Abstract
Kindlin-1 is an intracellular focal adhesion protein that regulates actin cytoskeleton. Patients suffering from Kindler syndrome have a homologous mutation of the kindlin-1 gene and develop skin blisters, periodontal disease and intestinal complications because of deficient adhesion of the basal epithelial cells. We investigated kindlin-1 localization in periodontal tissue and its functions in cultured keratinocytes and showed that kindlin-1 co-localizes with migfilin and paxillin in the basal epithelial cells of oral mucosa and in cultured keratinocytes. The kindlin-1 deficient oral mucosal tissue from a Kindler syndrome patient showed a complete lack of paxillin and reduced migfilin immunostaining in the basal keratinocytes. Co-immunoprecipitation showed that migfilin directly interacted with kindlin-1. RNA interference-induced kindlin-1 deficiency in keratinocytes led to an altered distribution of migfilin containing focal adhesions, reduced cell spreading, decreased cell proliferation and decelerated cell migration. Disruption of microtubules in the kindling-1-deficient cells further reduced cell spreading, suggesting that microtubules can partially compensate for kindlin-1 deficiency. Kindlin-1 supported mature cell-extracellular matrix adhesions of keratinocytes, as downregulation of kindlin-1 expression significantly reduced cell adhesion strength. In summary, kindlin-1 interacts with migfilin and plays a crucial role in actin-dependent keratinocyte cell adhesion essential for epidermal and periodontal health.
Keywords: Kindler syndrome, periodontal disease, kindlin-1, migfilin, paxillin, keratinocyte, actin cytoskeleton
Introduction
Kindler syndrome is an autosomal recessive disorder with alterations in skin, including congenital acral blisters, blisters after trauma or sun exposure and hyper- and hypopigmentation of the skin (1). Additional features such as hyperkeratosis of palms and soles, skin atrophy, and mucosal alterations, including urethral, vaginal, anal, esophageal and oral commisure stenosis have been described. In addition, patients with Kindler syndrome show early onset, rapidly progressive aggressive peridotontitis (2, 3). Blisters in gingival tissue of Kindler syndrome patients form at the level of lamina lucida (4). Furthermore, junctional epithelial adhesion to the tooth may be severely compromised in these patients (5). Other ultrastructural findings in Kindler syndrome patients include duplication of the lamina densa, presence of breaks in the basement membrane zone and altered expression of type VII collagen (4).
Kindler syndrome is caused by loss-of-function mutations targeting different sites in the KIND1 gene encoding kindlin-1 protein (6–12). Kindlin-1 (also known as Unc-112 Related Protein 1 or URP1) belongs to a three-member kindlin-family of focal adhesion proteins (kindlin-1, -2 and -3) (13). Kindlin-1 is highly expressed in epithelial cells, including keratinocytes (7, 14, 15). A splice variant potentially producing a truncated protein has been detected in kidney, colon and small intestine (15, 16). Kindlin-2 (also known as mitogen-induced gene-2 or Mig-2) is expressed widely in different tissues while kindlin-3 (URP2) is expressed only in hematopoietic cells (15). Proliferation of keratinocytes from Kindler patients is reduced, and kindlin-1 deficient keratinocytes show increased apoptosis both in vitro and in vivo (17). Interestingly, kindlin-1 is significantly over-expressed in lung and colon carcinomas (18). It is composed of FERM (filopodin and ezrin/radixin/moesin) domains interrupted by a pleckstrin homology (PH) domain (13). Kindlin-1 can bind and activate β1 and β3 integrin cytoplasmic tails though the F3 FERM domain (14, 19). Kindlin-1 deficiency causes lethality in mice due to intestinal problems relating to epithelial detachment and inflammation due to defective integrin activation (20).
Kindlin-2 is the closest homolog to kindlin-1 with 62% amino acid identity (7, 21). It also localizes to focal adhesions and is required for cell shape modulation (22). Kindlin-2 binds to integrins (23) and migfilin, which in turn interacts with filamin, an actin-binding protein, and appears to recruit migfilin into cell-extracellular matrix adhesions (22). Kindlin-2 deficiency causes lethality due to failed integrin signaling (24). In fibroblasts, kindlin-1 and -2 appear to localize to separate adhesion sites (15).
In the present study, we investigated kindlin-1 localization in the periodontal tissues and its potential functions in cultured cells.
Materials and methods
Antibodies
Monoclonal antibodies against kindlin-1, kindlin-2, and migfilin were prepared in the laboratory (22, 25–27). ILK-1 antibody was purchased from Upstate Cell Signaling Solutions (Lake Placid, NY, USA). Actin-binding Phalloidin [tetramethylrhodamine isothiocyanate- (TRITC)-labeled] was purchased from Sigma Chemical Co (St. Louis, MO, USA). Anti-paxillin antibody was purchased from BD Transduction Laboratories (BD Biosciences, San Jose, CA, USA). Anti-β-actin antibody was from Abcam (Cambridge, MA, USA) Secondary antibodies used for immunofluorescence staining were Alexa Fluor 488 and Alexa Fluor 594 (Invitrogen, Detection Technologies, Eugene, OR, USA). Horseradish peroxidase-conjugated IgGs (Amersham, GE Healthcare, Little Chalfont, UK) were used as secondary antibodies in immunoblotting.
Immunolocalization of kindlin-1, kindlin-2, migfilin, ILK-1, paxillin in periodontal tissues
The oral mucosal specimens were obtained from healthy volunteers and from one Kindler syndrome patient. Procedures were approved by the Clinical Research Ethics Board of the University of British Columbia and were in accordance with the Declaration of Helsinki. Immediately after collection, samples were snap-frozen in liquid nitrogen, sectioned at 8–10 µm thickness in a cryostat and stored at −86°C until use. Just before staining, sections were allowed to thaw by air-drying at room temperature and then fixed with −20°C acetone for 5 minutes. Sections were then re-hydrated in phosphate buffered saline (PBS) containing 0.01% Triton-X-100 for 5 minutes, and incubated first in normal blocking serum (Vectastain ABC Kit, Vector Laboratories, Burlingame, CA, USA) for 20 minutes at room temperature and then with primary antibodies diluted in PBS containing 1 mg/ml of BSA and 0.01% Triton-X-100 in a humidified chamber overnight at 4°C. Sections were washed three times and then incubated with a biotinylated anti-mouse secondary antibody (Vectastain ABC Kit) for 1 hour at room temperature. After washing, the sections were incubated with ABC avidin/peroxidase reagent (Vectastain ABC Kit) for 30 minutes and then reacted with Vector VIP substrate (Vector VIP Peroxidase Substrate Kit, Vector Laboratories). The sections were mounted using Vecta Mount™ Permanent Mounting Medium (Vector Laboratories). Control stainings were performed by incubation with an appropriate non-immune serum or by omitting the primary antibody incubation step and gave negligible background staining (not shown).
Cell culture
The immortalized epidermal keratinocyte cell line, HaCaT was obtained as a generous gift from Dr. Norbert Fusenig (German Cancer Centre, Heidelberg, Germany). The cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco BRL, Life Technologies, Rockville, MD, USA) supplemented with 23 mM sodium bicarbonate, 20 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), antibiotics (50 µg/ml streptomycin sulfate, 100 U/ml penicillin) and 10% heat-inactivated fetal bovine serum (FBS; Gibco).
Downregulation of kindlin-1 by siRNA transfection
In order to investigate the effect of kindlin-1 on HaCaT cell functions, kindlin-1 expression was downregulated by using siRNA transfection. The 21-bp siRNA against human kindlin-1 has been described previously (14; sense sequence: 5’-GAA GUU ACU ACC AAA AGC UdTdT-3’; synthesized by Invitrogen, Burlington, ON, Canada). A 21-bp non-specific siRNA (IR; sense sequence: 5’-ACU UCG ACA CAU CGA CUG CdTdT-3’; designed by us and synthesized by Invitrogen) and BLOCK-IT Fluorescent control siRNA (F-siRNA; Invitrogen), which are not homologous to any human genes, were used as negative controls. SiRNA transfection protocol was optimized by using F-siRNA and measuring the incorporated fluorescence by flow cytometry. For siRNA transfections, HaCaT cells were trypsinized and counted. 50 pmol of siRNA oligonucleotide dimers (final concentration 100 nM) and 1.5 µl of Lipofectamine 2000 (Invitrogen) were diluted separately with Opti-MEM (Gibco) to a total volume of 50 µl each, mixed according to the manufacturer’s instructions and added to cells (2 × 105) in suspension in 400 µl of serum-free, antibiotic-free DMEM. The cells were gently shaken with the liposome-siRNA complexes at room temperature for 30 minutes and then plated on 24-well plates. Alternatively, 25 pmol of siRNA dimers (final concentration 50 nM) and 0.75 µl of Lipofectamine RNAiMAX (Invitrogen) were used. When using other plate sizes, the transfection mixture volumes and cell numbers were adjusted accordingly. After three-hour incubation and attachment at 37°C (5% CO2), FBS was added to the cells (final concentration 8%). Every two days thereafter, the cells were provided with fresh DMEM containing 10% FBS. Kindlin-1 downregulation was verified by quantitative real-time PCR (qPCR) and Western blotting as explained below. The cells were used for cell attachment, spreading, proliferation and migration assays and for immunostaining experiments as described below.
