Abstract.
During cardiogenesis, the epicardium grows from the proepicardial organ to form the outermost layer of the early heart. Part of the epicardium undergoes epithelial-mesenchymal transformation, and migrates into the myocardium. These epicardium- derived cells differentiate into interstitial fibroblasts, coronary smooth muscle cells, and perivascular fibroblasts. Moreover, epicardium-derived cells are important regulators of formation of the compact myocardium, the coronary vasculature, and the Purkinje fiber network, thus being essential for proper cardiac development. The fibrous structures of the heart such as the fibrous heart skeleton and the semilunar and atrioventricular valves also depend on a contribution of these cells during development. We hypothesise that the essential properties of epicardium-derived cells can be recapitulated in adult diseased myocardium. These cells can therefore be considered as a novel source of adult stem cells useful in clinical cardiac regeneration therapy.
Keywords. Stem cells, epicardium, epicardium-derived cells, embryonic development, heart
Introduction
The epicardium consists of mesothelial epithelial tissue that forms the outermost layer of the heart. It has many functions during embryonic development and adult life, which were unknown until 40 years ago. Covering the myocardium, the epicardium serves as a smooth layer which enables the heart to slide over the outer pericardial epithelium. During embryogenesis, the epicardium gives rise to all cellular elements of the subepicardial layer, to interstitial and perivascular fibroblasts, and to smooth muscle cells of the coronary arteries. Moreover, recent data demonstrated that epicardium and epicardium-derived cells (EPDCs) have a crucial stimulatory role in the development of the embryonic compact myocardium, the coronary vasculature and the Purkinje fiber system. Their role in valve and fibrous heart skeleton differentiation is still unresolved. In this review we will discuss the origin of the epicardium and the function of EPDCs and their derivatives in embryonic cardiac development. We thereafter postulate that EPDCs might recapitulate their embryonic capacities when in contact with adult diseased myocardium. In this way they can serve as an adult stem cell for cardiac regeneration.
Origin of the epicardium
Initially, the primary heart develops from two cardiogenic fields of splanchnopleuric mesoderm that differentiate into a myocardial tube, lined on the inside by endocardium [1]. Between these layers the cardiac jelly is produced. This structure is called the primary heart tube [2] and protrudes into the coelomic cavity, referred to as the pericardio-peritoneal canal. The dorsal mesocardium, during development separated to form arterial and venous pole connections, links the primary heart tube to the dorsal body wall. Later on, the primary heart tube is covered by a layer of epicardium, which arises at the venous pole.
Our current view on epicardial origin was already proposed by Kurkiewicz in 1909. He reported that the myocardium consisted solely of cardiomyoblasts, and that the epicardium was derived from an extracardiac source [3]. His findings were disregarded and overlooked for a long time. The prevailing dogma in the middle of last century was that the epicardium formed an inert layer, basically functioning to protect the myocardium, and itself being derived from the myocardium. This layer was also referred to as the epimyocardium [4,5]. Manasek showed, using light and transmission electron microscopy, that the early myocardium consisted of cardiomyoblasts only, and thus did not contain epicardial cells. He hypothesised that the epicardium originated from an extracardiac source, but he did not exclude that the myocardium could dedifferentiate into epicardial cells as well [6,7]. Viragh gave the solution, by studying mouse embryos with light and transmission electron microscopy. He observed that epicardial cells migrated to the heart from somatopleural cells of the transverse septum [8]. Ho and Shimada supported the research from Viragh by demonstrating with scanning electron microscopy (SEM) that epicardial cells and cardiomyocytes were cytologically different. They did not find transitional cardiomyocytes, thereby dismissing the possibility that cardiomyocytes would dedifferentiate into epicardial cells [9]. By broader use of SEM, many studies clarified the origin of the epicardium. It was shown in amphibians, reptiles, birds and mammals that epicardial cells are derived from villous protrusions in the region of the venous pole of the heart near the developing transverse septum [9–14]. Further knowledge about the origin and attachment of epicardial cells to the heart, their spreading pattern, transformation of the cells into mesenchymal cells, and their derivatives, was derived from experiments in the last decade of the 20th century.
The term ‘proepicardial organ’ (PEO) was coined to describe the previously mentioned villous protrusions because of its heterogeneous cell structure [15], although it is not a real organ. The PEO arises from the coelomic serosa and its immediately underlying mesoderm. This area is also the source of sinus venosus myocardium. We adhere in this review to the use of the term ‘PEO’ solely for the transient cauliflower like strucutre which has already differentiated into a purely epicardial direction. In mammals, the PEO consists of bilaterally and symmetrically distributed clusters of mesothelial protrusions and villi, covering the transverse septum [10–12]. In avian embryos, the PEO first consists of bilateral protrusions of the right and left sinus horns. Preceded by right-sided asymmetric gene expression [16], the left part ceases to develop, leaving only the right protrusion to form into a cauliflower-like structure consisting of mesothelial villi covered by squamous cells [17].
The way in which the epicardial cells translocate from the proepicardial serosa to the heart differs between species. In avian embryos the main pathway through which epicardial cells reach the heart is a tissue bridge between the ventral side of sinus venosus and the dorsal surface of the developing ventricles [14,18,19]. This tissue is positioned around, and probably guided by, a bridge of extracellular matrix [20]. In mammalian and fish embryos such a sino-ventricular ligament is absent. Free-floating epicardial cell aggregates fuse together to give rise to the epicardial sheet that covers the heart [10–12, 21].
The epicardial cells cover the developing heart in a spatiotemporal pattern comparable for various species [9,11,13,14,19,22]. Embryo stages described for quail can be extrapolated to corresponding stages in other species. At Hamburger Hamilton stage 14 (HH14) [23], the PEO of the quail embryo starts to develop at the ventral surface of the proepicardial serosa [15] (Fig. 1a,b).Villi protrude from the surface at HH 15 and 16, giving it its cauliflower-like appearance [10,15,19]. At HH17, the tips of the epicardial protrusions reach the dorsal surface of the early heart tube at the atrioventricular sulcus, and form a circular patch of epicardial cells as they migrate radially over the myocardium at stage 18 [19]. At HH18–HH20 the epicardial cells spread ventrally along the left and right side of the atrioventricular canal to the inner curvature of the heart, and caudoventrally over the ventricular inlet segment. Spreading proceeds at HH20–HH24 from the inner curvature, over the outflow tract, towards the ventriculo-arterial junction. The right atrium is completely covered between HH23 and HH24. At HH25 the left atrium and a part of the outflow tract are the only parts of the heart that are still uncovered. Whole-mount cytokeratin staining patterns show that these parts are covered at HH26, by which stage the epicardial covering of the heart is complete [19], and after which proepicardial structures are no longer seen [15]. There is some conflicting evidence on timing and source of epicardium at the ventriculo-arterial junction, which may be tracing technique dependent. Whole-mount cytokeratin studies show complete covering of the myocardial outflow tract by PEO-derived epicardium at HH26 [19]. After complete PEO ablation, arterial pole-derived mesothelial cells, also referred to as cephalic pericardium, cover a myocardial collar of the outflow tract at HH28 [24, 25]. This might be explained by the concurrent addition of secondary heart field myocardium to the outflow tract (for review see [26]). From quail-chick chimera techniques, it has been described that the distal part of the outflow tract is covered by a mixed population of arterial pole-derived mesothelium and venous pole-derived epicardium at least until HH35[24, 25, 27, 28].
Figure 1.
