Abstract
A light-activated NO donor, [Mn(PaPy3)(NO)]ClO4 (1a), has been incorporated into HEMA-based polymer hydrogel and the nitrosyl-polymer conjugate materials 1ax · HG and 1ax · HGMB have been characterized. The NO releasing properties and antibacterial capabilities of these materials in conjunction with growth attenuators such as hydrogen peroxide and methylene blue (MB) are reported. Since the nitrosyl releases NO only upon exposure to light, materials like 1ax · HGMB could be used as wound dressings that deliver NO under controlled conditions.
Introduction
In the treatment of wounds (breaks in the skin), it is important to protect the wound from dehydration, bacterial contamination, and allow drainage of exudates, while at the same time permitting oxygen to reach the affected area [1]. Hydrogels based on poly(2-hydroxyethyl methacrylate) (pHEMA) are widely studied for biomedical applications due to their swellability, oxygen permeability, and biocompatibility [2–4]. Addition of an antibacterial agent is however necessary for such hydrogels to control bacterial infection. Recently, pHEMA hydrogels containing the antibiotic ciprofloxacin have been characterized for the dressing of burns [5]. As in most passive drug delivery systems, delivery of ciprofloxacin is diffusion controlled and can be modulated to some degree by varying the degree of cross-linking within the polymer matrix. To address the controllability of drug release, in the present work, we report simple fabrication of a hybrid material consisting of a pHEMA core containing a light activated nitric oxide donor (NO donor) and a polyurethane (PU) coating. NO was selected as the antibiotic agent to avoid concern over the use of antibiotics and the emergence of antibiotic resistant strains of bacteria in hospital and community environments [6–8]. The role of NO in the non-specific immune response [9], the utility of NO/NO-donors in mimicking innate immunity [10], as well as the role of NO in wound repair [11–13] have been well established in recent years. Upregulation and expression of inducible Nitric Oxide Synthase (iNOS) in macrophages has been observed in conjunction with inflammation and the immune response to infection. NO and its congeners (such as peroxynitrite, ONOO−; nitrogen dioxide, NO2, and dinitrogen trioxide, N2O3) deaminate DNA and modify proteins and lipids [9]. This type of general oxidative stress is in contrast with the mode of action of other antibiotics, most of which fall into a few structurally (and functionally) related classes.
Numerous studies have been performed to explore the antimicrobial properties of NO from various NO-donors [9]. The antibiotic utility of these NO donors seems to vary widely depending on the source of NO, the strain of bacteria, and the experimental conditions. Selective antimicrobial action of dilute NO gas (200 ppm) has also been demonstrated in Staphylococcus aureus (S. aureus), methicillin-resistant S. aureus, Escherichia coli (E.coli), Group B Streptococcus, Pseudomonas aeurginosa (P. aeurginosa), and Candida albicans(C. albicans) [14]. This dose was not found to adversely affect fibroblast cultures. Since NO is rapidly oxidized in presence of oxygen, both handling and controlling of NO administration are more easily accomplished via employment of suitable NO donors. Schoenfisch and coworkers have demonstrated decreased bacterial adhesion to implanted devices and sensors coated with diazeniumdiolate conjugated xerogels [15, 16, and references therein]. Diazeniumdiolates however release NO upon hydration. Hence, once introduced to the physiological milieu, hydrolysis of NO from the parent material is almost diffusion controlled. While the lack of control over NO release does not affect the utility of the xerogels in the stated applications, systems like this can hardly be employed to deliver NO to a targeted area.
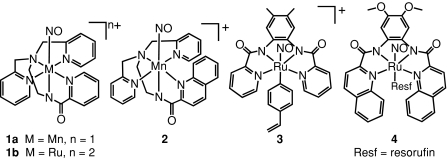
With the goal of isolating site specific NO donors in mind, we have developed a series of photoactive NO donors that can deliver NO to a variety of biological targets. These photoactive NO-donors include the Ru and Mn nitrosyls (NO complexes) [Mn(PaPy3)(NO)]+ (1a), [Ru(PaPy3)(NO)]2+ (1b), [Mn(PaPy2Q)(NO)]+ (2), [(Me2bpb)Ru(NO)(4-vpy)]+ (3), and [((OMe)2bQb)Ru(NO)(Resf)] (4, Fig. 1 1). Solutions of these nitrosyls have been used to deliver NO to proteins, enzymes, cells and tissues [17, 18, 19]. In order to control the timing and site specificity of light-triggered NO-release with these nitrosyls, the complexes 1a and [Mn(PaPy2Q)(NO)]+ (2) have been incorporated into polyurethane (PU) coated sol–gel hybrid materials [20, 21]. The ruthenium nitrosyl [(Me2bpb)Ru(NO)(4-vpy)]+ (3, 4-vpy = 4-vinylpyridine) was covalently incorporated into a hydrogel material via copolymerization with 2-hydroxyethyl methacrylate (HEMA) and ethyleneglycol dimethacrylate (EGDMA, cross-linker) [22]. All these materials demonstrate the ability to transfer NO to reduced myoglobin upon illumination with UV light. In biomedical applications, it is often desirable to avoid the use of UV light. Along this line, the dye-tethered nitrosyl 4 (Resf = resorufin dye aborbing strongly at ~500 nm) acts as a NO donor under illumination with visible light and promotes NO-induced apoptosis in human breast cancer cells [23].