To assess the level of mRNA downregulation, siRNA treated cells were cultured in a 12-well plate up to four days. The cells were trypsinized, and total RNA was extracted using NucleoSpin RNA II kit (Macherey-Nagel, Inc., Bethlehem, PA, USA). RNA integrity was checked by agarose gel electrophoresis and concentration was measured by spectrophotometry at 260 nm. Total RNA (1 µg) was reverse transcribed (RT) with oligo(dT) primers using iScript Select cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer’s instructions. The primers used for qPCR for β-actin were 5’-CTGTGGCATCCACGAAAC-3’ and 5’-CAGACAGCACTGTGTTGG-3’ corresponding to 886–903 and 957–974 of β-actin cDNA, respectively. The primers for kindlin-1 were 5’-ACTTGAACAAGGAGAACCAC-3' and 5’-CATTCATACCATCGGCAACA-3', and they were expected to generate a 124-bp product. These primers were designed and verified using PerlPrimer v1.1.9 software (www.bioinfo.rpi.edu/applications/mfold/), and primer sequences were analyzed by BLASTn for their specificity. Efficiency of target amplification was optimized up to 95% for each primer set using a 10-fold dilution series of cDNA while standard curves were made. For the reaction, 5 µl of diluted RT products were mixed with 10 µl of 2 X iQ SYBR Green I Supermix (Bio-Rad) and primers (15 pmol of β-actin primers or 5 pmol of kindlin-1 primers) in a final volume of 20 µl. The RT products were diluted to a concentration such that their CT values were well within the range of the standard curve. qPCR amplification was performed on the MiniOpticon Real-Time System (Bio-Rad) on the program of 3 min at 95°C, followed by 35 cycles of 15s at 94°C, 15s at 58°C, 15s at 72°C, and completion with reading plate and a melt curve analysis from 50°C to 90°C, 2s for each 1°C. Amplification reaction was conducted for kindlin-1 gene using β-actin as a reference gene and replicated three times in each experiment for each sample. The data were analyzed with Gene Expression Analysis for iCycler iQ Real-Time PCR Detection System (Bio-Rad).
Western blot analysis was used to confirm the knockdown of the kindlin-1 protein. siRNA-treated cells were cultured in a 12-well plate for up to four days. Cell lysates were collected, and Western blotting of the kindlin-1 protein was performed under non-reducing conditions using β-actin as a loading control as previously described (28).
Interaction of kindlin-1 with migfilin
The cDNA encoding the full-length human kindlin-1 was inserted into the pGEX-5x-1 vector to create a kindlin-1-glutathione-S-transferase (GST) fusion protein (Pharmacia Biotech, Uppsala, Sweden) and the recombinant pGEX-kindlin-1 vector was used to transform Escherichia coli BL21 (DE3). The expression of GST-kindlin-1 was induced at room temperature with IPTG (isopropyl 1-thio- β-D-galactopyranoside) for 16 h, and it was purified with glutathione-Sepharose 4B beads. Human 293 cells were lysed with 20 mM Tris-buffered saline (pH 7.5) containing 1% Triton X-100 (TX-100) and proteinase and protein phosphatase inhibitors (5 mM EDTA, 1 mM Na3VO4, 2 mM AEBSF, 5 µg/ml pepstatin A, 10 µg/ml aprotinin and 10 µg/ml leupeptin). 250 µg of cell lysate was incubated with GST-kindlin-1-bound glutathione-Sepharose beads, or GST-bound glutathione-Sepharose beads as a control for overnight at 4°C. The beads were washed with PBS containing 0.2% TX-100, and the proteins bound to the beads were analyzed by SDS-PAGE and Western blotting with a monoclonal anti-migfilin antibody (25).
Cell attachment and spreading assays
Cell spreading assays were performed essentially as described (29). After siRNA transfection with kindlin-1, IR- or F-siRNA, HaCaT cells were trypsinized and seeded into 24-well plates (5 × 104 cells per well; Becton Dickinson, Franklin Lakes, NJ, USA) pre-coated with bovine fibronectin (20 µg/ml, Chemicon, Temencula, CA, USA), bovine type I collagen (20 µg/ml, Vitrogen) or laminin 10/11 (5 µg/ml, Gibco) and blocked with heat-denatured BSA (10 mg/ml, Sigma-Aldrich Co., St. Louis, MO, USA) or left uncoated. The cells were allowed to spread on coated wells for up to 4 hours and fixed with formaldehyde fixative (PBS containing 4% formadehyde and 5% sucrose). The percentage of spread cells out of total number of cells was then calculated.
In order to investigate the effect of kindlin-1 on HaCaT cell spreading exclusively via the actin cytoskeleton, kindlin-1 level was downregulated by siRNA transfection as above for four days. HaCaT cells were trypsinized, seeded into fibronectin-coated wells (104 cells per well) in the presence or absence of colchicine (1 µM; Biomol, Plymouth Meeting, PA, USA), allowed to spread up to four hours at 37°C and then fixed with formaldehyde fixative. IR siRNA-transfected cells were used as control. The percentage of spread cells was counted as above.
To investigate the effect of kindlin-1 deficiency on mature extracellular matrix contacts, HaCaT cells were transfected with kindlin-1 or control siRNAs as described above, and mature extracellular matrix contacts were then allowed to form for four days. The adhesion strength was measured by detaching the cells using diluted trypsin (0.25x in PBS) for up to 30 min at room temperature. Detached cells were removed carefully by rinsing with PBS, and the attached cells fixed with formaldehyde fixative. The cells were stained with crystal violet, and the dye was extracted with acetic acid as described previously (30). The absorbance was measured with an ELISA reader (Model 3550, Bio-Rad) at 595 nm.
Cell proliferation assay
To assess the effect of kindlin-1 deficiency on HaCaT cell proliferation, kindlin-1 and IR siRNA transfected cells were seeded in 96-well plates in DMEM and allowed to attach for three hours. FBS was then added to the media to a final concentration of 10%. The proliferation assay was terminated after 6, 9, 12, 24, 48 and 72 hours. To analyze the number of viable cells, Promega reagent (Promega CellTiter 96 Non-Radioactive Cell Proliferation Assay, Promega, Madison, WI, USA) was added to the cells according to the instructions of the manufacturer, and the cell numbers were measured by reading the absorbance at 595 nm using an ELISA reader.
Cell migration assay
Scratch-wound migration assays were performed as described previously (28). HaCaT cells were transfected with siRNAs as described above and seeded in 24-well plates (2 × 105 cells per well) in DMEM containing 10% FBS. The cells were grown to confluence for two days after which the cultures were scratch-wounded with a pipette tip. Loose cells were removed by washing with PBS. 300 µl of Ca+2-free minimum essential medium (EMEM; Bio-Whittaker, Walkersville, MD, USA) supplemented with 1% Ca+2-free FBS (31) and 1 ng/ml of heparin-binding epidermal growth factor-like growth factor (HB-EGF; Oncogene Research Products; San Diego, CA, USA) was added to the cells, which were then allowed to migrate across the wound. After 24 h, the cells were fixed, stained with crystal violet and photographed using a phase contrast microscope equipped with a 10x objective. The number of cells in eight standardized fields per treatment group was counted from the digitized images. The experiment was repeated three times. Treatment group comparison was made between kindlin-1 siRNA transfected versus control siRNA transfected cells.
Immunolocalization of kindlin-1, kindlin-2, migfilin, ILK-1 and paxillin in cultured keratinocytes
Immunolocalization of focal adhesion proteins was carried out as described previously (32). HaCaT cells were either transfected with kindlin-1, or IR-siRNA as described above or left non-transfected (control). The cells were trypsinized 48 hours after transfection, seeded on glass cover slips and allowed to spread overnight at 37°C in the presence or absence of transforming growth factor β1 (TGFβ1; 10 ng/ml; Chemicon, Temencula, CA, USA). The cells were washed twice with PBS and then fixed with formaldehyde fixative for 20 minutes at room temperature. Non-specific protein binding sites were blocked with PBS containing 10 mg/ml of BSA for 30 minutes. Coverslips were then washed with PBS and incubated with primary antibodies against focal adhesion proteins kindlin-1, kindlin-2, migfilin, ILK-1 or paxillin for 1 hour at room temperature followed by rinsing and incubation with an Alexa Fluor 594-conjugated secondary antibody. For double immunostaining of focal adhesion proteins and actin, the specimens were reacted first for 1 hour with kindlin-1 or migfilin antibody followed by an Alexa 488-conjugated secondary antibody and then with TRITC-phalloidin for 1 hour. The cells were then washed and the coverslips mounted onto microscope slides. Negative control stainings were performed by omitting the primary antibody incubation step.