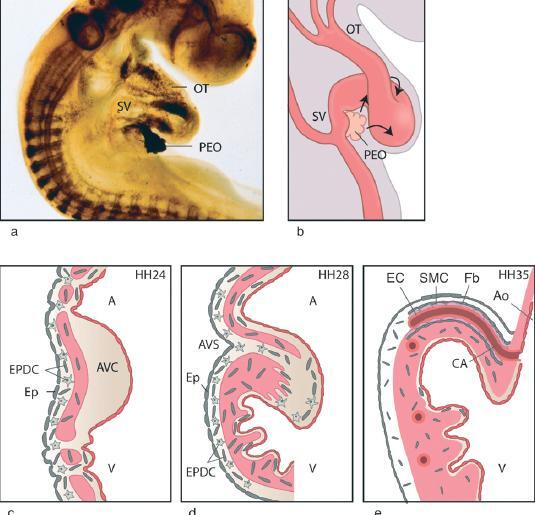
Spreading and migration of EPDCs. (a) Whole-mount quail embryo (HH16) stained for HNK1, showing a clearly demarcated proepicardial organ (PEO) at the venous pole of the heart, (b) Schematic representation indicating (arrows) the direction of growth of the epicardium over the myocardial tube. (c–e) Schematic drawing of increasing ages with the migration pattern of the EPDCs. (c) HH24: epicardial cells cover the heart tube, and EPDCs (star shaped, grey) enter the myocardium and, through gaps, the subendocardial layer. The endocardial cushion is still devoid of EPDCs. (d) HH28: the compact myocardium is formed, and EPDCs have entered all cardiac components. Note the contribution to the formed atrioventricular sulcus and the endocardial cushions, (e) HH35: the coronary vasculature has grown into the aorta, and EPDCs through EMT (cuboid cells) now also contribute to the coronary arterial vascular wall. OT, outflow tract; SV, sinus venosus; PEO, proepicardial organ; AVC, atrioventricular cushion; EPDC, epicardium-derived cell; Ep, epicardium; V, ventricle; A, atrium; AVS, atrioventricular sulcus; EC, endothelial cell; SMC, smooth muscle cell; Fb, fibroblast, CA, coronary artery; Ao, aorta.
Origin of epicardium-derived cells (EPDCs)
After the primitive heart has been covered by a layer of epicardial cells, part of the epicardial cells undergoes epithelial-mesenchymal transformation (EMT), thereby acquiring the ability to migrate. Gittenberger-de Groot and co-workers called these cells that undergo EMT epicardium-derived cells or, for short, EPDCs [29]. The EPDCs migrate into the, originally acellular, subepicardial space and subsequently into the myocardium, where they differentiate into various cell types. EMT involves cytoskeletal reorganisation, observed in epicardial cells both in vivo [30–32] and in vitro [33]. Proepicardial and epicardial cells contain the keratin tonofilament bundles ‘cytokeratin’ [15,19]. These bundles are replaced by filaments of vimentin during the process of transformation. This substitution process is not instantaneous. Therefore, a coexpression of vimentin and cytokeratin is observed in the proepicardial cells that will undergo EMT, and in the EPDCs recently derived from the epicardial layer [30,31].
Insight into timing of EPDC invasion into the developing heart can be gained by quail-chick chimera studies [34,35], viral tracing experiments [33,36], and EPDC reporter gene studies in mice [37]. The results of the tracing studies, however, focus mostly on subsequent EPDC differentiation, which will be dealt with later on in this review. Normal quail-chick chimera experiments in which quail PEO is added to chick PEO [34, 35] are superior to blocking of the PEO by an eggshell membrane [18], because delay in PEO outgrowth is initiated in the latter. Quail-chick experiments provide evidence that already at HH19, immediately after the onset of spreading over the myocardial surface, EMT is seen and EPDCs migrate into the inner curvature myocardium. This area seems to be specifically permissive at this time point, as other mycoardial areas are not yet invaded [35]. Thereafter, invasion of the still thin atrial and ventricular myocardium is seen, with a specific migration to the subendocardial layer through myocardial gaps from HH20–24 [29] (Fig. 1c). With formation of compact myocardium these gaps disappear and EPDCs are found throughout both the compact and trabecular myocardium (Fig. 1d). At HH28, invasion of the atrioventricular endocardial cushions is seen as well as abundant filling of atrioventricular and periarterial mesenchyme [27, 29]. These data are recently supported by results from studies with epicardium-restricted LacZ expression in transgenic mice [37]. At the time of ingrowth of the coronary vasculature into the aorta (HH32) (Fig. 1e), abundant EMT is seen adjacent to the developing coronary orifices [32]. It is unknown whether this process of EMT continues throughout development, initial hatching or birth, or even into postnatal stages.
Molecular processes involved in epicardium and EPDC formation
Although important regulators of EMT and differentiation of EPDCs have been recently described, only little is known about these processes. Most of the factors discovered to be important for epicardial outgrowth and EPDC formation were used as manipulative targets to study the role of the EPDC in cardiac development. In this paragraph we will discuss the prinicipal molecular processes known to date.
Factors involved in adhesion of epicardial cells
Interaction between vascular cell adhesion molecule (VCAM-1) and α4 integrin is essential for adhesion and spreading of the epicardium [38–10]. These surface molecules are expressed in a reciprocal fashion in the myocardium and epicardium, respectively, and mediate cell-cell adhesion. VCAM-1 and α4 integrin null mice show a remarkably comparable phenotype, being absence of epicardium, absence of subepicardial vessels with subsequent cardiac hemorrhage [38, 39], and hampered compaction of the ventricular myocardium [38]. Yang et al. showed that α4 integrin is not essential for initial adhesion of epicardium to the myocardium, but that it is crucial for the maintenance of epicardial integrity. In contrast, a more recent study showed that α4 integrin is not only essential for maintaining the epicardium, but that it is also involved in the earlier process of outgrowth of the epicardium from the PEO and the subsequent spreading of the epicardium over the heart [40]. It was also described that normal levels of α4 integrin promote adhesion of epicardial cells and restrain EMT and migration, while inhibition of α4 integrin leads to stimulation of EMT [41]. Spreading of epicardial cells and maintenance of epicardial integrity therefore depend on a balanced interaction between VCAM-1 and α4 integrin.
Factors involved in outgrowth and differentiation of EPDCs
Essential for the initial steps in EMT are the homologous transcription factors Snail and Slug, expressed in mammalian and avian embryos, respectively [42–44]. Slug, expressed by the proepicardium, epicardium and undifferentiated EPDCs [45], can trigger EMT in epithelial cells by repression of cell adhesion molecules, including E-cadherin [43, 44, 46]. It would be interesting to study the relation between Slug and α4 integrin, because it has been demonstrated as mentioned above that inhibition of α4 integrin also stimulates EMT, migration, and invasion of epicardial cells [41].
The exact role of the transcription factor WT-1 in EPDC formation is still unclear, although it is essential, as was shown in WT1 null mice [47]. WT-1 expression is found in proepicardial cells, epicardial cells, and EPDCs in the subepicardial space, but not in fully differentiated EPDCs [48–50]. Interestingly, the areas of Slug and WT-1 expression are highly similar, except for EPDCs in the myocardium, which are positive for WT-1, while negative for Slug [50]. It has been suggested that WT-1 keeps EPDCs in an undifferentiated state, enabling early differentiation of EPDCs in the absence of WT-1 [48], although in WT-1−/− embryos no invaded, differentiated EPDCs are found [48]. This seems contradictory, as differentiation is normally associated with invasion.
Ets-1 and ets-2 are zinc finger transcription factors, similar to WT-1 and Slug/Snail, and are known to activate the expression of proteolytic enzymes, resulting in degradation of extracellular matrix, a process necessary to enable migration [51]. From an antisense study we now know that ets-1 and ets-2 are key regulators of epicardial EMT, and thereby essential for the development of EPDCs [52].
Fibroblast growth factor (FGF) and the tissue growth factor TGFβ are generally accepted to be stimulators of epicardial EMT [53–55]. TGFβ is also known to be an inducer of smooth muscle cell differentiation from epicardial cells [56]. However, Morabito et al. described an inhibitory role of TGFβ in epicardial EMT. They demonstrated that TGFβ3 was actively produced by myocardium, thereby postulating that TGFβ exerts a paracrine effect on epicardial cells, inhibiting EMT, and retaining them in the epicardium [57]. Based on expression studies, a role for PDGF receptor-β signalling upon stimulation by PDGF-B in the differentiation of EPDCs into coronary smooth muscle cells seems likely [58].
Retinoic acid, its receptor RXRα and RALDH2 — the key embryonic retinaldehyde dehydrogenase in retinoic acid synthesis — are critical for heart morphogenesis, with RXRα−/− embryos dying early from ventricular myocardial thinning [59–63]. Retinoic acid signalling in the epicardium is important for initial epicardial outgrowth, as RXRα−/− embryos exhibit a delay in the outgrowth of the epicardium from the PEO [64]. Furthermore, it is known to be a critical regulator of cardiomyocyte proliferation, which will be discussed later in this review.