Fig. 1.
Designed metal nitrosyls that release NO upon exposure to light
In order to explore the bactericidal activity of these designed metal nitrosyls, we have now looked into the ability of hydrogel disks consisting of a pHEMA core containing 1a (1ax · HG) coated with PU. Initial modified dilution assay with such materials indicated that NO generated by these materials alone is generally insufficient to cause significant bacterial death in case of E.coli or P. aeurginosa. The levels of NO photoreleased from the disks most possibly are not sufficient enough to cause serious damage to the microorganisms in the growth media. Pacelli and coworkers have circumvented situations like this by potentiating the detrimental effects of NO (delivered by diethylamine(DEA)/NO) on bacterial suspension by addition of a secondary agent namely, H2O2 [24]. The synergistic cytotoxicity arising from the combination of reactive oxygen species (ROS) and NO was sufficient to suppress the bacterial growth by several orders of magnitude in their experiment. Wright and coworkers have demonstrated photobacterial activity of phenothiazinium dyes (such as methylene blue, MB) against methicillin-resistant S. aureus [25]. We therefore decided to augment the photobacterial activity of the 1ax · HG by addition of growth attenuators like H2O2 and MB. In this paper, we report the results of studies on the efficacy of 1ax · HG toward killing E. coli and P. aeurginosa. In particular, we describe (a) the synthesis and characterization of 1ax · HG and 1ax · HGMB, the hydrogel containing the Mn-nitrosyl 1a of different concentrations x and (b) an assay to determine the enhancement of antibacterial power of 1ax · HG via photorelease of NO in the presence of hydrogen peroxide (H2O2) and methylene blue (MB). The results described below show an interesting strain-specificity in response to the photoreleased NO under the experimental conditions.
Materials and methods
Praparation of pHEMA hydrogels
A batch of 3.4 g (26 mmol) of medical grade HEMA (Sigma-Aldrich, St. Louis, MO) and a batch of 0.18 g (0.91 mmol) of EGDMA (Sigma-Aldrich, St. Louis, MO) were added sequentially to 0.96 ml of deionized water and stirred until homogeneous. The prepolymer mixture was degassed by three freeze-pump-thaw cycles. A batch of 0.005 g (0.022 mmol) of ammonium persulfate and a batch of 0.005 g (0.026 mmol) of sodium metabisulfite were dissolved in 0.96 ml deionized water and deoxygenated by bubbling nitrogen through the solution. The resulting solution of initiators was then added to the prepolymer mixture under nitrogen. The mixture was transferred to ten Eppendorf tubes (0.5 ml each) and refrigerated (0°C) in an ice bath overnight. For the nitrosyl-containing hydrogels (1ax · HG), 0.05 g (0.08 mmol) or 0.1 g (0.16 mmol) of [Mn(PaPy3)(NO)]ClO4 (1a, [26], the subscript x refers to the molarity of 1 in the prepolymer) was added to the prepolymer mixture before degassing. The concentrations of 1 in the resulting gels were 16 mM and 32 mM, respectively. To make gels containing 0.2 g (0.32 mmol) 64 mM) 1, the amount of crosslinker (EGDMA) was doubled (0.36 g, and the amount of HEMA reduced to 3.2 g) to give gels of similar rigidity. The concentration of 1a was 64 mM (x = 64) in this material. The blank hydrogels were completely clear and the nitrosyl-containing hydrogels were transparent and dark green. The hydrogels were easily sliced by razor blades. The hydrogels were sliced into 1 mm thick disks with the assistance of a custom-made gel-slicer. The 1 mm disks were then coated twice with a solution of 1 g polyurethane (Tecoflex SG-80A, PU) dissolved in 10 ml of THF. A set of disks was coated with a solution of PU (1 g) containing 1 mM methylene blue trihydrate (0.0037 g) in 10 ml methylene chloride. These materials are denoted with the superscript MB to the right of HG (e.g. 116 · HGMB). Coated gels were stored in humidity chambers (60%) at 0°C until use.