To determine whether kindlin-1 co-localizes with migfilin or paxillin using two mouse monoclonal antibodies, we labeled kindlin-1 first using the indirect method and Alexa Fluor 488 as a secondary antibody as described above. Migfilin and paxillin antibodies were directly coupled with the secondary antibody (Alexa Fluor 594) in a test tube at room temperature for 1 hour in the dark. Excess secondary antibodies not conjugated to the primary antibody were quenched by adding IgG from murine serum (1 mg/ml; Sigma-Aldrich) for 1 hours. Appropriate tests were performed to verify that the quenching was sufficient to block all free secondary antibody. Finally, the pre-formed primary-secondary conjugate mixture was added to kindlin-1-labeled cells and incubated for a further 3 hours.
Statistical analysis
All graphical and tabular data are expressed as the mean ± standard error of the mean (SEM) of parallel experiments. Differences between two individual means were calculated using unpaired t-tests with Welch’s correction. One-way analysis of variance (ANOVA) with Bonferroni’s post-test was used to compare means of multiple treatment groups. Differences between means were considered statistically significant if the p-value was ≤ 0.05. All statistical calculations were made using Prism® software (Version 2; GraphPad Software Inc, San Diego, CA, USA).
Results
Kindlin-1 co-localizes with migfilin and paxillin in the basal keratinocytes of oral mucosa
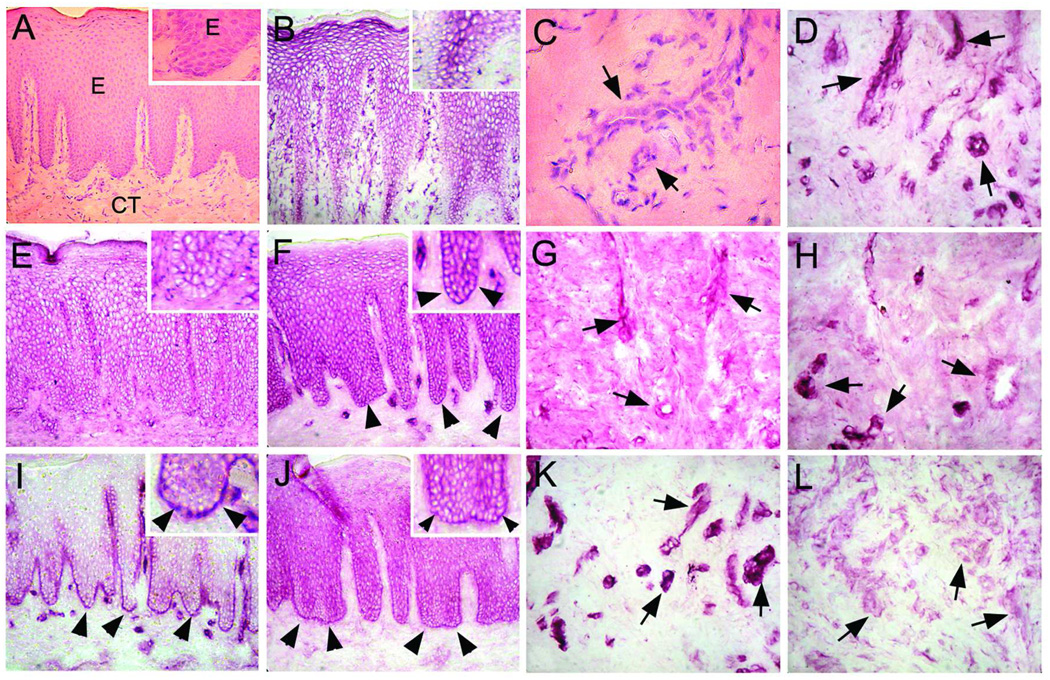
To investigate the localization of kindlin-1 in periodontal tissues, we performed immunolocalization studies using human palatal mucosa and gingival tissue. In addition to kindlin-1 protein, we localized other focal adhesion proteins that have similar functions (kindlin-2), potentially bind to kindlin-1 (migfilin) or localize to the same adhesion structures (paxillin, integrin-linked kinase [ILK]). Kindlin-1 was localized in the basal aspect of the basal keratinocytes with some diffuse staining in the suprabasal cell layers (Fig. 1J). Its immunostaining in connective tissue cells was almost negligible (Fig. 1L). Migfilin localized in cell-cell contacts of the basal and suprabasal cell layers as well as at the basal aspect of the basal keratinocytes, thus partially co-localizing with kindlin-1 (Fig. 1F). Migfilin was also present in endothelial cells in some blood vessels (Fig. 1H). Paxillin was exclusively localized at the basal aspect of the basal keratinocytes and in the endothelial cells surrounding blood vessels (Fig. 1I and K). Kindlin-2 was localized in cell-cell contacts of the suprabasal cell layers but not against the basement membrane (Fig. 1E). Its immunostaining intensity in the endothelial cells was weak (Fig. 1G). ILK-1 was strongly present in cell-cell contacts of suprabasal keratinocytes and in endothelial cells but not in the basal keratinocytes (Fig. 1B and D). In summary, kindlin-1 co-localized with migfilin and paxillin in the basal keratinocytes facing the basement membrane. This co-localization pattern suggests that kindlin-1 associates with migfilin-and paxillin-containing adhesions in vivo.
Fig. 1.
Immunolocalization (VIP Staining) of ILK-1 (B, D), kindlin-2 (E, G), migfilin (F, H), paxillin (I, K), and kindlin-1 (J, L) in human palatal oral epithelium and connective tissue. Panels A and C represent hematoxylin-eosin stained sections from the corresponding area. A magnified view of the basement membrane zone (BMZ) and basal cell layer is provided in the top-right corner of the left side panels. ILK-1 (B) and kindlin-2 (E) do not localize to the basement membrane zone or the basal cells, but are found in between cells in the suprabasal layers and blood vessels (D and G, respectively; arrows). Migfilin (F), paxillin (I), and kindlin-1 (J) localize strongly to the basement membrane zone (arrowheads) and between cells throughout all layers of the epithelium with only migfilin (H) and paxillin (K) localizing to blood vessels (arrows). Kindlin-1 (L) expression is weak in blood vessels (arrows).
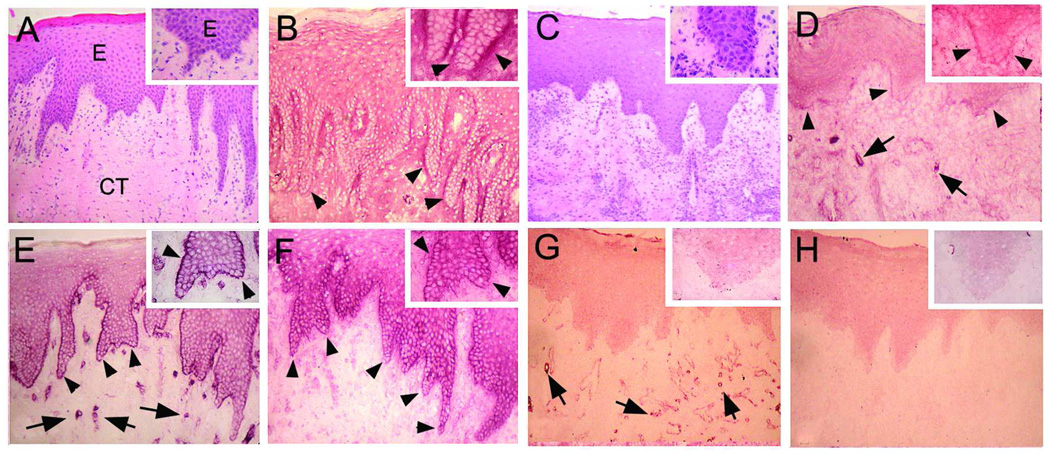
We next tested whether the localization of migfilin or paxillin is altered in kindlin-1-deficient oral mucosa. Mucosal tissue of a well-characterized case with Kindler syndrome (2, 5) showed no immunoreactivity of kindlin-1 (Fig. 2H) and revealed notable differences in the immunolocalization of paxillin and migfilin compared to the tissue from healthy subjects (Fig. 2). While some migfilin was still present facing the basement membrane zone, its abundance was reduced in basal keratinocytes (Fig. 2D). Interestingly, the staining intensity of paxillin was totally downregulated in basal keratincoytes while it was still present in endothelial cells of the blood vessels (Fig. 2G). Thus, kindlin-1 deficiency leads to reduced migfilin and paxillin abundance in the basal keratinocytes, suggesting that these proteins participate together in cell-basement membrane adhesions of basal keratinocytes.