Erythropoietin is essential for cardiac development, with erythropoietin−/− and erythropoietin receptor−/− mice suffering from a thin ventricular myocardium and abnormal coronary vessel formation, besides a severly disturbed epicardium. The erythropoietin receptor is expressed in epicardium and endocardium but not in the myocardium. Erythropoietin is thus another important factor for epicardial and/or EPDC formation [65].
Friend of GATA-2 (FOG-2), a cofactor for the GATA transcription factors, is expressed in the myocardium and is crucial for EMT of epicardial cells. FOG-2−/− embryos have an intact epicardial layer, but no EPDCs, resulting in severe cardiac malformations, as mentioned before. Re-expression of FOG-2 in cardiomyocytes results in EPDC formation and rescue of the phenotype, demonstrating that FOG-2 in cardiomyocytes is required for epicardial EMT and EPDC differentiation, revealing the importance of myocardial to epicardial signalling pathways in epicardial development [66].
Derivatives of EPDCs
Components of coronary vessels (Fig. 2)
Figure 2.
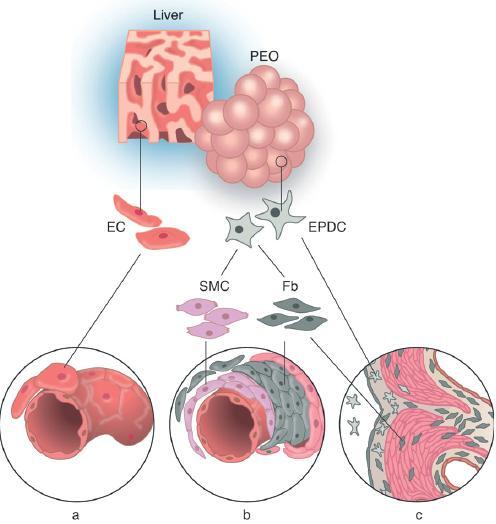
Differentiation of EPDCs. (a) The coronary endothelial cells (EC, pink) are derived from liver sinusoidal cells and grow into the myocardium along with the EPDCs. (b) The epicardium is derived from the proepicardial organ (PEO), and EPDCs are formed through epithelial mesenchymal transformation. The EPDCs (star shaped, grey) are still undifferentiated and have the potential to form smooth muscle cells (SMC, purple) and fibroblasts (Fb, grey). These cells form the media and adventitia of the coronary vessels, (c) The fibroblasts form the interstitial cells of the myoardium as well as the cells of the fibrous skeleton of the heart. Their role in valve differentiation is unknown. PEO, proepicardial organ; EC, endothelial cell, EPDC, epicardium-derived cell; SMC, smooth muscle cell; Fb, fibroblast.
Investigation of the fate and differentiation of EPDCs requires sophisticated tracing experiments and subsequent use of differentiation markers. These techniques include retroviral tracing [36,67], adenoviral and vital dye labeling [33], immunohistochemical analysis [15,19], quail-chick chimeras [27,29,32,34], mechanically inhibited embryos [18, 24, 28, 68], knockout models [37–39, 60, 66], knock-down models [52], and an epicardium-restricted LacZ expression model [37]. From these experiments it became evident that EPDCs give rise to the smooth muscle cells of the coronary vascular system as well as their surrounding adventitial fibroblasts [27, 29, 32, 33, 36]. There is some discussion of whether there are species differences in the origin of the proximal smooth muscle cells of the coronary arteries, as in Wnt LacZ mouse reporter studies they seem to derive from neural crest cells [69]. These latter cells provide most of the smooth muscle cells of the main great arteries in the thorax [70, 71]. As indicated by initial expression of smooth muscle cell markers, EPDCs do not differentiate into smooth muscle cells until the putative coronary arteries have grown from the periarterial plexus [32–34] into the aorta. Smooth muscle cell differentiation therefore appears to be triggered by the onset of arterial flow [32]. Development of coronary vessels highly depends on proper EPDC migration and differentiation. Coronary vasculature development can be blocked altogether in the absence of EPDCs [24, 38, 39, 66], resulting in embryonic death. In less severe abnormalities of EPDC formation, ingrowth of main coronary arteries was absent or abnormal [28, 37, 52, 68], with in some cases development of coronary ventricular fistulae [28, 52].
While it is generally accepted that smooth muscle cells and fibroblasts of the coronary vessels derive from the EPDCs, the origin of coronary endothelial cells is still a subject of debate. In several studies the presence of quail-derived endothelial cells in proepicardial quail-chick chimeras was employed to argue that EPDCs are also the source of coronary endothelial cells [27, 72, 73]. However, in these studies the PEO was isolated from an HH16–17 quail embryo, a stage at which the PEO already contains endothelial precursor cells [35]. Furthermore, it cannot be excluded that a piece of liver was excised together with the PEO, which is common in the generation of quail-chick chimeras. In that case it is to be expected that endothelial cells of quail origin be found [34]. On the other hand, double positive cells for quail endothelial marker and several epicardial markers have been reported in chimera [73] and quail PEO culture studies [74]. However, WT-1, cytokeratin, and RALDH2, which were used as epicardial indicators, are normally not only expressed in the epicardium but also in the dorsal mesoderm. This implies that liver-derived endothelial cells might express these markers as well [50, 75, 76]. Other chimera studies [34], fate-mapping studies [33], and genetically manipulated mouse models [37] dit not find coronary endothelial cells being derived from EPDCs. Merki et al. used a murine model in which the epicardial GATA-5/Cre transgenic mouse was crossed with the floxed ROSA26 LacZ reporter mouse to generate mice expressing LacZ in the epicardium and its derivatives. In this model, no LacZ expression was observed in the coronary endothelium [37]. However, it was not demonstrated that indeed all epicardial cells were labelled by this Cre-line. Thus, there is no conclusive evidence about the origin of coronary endothelial cells. Further research is needed to elucidate the possible contribution of EPDCs to the coronary endothelium.
Components of the fibrous skeleton of the heart and cushion mesenchyme (Fig. 2)
The differentiation of EPDCs into interstitial fibroblasts of the myocardium has not attracted much attention. From quail-chick chimera studies it is known that EPDCs in the subepicardium, subendocardium, and myocardium express pro-collagen I [29], indicating a fibrous differentiation pathway. Current unpublished data from our group postulate an active role for EPDCs in the formation of the fibrous heart skeleton by inducing specifically localized cardiomyocyte-fibroblast transformation, which is essential in the insulation of atrial and ventricular mycoardium. EPDCs that are found in the endocardial cushion tissue have not been traced by differentiation markers into a fibrous or other cell lineage [27,29]. Interestingly, late-stage quail-chick chimera studies demonstrate only a minor material contribution of EPDCs to finally formed valve leaflets [77], suggesting a regulatory role instead of a physical contribution of EPDCs to cushion tissue, which will be discussed later in this review.
The differentiation of EPDCs into cardiomyocytes has not been supported by chimera studies [27, 29]. There is, however, convincing evidence that the coelomic wall and adjacent mesoderm provide a common progenitor for epicardium and venous pole myocardium [78]. The diversification of differentiation of these two lineages is highly dependent on BMP and FGF signalling [16, 78].
Modulatory roles of EPDCs and their derivatives
EPDCs do not only physically contribute to the developing heart, they also have a regulatory role that is essential for proper cardiac development.
Myocardial compaction
Both mechanically inhibited [24, 28, 68], and knockout [37, 38, 47, 59, 60, 66] and knock-down embryos [52] suffer from a thin ventricular myocardium due to absence of EPDCs in the myocardium, as mentioned earlier. As EPDCs do not give rise to cardiomyocytes themselves, the cause of this phenomenon must originate in the regulatory influence of EPDCs on cardiomyocytes.
