Abstract
The directed differentiation of forebrain neuronal types from human embryonic stem cells (hESCs) has not been achieved. Here, we show that hESCs differentiate to telencephalic progenitors with a predominantly dorsal identity in a chemically defined medium without known morphogens. This is attributed to endogenous Wnt signaling, which upregulates the truncated form of GLI3, a repressor of sonic hedgehog (SHH). A high concentration of SHH, or the inhibition of Wnt by dickkopf 1 (DKK1) together with a low concentration of SHH, almost completely converts the primitive dorsal precursors to ventral progenitors, which is partially achieved through both downregulation of the truncated GLI3 and upregulation of full-length GLI3 expression. These dorsal and ventral telencephalic progenitors differentiate to functional glutamatergic and GABAergic neurons, respectively. Thus, although hESCs generate dorsal telencephalic cells, as opposed to ventral progenitors in other vertebrates, in the absence of exogenous morphogens, human cells use a similar molecular mechanism to control the dorsal versus ventral fate. The coordination of Wnt and SHH signaling through GLI3 represents a novel mechanism that regulates ventral-dorsal patterning in the development of forebrain neuronal subtypes.
Keywords: Human embryonic stem cells, Neural patterning, Neural differentiation, Sonic hedgehog, Wnt signaling
INTRODUCTION
The development of diverse neuronal subtypes in the telencephalon begins with the specification of regional progenitors through inductive signals that are secreted from the patterning centers. The cerebral cortex on the dorsal side is patterned by Wnt and bone morphogenetic proteins (BMPs) that are derived from the cortical hem (Campbell, 2003; Grove and Fukuchi-Shimogori, 2003). BMPs are concentrated in the dorsal-medial portions of the telencephalon and are involved in the formation of dorsal midline derivatives, whereas Wnts are expressed throughout the pallium and are crucial for the generation of the cerebral cortex (Hebert et al., 2002). Studies using chick explant cultures (Gunhaga et al., 2003) further revealed that Wnt3a or Wnt8, but not BMPs, convert the Mash1+ and Nkx2-1+ ventral telencephalic cells (Sussel et al., 1999) into the Nkx2-1-, Pax6+ and Ngn2+ prospective dorsal telencephalic cells. By contrast, the specification of ventral telencephalic progenitors is regulated by sonic hedgehog (SHH), a glycoprotein that is secreted from the node (Gunhaga et al., 2003). Shh-null mice lack the development of Nkx2-1+ cells in the medial ganglionic eminence (MGE), a ventral structure in the telencephalon (Chiang et al., 1996), indicating the SHH-dependent specification of MGE progenitors. The dorsal and ventral progenitors are fated mainly to glutamatergic and gamma-aminobutyric acid (GABA) neurons in the telencephalon, respectively (Olsson et al., 1997; Olsson et al., 1998; Marin and Rubenstein, 2001; Stuhmer et al., 2002), although the mechanism underlying this process remains largely unknown.
Consistent with in vivo neural development, mouse embryonic stem cells (mESCs), under serum-free culture conditions without the presence of morphogens, differentiate into anterior neural precursor cells (Wichterle et al., 2002; Ying et al., 2003; Watanabe et al., 2005; Gaspard et al., 2008). The majority exhibit a ventral phenotype, which is attributed to predominant SHH signaling (Gaspard et al., 2008). The inhibition of SHH signaling promotes the generation of dorsal telencephalic progenitors that ultimately differentiate into neurons of the cerebral cortex (Gaspard et al., 2008). Thus, in vitro differentiation of mESCs recapitulates fundamental aspects of in vivo neural development.
Under a similar serum-free condition without known morphogens, human embryonic stem cells (hESCs) differentiate into a synchronized population of neuroepithelial cells that organize into neural-tube-like rosettes in 2 weeks (Zhang et al., 2001), a time corresponding to the development of the neural plate/tube in a human embryo. The hESC-derived neuroepithelial cells uniformly express anterior transcription factors, including OTX2, SIX3 and LHX2, but not Hox proteins (Li et al., 2005; Pankratz et al., 2007). Even when the hESCs are differentiated through co-culture with stroma cells, a large population of the neural precursors exhibits anterior characteristics (Elkabetz et al., 2008). These anterior precursors, however, can be efficiently patterned into midbrain dopaminergic neurons and spinal motoneurons with the appropriate set of morphogens through the use of secreted morphogens and growth factors (Perrier et al., 2004; Li et al., 2005; Singh et al., 2005; Yan et al., 2005; Roy et al., 2006; Lee et al., 2007). These results suggest that hESCs, like mESCs, differentiate into region-specific progenitors and corresponding functional neurons following the same developmental principles that have been learned from animal studies. It is currently unknown whether the human forebrain neuroepithelia can be efficiently directed to dorsal and ventral forebrain progenitors without inducing caudalization in minimal medium and whether these progenitors mature into functional neuronal subtypes including glutamatergic and GABAergic neurons.