Fig 2.
Immunolocalization (VIP Staining) of migfilin (B, D), paxillin (E, G), and kindlin-1 (F, H) in the oral epithelium of normal (B, E, F) or kindlin-1 deficient gingiva (D, G, H). Panels A and C represents hematoxylin-eosin stained sections from the corresponding areas. A magnified view of the BMZ and basal cell layer is provided in the top-right corner. Migfilin and paxillin fail to localize to the BMZ in kindlin-1 deficient gingiva. Arrowheads point to the BMZ and arrows to blood vessels, respectively.
Kindlin-1 interacts with migfilin with co-localization in focal adhesions
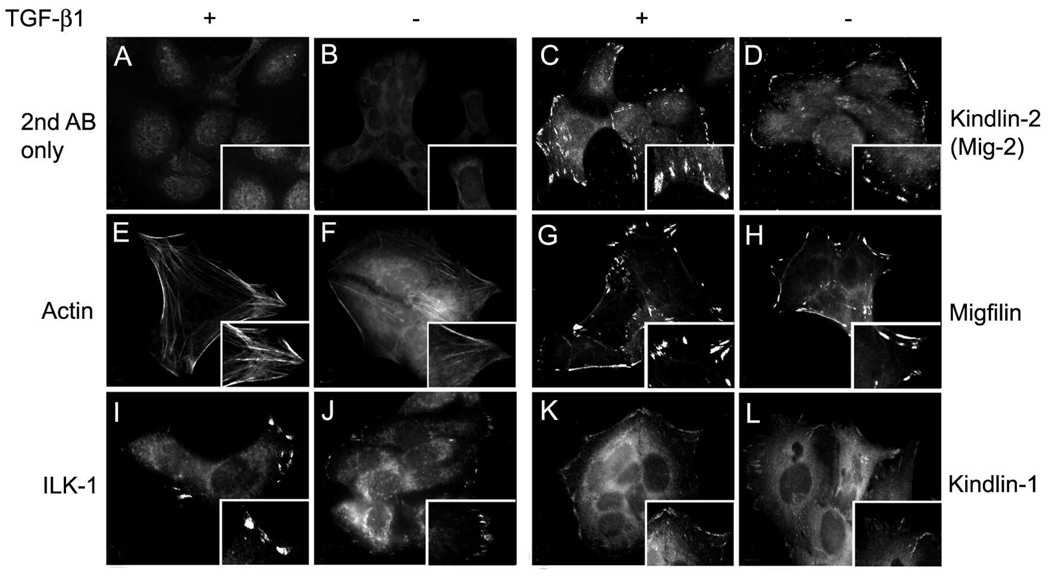
To establish whether kindlin-1 interacts with migfilin and paxillin, we investigated the localization of these proteins in cultured keratinocytes. Immunofluorescence staining of HaCaT cells demonstrated that kindlin-1 kindlin-2, migfilin and ILK-1 all localized to focal adhesion-like structures (Fig. 3). The staining pattern was similar for kindlin-1 and -2 and for migfilin while ILK staining was present in fewer positively stained focal adhesions. We then repeated the experiment by treating the cells with TGFβ1, known to increase kindlin-1 levels (14). Addition of TGFβ1 to the cells resulted in an increase in the number of focal adhesions containing ILK-1, kindlin-1, kindlin-2 and migfilin and their staining intensity when compared to non-TGFβ1-treated controls (Fig. 3). Even in these stimulated conditions, only sparse ILK-1 positive focal adhesions were present. These results suggest that, in keratinocytes, kindlin-1 and kindlin-2, but not ILK-1, are likely localized in the same focal adhesions with migfilin.
Fig. 3.
Immunolocalization of filamentous actin (E, F) and focal adhesion proteins ILK-1 (I, J), kindlin-2 (C, D), migfilin (G, H), and kindlin-1 (K, L) in HaCaT keratinocytes spread for 16 hours in the presence (A, C, E, G, I, K) or absence (B, D, F, H, J, L) of TGF-β1. Secondary antibody alone (A, B) was used as a negative control. A magnified view of the focal adhesions is provided in the bottom-right corner of each panel. TGF-β promotes expression of actin filaments and an increase in the size of ILK-1, kindlin-2, migfilin, and kindlin-1 focal adhesions.
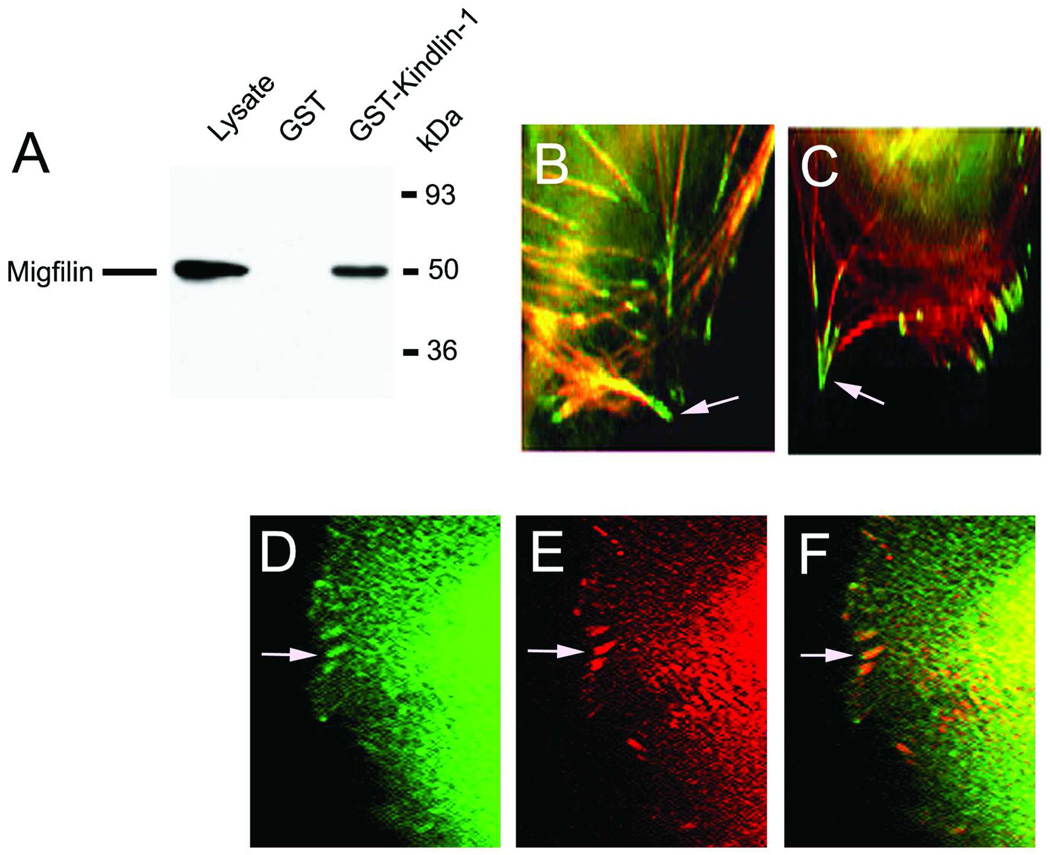
To find out whether kindlin-1 interacts with migfilin we generated a GST-kindlin-1 fusion protein that was bound to glutathione-Sepharose beads. When cell lysates of human 293 cells were incubated with the beads containing the GST tag alone, no bands were detected in Western blots using anti-migfilin antibody (Fig. 4A). The lysate itself, however, gave a strong signal, demonstrating that migfilin was present in these cells (Fig. 4A). When GST-kindlin-1 beads were used as bait, migfilin readily co-precipitated from the cell lysate, demonstrating that kindlin-1 can physically interact with migfilin in vitro (Fig. 4A). Next, we studied the localization of kindlin-1 and migfilin in HaCaT keratinocytes. Both kindlin-1 and migfilin localized into focal adhesions at the termination points of actin filaments (Fig. 4B and C), suggesting that both kindlin-1 and migfilin are involved in the maintenance of the actin cytoskeleton in keratinocytes. To test whether kindlin-1 and migfilin localized to the same focal adhesions, we performed double immunofluorescence staining of these proteins. Kindlin-1 co-localized with migfilin in almost all focal adhesions at the cell periphery (Fig. 4D–F). Thus, kindlin-1 appears to physically interact with migfilin and localize to the same structures in cultured keratinocytes.