Characterization of the hybrid materials
The water content of the gels with different amounts of cross-linkers was measured by equilibrating the coated hydrogels in MilliQ purified water for 1 h. Longer immersion times did not increase the weight of the hydrated gels. This is most likely because only the PU coating absorbs water without transmitting it to the hydrogel core. Excess water was blotted away and the weight was recorded (W h). The gels were then lyophilized and the dry weight recorded (W d). The degree of swelling (q) was calculated according to the equation:
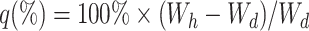 |
The NO releasing capacity of the gels was measured under aerobic conditions using an amiNO-2000 electrode (inNO Nitric Oxide Measuring System, Innovative Instruments, Inc.).
Bacterial cell culture
Strains of E. coli ATCC® 25922TM and Pseudomonas aeruginosa ATCC® 27853TM were received from the ATCC as freeze-dried cultures and revived per specifications into 5 ml cultures of trypticase soy broth (TSB), and streaked onto Trypticase soy plates. From these plates, colonies were picked and used to inoculate varying amounts (from 5 to 50 ml) of TSB, one colony per culture. The cultures were grown aerobically at 37°C for about 4 h, depending on the species and culture size, until the culture reached an A600 of 0.63 (UV-1601 Shimadzu spectrometer). The cultures were then put on ice for 15 min, and sterile-filtered (0.22 μm) DMSO was added to a final concentration of 7.5%. At this point the bacterial cultures were dispensed in to small aliquots, immediately frozen in liquid nitrogen, and then stored in a −80°C freezer until use.
Modified dilution assay
Sets of three hydrogels (e.g., HG (control), 1a16 · HG (low NO dose), and 1a32 · HG, (high NO dose)) were each placed at the bottom of wells in a 96 well plate. A bacterial suspension was prepared by diluting 5 μl of the thawed bacteria to a total volume of 1 ml with 995 μl of TSB (~1 × 107 cfu/ml). When applicable, aqueous preparations of 1 mM methylene blue or 1 mM H2O2 were added to the TSB prior to the addition of the bacteria. For P. aeruginosa, the concentration of MB or H2O2 was 5 μM; for E. coli, the concentration of MB or H2O2 was 500 μM, (optimization data not shown). A 100 μl aliquot of the attenuated bacteria was then transferred to the wells with the gels and either incubated at room temperature under ambient light (D) or illuminated with 100 mW light for 20 min (L). In order to assess the bacterial survival, D and L cultures were then plated onto TSB agar plates, incubated for 16 h, and the resultant densities of colonies were qualitatively compared. Each experiment was performed a minimum of three times.
Results and discussion
Morphology of the hybrid material
The hybrid materials were prepared by mixing the NO-donor (1a) in the prepolymer mixture, followed by radical polymerization, and lastly dip coating the resulting hydrogels with polyurethane to prevent leaching of the NO donor. The structure of the hybrid material allows diffusion of NO gas, while retaining the NO donor and its photoproducts within the core.