In the present study, we demonstrate that, in the absence of known morphogens, hESC-differentiated neural progenitors exhibited a dorsal telencephalic trait, which is attributed to endogenous Wnt signaling. The inhibition of Wnt proteins or activation of SHH signaling almost completely converts the primitive dorsal telencephalic precursors to ventral progenitors. The specification of dorsal-ventral progenitors by Wnt and/or SHH is partially achieved through differentially regulating the expression of active and repressive forms of GLI3. The regionalized progenitors further differentiate into functional cortical glutamatergic neurons and telencephalic GABAergic neurons, respectively.
MATERIALS AND METHODS
Human ESC culture
H9 and H1 hESCs (passages 15 to 42) were cultured and passaged weekly on a feeder layer of irradiated mouse embryonic fibroblasts (MEFs) as previously described (Thomson et al., 1998). For quality control, the differentiated clones were picked up and removed when passaging the hESCs. The cultures were also routinely screened with VenorGeM (Sigma-Aldrich, St Louis, MO, USA) to ensure that they were free from mycoplasma contamination.
Culture of neuroepithelial cells and telencephalic neurons
The procedure for generating neuroepithelial (NE) cells from hESCs was described previously (Zhang et al., 2001). Briefly, hESC colonies were detached from feeder cells (day 0) and suspended in hESC medium for 4 days. Then, these ESC aggregates were cultured in a neural medium comprising Dulbecco's Modified Eagle Medium (DMEM)/F12, N2 supplement and heparin (2 μg/ml) without growth factors. After adhering to a plastic surface on day 6, primitive NE cells were generated at days 8-10, followed by definitive NE cells in the form of neural-tube-like rosettes at days 14-17 of differentiation (Pankratz et al., 2007).
To differentiate the region-specific progenitors, SHH and dickkopf 1 (DKK1), DKK1-conditioned medium (1×) and/or WNT3A-conditioned medium (1×) were added to the primitive NE cells at day 10 over a total 20-day period (see Results). Definitive NE cells were maintained in suspension cultures as neuroepithelial clusters in the above neural medium. For neuronal differentiation, neural progenitor clusters were dissociated with Accutase (Innovative Cell Technologies) and plated onto ornithine/laminin-coated coverslips in Neurobasal medium (Gibco). Trophic factors, such as brain-derived neurotrophic factor (BDNF), glial-derived neurotrophic factor (GDNF) and insulin-like growth factor 1 (IGF1) (all 10 ng/ml, PeproTech), were supplemented to the medium in the presence of N2 and B27.
Preparation of WNT3A- and DKK1-conditioned media
WNT3A- and DKK1-expresssing cell lines were obtained from Dr Randall Moon's laboratory at University of Washington. Cells were grown in a T75 flask at a density of 2×105 per flask in DMEM containing 10% fetal bovine serum and passaged twice a week. After 2 days, when cells grew to 70% confluence, they were washed with DMEM (to remove serum) and further cultured with the above neural medium comprising F12/DMEM, with N2 supplement for another 2 days. The medium was then collected and centrifuged at 600 g for 10 minutes and the supernatant was stored at 4°C. To concentrate the conditioned medium, the original conditioned medium (1×) was placed in a centrifugal filter device (Millipore) and centrifuged at 1900 g for 1 hour. The concentrated medium was collected in a culture hood and stored at 4°C (~5 ml of medium was obtained from 50 ml of original medium, 10× concentrated).