Fig. 4.
Interaction of migfilin with kindlin-1. A, GST-kindlin-1 specifically binds to migfilin in human 293 cells. GST-kindlin-1-bound glutathione-Sepharose or control beads were incubated with cell lysates and subjected to Western blotting using anti-migfilin antibody. Lane 1, cell lysate; lane 2, cell lysate incubated with GST-Sepaharose beads; lane 3, cell lysate incubated with GST-kindlin-1 beads. B, Immunolocalization of kindlin-1 (green) at the end of the actin filaments (red) in keratinocytes; C, immunolocalization of migfilin (red) at the end of actin filaments in keratinocytes; D–F, double immunolocalization of kindlin-1 (D, green) and migfilin (E, red) in same focal adhesions (F, orange). Arrows point to the focal adhesions.
Distribution of migfilin containing focal adhesions is altered in kindlin-1 deficient cells
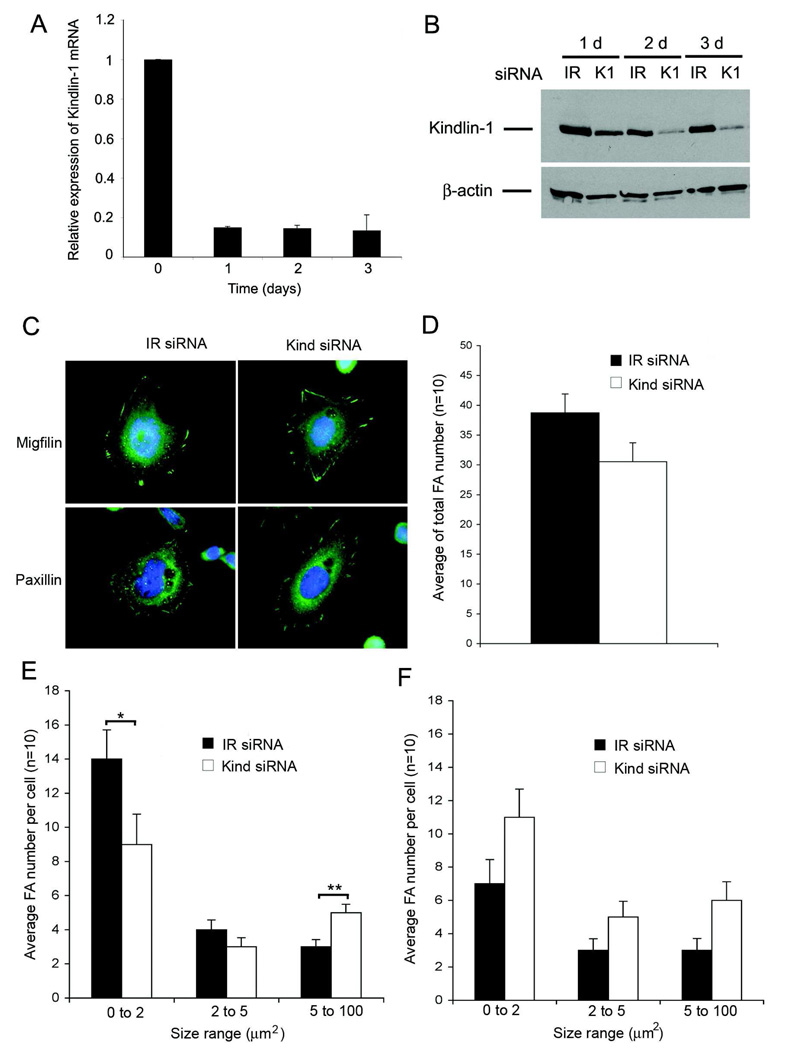
To investigate whether kindlin-1 regulates migfilin accumulation in focal adhesions, we knocked down kindlin-1 expression in keratinocytes by siRNA transfection (Fig. 5). Knockdown reduced kindlin mRNA levels by about 90% in 24 hours and remained at that level until day 3 (Fig. 5A). The corresponding protein levels were also reduced by about 90% by 48 hours (Fig. 5B). In kindlin-1-deficient cells, migfilin was still present in focal adhesions with no significant reduction in the number of focal adhesions per cell (Fig. 5C and D). We then investigated the size distribution of migfilin- and paxillin-positive focal adhesions. Kindlin-1 deficient cells contained significantly more migfilin-positive larger focal adhesions (over 5 µm2) and a correspondingly reduced number of smaller focal adhesions (0–2 µm2; Fig. 5E). The distribution of paxillin-positive focal adhesions was not significantly altered in kindlin-1 deficient cells (Fig. 5F). Taken together, kindlin-1-deficient keratinocytes show altered migfilin distribution in focal adhesions, suggesting that kindlin-1 regulates migfilin dynamics in focal adhesions.
Fig. 5.
Effect of kindlin-1 knockdown on migfilin and paxillin containing focal adhesions. A, expression of kindlin-1 mRNA after 0–3 days following kindlin-1 siRNA transfection relative to transfection with the control siRNA; B, expression of kindlin-1 protein 1–3 days after transfection with the kindlin-1 siRNA (K1) or irrelevant control siRNA (IR). β-actin was used as a loading control; C, Immunolocalization of migfilin and paxillin containing focal adhesions after transfection with kindlin-1 or IR siRNA; D, average number of migfilin containing focal adhesions per cell after siRNA transfections; E–F, distribution of migfilin (E) and paxillin (F) containing focal adhesions according to the size (0–2 µm, 2–5 µm, 5–100 µm) after siRNA transfection. All histograms show the mean +/− SEM of three parallel experiments (* p<0.05, **p<0.01).
Kindlin-1 is required for optimal keratinocyte adhesion
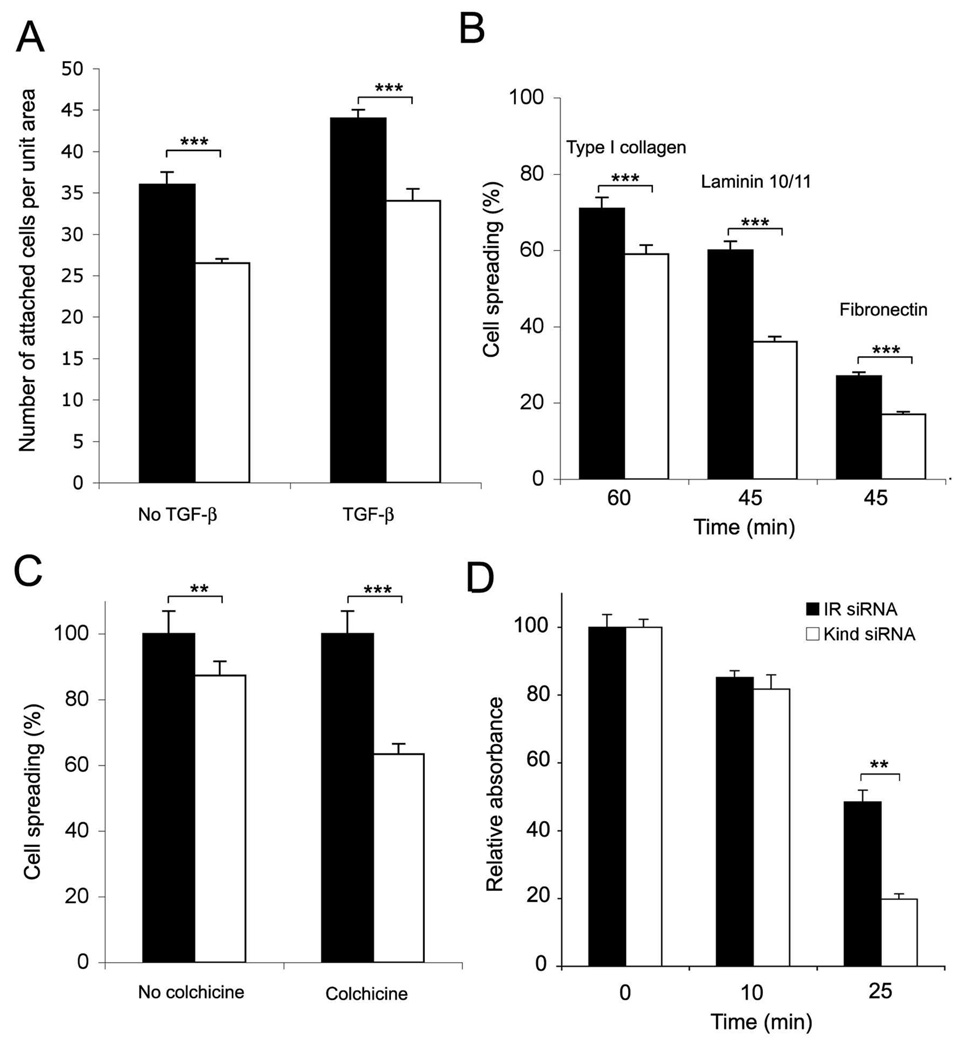
To test the functional consequences of kindlin-1 deficiency and altered migfilin dynamics in keratinocytes, we investigated cell adhesion and spreading. Kindlin-1-deficient HaCaT keratinocytes displayed a decrease in cell adhesion on glass coverslips in the presence or absence of TGFβ1 (Fig. 6A). In addition, cell spreading on various extracellular matrix molecules, namely type I collagen, laminin 10/11 and fibronectin was reduced (Fig. 6B). Overall, kindlin-1-deficient cells demonstrated significant but modest deficiencies in their cell spreading. Cultured HaCaT keratinocytes use mostly their microtubule cytoskeleton for cell spreading (33). Therefore, we repeated the spreading experiments in the presence of microtubule disruptor colchicine. Kindlin-1 siRNA transfection decreased the spreading of HaCaT cells on uncoated cell culture plates by about 20% in 4 hours compared to controls (Fig. 6C). In the presence of colchicine, the cell spreading deficiency was almost doubled, and the kindlin-1-deficient cells showed about 38% reduction in cell spreading (Fig. 6C). These results suggest that, in cell culture, microtubules can compensate for the adhesion deficiency caused by the lack of kindlin-1. The fact that 62% of the cells spread without microtubules and regardless of kindlin-1 knock down illustrates that other focal adhesion proteins can compensate for the lack of kindlin-1 in focal adhesions in vitro.