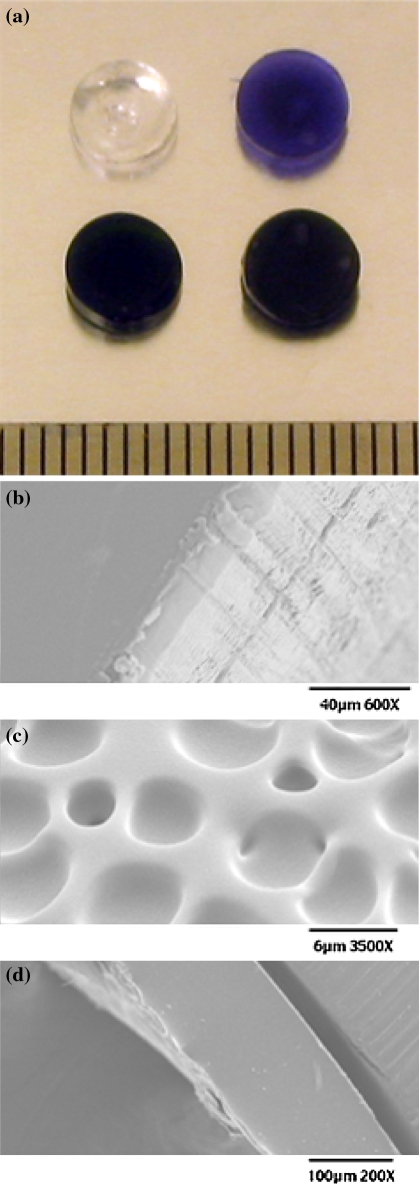
Pictures of representative hybrid materials are shown in Fig. 2a. The control disks containing no 1a are clear. The hybrid materials containing 1a are transparent and dark green. In the case of gels coated with the mixture of methylene blue and polyurethane (but no 1a inside), the disks are vibrantly blue and translucent. The approximate dimensions are 5.9 ± 0.1 mm in diameter and 1.3 ± 0.1 mm in thickness. For SEM imaging, the disks were first frozen in liquid N2 and then lyophilized. As shown in Fig. 2b, no porous texture is observed in the lyophilized hydrogel material at the micrometer scale. Interestingly, pockets can be seen in the polyurethane coating (Fig. 2c). The thickness of the coating was evaluated by SEM imaging (Fig. 2d). The thickness is approximately 150 microns and does not vary appreciably due to formulation with MB.
Fig. 2.
a Photographs of HG, HGMB, 1a32 · HGMB, and 1a32 · HG (clockwise from top left); representative SEM micrographs: b smooth surface of lyophilized hydrogel HG c pocketed surface of PU and d image of the hydrogel-PU interface
Composition of the materials
The water contents of the gels are summarized in Table 1. They were calculated using the swelling efficiency (% water/dry mass). The water content varies from 19 to 29% of the total dry mass. The standard deviations are however large (3–6%). We suspect that the thin gels lose a small amount of water during the dip-coating step. The concentration of 1a appears to have no effect on the water content of the gels. Interestingly, doubling the concentration of EGDMA does not seem to affect the water content of 1a64 · HG and 1a64 · HGMB. Usually one expects that increase in the amount of the cross-linker should decrease the degree of swelling. However, because of the polyurethane coating, we did not observe any change in the water content of 1a64 · HG and 1a64 · HGMB. The polyurethane constitutes 13–16% of the total dry mass, giving the coatings an approximate thickness of ~150 μm. Inspection of the data of Table 1 indicates that the presence of MB in the PU coat does not affect its water permeability.
Table 1.
Degree of swelling (q) and percent polyurethane (%PU) coating in the hybrid materials
| q | % PU | q | % PU | ||
|---|---|---|---|---|---|
| HG | 26 ± 3 | 13 ± 3 | HGMB | 25 ± 3 | 13 ± 3 |
| 1a16 · HG | 19 ± 4 | 14 ± 4 | 1a16 · HGMB | 20 ± 3 | 14 ± 4 |
| 1a32 · HG | 29 ± 6 | 15 ± 3 | 1a32 · HGMB | 29 ± 4 | 15 ± 4 |
| 1a64 · HG | 26 ± 3 | 16 ± 3 | 1a64 · HGMB | 29 ± 4 | 16 ± 5 |
Some leakage of methylene blue was observed from the hybrid materials coated with MB-impregnated PU. During the 1hour equilibration period, the 1.5 ml MilliQ water became 0.3 ± 0.1 μM in MB. During the NO amperogram studies, an additional portion of MB diffused into the 100 μl aliquot of water giving concentrations of ~5 nM. This is significantly lower than the upper limit of tolerance reported in cases of intravenous administration of MB (5 mg/kg) [27].
NO-release profile
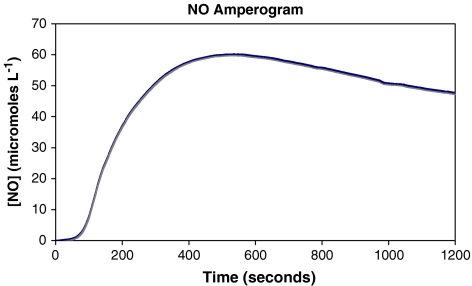
Over the course of the 20 min illumination time, the concentration of NO released into solution increases gradually for the first 3 to 5 min before reaching near steady-state NO flux. In a typical measurement with 1a32 · HG, the steady-state concentration of NO reaches a maximum at ~60 μM NO after 10 min of illumination before tapering off to ~50 μM by the end of the illumination time (Fig. 3). Since very similar NO flux was noted with 1a64 · HG and 1a64 · HGMB, these gels were not used further in the antibiotic efficacy assay. In case of 1a16 · HG, the steady-state concentration of NO was ~5 μM.