It was demonstrated that the onset of formation of the compact myocardium coincides with invasion by EPDCs in that specific area [29]. This spatial relationship supports the generally accepted effect of EPDCs on cardiomyocyte differentiation and proliferation [24,79]. The signalling molecules that are responsible for this interplay between EPDCs and cardiomyocytes are largely unknown, although retinoic acid signalling is shown to contribute to this phenomenon. In vivo, EPDCs express RALDH2 during their invasion [63], but RALDH2 expression disappears after the EPDCs have differentiated [49], suggesting that retinoic acid is produced by undifferentiated EPDCs. Since absence of RXRα specifically in the myocardium does not disturb cardiac development, we can conclude that it is not the retinoic acid secreted by EPDCs that induces signalling in cardiomyocytes to promote compaction, but a more complicated process [37]. Indeed, specific removal of epicardial RXRα expression did result in ventricular thinning [37], suggesting that retinoic acid signalling works in an autocrine loop on the EPDCs. In vitro experiments showed that epicardial cells secrete trophic factors that drive fetal cardiomyocyte proliferation in response to retinoic acid signalling in EPDCs [80]. It has been demonstrated in vivo that FGF constitutes to this epicardial factor that is known to regulate myocardial growth and differentiation [81, 82]. Two redundantly acting receptors on cardiomyocytes, FGF receptor 1 and 2 (FGFR-1 and FGFR-1), receive the essential FGF signals [81].
Another factor involved in EPDC-cardiomyocyte interaction is endothelin (ET). ET is known to have a positive inotropic effect on cardiomyocytes and to induce cellular hypertrophy [83]. As ET is released by epicardial cells [84], it is likely that ET contributes to the ‘epicardial factor’ [79] that is responsible for myocardial compaction.
As there is an influential signalling of EPDCs to cardiomyocytes, it would be expected that there are also factors produced by the myocardium regulating EPDC development. In fact, FOG-2 is such a factor, as was described earlier. It is produced in cardiomyocytes and is essential for EMT of the epicardium [66]. This field of research is largely unexplored, and therefore interesting for further research.
Purkinje fiber development
Cells of the avian Purkinje fiber network of the ventricular conduction system and cardiomyocytes develop from a common progenitor [85]. Gittenberger-de Groot and colleagues postulated an intermediary role for EPDCs in Purkinje cell differentiation, showing a close spatiotemporal relationship between EPDCs and Purkinje cell differentiation [29], which had already been demonstrated to take place in the immediate environment of perfused coronary arteries [86]. Endothelin might play a role in this conversion process, as cultured embryonic myocytes can respond to this paracrine factor and exhibit a Purkinje fiber phenotype [87].
Inhibition of endocardial EMT
Because of their close spatiotemporal relationship, it is to be expected that EPDCs also have a function in endocardial EMT [29,49]. Initially, EPDCs are found in the myocardium and the subendocardial region, places without endocardial EMT, but not in the AV cushion tissue. Later on, when endocardial EMT has resulted in mesenchymal cushion cells, EPDCs invade the AV cushion tissue. Because of this reciprocal spatiotemporal relationship, it has been suggested that EPDCs have an inhibitory effect on EMT in adjacent endocardial cells [29]. This supposed inhibitory influence of EPDCs might function through inhibtion of JB3 and ES/130 expression in adjacent cells. JB3 is a protein which is known to be important for endocardial EMT, and is expressed in endocardial cells of the cushion tissue, but not in ventricular endocardium [88]. Expression of ES/130, another endocardial transformation molecule, is found only in endocardial cells and cardiomyocytes in the region where the cushions develop [89]. After endocardial EMT is complete, and thus when the EPDCs have invaded the cushion tissue, ES/130 expression is downregulated [90].
Development of coronary vessels
Besides their considerable physical contribution, EPDCs constitute a signaling center for coronary vessel development. Upon activation of FGFR-1 and -2 on cardiomyocytes, endocardial and epicardial derived FGF signals regulate Hedgehog (HH) activation. HH signaling in turn induces vascular endothelial growth factor (VEGF) and angiopoietin (Ang) expression, which results in coronary vessel formation [91]. On the other hand, EPDCs enable the ingrowth of coronary arteries into the aorta through induction of apoptosis [28, 92, 93]. They do so by production of Fas ligand, which is known to induce apoptosis in cardiomyocytes [94], specifically at the sites of coronary ingrowth [28].
Modulatory role also observed in adult epicardial cells
Eid et al. demonstrated that even adult epicardial cells have a regulatory effect on adult cardiomyocyte phenotype and function. When cocultured with adult rat epicardial cells, the dedifferentiation process that normally occurs in long-term monoculture of adult rat ventricular cardiomyocytes is delayed, or maybe even reversed [95, 96]. This appeared to be dependent on cell-cell interaction between epicardial cells and cardiomyocytes [96]. In conclusion, there is an essential regulatory role of EPDCs on the developing heart, but further investigation is needed to unravel the mechanisms behind this process.
EPDCs as stem cells
During embryonic development EPDCs are crucial for proper cardiogenesis both because of their physical contribution and their modulatory role. There is hardly any information on the role of EPDCs during fetal and postnatal stages of cardiac maturation and growth. We do not know whether there is still active in vivo EMT and continuous recruitment of new EPDCs. It is assumed that during the phase of myocardial hyperplasia the interstitial fibroblast follows this growth pattern, and similar assumptions are made for coronary vascular growth. Studies from our group have shown that during active myocardial growth, coronary splitting or intussusception is the most effective and rapid way for addition of vasculature [97]. It has also been recently shown that Purkinje fiber differentiation continues during late development, and as such EPDC-derived fibroblasts or undifferentiated EPDCs might still play a role. The recent data providing evidence for the existence of a population of myocardial progenitor cells present in the adult heart that can divide and differentiate into mature cardiomyocytes [98, 99] triggers the question as to a potential role of (adult) EPDCs in this process.
In this respect some recent data are of importance. In rat studies it has been shown that adult EPDCs can still undergo EMT and differentiate into smooth muscle cells [100]. Eid et al. demonstrated that adult epicardial rat cells still have the capacity to positively modify cardiomyocyte phenotype and function [96]. As mentioned before, these epicardial cells can produce ET [84], which is known to increase cardiomyocyte contractility [83]. Moreover, it has been demonstrated that WT1, expressed in undifferentiated EPDCs and not in EPDCs incorporated in the coronary vessel wall [48–50], is switched on de novo in the coronary vessels of adult hearts in case of hypoxia. A colocalisation of WT1 and a proliferative marker was described [101]. These findings suggest that adult EPDCs can reactivate embryonic genetic transcription.
On the basis of the embryonic potential of EPDCs, these cells can be considered to be relatively undifferentiated cells that can give rise to a differentiated progeny of at least smooth muscle cells and fibroblasts. This classifies them as cells with stem cell capacity [102], having also a variety of modulatory functions. We hypothesised, also on the basis of novel data on the regenerative potential of adult myocardium [98, 99], that EPDCs might recapitulate their stem cell capacities in the diseased adult myocardium. As recruitment of embryonic human EPDCs is both technically and ethically almost impossible, we investigated the in vitro growth and differentiation potential of adult human EPDCs. In vitro culture of adult human EPDCs, harvested from atrial biopsy material, is relatively easy. These epitheloid cells soon show EMT to a spindle-shaped cell type. In vitro characterisation shows that they acquire a phenotype that is reminiscent of human mesenchymal stem cells [103]. Currently we are determining the effect of injected cultured adult human EPDCs on infarcted ventricular myocardium. The initial results are promising, with a high survival rate of the injected cells (Fig. 3). We hypothesise that the engrafted adult EPDCs will reactivate part of their embryonic program, and will rescue hibernating myocardial cells, stimulate mycoardial progenitor cells to differentiation, and ensure neovascularisation with the required arteriogenesis. If these capacities can be proven, the adult EPDC might qualify as a novel autologous adult stem cell that can be useful for treatment of cardiovascular disease.
Figure 3.