Immunocytochemistry and quantification
Coverslip cultures were fixed in 4% paraformaldehyde and immunostaining was performed as previously described (Zhang et al., 2001; Li et al., 2005). Antigen-antibody reactions were developed by appropriate fluorescencein-conjugated secondary antibodies. Nuclei were stained with Hoechst. The primary antibodies used in this study included GAD65/67 (1:5000, rabbit IgG, Chemicon), GABA (1:5000, rabbit IgG, Chemicon), DARPP32 (1:500, rabbit IgG, Chemicon), vesicular glutamate transporter 1 (1:1000, rabbit IgG, Synaptic Systems), TBR1 (1:2000, rabbit IgG, Chemicon), NKX2-1 (1:200, mouse IgG, Chemicon), βIII-tubulin (1:5000, rabbit IgG, Covance), CTIP2 (1:2000, rat IgG, Chemicon), mono-βIII-tubulin (1:1000, mouse IgG, Sigma), PAX6 [1:5000, Developmental Studies Hybridoma Bank (DSHB), Iowa City, IA, USA] and HOXB4 (1:50, DSHB). Polyclonal FOXG1 antibody was kindly provided by Dr Y. Sasai (Kobe, Japan). Images were collected using a Spot digital camera mounted onto a Nikon TE600 fluorescence microscope or a confocal microscope (Nikon, Tokyo, Japan).
The population of FOXG1, PAX6, NKX2-1, CTIP2, ISLET1 and TBR1-expressing cells among total differentiated cells (Hoechst-labeled) was counted as described previously (Li et al., 2005). A Nikon fluorescence microscope (Nikon, Melville, NY, USA) was used to capture images. At least five fields of each coverslip were chosen and counted using Metamorph software (Universal Imaging, Downingtown, PA, USA) by an observer blinded to the experimental conditions. Three to four coverslips in each group were counted. Data are expressed as mean±s.d.
RNA Isolation and RT-PCR
Total RNA was extracted from hESCs and neural differentiation cultures using RNA STAT-60 (Tel-Test, Friendswood, TX, USA). cDNA was synthesized using the SuperScript III First-Strand Synthesis System (Invitrogen, Carlsbad, CA, USA) according to the supplier's protocol and was used as a template for the quantitative PCR (qPCR) reaction. qPCR was performed in a 20 μl mixture containing cDNA, primers and 1× SYBR Green PCR Master Mix (Applied Biosystems, Warrington, UK). Standard curves and melting curves were measured for each set of primers to confirm that only one amplicon was generated at the same efficiency as glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a house keeping gene. The expression levels of the mRNA were calculated using the comparative CT method. The primers used are listed in Table S1 in the supplementary material. Primers for GAPDH recognize both the human and mouse genes.