Fig. 3.
Profile of NO photorelease from 1a32 · HG
Antibiotic properties of the hybrid materials
In the absence of any standardized assay for determining the antibiotic capacity of a gas-emitting hybrid material, we needed to develop an assay to determine the antibiotic efficacy of our NO-releasing material. Antibiotic susceptibility is generally evaluated by using the disk-diffusion (Kirby-Bauer) method or dilution assays. In the disk-diffusion assay, the diameter of the zone of inhibition (DIZ) depends on the diffusivity of the antibiotic as well as the bacterial strain. For example, a particular antibiotic with a low diffusivity will give smaller zones of inhibition, but the bacteria under investigation may be susceptible. This assay however has the advantage that it mimics the nutrient rich surface of a wound. Unfortunately, the gaseous nature of NO allows it to diffuse in all directions, not simply into the agar as in the disk-diffusion assay. Furthermore, in aerobic environments, rapid oxidation of NO results in quick conversion of NO to nitrogen dioxide (and other gases). In aqueous environments, NO is eventually converted into nitrite and nitrate. Indeed, placing our hydrogel disks on an agar surface seeded with bacteria, followed by illumination did not result in a measurable decrease in bacterial growth. Since we did not have a means to assess the concentration of NO released in the agar or the air above, we could not trace the fate of the NO released from the gels. In dilution assays, the compound of interest is dissolved (in different concentrations) directly in a suspension of bacteria. The suspensions are then plated to assess the degree of colony survival. The concentration at which no more bacteria grow is the minimum inhibitory concentration (MIC). Because we have access to the NO electrode, we can measure in real time the concentration of NO released by our gels in solution. As mentioned before, the concentrations of NO generated by our hybrid materials (~60 μM) were insufficient to diminish bacterial growth. We therefore employed H2O2 [24] and MB [25] as auxiliary growth attenuators. Wainwright and coworkers have established the efficacy of MB as an antibiotic agent in solution [25, 28, 29] as well as when incorporated into polymer films [30]. The assay that we have employed in the present study is essentially a modified dilution assay in combination with H2O2 or MB as growth attenuators.
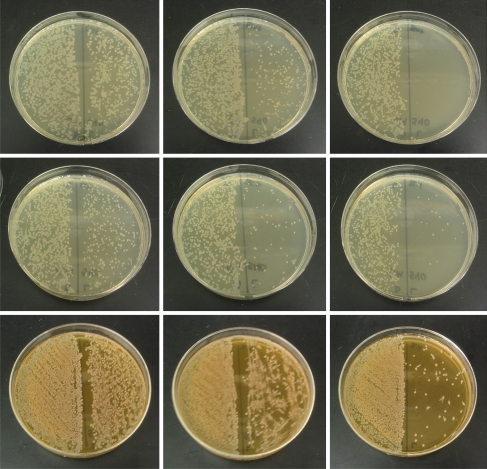
The results of antiobiotic efficacy studies with HG, 1a16 · HG, and 1a32 · HG are shown in Fig. 4. As shown in the left plate of top row of Fig. 4, the growth of P. aeruginosa was slightly affected when the bacterial growth media was illuminated in the presence of a hydrogel disk with MB only (HGMB). However, when the disk contained 1a16 · HGMB (MB in its coat), exposure to light caused a significant decrease in the number of colonies of the surviving bacteria (middle slide, top row). That the decrease is due to photoreleased NO is confirmed by the complete killing of the bacteria when 1a32 · HGMB was employed in the bacterial growth medium (right slide, top row).
Fig. 4.