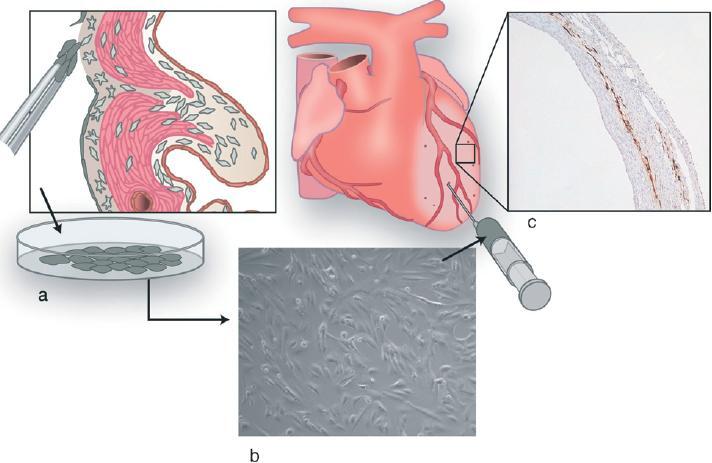
Illustration of EPDC transplantation experiments, (a) Adult epicardial cells are cultured, (b) Cultured epicardial cells are injected into the ischemic area and border zone of the left ventricular wall, (c) Histological section (10×) of the ischemic left ventricular wall after immunohistochemical staining against enhanced green fluorescent protein (eGFP), showing injected eGFP-transduced EPDCs.
References
- 1.DeRuiter M. C., Poelmann R. E., VanderPlas-de Vries I., Mentink M. M. T., Gittenberger-de Groot A. C. The development of the myocardium and endocardium in mouse embryos. Fusion of two heart tubes? 1992;185:461–473. doi: 10.1007/BF00174084. [DOI] [PubMed] [Google Scholar]
- 2.Gittenberger-de Groot A. C., Bartelings M. M., DeRuiter M. C., Poelmann R. E. Basics of cardiac development for the understanding of congenital heart malformations. Pediatr. Res. 2005;57:169–176. doi: 10.1203/01.PDR.0000148710.69159.61. [DOI] [PubMed] [Google Scholar]
- 3.Kurkiewicz, T. (1909) [Zur Histogenese des Hersmuzkels der Wirbeltiere]. Bull. Int. Acad. Sci. Cracovie 148–191.
- 4.Kölliker A. Entwicklungsgeschichte des Menschen und der Thiere. Leipzig: Engelmann; 1879. [Google Scholar]
- 5.De Haan R. L. In: Organogenesis. De Haan R. L., Ursprung H., editors. New York: Holt, Rinehart and Winston; 1965. [Google Scholar]
- 6.Manasek F. J. Embryonic development of the heart. I. A light and electron microscopic study of myocardial development in the early chick embryo. J. Morphol. 1968;125:329–365. doi: 10.1002/jmor.1051250306. [DOI] [PubMed] [Google Scholar]
- 7.Manasek F. J. Embryonic development of the heart. II. Formation of the epicardium. J. Embryol. Exp. Morphol. 1969;22:333–348. [PubMed] [Google Scholar]
- 8.Viragh S., Challice C.E. Origin and differentiation of cardiac muscle cells in the mouse. J. Ultrastruct. Res. 1973;42:1–24. doi: 10.1016/s0022-5320(73)80002-4. [DOI] [PubMed] [Google Scholar]
- 9.Ho E., Shimada Y. Formation of the epicardium studied with the scanning electron microscope. Dev. Biol. 1978;66:579–585. doi: 10.1016/0012-1606(78)90263-4. [DOI] [PubMed] [Google Scholar]
- 10.Viragh S., Challice C. E. The origin of the epicardium and the embryonic myocardial circulation in the mouse. Anat. Rec. 1981;201:157–168. doi: 10.1002/ar.1092010117. [DOI] [PubMed] [Google Scholar]
- 11.Komiyama M., Ito K., Shimada Y. Origin and development of the epicardium in the mouse embryo. Anat. Embryol. 1987;176:183–189. doi: 10.1007/BF00310051. [DOI] [PubMed] [Google Scholar]
- 12.Kuhn H. J., Liebherr G. The early development of the epicardium in Tupaia belangeri. Anat. Embryol. 1988;177:225–234. doi: 10.1007/BF00321133. [DOI] [PubMed] [Google Scholar]
- 13.Hiruma T., Hirakow R. Epicardial formation in embryonic chick heart: computer-aided reconstruction, scanning, and transmission electron microscopic studies. Am. J. Anat. 1989;184:129–138. doi: 10.1002/aja.1001840204. [DOI] [PubMed] [Google Scholar]
- 14.Männer J. The development of pericardial villi in the chick embryo. Anat. Embryol. (Berl) 1992;186:379–385. doi: 10.1007/BF00185988. [DOI] [PubMed] [Google Scholar]
- 15.Viragh S., Gittenberger-de Groot A.C., Poelmann R. E., Kalman F. Early development of quail heart epicardium and associated vascular and glandular structures. Anat. Embryol. (Berl) 1993;188:381–393. doi: 10.1007/BF00185947. [DOI] [PubMed] [Google Scholar]
- 16.Schlueter J., Manner J., Brand T. BMP is an important regulator of proepicardial identity in the chick embryo. Dev. Biol. 2006;295:546–558. doi: 10.1016/j.ydbio.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 17.Männer J., Perez-Pomares J. M., Macias D., Munoz-Chapuli R. The origin, formation and developmental significance of the epicardium: a review. Cells Tissues Organs. 2001;169:89–103. doi: 10.1159/000047867. [DOI] [PubMed] [Google Scholar]
- 18.Männer J. Experimental study on the formation of the epicardium in chick embryos. Anat. Embryol. (Berl) 1993;187:281–289. doi: 10.1007/BF00195766. [DOI] [PubMed] [Google Scholar]
- 19.Vrancken Peeters M.-P. F.M., Mentink M. M. T., Poelmann R. E., Gittenberger-de Groot A. C. Cytokeratins as a marker for epicardial formation in the quail embryo. Anat. Embryol. 1995;191:503–508. doi: 10.1007/BF00186740. [DOI] [PubMed] [Google Scholar]
- 20.Nahirney P. C., Mikawa T., Fischman D. A. Evidence for an extracellular matrix bridge guiding proepicardial cell migration to the myocardium of chick embryos. Dev. Dyn. 2003;227:511–523. doi: 10.1002/dvdy.10335. [DOI] [PubMed] [Google Scholar]
- 21.Munoz-Chapuli R., Macias D., Ramos C., Fernandez B., Sans-Coma V. Development of the epicardium in the dogfish (Scyliorhinus canicula) Acta Zool. 1997;78:39–46. doi: 10.1111/j.1463-6395.1997.tb01124.x. [DOI] [Google Scholar]
- 22.Fransen M. E., Lemanski L. F. Epicardial development in the axolotl, Ambystoma mexicanum. Anat. Rec. 1990;226:228–236. doi: 10.1002/ar.1092260212. [DOI] [PubMed] [Google Scholar]
- 23.Hamburger V., Hamilton H. L. A series of normal stages in the development of the chick embryo. J. Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- 24.Gittenberger-de Groot A. C., Vrancken Peeters M. P., Bergwerff M., Mentink M. M., Poelmann R. E. Epicardial outgrowth inhibition leads to compensatory mesothelial outflow tract collar and abnormal cardiac septation and coronary formation. Circ. Res. 2000;87:969–971. doi: 10.1161/01.res.87.11.969. [DOI] [PubMed] [Google Scholar]
- 25.Perez-Pomares J.M., Phelps, Sedmerova M., Wessels A. Epicardial-like cells on the distal arterial end of the cardiac outflow tract do not derive from the proepicardium but are derivatives of the cephalic pericardium. Dev. Dyn. 2003;227:56–68. doi: 10.1002/dvdy.10284. [DOI] [PubMed] [Google Scholar]
- 26.Kelly R. G. Molecular inroads into the anterior heart field. Trends Cardiovasc. Med. 2005;15:51–56. doi: 10.1016/j.tcm.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Männer J. Does the subepicardial mesenchyme contribute myocardioblasts to the myocardium of the chick embryo heart? A quail-chick chimera study tracing the fate of the epicardial primordium. Anat. Rec. 1999;255:212–226. doi: 10.1002/(sici)1097-0185(19990601)255:2<212::aid-ar11>3.3.co;2-o. [DOI] [PubMed] [Google Scholar]
- 28.Eralp I., Lie-Venema H., DeRuiter M. C., Van Den Akker N. M., Bogers A. J., Mentink M. M., Poelmann R. E., Gittenberger-de Groot A. C. Coronary artery and orifice development is associated with proper timing of epicardial outgrowth and correlated Fas-ligand-associated apoptosis patterns. Circ. Res. 2005;96:526–534. doi: 10.1161/01.RES.0000158965.34647.4e. [DOI] [PubMed] [Google Scholar]
- 29.Gittenberger-de Groot A. C., Vrancken Peeters M. P., Mentink M. M., Gourdie R. G., Poelmann R. E. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circ. Res. 1998;82:1043–1052. doi: 10.1161/01.res.82.10.1043. [DOI] [PubMed] [Google Scholar]