The statistical significance in the comparison of multiple sample sets versus the control group (Fig. 2E) was analyzed with Dunnett's test. The statistical significance in mean values among multiple sample groups was examined with Tukey's studentized range test after a one-way ANOVA test (Fig. 4). The significance level was defined as P<0.05 and all significance tests were conducted using SAS 9.1 (SAS Institute, Cary, NC, USA).
Fig. 2.
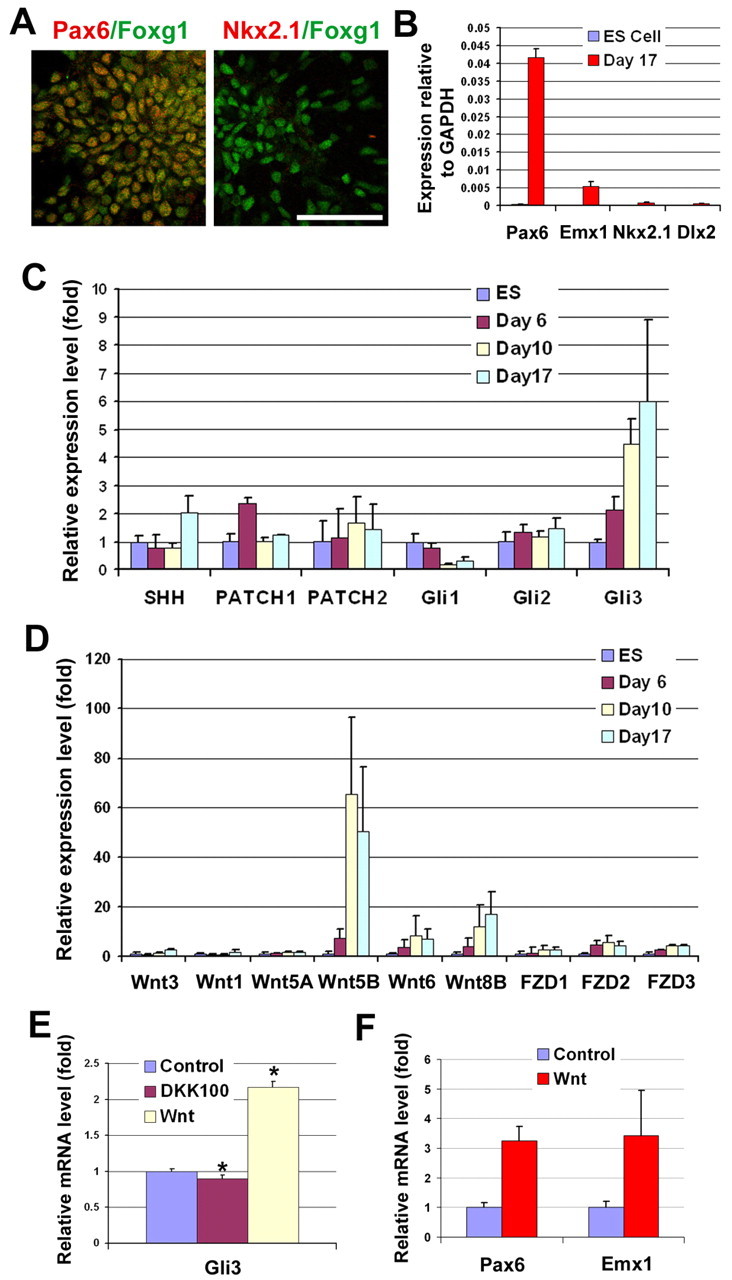
Neuroepithelial cells display a dorsal identity. (A) At 1 month after differentiation in the absence of exogenous morphogens, the majority of cells were positive for both FOXG1 and PAX6, whereas almost no cells were NKX2-1+. (B) qPCR showed the high expression level of PAX6 and EMX1 mRNA, but low level of NKX2-1 and DLX2 mRNA by NE cells at day 17. (C) Microarray analysis indicated little change in SHH signaling components except a significant increase in GLI3. (D) There is an increase in Wnt proteins and frizzled (FZD) proteins after day 6 of differentiation. (E) WNT3A-conditioned medium upregulated, whereas DKK1 (100 ng/ml, from day 10 to day 17) decreased, GLI3 expression at the mRNA level as revealed by qPCR. (F) Addition of WNT3A-conditioned medium from day 10 to day 17 resulted in an increase in the expression of PAX6 and EMX1 mRNA. Data are presented as mean±s.d. *, P<0.05 versus the control group, n=4. Scale bar: 50 μm.
Fig. 4.
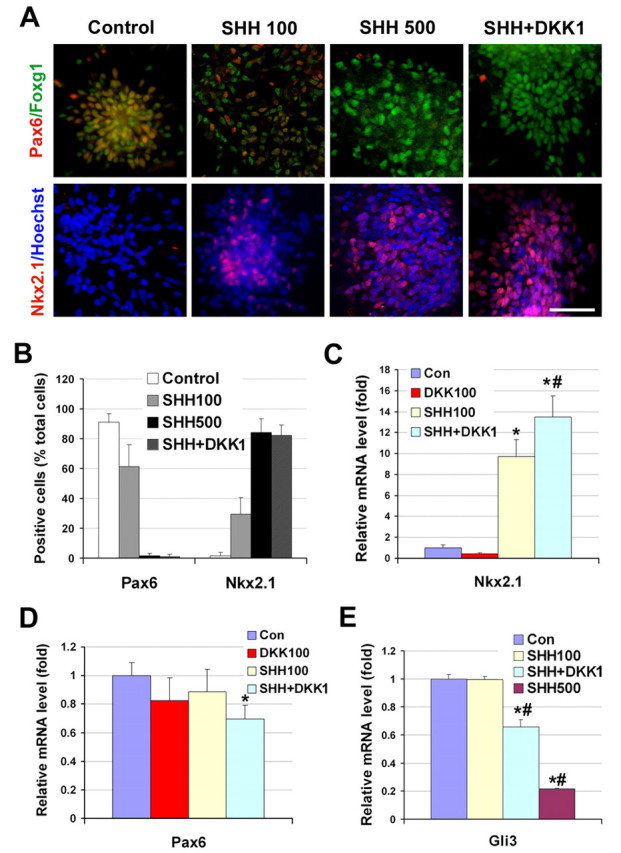
Specification of dorsal and ventral telencephalic progenitors. (A,B) Primitive neuroepithelial (NE) cells (day 10) were treated with SHH and/or DKK1 and the populations of NKX2-1+ and PAX6+ cells were compared at day 28. A high concentration of SHH, or SHH and DKK1 together, converted PAX6-expressing cells to NKX2-1+ cells. (C-E) The expression of NKX2-1 (C), PAX6 (D) and GLI3 (E) was measured by qRT-PCR 7 days after the primitive NE cells were cultured in basic medium (control) or in the presence of SHH (100 or 500 ng/ml), DKK1 (100 ng/ml), or DKK1 (100 ng/ml) plus SHH (100 ng/ml). Data are presented as mean±s.d. *, P<0.05 versus the control group, #, P<0.05 versus the SHH-treated (100 ng/ml) group, n=4. Blue, Hoechst-stained nuclei. Scale bar: 50 μm.