Top row: plates of P. aeruginosa after incubation with HGMB, 1a16 · HGMB, and 1a32 · HGMB (left to right); middle row: plates of P. aeruginosa after incubation with 5 μM H2O2 and: HG, 1a16 · HG, and 1a32 · HG (left to right); bottom row: plates of E. coli after incubation with 500 μM H2O2 and: HG, 1a16 · HG, and 1a32 · HG (left to right). In each case, the left half of the plate was streaked with cultures kept in the dark while cultures exposed to light were used to streak the right side to show the effect of light
Similar results were obtained when H2O2 was used as the growth attenuator. When P. aeruginosa was incubated to HG (no MB in the coating) in presence of 5 μM H2O2 in the dark, no noticeable change in the growth was observed. Exposure to light caused minimal damage (left plate, middle row). Use of 1a16 · HG instead of HG however resulted in significant decrease in colony density (middle plate, middle row) and with 1a32 · HG, the growth was further arrested (right plate, middle row). In case of E. coli, the initial bacterial growth medium was first treated with 500 μM H2O2 because of the robust nature of the microorganism. As shown in the left plate of bottom row of Fig. 4, such treatment with H2O2 did not stress the microorganism to a great extent either in the dark or under illumination. However, presence of 1a16 · HG in the growth medium (in addition to 500 μM H2O2) clearly resulted in significant decrease in colony density (middle plate, bottom row) and further loss was evident when 1a32 · HG was employed (right plate, bottom row).
Our results (Fig. 4) clearly indicate that in presence of H2O2 as growth attenuator, photoreleased NO does diminish the colony-formation ability of both P. aeruginosa and E. coli (both gram-negative). Although 500 μM H2O2 alone was not sufficient to kill E. coli, combination of 1a32 · HG and 500 μM H2O2 almost abolished their colony-forming ability when exposed to light. We therefore conclude that the bactericidal effect in these cases arises from the combination of ROS and NO. It is noteworthy that only 5 μM H2O2 was required to kill P. aeruginosa with 1a32 · HG. Since MB also produces ROS in presence of light, its presence in the coating of 1a16 · HGMB and 1a32 · HGMB brings about complete elimination of P. aeruginosa. In case of E. coli, the concentration of MB in the coating of 1a32 · HGMB was not sufficient enough to cause significant reduction in the number of colonies.
The activation of macrophages and subsequent NO generation has been implicated in fighting colonizing microorganisms [9]. Although the exact mechanism of how NO exerts this microbicidal effect is still not fully understood, it is generally believed that the interaction of NO with oxygen and ROS results in reactive intermediates (such as ONOO− and N2O3) with antimicrobial properties. The present results provide support to this hypothesis. The photoreleased NO clearly accentuates the antimicrobial activity when ROS (originating from H2O2) or singlet oxygen (arising from MB and light) is present in the growth media. Interestingly, in case of E. coli, the bactericidal effect is observed only when a higher dose of H2O2 (500 μM) is employed since the bacterium is known to scavenge NO more readily via respiratory detoxification of NO by a cytochrome c nitrite reductase [31]. The absence of such a pathway in P. aeruginosa makes it more susceptible to the combination of NO and ROS (from low doses of H2O2) as evident by the results of this work.
Conclusions
Since NO has been associated with the prevention of microbial invasion [9] and wound healing [13], the fabrication of a hydrogel wound dressing that can deliver NO along with other growth attenuators (such as H2O2 or MB) is a very desired goal. The present results strongly suggest that materials such as 1a32 · HG and 1a32 · HGMB could be used for such a purpose. One major advantage of these materials is the fact that release of NO can be conveniently controlled by exposure to light. As a consequence, the dressing can be illuminated from time to time to deliver NO only to the wound site and maintain antiseptic conditions. This approach might be superior to simple wash of the affected area with 3% solution of H2O2.
Acknowledgments
Financial support from the NSF Grant CHE-0553405 is gratefully acknowledged. G.M.H. and K.A.O. were supported by NIH IMSD Grant No. GM58903.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
PaPy−3 is the deprotonated form of the pentadentate ligand, N,N-bis(2-pyridylmethyl)amine-N-ethyl-2-pyridinecarboxamide (PaPy3H); The other ligands are: N,N-bis(2-pyridylmethyl)amine-N-ethyl-2-quinolinecarboxamide (PaPy2QH), 1,2-bis(pyridine-2-carboxamido)-4,5-dimethylbenzene (H2Me2bpb); 1,2-bis(quinoline-2-carboxamido)-4,5-dimethoxybenzene (H2(OMe2)bQb).