- 30.Perez-Pomares J. M., Macias D., Garcia-Garrido L., Munoz-Chapuli R. Contribution of the primitive epicardium to the subepicardial mesenchyme in hamster and chick embryos. Dev. Dyn. 1997;210:96–105. doi: 10.1002/(SICI)1097-0177(199710)210:2<96::AID-AJA3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 31.Perez-Pomares J. M., Macias D., Garcia-Garrido L., Munoz-Chapuli R. The origin of the subepicardial mesenchyme in the avian embryo: an immunohistochemical and quail-chick chimera study. Dev. Biol. 1998;200:57–68. doi: 10.1006/dbio.1998.8949. [DOI] [PubMed] [Google Scholar]
- 32.Vrancken Peeters M. P., Gittenberger-de Groot A. C., Mentink M. M., Poelmann R. E. Smooth muscle cells and fibroblasts of the coronary arteries derive from epithelial-mesenchymal transformation of the epicardium. Anat. Embryol. (Berl) 1999;199:367–378. doi: 10.1007/s004290050235. [DOI] [PubMed] [Google Scholar]
- 33.Dettman R.W., Denetclaw W., Jr., Ordahl C. P., Bristow J. Common epicardial origin of coronary vascular smooth muscle, perivascular fibroblasts, and intermyocardial fibroblasts in the avian heart. Dev. Biol. 1998;193:169–181. doi: 10.1006/dbio.1997.8801. [DOI] [PubMed] [Google Scholar]
- 34.Poelmann R. E., Gittenberger-de Groot A. C., Mentink M. M., Bokenkamp R., Hogers B. Development of the cardiac coronary vascular endothelium, studied with antiendothelial antibodies, in chicken-quail chimeras. Circ. Res. 1993;73:559–568. doi: 10.1161/01.res.73.3.559. [DOI] [PubMed] [Google Scholar]
- 35.Lie-Venema H., Eralp I., Maas S., Gittenberger-de Groot A. C., Poelmann R. E., DeRuiter M. C. Myocardial heterogeneity in permissiveness for epicardium-derived cells and endothelial precursor cells along the developing heart tube at the onset of coronary vascularization. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2005;282:120–129. doi: 10.1002/ar.a.20154. [DOI] [PubMed] [Google Scholar]
- 36.Mikawa T., Gourdie R. G. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev. Biol. 1996;174:221–232. doi: 10.1006/dbio.1996.0068. [DOI] [PubMed] [Google Scholar]
- 37.Merki E., Zamora M., Raya A., Kawakami Y., Wang J., Zhang, Burch J., Kubalak S.W., Kaliman P., Belmonte J. C., Chien K. R., Ruiz-Lozano P. Epicardial retinoid X receptor alpha is required for myocardial growth and coronary artery formation. Proc. Natl. Acad. Sci. USA. 2005;102:18455–18460. doi: 10.1073/pnas.0504343102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwee L., Baldwin H. S., Shen H. M., Stewart C. L., Buck C., Buck C. A., Labow M. A. Defective development of the embryonic and extraembryonic circulatory systems in vascular cell adhesion molecule (VCAM-1) deficient mice. Development. 1995;121:489–503. doi: 10.1242/dev.121.2.489. [DOI] [PubMed] [Google Scholar]
- 39.Yang J. T., Rayburn H., Hynes R. O. Cell adhesion events mediated by alpha 4 integrins are essential in placental and cardiac development. Development. 1995;121:549–560. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]
- 40.Sengbusch J. K., He W., Pinco K. A., Yang J. T. Dual functions of [alpha]4[beta]1 integrin in epicardial development: initial migration and long-term attachment. J. Cell Biol. 2002;157:873–882. doi: 10.1083/jcb.200203075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dettman R.W., Pae S.H., Morabito C., Bristow J. Inhibition of alpha4-integrin stimulates epicardial-mesenchymal transformation and alters migration and cell fate of epicardially derived mesenchyme. Dev. Biol. 2003;257:315–328. doi: 10.1016/s0012-1606(03)00064-2. [DOI] [PubMed] [Google Scholar]
- 42.Sefton M., Sanchez S., Nieto M. A. Conserved and divergent roles for members of the Snail family of transcription factors in the chick and mouse embryo. Development. 1998;125:3111–3121. doi: 10.1242/dev.125.16.3111. [DOI] [PubMed] [Google Scholar]
- 43.Batlle E., Sancho E., Franci C., Dominguez D., Monfar M., Baulida J., Garcia D. H. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 44.Cano A., Perez-Moreno M. A., Rodrigo I., Locascio A., Blanco M. J., del Barrio M. G., Portillo F., Nieto M. A. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 45.Carmona R., Gonzalez-Iriarte M., Macias D., Perez-Pomares J. M., Garcia-Garrido L., Munoz-Chapuli R. Immunolocalization of the transcription factor Slug in the developing avian heart. Anat. Embryol. (Berl) 2000;201:103–109. doi: 10.1007/pl00008230. [DOI] [PubMed] [Google Scholar]
- 46.Huber O., Bierkamp C., Kemler R. Cadherins and catenins in development. Curr. Opin. Cell Biol. 1996;8:685–691. doi: 10.1016/s0955-0674(96)80110-4. [DOI] [PubMed] [Google Scholar]
- 47.Kreidberg J. A., Sariola H., Loring J. M., Maeda M., Pelletier J., Housman D., Jaenisch R. WT-1 is required for early kidney development. Cell. 1993;74:679–691. doi: 10.1016/0092-8674(93)90515-r. [DOI] [PubMed] [Google Scholar]
- 48.Moore A. W., McInnes L., Kreidberg J., Hastie N. D., Schedl A. YAC complementation shows a requirement for Wt1 in the development of epicardium, adrenal gland and throughout nephrogenesis. Development. 1999;126:1845–1857. doi: 10.1242/dev.126.9.1845. [DOI] [PubMed] [Google Scholar]
- 49.Perez-Pomares J. M., Phelps A., Sedmerova M., Carmona R., Gonzalez-Iriarte M., Munoz-Chapuli R., Wessels A. Experimental studies on the spatiotemporal expression of WT1 and RALDH2 in the embryonic avian heart: a model for the regulation of myocardial and valvuloseptal development by epicardially derived cells (EPDCs) Dev. Biol. 2002;247:307–326. doi: 10.1006/dbio.2002.0706. [DOI] [PubMed] [Google Scholar]
- 50.Carmona R., Gonzalez-Iriarte M., Perez-Pomares J.M., Munoz-Chapuli R. Localization of the Wilm’s tumour protein WT1in avian embryos. Cell Tissue Res. 2001;303:173–186. doi: 10.1007/s004410000307. [DOI] [PubMed] [Google Scholar]
- 51.Wasylyk B., Hahn S. L., Giovane A. The Ets family of transcription factors. Eur. J. Biochem. 1993;211:7–18. doi: 10.1007/978-3-642-78757-7_2. [DOI] [PubMed] [Google Scholar]
- 52.Lie-Venema H., Gittenberger-de Groot A.C., van Empel L. J., Boot M. J., Kerkdijk H., de Kant E., DeRuiter M. C. Ets-1 and Ets-2 transcription factors are essential for normal coronary and myocardial development in chicken embryos. Circ. Res. 2003;92:749–756. doi: 10.1161/01.RES.0000066662.70010.DB. [DOI] [PubMed] [Google Scholar]
- 53.Kalluri R., Neilson E. G. Epithelial-mesenchymal transition and its implications for fibrosis. J. Clin. Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Camenisch T. D., Molin D. G., Person A., Runyan R. B., Gittenberger-de Groot A.C., McDonald J.A., Klewer S. E. Temporal and distinct TGFbeta ligand requirements during mouse and avian endocardial cushion morphogenesis. Dev. Biol. 2002;248:170–181. doi: 10.1006/dbio.2002.0731. [DOI] [PubMed] [Google Scholar]