Fig. 6.
Effect of kindlin-1 knockdown on keratinocyte cell adhesion. A, attachment of siRNA treated HaCaT keratinocytes on glass coverslips over 24 hours in the presence or absence of 10 ng/ml TGFβ1; B, spreading of siRNA treated keratinocytes on extracellular matrix proteins (type I collagen, laminin10/11 and fibronectin) for 45–60 minutes; C, spreading of siRNA treated keratinocytes on fibronectinin the presence of absence of 1 µM colchicine. Relative spreading percentage to no drug treatment (control, 100%) is shown; D, detachment of established keratinocyte cultures (confluent siRNA treated cells cultured for 4 days) with diluted (1:4) trypsin at various time points (0–25 minutes). Representative result (mean +/− SEM of triplicate samples) from three parallel experiments is shown (**p<0.01; *** p<0.001).
We then tested the importance of kindlin-1 in mature cell-matrix contacts formed during four days of cell culturing. Kindlin-1-deficient and control keratinocytes were treated with diluted trypsin to assess the strength of cell adhesion. After 25 minutes of incubation at room temperature, significantly more kindlin-1 deficient cells were detached compared to controls (Fig. 6D), suggesting that kindlin-1 is an important adhesion protein in mature keratinocyte-matrix adhesions.
Kindlin-1 deficient cells have reduced proliferation and migration rate
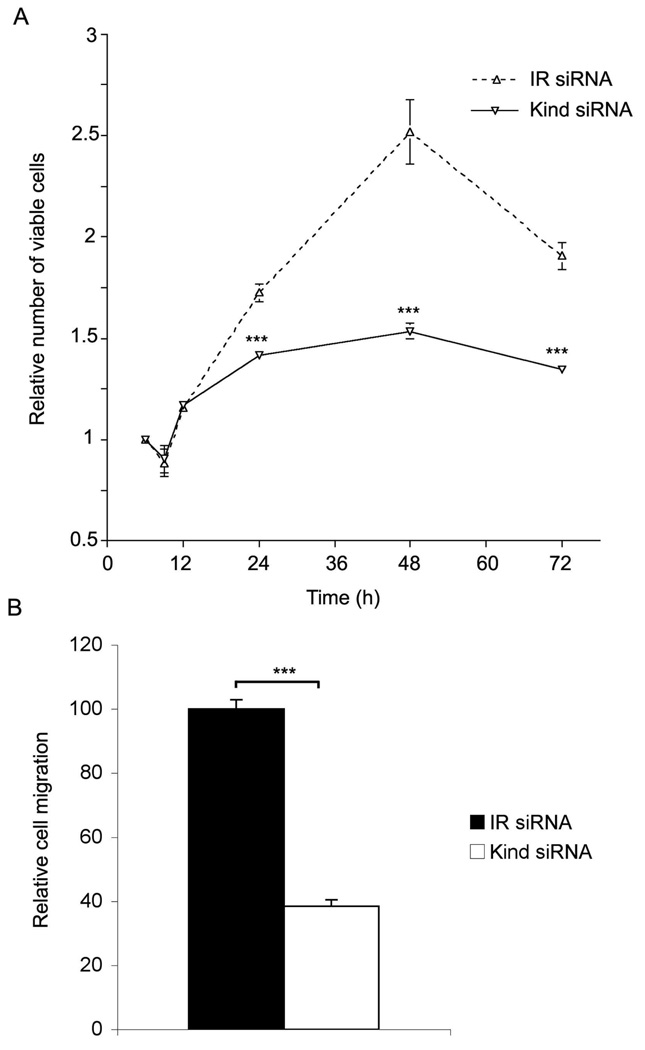
Next, the effect of kindlin-1 deficiency to HaCaT cell proliferation and migration was investigated. Kindlin-1-deficiency resulted in a significant decrease in HaCaT keratinocyte proliferation after 24, 48 and 72 hours (Fig. 7). To investigate the cell migration rates of the kindlin-1 deficient keratinocytes, we performed classical scratchwounding assays in which wound closure was stimulated with HB-EGF (28). Kindlin-1 deficient cells demonstrated a significant reduction in the wound closure at 73.4% (p<0.0001) compared to control siRNA-transfected cells (Fig. 7).
Fig. 7.
Effect of kindlin-1 knockdown on keratinocyte proliferation and migration. A, number of viable kindlin-1 (kind siRNA) and control (IR siRNA) siRNA transfected HaCaT keratinocytes relative to the seeding density at 3 hours. Representative result from three parallel experiments is shown; B, migration of kindlin-1 and IR siRNA transfected HaCaT keratinocytes for 24 hours into the wounds made by scratching with pipette tip. The number of cells migrated into the wound per unit area was calculated. The histogram shows the relative mean +/− SEM of three parallel experiments (***p<0.0001).
Discussion
In the present study, we demonstrated that kindlin-1 localizes against the basement membrane zone in periodontal tissues and that it binds to and regulates migfilin dynamics. In addition, kindlin-1 appears to regulate paxillin distribution in vivo but not in vitro. These observations shed new light to our earlier observations that the adhesion of gingival junctional epithelium to the tooth enamel may be affected in Kindler patients who demonstrate advanced periodontal disease (3, 5). Our results show that migfilin and kindlin-1 bind each other which supports recent findings (34). This study investigated overall migfilin distribution and did not search, however, for differences in migfilin dynamics in kindlin-1 deficient cells. Interestingly both kindlin-1 and migfilin can regulate integrin activation (20, 35). Kindlin-1 deficiency reduces cell adhesion to β1 integrin ligands such as fibronectin, laminin10/11 and type I collagen and affects cell migration and proliferation as demonstrated in the present and previous studies (14, 17). Kindlin-2 does not seem to compensate to restore integrin activation in keratinocytes as kindlin-2 knockdown did not add to the adhesion deficiency phenotype (not shown). In addition, expression of kindlin-2 is minimal in basal keratinocytes in vivo. It is possible that kindlin-2 and kindlin-1 accumulate in distinct adhesion sites in keratinocytes as they do in transfected fibroblasts (15).
Activation of integrins by kindlin-1 in basal keratinocytes results from direct binding of the cytoplasmic tail of the β1 integrin (14, 19). This binding leads to a conformational change in the integrin extracellular domain and renders it to a high affinity state (13). Migfilin likely regulates integrin activity through an indirect mechanism (35). Migfilin does not directly bind to the integrin tail but instead to filamin (26). Filamin can prevent integrin binding to talin that is required for integrin activation (36). Therefore, migfilin serves as a molecular switch by removing filamin from the integrin tail, allowing talin to interact with it (35). How migfilin regulates kindlin-1 function in keratinocytes remains unknown. Interestingly, it appears that kindlin-1 can also regulate migfilin dynamics as the distribution of migfilin focal adhesions was changed and migfilin abundance was strongly reduced in basal keratinocytes of a Kindler patient that is consistent with the direct interaction between kindlin-1 and migfilin. Kindlin-1 knockdown in keratinocytes leads to an increase in the number of large (mature) migfilin-positive focal adhesions. This observation suggests that in the absence of kindlin-1, migfilin accumulation to small focal adhesions (early contacts) is reduced in favor of the more mature cell-matrix binding sites.