References
- 1.DiPietro LA, Burns AL, editors. Wound healing: methods and protocols. Totowa: Humana Press; 2003. [Google Scholar]
- 2.Peppas NA, editor. Hydrogels in medicine and pharmacy. Boca Raton: CRC Press; 1987. [Google Scholar]
- 3.Sun Y-M, Huang J-J, Lin F-C, Lai J-Y. Composite poly(2-hydroxyethyl methacrylate) membranes for transdermal applications. Biomaterials. 1997;18:527–533. doi: 10.1016/S0142-9612(96)00166-4. [DOI] [PubMed] [Google Scholar]
- 4.Compañ V, Guzmán J, Riande E. A potentiostatic study of oxygen transmissibility and permeability through hydrogel membranes. Biomaterials. 1998;19:2139–2145. doi: 10.1016/S0142-9612(98)00113-6. [DOI] [PubMed] [Google Scholar]
- 5.Tsou T-L, Tang S-T, Huang Y-C, Wu J-R, Young J-J, Wang H-J. Poly(2-hydroxyethyl methacrylate) wound dressing containing ciprofloxacin and its drug release studies. J Mater Sci Mater Med. 2005;16:95–100. doi: 10.1007/s10856-005-5954-2. [DOI] [PubMed] [Google Scholar]
- 6.Barrett CT, Barrett JF. Antibacterials: are the new entries enough to deal with the emerging resistance problems? Curr Opin Biotechnol. 2003;14:621–626. doi: 10.1016/j.copbio.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Bearman GML, Munro C, Sessler CN, Wenzel RP. Infection control and prevention of nosocomial infection in the intensive care unit. Semin Respir Crit Care Med. 2006;27:310–324. doi: 10.1055/s-2006-945534. [DOI] [PubMed] [Google Scholar]
- 8.Wenzel RP, Bearman G, Edmond MB. Community-acquired methicillin-resistant Stphylococcus aureus (MRSA): new issues for infection control. Int J Antimicrob Agents. 2007;30:210–212. doi: 10.1016/j.ijantimicag.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Fang FC, editor. Nitric oxide and infection. New York: Kluwer Academic/Plenum Publishers; 1999. [Google Scholar]
- 10.Singh R, Manjunatha U, Boshoff HIM, Ha YH, Niyomrattanakit P, Lidwidge R, Dowd CS, Lee IY, Kim P, Zhang L, Kang S, Keller TH, Jiricek J, Barry CE. PA-824 kills nonreplicating Mycobacterium tuberculosis by intracellular NO release. Science. 2008;322:1392–1395. doi: 10.1126/science.1164571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schäffer MR, Tantry U, van Wesep RA, Barbul A. Nitric oxide metabolism in wounds. J Surg Res. 1997;71:25–31. doi: 10.1006/jsre.1997.5137. [DOI] [PubMed] [Google Scholar]
- 12.Stallmeyer B, Kampfer H, Kolb N, Pfeilschifter J, Frank S. The funcion of nitric oxide in wound repair: inhibition of inducible nitric oxide-synthase severely impairs wound reepithelialization. J Invest Dermatol. 1999;113:1090–1098. doi: 10.1046/j.1523-1747.1999.00784.x. [DOI] [PubMed] [Google Scholar]
- 13.Bohl Masters KS, Leibovich SJ, Belem P, West JL, Poole-Warren LA. Effects of nitric oxide releasing poly(vinyl alcohol) hydrogel dressings on dermal wound healing in diabetic mice. Wound Rep Reg. 2002;10:286–294. doi: 10.1046/j.1524-475X.2002.10503.x. [DOI] [PubMed] [Google Scholar]
- 14.Ghaffari A, Miller CC, McMullin B, Ghahary A. Potential application of gaseous nitric oxide as a topical antimicrobial agent. Nitric Oxide. 2006;14:21–29. doi: 10.1016/j.niox.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Hetrick EM, Schoenfisch MH. Reducing implant-related infections: active release strategies. Chem Soc Rev. 2006;35:780–789. doi: 10.1039/b515219b. [DOI] [PubMed] [Google Scholar]
- 16.Shin JH, Shoenfisch MH. Improving the biocompatibility of in vivo sensors via nitric oxide release. Analyst (Lond) 2006;131:609–615. doi: 10.1039/b600129g. [DOI] [PubMed] [Google Scholar]