- 55.Molin D. G., Bartram U., Van der H. K., Van Iperen L., Speer C. P., Hierck B. P., Poelmann R. E., Gittenbergerde-Groot A. C. Expression patterns of Tgfbeta1-3 associate with myocardialisation of the outflow tract and the development of the epicardium and the fibrous heart skeleton. Dev. Dyn. 2003;227:431–444. doi: 10.1002/dvdy.10314. [DOI] [PubMed] [Google Scholar]
- 56.Compton L. A., Potash D. A., Mundell N. A., Barnett J. V. Transforming growth factor-beta induces loss of epithelial character and smooth muscle cell differentiation in epicardial cells. Dev. Dyn. 2006;235:82–93. doi: 10.1002/dvdy.20629. [DOI] [PubMed] [Google Scholar]
- 57.Morabito C. J., Dettman R.W., Kattan J., Collier J. M., Bristow J. Positive and negative regulation of epicardial-mesenchymal transformation during avian heart development. Dev. Biol. 2001;234:204–215. doi: 10.1006/dbio.2001.0254. [DOI] [PubMed] [Google Scholar]
- 58.Van Den Akker N. M., Lie-Venema H., Maas S., Eralp I., DeRuiter M. C., Poelmann R. E., Gittenberger-de Groot A. C. Platelet-derived growth factors in the developing avian heart and maturating coronary vasculature. Dev. Dyn. 2005;233:1579–1588. doi: 10.1002/dvdy.20476. [DOI] [PubMed] [Google Scholar]
- 59.Sucov H. M., Dyson E., Gumeringer C. L., Price J., Chien K. R., Evans R.M. RXRα mutant mice establish a genetic basis for vitamin A signaling in heart morphogenesis. Genes Dev. 1994;8:1007–1018. doi: 10.1101/gad.8.9.1007. [DOI] [PubMed] [Google Scholar]
- 60.Kastner P., Grondona J. M., Mark M., Gansmuller A., LeMeur M., Decimo D., Vonesch J. L., Dolle P., Chambon P. Genetic analysis of RXR alpha developmental function: convergence of RXR and RAR signaling pathways in heart and eye morphogenesis. Cell. 1994;78:987–1003. doi: 10.1016/0092-8674(94)90274-7. [DOI] [PubMed] [Google Scholar]
- 61.Kastner P., Messaddeq N., Mark M., Wendling O., Grondona J. M., Ward S., Ghyselinck N., Chambon P. Vitamin A deficiency and mutations of RXRalpha, RXRbeta and RARalpha lead to early differentiation of embryonic ventricular cardiomyocytes. Development. 1997;124:4749–4758. doi: 10.1242/dev.124.23.4749. [DOI] [PubMed] [Google Scholar]
- 62.Kubalak S. W., Hutson D. R., Scott K. K., Shannon R. A. Elevated transforming growth factor beta2 enhances apoptosis and contributes to abnormal outflow tract and aortic sac development in retinoic X receptor alpha knockout embryos. Development. 2002;129:733–746. doi: 10.1242/dev.129.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xavier-Neto J., Shapiro M.D., Houghton L., Rosenthal N. Sequential programs of retinoic acid synthesis in the myocardial and epicardial layers of the developing avian heart. Dev. Biol. 2000;219:129–141. doi: 10.1006/dbio.1999.9588. [DOI] [PubMed] [Google Scholar]
- 64.Jenkins S. J., Hutson D. R., Kubalak S. W. Analysis of the proepicardium-epicardium transition during the malformation of the RXRalpha−/− epicardium. Dev. Dyn. 2005;233:1091–1101. doi: 10.1002/dvdy.20393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu H., Lee S. H., Gao J., Liu X., Iruela-Arispe M. L. Inactivation of erythropoietin leads to defects in cardiac morphogenesis. Development. 1999;126:3597–3605. doi: 10.1242/dev.126.16.3597. [DOI] [PubMed] [Google Scholar]
- 66.Tevosian S. G., Deconinck A. E., Tanaka M., Schinke M., Litovsky S.H., Izumo S., Fujiwara Y., Orkin S. H. FOG-2, a cofactor for GATA transcription factors, is essential for heart morphogenesis and development of coronary vessels from epicardium. Cell. 2000;101:729–739. doi: 10.1016/s0092-8674(00)80885-5. [DOI] [PubMed] [Google Scholar]
- 67.Mikawa T., Fischman D. A. Retroviral analysis of cardiac morphogenesis: discontinuous formation of coronary vessels. Proc. Natl. Acad. Sci. USA. 1992;89:9504–9508. doi: 10.1073/pnas.89.20.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Perez-Pomares J. M., Phelps A., Munoz-Chapuli R., Wessels A. The contribution of the proepicardium to avian cardiovascular development. Int. J. Dev. Biol. 2001;45:S155–S156. [Google Scholar]
- 69.Jiang X., Rowitch D. H., Soriano P., McMahon A. P., Sucov H. M. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- 70.Bergwerff M., Verberne M. E., DeRuiter M. C., Poelmann R. E., Gittenberger-de Groot A. C. Neural crest cell contribution to the developing circulatory system: implications for vascular morphology? Circ. Res. 1998;82:221–231. doi: 10.1161/01.res.82.2.221. [DOI] [PubMed] [Google Scholar]
- 71.Waldo K. L., Hutson M. R., Ward C. C., Zdanowicz M., Stadt H. A., Kumiski D., Abu-Issa R., Kirby M. L. Secondary heart field contributes myocardium and smooth muscle to the arterial pole of the developing heart. Dev. Biol. 2005;281:78–90. doi: 10.1016/j.ydbio.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 72.Perez-Pomares J. M., Carmona R., Gonzalez-Iriarte M., Atencia G., Wessels A., Munoz-Chapuli R. Origin of coronary endothelial cells from epicardial mesothelium in avian embryos. Int. J. Dev. Biol. 2002;46:1005–1013. [PubMed] [Google Scholar]
- 73.Guadix J. A., Carmona R., Munoz-Chapuli R., Perez-Pomares J. M. In vivo and in vitro analysis of the vasculogenic potential of avian proepicardial and epicardial cells. Dev. Dyn. 2006;235:1014–1026. doi: 10.1002/dvdy.20685. [DOI] [PubMed] [Google Scholar]
- 74.Perez-Pomares J.M., Mironov V., Guadix J. A., Macias D., Markwald R. R., Munoz-Chapuli R. In vitro self-assembly of proepicardial cell aggregates: an embryonic vasculogenic model for vascular tissue engineering. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2006;288:700–713. doi: 10.1002/ar.a.20338. [DOI] [PubMed] [Google Scholar]
- 75.Yanai M., Tatsumi N., Endo F., Yokouchi Y. Analysis of gene expression patterns in the developing chick liver. Dev. Dyn. 2005;233:1116–1122. doi: 10.1002/dvdy.20413. [DOI] [PubMed] [Google Scholar]
- 76.Berggren K., McCaffery P., Drager U., Forehand C. J. Differential distribution of retinoic acid synthesis in the chicken embryo as determined by immunolocalization of the retinoic acid synthetic enzyme, RALDH-2. Dev. Biol. 1999;210:288–304. doi: 10.1006/dbio.1999.9286. [DOI] [PubMed] [Google Scholar]
- 77.de Lange F. J., Moorman A. F., Anderson R.H., Manner J., Soufan A. T., Gier-de Vries C., Schneider M. D., Webb S., van den Hoff M. J., Christoffels V.M. Lineage and morphogenetic analysis of the cardiac valves. Circ. Res. 2004;95:645–654. doi: 10.1161/01.RES.0000141429.13560.cb. [DOI] [PubMed] [Google Scholar]
- 78.Kruithof B. P., van Wijk B., Somi S., Kruithof-de Julio M., Perez Pomares J.M., Weesie F., Wessels A., Moorman A. F., van den Hoff M. J. BMP and FGF regulate the differentiation of multipotential pericardial mesoderm into the myocardial or epicardial lineage. Dev. Biol. 2006;295:507–522. doi: 10.1016/j.ydbio.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 79.Kang J. O., Sucov H.M. Convergent proliferative response and divergent morphogenic pathways induced by epicardial and endocardial signaling in fetal heart development. Mech. Dev. 2005;122:57–65. doi: 10.1016/j.mod.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 80.Chen T. H., Chang T. C., Kang J.O., Choudhary B., Makita T., Tran C. M., Burch J. B., Eid H., Sucov H. M. Epicardial induction of fetal cardiomyocyte proliferation via a retinoic acid-inducible trophic factor. Dev. Biol. 2002;250:198–207. doi: 10.1006/dbio.2002.0796. [DOI] [PubMed] [Google Scholar]