Kindlin-1 shows fairly close homology to the C. elegans protein UNC-112 (7). Loss-of-function mutations of UNC-112 cause the PAT phenotype in nematodes in which myosin and actin fail to organize into sarcomeres in the body wall muscle cells. This defect is due to a failure to organize PAT-3/β-integrin into focal adhesion-like structures (37). During the formation of these structures, UNC-112 also binds PAT-4, the worm homolog of ILK (38). It remains to be determined whether directly binds kindlin-1. The localization of ILK-1 and kindlin-1 appeared distinct in cultured and in vivo human keratinocytes. Interestingly, however, ILK is highly expressed in murine epidermis and ILK-deficient mice exhibit blistering and abnormal hair follicle morphogenesis accompanied with impaired keratinocyte adhesion and migration suggesting that defects in several focal adhesion proteins can lead to reduced epidermal adhesion (39, 40).
Surprisingly, paxillin co-localized with kindlin-1 in keratinocytes (not shown) and its abundance was strongly reduced in basal keratinocytes of Kindler patient tissue. Paxillin is a focal adhesion protein that binds to a number of partners directly but not to the integrin tail (41). It can bind, however, indirectly to other focal adhesion proteins via focal adhesion kinase and to actin cytoskeleton via actopaxin and vinculin (41). Extracellular matrix contacts between hemidesmosomes are believed to be mediated by integrins that link to actin cytoskeleton mimicking focal adhesions (42). Interestingly, podosome-like actin-rich adhesions that contain α3β1 integrin and paxillin have been observed between hemidesmosomes in keratinocytes (43). It is possible, therefore, that kindlin-1 could regulate both migfilin and paxillin dynamics and thereby integrin activation in basal keratinocytes that mediate junctional epithelial adhesion to the tooth.
Taken together, kindlin-1 localizes to basal keratinocytes and regulates migfilin and paxillin abundance. Advanced periodontal disease in Kindler syndrome patients is the first oral condition directly linked to integrin activation in basal keratinocytes of the oral tissues.
Acknowledgements
This study was supported by a grant from National Institutes of Health (DE016099).
Footnotes
The authors declare no conflict of interest.
References
- 1.Kindler T. Congenital poikiloderma with traumatic bulla formation and progressive cutaneous atrophy. Br J Dermatol. 1954;66:104–111. doi: 10.1111/j.1365-2133.1954.tb12598.x. [DOI] [PubMed] [Google Scholar]
- 2.Wiebe CB, Silver JG, Larjava HS. Early-onset periodontitis associated with Weary-Kindler syndrome: a case report. J Periodontol. 1996;67:1004–1010. doi: 10.1902/jop.1996.67.10.1004. [DOI] [PubMed] [Google Scholar]
- 3.Wiebe CB, Penagos H, Luong N, Slots J, Epstein E, JR, Siegel D, HÄkkinen L, Putnins EE, Larjava HS. Clinical and microbiologic study of periodontitis associated with Kindler syndrome. J Periodontol. 2003;74:25–31. doi: 10.1902/jop.2003.74.1.25. [DOI] [PubMed] [Google Scholar]
- 4.Wiebe CB, Larjava HS. Abnormal deposition of type VII collagen in Kindler syndrome. Arch Dermatol Res. 1999;291:6–13. doi: 10.1007/s004030050377. [DOI] [PubMed] [Google Scholar]
- 5.Wiebe CB, Petricca G, HÄkkinen L, Jiang G, Wu C, Larjava HS. Kindler syndrome and periodontal disease: Review of the literature and 12-year followup case. J Periodontol. 2008;79:961–966. doi: 10.1902/jop.2008.070167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jobard F, Bouadjar B, Caux F, Hadj-Rabia S, Has C, Matsuda F, Weissenbach J, Lathrop M, Prud'Homme JF, Fischer J. Identification of mutations in a new gene encoding a FERM family protein with a pleckstrin homology domain in Kindler syndrome. Hum Mol Genet. 2003;12:925–935. doi: 10.1093/hmg/ddg097. [DOI] [PubMed] [Google Scholar]
- 7.Siegel DH, Ashton GH, Penagos HG, Lee JV, Feiler HS, Wilhelmsen KC, South AP, Smith FJ, Prescott AR, Wessagowit V, Oyama N, Akiyama M, Al Aboud D, Al Aboud K, Al Githami A, Al Hawsawi K, Al Ismaily A, Al-Suwaid R, Atherton DJ, Caputo R, Fine JD, Frieden IJ, Fuchs E, Haber RM, Harada T, Kitajima Y, Mallory SB, Ogawa H, Sahin S, Shimizu H, Suga Y, Tadini G, Tsuchiya K, Wiebe CB, Wojnarowska F, Zaghloul AB, Hamada T, Mallipeddi R, Eady RA, McLean WH, McGrath JA, Epstein EH. Loss of Kindlin-1, a human homolog of the Caenorhabditis elegans actin-extracellular matrix linker protein UNC-112, causes Kindler syndrome. Am J Hum Genet. 2003;73:174–187. doi: 10.1086/376609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashton GHS, McLean WHI, South AP, Oyama N, Smith FJD, Al-Suwaid R, Al-Ismaily A, Atherton DJ, Harwood CA, Leigh IM, Moss C, Didona B, Zambruno G, Patrizi A, Eady RA, McGrath JA. Recurrent mutations in kindlin-1, a novel keratinocyte focal contact protein, in the autosomal recessive skin fragility and photosensitivity disorder, Kindler syndrome. J Invest Dermatol. 2004;122:78–83. doi: 10.1046/j.0022-202X.2003.22136.x. [DOI] [PubMed] [Google Scholar]
- 9.Fassihi H, Wessagowit V, Jones C, Dopping-Hepenstal P, Denyer J, Mellerio JE, Clarck S, McGrat JA. Neonatal diagnosis of Kindler syndrome. J Dermatol Sci. 2005;39:183–185. doi: 10.1016/j.jdermsci.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Sethuraman G, Fassihi H, Ashton GH, Bansal A, Kabra M, Sharma VK, McGrath JA. An Indian child with Kindler syndrome resulting from a new homozygous nonsense mutation (C468X) in the KIND1 gene. Clin Exp Dermatol. 2005;30:286–288. doi: 10.1111/j.1365-2230.2004.01712.x. [DOI] [PubMed] [Google Scholar]
- 11.Has C, Wessagowit V, Pascucci M, Baer C, Didona B, Wilhelm C, Pedicelli C, Locatelli A, Kohlhase J, Ashton GH, Tadini G, Zambruno G, Bruckner-Tuderman L, McGrath JA, Castiglia D. Molecular basis of Kindler syndrome in Italy: novel and recurrent Alu/Alu recombination, splice site, nonsense, and frameshift mutations in the KIND1 gene. J Invest Dermatol. 2006;126:1776–1783. doi: 10.1038/sj.jid.5700339. [DOI] [PubMed] [Google Scholar]
- 12.Sadler E, Klausegger A, Muss W, Deinsberger U, Pohla-Gubo G, Laimer M, Lanschuetzer C, Bauer JW, Hintner H. Novel KIND1 gene mutation in Kindler syndrome with severe gastrointestinal tract involvement. Arch Dermatol. 2006;142:1619–1624. doi: 10.1001/archderm.142.12.1619. [DOI] [PubMed] [Google Scholar]
- 13.Larjava H, Plow EF, Wu C. Kindlins: essential regulators of integrin signalling and cell-matrix adhesion. EMBO Rep. 2008;9:1203–1208. doi: 10.1038/embor.2008.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kloeker S, Major MB, Calderwood DA, Ginsberg MH, Jones DA, Beckerle MC. The kindler syndrome protein is regulated by transforming growth factor-B and involved in integrin-mediated adhesion. J Biol Chem. 2004;279:6824–6833. doi: 10.1074/jbc.M307978200. [DOI] [PubMed] [Google Scholar]
- 15.Ussar S, Wang HV, Linder S, FÄssler R, Moser M. The Kindlins: subcellular localization and expression during murine development. Exp Cell Res. 2006;312:3142–3151. doi: 10.1016/j.yexcr.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 16.Kern JS, Herz C, Haan E, Moore D, Nottelmann S, Von Lilien T, Greiner P, Schmitt-Graeff A, Opitz OG, Bruckner-Tuderman L, Has C. Chronic colitis due to an epithelial barrier defect: the role of kindlin-1 isoforms. J Pathol. 2007;213:462–470. doi: 10.1002/path.2253. [DOI] [PubMed] [Google Scholar]