- 17.Afshar RK, Patra AK, Mascharak PK. Light-induced inhibition of papain by a {Mn-NO}6 nitrosyl: identification of papain-SNO adduct by mass spectrometry. J Inorg Biochem. 2005;99:1458–1464. doi: 10.1016/j.jinorgbio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Madhani M, Patra AK, Miller TW, Eroy-Reveles AA, Hobbs AJ, Fukuto JM, Mascharak PK. Biological activity of designed photolabile metal nitrosyls: light-dependent activation of soluble guanylate cyclase and vasorelaxant properties in rat aorta. J Med Chem. 2006;49:7325–7330. doi: 10.1021/jm0604629. [DOI] [PubMed] [Google Scholar]
- 19.Szundi I, Rose MJ, Sen I, Eroy-Reveles AA, Mascharak PK, Einarsdóttir Ó. A new approach for studying fast biological reactions involving nitric oxide: generation of NO using photolabile ruthenium and manganese NO donors. Photochem Photobiol. 2006;82:1377–1384. doi: 10.1562/2006-07-25-RC-984. [DOI] [PubMed] [Google Scholar]
- 20.Eroy-Reveles AA, Leung Y, Mascharak PK. Release of nitric oxide from a sol-gel hybrid material containing a photoactive manganese nitrosyl upon illumination with visible light. J Am Chem Soc. 2006;128:7166–7167. doi: 10.1021/ja061852n. [DOI] [PubMed] [Google Scholar]
- 21.Eroy-Reveles AA, Leung Y, Beavers CM, Olmstead MM, Mascharak PK. Near-infrared light activated release of nitric oxide from designed photoactive manganese nitrosyls: strategy, design, and potential as NO donors. J Am Chem Soc. 2008;130:4447–4458. doi: 10.1021/ja710265j. [DOI] [PubMed] [Google Scholar]
- 22.Halpenny GM, Olmsead MM, Mascharak PK. Incorporation of a designed ruthenium nitrosyl in polyHEMA hydrogel and light-activated delivery of NO to myoglobin. Inorg Chem. 2007;46:6601–6606. doi: 10.1021/ic700694b. [DOI] [PubMed] [Google Scholar]
- 23.Rose MJ, Fry NL, Marlow R, Hinck L, Mascharak PK. Sensitization of ruthenium nitrosyls to visible ligh via direct coordination of the dye resorufin: trackable NO donors for light-triggered NO delivery to cellular targets. J Am Chem Soc. 2008;130:8834–8846. doi: 10.1021/ja801823f. [DOI] [PubMed] [Google Scholar]
- 24.Pacelli R, Wink DA, Cook JA, Krishna MC, DeGraff W, Friedman N, Tsokos M, Samuni A, Mitchell JB. Nitric oxide potentiates hydrogen peroxide-induced killing of Escherichia coli. J Exp Med. 1995;182:1469–1479. doi: 10.1084/jem.182.5.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wainwright M, Phoenix DA, Laycock SL, Wareing DRA, Wright PA. Photobactericidal activity of phenothiazinium dyes against methicillin-resistant strains of Staphylococcus aureus. FEMS Microbiol Lett. 1998;160:177–181. doi: 10.1111/j.1574-6968.1998.tb12908.x. [DOI] [PubMed] [Google Scholar]
- 26.Ghosh K, Eroy-Reveles AA, Avila B, Holman TR, Olmstead MM, Mascharak PK. Reactions of NO with Mn(II) and Mn(III) centers coordinated to carboxamido nitrogen: synthesis of a manganese nitrosyl with photolabile NO. Inorg Chem. 2004;43:2988–2997. doi: 10.1021/ic030331n. [DOI] [PubMed] [Google Scholar]
- 27.Gillman PK. Methylene blue implicated in potentially fatal serotonin toxicity. Anaesthesia. 2006;61:1013–1014. doi: 10.1111/j.1365-2044.2006.04808.x. [DOI] [PubMed] [Google Scholar]
- 28.Phoenix DA, Sayed Z, Hussain S, Harris F, Wainwright M. The phototoxicity of pheothiazinium derivatives against Escherichia coli and Stapylococcus aureus. FEMS Immunol Med Microbiol. 2003;39:17–22. doi: 10.1016/S0928-8244(03)00173-1. [DOI] [PubMed] [Google Scholar]
- 29.Wainwright M, Mohr H, Walker WH. Phenothiazinium derivatives for pathogen inactivation in blood products. J Photochem Photobio B Bio. 2007;86:45–58. doi: 10.1016/j.jphotobiol.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Wainwright M, Byrne MN, Gattrell MA. Phenothiazinium-based photobactericidal materials. J Photochem Photobio B Bio. 2006;84:227–230. doi: 10.1016/j.jphotobiol.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Poock SR, Leach ER, Moir JWB, Cole JA, Richardson DA. Respiratory detoxification of nitric oxide by the cytochrome c reductase of Escherichia coli. J Biochem. 2002;26:23664–23669. doi: 10.1074/jbc.M200731200. [DOI] [PubMed] [Google Scholar]