- 81.Lavine K. J., Yu K., White A. C., Zhang X., Smith C., Partanen J., Ornitz D. M. Endocardial and epicardial derived FGF signals regulate myocardial proliferation and differentiation in vivo. Dev. Cell. 2005;8:85–95. doi: 10.1016/j.devcel.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 82.Pennisi D. J., Ballard V. L., Mikawa T. Epicardium is required for the full rate of myocyte proliferation and levels of expression of myocyte mitogenic factors FGF2 and its receptor, FGFR-1, but not for transmural myocardial patterning in the embryonic chick heart. Dev. Dyn. 2003;228:161–172. doi: 10.1002/dvdy.10360. [DOI] [PubMed] [Google Scholar]
- 83.Kelly R.A., Eid H., Kramer B.K., O’Neill M., Liang B. T., Reers M., Smith T. W. Endothelin enhances the contractile responsiveness of adult rat ventricular myocytes to calcium by a pertussis toxin-sensitive pathway. J. Clin. Invest. 1990;86:1164–1171. doi: 10.1172/JCI114822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eid H., de Bold K., Chen J. H., de Bold A. J. Epicardial mesothelial cells synthesize and release endothelin. J. Cardiovasc. Pharmacol. 1994;24:715–720. doi: 10.1097/00005344-199424050-00005. [DOI] [PubMed] [Google Scholar]
- 85.Gourdie R. G., Mima T., Thompson R. P., Mikawa T. Terminal diversification of the myocyte lineage generates purkinje fibers of the cardiac conduction system. Development. 1995;121:1423–1431. doi: 10.1242/dev.121.5.1423. [DOI] [PubMed] [Google Scholar]
- 86.Hyer J., Johansen M., Prasad A., Wessels A., Kirby M. L., Gourdie R. G., Mikawa T. Induction of Purkinje fiber differentiation by coronary arterialization. Proc. Natl. Acad. Sci. USA. 1999;96:13214–13218. doi: 10.1073/pnas.96.23.13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gourdie R. G., Wei Y., Kim D., Klatt S. C., Mikawa T. Endothelin-induced conversion of embryonic heart muscle cells into impulse-conducting purkinje fibers. Proc. Natl. Acad. Sci. USA. 1998;95:6815–6818. doi: 10.1073/pnas.95.12.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wunsch A. M., Little C. D., Markwald R. R. Cardiac endothelial heterogeneity defines valvular development as demonstrated by the diverse expression of JB3, an antigen of the endocardial cushion tissue. Biol. 1994;165:585–601. doi: 10.1006/dbio.1994.1278. [DOI] [PubMed] [Google Scholar]
- 89.Ramsdell A. F., Moreno-Rodriguez R. A., Wienecke M.M., Sugi Y., Turner D. K., Mjaatvedt C. H., Markwald R. R. Identification of an autocrine signaling pathway that amplifies induction of endocardial cushion tissue in the avian heart. Acta Anat. (Basel) 1998;162:1–15. doi: 10.1159/000046463. [DOI] [PubMed] [Google Scholar]
- 90.Rezaee M., Isokawa K., Halligan N., Markwald R. R., Krug E. L. Identification of an extracellular 130-kDa protein involved in early cardiac morphogenesis. J. Biol. Chem. 1993;268:14404–14411. [PubMed] [Google Scholar]
- 91.Lavine K. J., White A. C., Park C., Smith C. S., Choi K., Long F., Hui C. C., Ornitz D. M. Fibroblast growth factor signals regulate a wave of Hedgehog activation that is essential for coronary vascular development. Genes Dev. 2006;20:1651–1666. doi: 10.1101/gad.1411406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bogers A. J. J. C., Gittenberger-de Groot A. C., Poelmann R. E., Péault B. M., Huysmans H. A. Development of the origin of the coronary arteries, a matter of ingrowth or outgrowth? Anat. Embryol. 1989;180:437–441. doi: 10.1007/BF00305118. [DOI] [PubMed] [Google Scholar]
- 93.Rothenberg F., Hitomi M., Fisher S. A., Watanabe M. Initiation of apoptosis in the developing avian outflow tract myocardium. Dev. Dyn. 2002;223:469–482. doi: 10.1002/dvdy.10077. [DOI] [PubMed] [Google Scholar]
- 94.Sallee D., Qiu Y., Liu J., Watanabe M., Fisher S. A. Fas ligand gene transfer to the embryonic heart induces programmed cell death and outflow tract defects. Dev. Biol. 2004;267:309–319. doi: 10.1016/j.ydbio.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 95.Eppenberger M. E., Hauser I., Baechi T., Schaub M. C., Brunner U. T., Dechesne C. A., Eppenberger H. M. Immunocytochemical analysis of the regeneration of myofibrils in long-term cultures of adult cardiomyocytes of the rat. Dev. Biol. 1988;130:1–15. doi: 10.1016/0012-1606(88)90408-3. [DOI] [PubMed] [Google Scholar]
- 96.Eid H., Larson D. M., Springhorn J. P., Attawia M. A., Nayak R. C., Smith T. W., Kelly R. A. Role of epicardial mesothelial cells in the modification of phenotype and function of adult rat ventricular myocytes in primary coculture. Circ. Res. 1992;71:40–50. doi: 10.1161/01.res.71.1.40. [DOI] [PubMed] [Google Scholar]
- 97.van Groningen J. P., Wenink A. C., Testers L. H. Myocardial capillaries: increase in number by splitting of existing vessels. Anat. Embryol. (Berl) 1991;184:65–70. doi: 10.1007/BF01744262. [DOI] [PubMed] [Google Scholar]
- 98.Urbanek K., Rota M., Cascapera S., Bearzi C., Nascimbene A., De Angelis A., Hosoda T., Chimenti S., Baker M., Limana F., Nurzynska, et al. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ. Res. 2005;97:663–673. doi: 10.1161/01.RES.0000183733.53101.11. [DOI] [PubMed] [Google Scholar]
- 99.Beltrami A. P., Barlucchi L., Torella D., Baker M., Limana F., Chimenti S., Kasahara H., Rota M., Musso E., Urbanek K., Leri A., et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 100.Wada A. M., Smith T. K., Osler M. E., Reese D. E., Bader D. M. Epicardial/Mesothelial cell line retains vasculogenic potential of embryonic epicardium. Circ. Res. 2003;92:525–531. doi: 10.1161/01.RES.0000060484.11032.0B. [DOI] [PubMed] [Google Scholar]
- 101.Wagner K. D., Wagner N., Bondke A., Nafz B., Flemming B., Theres H., Scholz H. The Wilms’ tumor suppressor Wt1 is expressed in the coronary vasculature after myocardial infarction. FASEB J. 2002;16:1117–1119. doi: 10.1096/fj.01-0986fje. [DOI] [PubMed] [Google Scholar]
- 102.Wessels A., Perez-Pomares J. M. The epicardium and epicardially derived cells (EPDCs) as cardiac stem cells. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2004;276:43–57. doi: 10.1002/ar.a.10129. [DOI] [PubMed] [Google Scholar]
- 103.van Tuyn, J., Atsma, D. E., Winter, E. M., van der Velde-van Dijke, I., Pijnappels, D.A., Bax, N. A. M., Knaan-Shanzer, S., Gittenberger-de Groot, A. C., Poelmann, R. E., van der Laarse, A., van der Wall, E. E., Schalij, M. J. and de Vries, A. A. F. (2006) Epicardial cells of human adults can undergo an epithelial-to-mesenchymal transition and obtain characteristics of smooth muscle cells in vitro. Stem Cells. On line. [DOI] [PubMed]


