive target for the development of improved prognostic classification of colorectal and other carcinomas.
Acknowledgments
This work was supported by Lewis Trust to LGD and Cancer Research UK to RAE and JT.
Footnotes
Statement of Translational Relevance
The work presented here is the first comprehensive screen of NKG2D ligand expression on a large cohort of primary human colorectal cancers. Using tissue microarray technology our analysis has demonstrated that ligand expression is heterogenous and appears to have a co-operative benefit towards improved prognosis. We describe the statistical analysis of ligand expression, highlighting those situations that show statistical significance to patient survival. Mathematical modelling has identified a group of at-risk patients that has not been previously described. This group of early stage cancer patients would likely benefit from adjuvant therapy in addition to the currently accepted regimen of surgical resection for stage I patients.
References
- 1.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781–90. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 2.Eagle RA, Trowsdale J. Promiscuity and the single receptor: NKG2D. Nat Rev Immunol. 2007;7:737–44. doi: 10.1038/nri2144. [DOI] [PubMed] [Google Scholar]
- 3.Bauer S, Groh V, Wu J, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–9. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 4.Cosman D, Mullberg J, Sutherland CL, et al. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123–33. doi: 10.1016/s1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- 5.Radosavljevic M, Cuillerier B, Wilson MJ, et al. A cluster of ten novel MHC class I related genes on human chromosome 6q24.2-q25.3. Genomics. 2002;79:114–23. doi: 10.1006/geno.2001.6673. [DOI] [PubMed] [Google Scholar]
- 6.Chalupny NJ, Sutherland CL, Lawrence WA, Rein-Weston A, Cosman D. ULBP4 is a novel ligand for human NKG2D. Biochem Biophys Res Commun. 2003;305:129–35. doi: 10.1016/s0006-291x(03)00714-9. [DOI] [PubMed] [Google Scholar]
- 7.Bacon L, Eagle RA, Meyer M, Easom N, Young NT, Trowsdale J. Two human ULBP/RAET1 molecules with transmembrane regions are ligands for NKG2D. J Immunol. 2004;173:1078–84. doi: 10.4049/jimmunol.173.2.1078. [DOI] [PubMed] [Google Scholar]
- 8.Diefenbach A, Jamieson AM, Liu SD, Shastri N, Raulet DH. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nat Immunol. 2000;1:119–26. doi: 10.1038/77793. [DOI] [PubMed] [Google Scholar]
- 9.Cerwenka A, Bakker AB, McClanahan T, et al. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity. 2000;12:721–7. doi: 10.1016/s1074-7613(00)80222-8. [DOI] [PubMed] [Google Scholar]
- 10.Carayannopoulos LN, Naidenko OV, Fremont DH, Yokoyama WM. Cutting edge: murine UL16-binding protein-like transcript 1: a newly described transcript encoding a high-affinity ligand for murine NKG2D. J Immunol. 2002;169:4079–83. doi: 10.4049/jimmunol.169.8.4079. [DOI] [PubMed] [Google Scholar]
- 11.Groh V, Bahram S, Bauer S, Herman A, Beauchamp M, Spies T. Cell stress-regulated human major histocompatibility complex class I gene expressed in gastrointestinal epithelium. Proc Natl Acad Sci U S A. 1996;93:12445–50. doi: 10.1073/pnas.93.22.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pende D, Rivera P, Marcenaro S, et al. Major histocompatibility complex class I-related chain A and UL16-binding protein expression on tumor cell lines of different histotypes: analysis of tumor susceptibility to NKG2D-dependent natural killer cell cytotoxicity. Cancer Res. 2002;62:6178–86. [PubMed] [Google Scholar]
- 13.Salih HR, Antropius H, Gieseke F, et al. Functional expression and release of ligands for the activating immunoreceptor NKG2D in leukemia. Blood. 2003;102:1389–96. doi: 10.1182/blood-2003-01-0019. [DOI] [PubMed] [Google Scholar]
- 14.Raffaghello L, Prigione I, Airoldi I, et al. Neoplasia. Vol. 6. New York, NY: 2004. Downregulation and/or release of NKG2D ligands as immune evasion strategy of human neuroblastoma; pp. 558–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–90. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boissel N, Rea D, Tieng V, et al. BCR/ABL oncogene directly controls MHC class I chain-related molecule A expression in chronic myelogenous leukemia. J Immunol. 2006;176:5108–16. doi: 10.4049/jimmunol.176.8.5108. [DOI] [PubMed] [Google Scholar]
- 17.Terme M, Borg C, Guilhot F, et al. BCR/ABL promotes dendritic cell-mediated natural killer cell activation. Cancer Res. 2005;65:6409–17. doi: 10.1158/0008-5472.CAN-04-2675. [DOI] [PubMed] [Google Scholar]
- 18.Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413:165–71. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cerwenka A, Baron JL, Lanier LL. Ectopic expression of retinoic acid early inducible-1 gene (RAE-1) permits natural killer cell-mediated rejection of a MHC class I-bearing tumor in vivo. Proc Natl Acad Sci U S A. 2001;98:11521–6. doi: 10.1073/pnas.201238598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guerra N, Tan YX, Joncker NT, et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28:571–80. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–60. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 22.Wiemann K, Mittrucker HW, Feger U, et al. Systemic NKG2D down-regulation impairs NK and CD8 T cell responses in vivo. J Immunol. 2005;175:720–9. doi: 10.4049/jimmunol.175.2.720. [DOI] [PubMed] [Google Scholar]