- 17.Herz C, Aumailley M, Schulte C, Schlotzer-Schrehardt U, Bruckner-Tuderman L, Has C. Kindlin-1 is a phosphoprotein involved in regulation of polarity, proliferation, and motility of epidermal keratinocytes. J Biol Chem. 2006;281:36082–36090. doi: 10.1074/jbc.M606259200. [DOI] [PubMed] [Google Scholar]
- 18.Weinstein EJ, Bourner M, Head R, Zakeri H, Bauer C, Mazzarella R. URP1: a member of a novel family of PH and FERM domain-containing membrane-associated proteins is significantly over-expressed in lung and colon carcinomas. Biochim Biophys Acta. 2003;1637:207–216. doi: 10.1016/s0925-4439(03)00035-8. [DOI] [PubMed] [Google Scholar]
- 19.Harburger DS, Bouaouina M, Calderwood DA. Kindlin-1 and-2 directly bind the C-terminal region of beta integrin cytoplasmic tails and exert integrin-specific activation effects. J Biol Chem. 2009;284:11485–11497. doi: 10.1074/jbc.M809233200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ussar S, Moser M, Widmaier M, Rognoni E, Harrer C, Genzel-Boroviczeny O, FÄssler R. Loss of Kindlin-1 causes skin atrophy and lethal neonatal intestinal epithelial dysfunction. PLoS Genet. 2008;4(12):e1000289. doi: 10.1371/journal.pgen.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wick M, Burger C, Brusselbach S, Lucibello FC, Muller R. Identification of serum-inducible genes: different patterns of gene regulation during G0 S and G1 S progression. J Cell Sci. 1994;107:227–239. doi: 10.1242/jcs.107.1.227. [DOI] [PubMed] [Google Scholar]
- 22.Tu Y, Li F, Goicoechea S, Wu C. The LIM-only protein PINCH directly interacts with integrin-linked kinase and is recruited to integrin-rich sites in spreading cells. Mol Cell Biol. 1999;19:2425–2434. doi: 10.1128/mcb.19.3.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi X, Ma YQ, Tu Y, Chen K, Wu S, Fukuda K, Qin J, Plow EF, Wu C. The MIG-2/integrin interaction strengthens cell-matrix adhesion and modulates cell motility. J Biol Chem. 2007;282:20455–20466. doi: 10.1074/jbc.M611680200. [DOI] [PubMed] [Google Scholar]
- 24.Montanez E, Ussar S, Schiffer M, Bosl M, Zent R, Moser M, Fassler R. Kindlin-2 controls bidirectional signaling of integrins. Genes and Dev. 2008;22:1325–1330. doi: 10.1101/gad.469408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tu Y, Li F, Huang Y, Zhang Z, Hua Y, Wu C. A new focal adhesion protein that interacts with integrin-linked kinase and regulates cell adhesion and spreading. J Cell Biol. 2001;153:585–598. doi: 10.1083/jcb.153.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tu Y, Wu S, Shi X, Chen K, Wu C. Migfilin and Mig-2 link focal adhesions to filamin and the actin cytoskeleton and function in cell shape modulation. Cell. 2003;13:37–47. doi: 10.1016/s0092-8674(03)00163-6. [DOI] [PubMed] [Google Scholar]
- 27.Papachristou DJ, Gkretsi V, Tu Y, Shi X, Chen K, Larjava H, Rao UN, Wu C. Increased cytoplasmic level of migfilin is associated with higher grades of human leiomyosarcoma. Histopathology. 2007;51:499–508. doi: 10.1111/j.1365-2559.2007.02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koivisto L, Jiang G, HÄkkinen L, Chan B, Larjava H. HaCaT keratinocyte migration is dependent on epidermal growth factor receptor signaling and glycogen synthase kinase-3alpha. Exp Cell Res. 2006;312:2791–2805. doi: 10.1016/j.yexcr.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Koivisto L, Larjava K, HÄkkinen L, Uitto VJ, Heino J, Larjava H. Different integrins mediate cell spreading, haptotaxis and lateral migration of HaCaT keratinocytes on fibronectin. Cell Adhes Commun. 1999;7:245–257. doi: 10.3109/15419069909010806. [DOI] [PubMed] [Google Scholar]
- 30.Kueng W, Silber E, Eppenberger U. Quantification of cells cultured on 96-well plates. Anal Biochem. 1989;182:16–19. doi: 10.1016/0003-2697(89)90710-0. [DOI] [PubMed] [Google Scholar]
- 31.HÄkkinen L, Koivisto L, Larjava H. An improved method for culture of epidermal keratinocytes from newborn mouse skin. Methods Cell Sci. 2001;23:189–196. doi: 10.1023/a:1016385109922. [DOI] [PubMed] [Google Scholar]
- 32.Larjava H, Peltonen J, Akiyama SK, Yamada SS, Gralnick HR, Uitto J, Yamada KM. Novel function of β1 integrins in keratinocyte cell-cell interactions. J Cell Biol. 1990;110:803–815. doi: 10.1083/jcb.110.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koivisto L, HÄkkinen L, Matsumoto K, McCulloch CA, Yamada KM, Larjava H. Glycogen synthase kinase-3 regulates cytoskeleton and translocation of Rac1 in long cellular extensions of human keratinocytes. Exp Cell Res. 2004;293:68–80. doi: 10.1016/j.yexcr.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 34.Lai-Cheong JE, Ussar S, Arita K, Hart IR, McGrath JA. Colocalization of kindlin-1, kindlin-2, and migfilin at keratinocyte focal adhesion and relevance to the pathophysiology of Kindler syndrome. J Invest Dermatol. 2008;128:2156–2165. doi: 10.1038/jid.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ithychanda SS, Das M, Ma YQ, Ding K, Wang X, Gupta S, Wu C, Plow EF, Qin J. Migfilin, a molecular switch in regulation of integrin activation. J Biol Chem. 2009;284:4713–4722. doi: 10.1074/jbc.M807719200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wegener KL, Partridge AW, Han J, Pickford AR, Liddington RC, Ginsberg MH, Campbell ID. Structural basis of integrin activation by talin. Cell. 2007;128:171–182. doi: 10.1016/j.cell.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 37.Rogalski TM, Mullen GP, Gilbert MM, Williams BD, Moerman DG. The UNC-112 gene in Caenorhabditis elegans encodes a novel component of cell-matrix adhesion structures required for integrin localization in the muscle cell membrane. J Cell Biol. 2000;150:253–264. doi: 10.1083/jcb.150.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacKinnon AC, Qadota H, Norman KR, Moerman DG, Williams BD. C. elegans PAT-4/ILK functions as an adaptor protein within integrin adhesion complexes. Curr Biol. 2002;12:787–797. doi: 10.1016/s0960-9822(02)00810-2. [DOI] [PubMed] [Google Scholar]
- 39.Nakrieko KA, Welch I, Dupuis H, Bryce D, Pajak A, Arnaud RS, Dedhar S, D'Souza SJ, Dagnino L. Impaired hair follicle morphogenesis and polarized keratinocyte movement upon conditional inactivation of integrin-linked kinase in the epidermis. Mol Biol Cell Mol Biol Cell. 2008;19:1462–1473. doi: 10.1091/mbc.E07-06-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lorenz K, Grashoff C, Torka R, Sakai T, Langbein L, Bloch W, Aumailley M, FÄssler R. Integrin-linked kinase is required for epidermal and hair follicle morphogenesis. J Cell Biol. 2007;177:501–513. doi: 10.1083/jcb.200608125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turner CE. Paxillin and focal adhesion signalling. Nat Cell Biol. 2000;2:E231–E236. doi: 10.1038/35046659. [DOI] [PubMed] [Google Scholar]
- 42.Kikkawa Y, Sanzen N, Sekiguchi K. Isolation and characterization of laminin-10/11 secreted by human lung carcinoma cells. Laminin-10/11 mediates cell adhesion through integrin alpha3 beta1. J Biol Chem. 1998;273:15854–15859. doi: 10.1074/jbc.273.25.15854. [DOI] [PubMed] [Google Scholar]
- 43.Spinardi L, Marchisio PC. Podosomes as smart regulators of cellular adhesion. Eur J Cell Biol. 2006;85:191–194. doi: 10.1016/j.ejcb.2005.08.005. [DOI] [PubMed] [Google Scholar]