- 23.Oppenheim DE, Roberts SJ, Clarke SL, et al. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat Immunol. 2005;6:928–37. doi: 10.1038/ni1239. [DOI] [PubMed] [Google Scholar]
- 24.Coudert JD, Zimmer J, Tomasello E, et al. Altered NKG2D function in NK cells induced by chronic exposure to NKG2D ligand-expressing tumor cells. Blood. 2005;106:1711–7. doi: 10.1182/blood-2005-03-0918. [DOI] [PubMed] [Google Scholar]
- 25.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–8. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 26.Castriconi R, Cantoni C, Della Chiesa M, et al. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc Natl Acad Sci U S A. 2003;100:4120–5. doi: 10.1073/pnas.0730640100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JC, Lee KM, Kim DW, Heo DS. Elevated TGF-beta1 secretion and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J Immunol. 2004;172:7335–40. doi: 10.4049/jimmunol.172.12.7335. [DOI] [PubMed] [Google Scholar]
- 28.Eagle RA, Traherne JA, Ashiru O, Wills MR, Trowsdale J. Regulation of NKG2D ligand gene expression. Hum Immunol. 2006;67:159–69. doi: 10.1016/j.humimm.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 29.Smyth MJ, Swann J, Cretney E, Zerafa N, Yokoyama WM, Hayakawa Y. NKG2D function protects the host from tumor initiation. J Exp Med. 2005;202:583–8. doi: 10.1084/jem.20050994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–7. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 31.Watson NF, Durrant LG, Scholefield JH, et al. Cytoplasmic expression of p27(kip1) is associated with a favourable prognosis in colorectal cancer patients. World J Gastroenterol. 2006;12:6299–304. doi: 10.3748/wjg.v12.i39.6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watson NF, Durrant LG, Madjd Z, Ellis IO, Scholefield JH, Spendlove I. Expression of the membrane complement regulatory protein CD59 (protectin) is associated with reduced survival in colorectal cancer patients. Cancer Immunol Immunother. 2006;55:973–80. doi: 10.1007/s00262-005-0055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watson NF, Spendlove I, Madjd Z, et al. Expression of the stress-related MHC class I chain-related protein MICA is an indicator of good prognosis in colorectal cancer patients. Int J Cancer. 2006;118:1445–52. doi: 10.1002/ijc.21510. [DOI] [PubMed] [Google Scholar]
- 34.Ullenhag GJ, Mukherjee A, Watson NF, Al-Attar AH, Scholefield JH, Durrant LG. Overexpression of FLIPL is an independent marker of poor prognosis in colorectal cancer patients. Clin Cancer Res. 2007;13:5070–5. doi: 10.1158/1078-0432.CCR-06-2547. [DOI] [PubMed] [Google Scholar]
- 35.Duncan TJ, Watson NF, Al-Attar AH, Scholefield JH, Durrant LG. The role of MUC1 and MUC3 in the biology and prognosis of colorectal cancer. World journal of surgical oncology. 2007;5:31. doi: 10.1186/1477-7819-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayne D, Brown RS, McCormack M, Quinn MJ, Payne HA, Babb P. Current trends in colorectal cancer: site, incidence, mortality and survival in England and Wales. Clinical oncology (Royal College of Radiologists (Great Britain)) 2001;13:448–52. doi: 10.1053/clon.2001.9311. [DOI] [PubMed] [Google Scholar]
- 37.NICE . Improving outcomes in colorectal cancers. Manual update: National Institute for Clinical Excellence; London: Jun 23, 2004. [Google Scholar]
- 38.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Reporting recommendations for tumor marker prognostic studies (remark) Experimental oncology. 2006;28:99–105. [PubMed] [Google Scholar]
- 39.Eagle RA, Flack G, Warford A, et al. Cellular expression, trafficking, and function of two isoforms of human ULBP5/RAET1G. PLoS ONE. 2009;4:e4503. doi: 10.1371/journal.pone.0004503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hue S, Monteiro RC, Berrih-Aknin S, Caillat-Zucman S. Potential role of NKG2D/MHC class I-related chain A interaction in intrathymic maturation of single-positive CD8 T cells. J Immunol. 2003;171:1909–17. doi: 10.4049/jimmunol.171.4.1909. [DOI] [PubMed] [Google Scholar]
- 41.Rakha EA, El-Sayed ME, Lee AH, et al. Prognostic significance of Nottingham histologic grade in invasive breast carcinoma. J Clin Oncol. 2008;26:3153–8. doi: 10.1200/JCO.2007.15.5986. [DOI] [PubMed] [Google Scholar]
- 42.Unni AM, Bondar T, Medzhitov R. Intrinsic sensor of oncogenic transformation induces a signal for innate immunosurveillance. Proc Natl Acad Sci U S A. 2008;105:1686–91. doi: 10.1073/pnas.0701675105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stern-Ginossar N, Gur C, Biton M, et al. Human microRNAs regulate stress-induced immune responses mediated by the receptor NKG2D. Nat Immunol. 2008;9:1065–73. doi: 10.1038/ni.1642. [DOI] [PubMed] [Google Scholar]
- 44.Armeanu S, Bitzer M, Lauer UM, et al. Natural killer cell-mediated lysis of hepatoma cells via specific induction of NKG2D ligands by the histone deacetylase inhibitor sodium valproate. Cancer Res. 2005;65:6321–9. doi: 10.1158/0008-5472.CAN-04-4252. [DOI] [PubMed] [Google Scholar]