Microarray
RNA samples were collected from H9 and H1 hESCs at different time-points (day 6, day 10 and day 17) after differentiation. All samples were examined using the Human Genome U133 Plus 2.0 Array. The data were deposited at the NIH Neuroscience Microarray Consortium (http://arrayconsortium.tgen.org/np2/home.do) and in the ArrayExpress database (accession number E-MEXP-2426).
Western blot
Cell pellets were resuspended in lysis buffer (1% NP40, 50 mM Tris-HCl pH 8.0, 0.5% sodium deoxycholate, 150 mm NaCl, 5 mM EDTA, 10 mM PMSF and 10 mM NaF with protease inhibitor cocktail) followed by passage through a 28.5 gauge needle and lysed overnight. The particulate fraction was removed by centrifugation. Proteins (10-30 μg) were resolved by 10-12% SDS-PAGE and subjected to western blot analysis with β-actin (1:1000, mouse IgG, Sigma), GLI3 H280 (1:200, rabbit IgG, Santa Cruz), TBR1, DARPP32 and GAD65/67 antibodies.
Lentivirus production and transduction of hESCs
Knockdown of GLI3 was achieved using lentivirus that contains the pLVTHM plasmid backbones (see Fig. S1 in the supplementary material). To produce high-titer lentivirus, 10 μg of lentiviral transfer vector, 7.5 μg of lentiviral vector psPAX2, and 5 μg of pMD2.G (VSV-G envelope protein) were cotransfected into HEK293FT cells (Invitrogen) using the calcium phosphate method. Sixty hours after transfection, the cell culture medium containing viral particles was collected and filtered through a 0.45 μm filter (Millipore). The viral particles were further concentrated by ultracentrifugation (SW28 rotor, Beckman) at 50,000 g for 2 hours. The pellet was resuspended in hESC medium. For transduction, hESCs were passaged normally and pelleted by brief centrifugation. Cell pellets were then incubated with 100 μl of concentrated virus (106 transducing units/ml) at 37°C for 30 minutes. The virus and cell mixture was then transferred to a MEF feeder layer overnight and the medium changed the next day. These hESCs, after expansion, were differentiated into neuroepithelial cells and telencephalic progenitors using the same culture system as described above.
Electrophysiology
Coverslips were placed in a bath solution containing 127 mM NaCl, 1.2 mM KH2PO4, 1.9 mM KCl, 26 mM NaHCO3, 2.2 mM CaCl2, 1.4 mM MgSO4, 10 mM glucose and 290 mM mOsm, and were continuously bubbled with 95% O2/5% CO2. Recording pipettes with resistances of 4-7 MΩ were filled with an intracellular recording solution containing 20 mM KCl, 121 mM K+-gluconate, 10 mM Na+-HEPES, 10 mM BAPTA, 4 mM Mg2+-ATP pH 7.2 and 290 mM mOsm. The drug application was achieved using a drug barrel mounted on a Sutter MM-33 micromanipulator. Drugs were diluted into extracellular solution and continuously bubbled with 95% O2/5% CO2 during the experiment. Neurons were visualized using an Olympus BX51WI microscope with differential interference contrast (DIC) optics at 40×. The voltage clamp and current clamp recordings were obtained using a MultiClamp 700B amplifier (Axon Instruments, Molecular Devices, Sunnyvale, CA, USA). Signals were filtered at 4 kHz and sampled at 100 kHz using a Digidata 1322A analog-digital converter (Axon Instruments). Data were analyzed with pClamp 9.0 (Axon Instruments). Capacitance and series resistance were compensated (50-80%). All recordings were performed at 21-23°C. All chemicals were obtained from Sigma.
RESULTS
Human ESCs differentiate to telencephalic progenitors in the absence of exogenous morphogens
Human ESCs adopt a primitive neuroepithelial (NE) fate after 8-10 days of differentiation in a chemically defined culture environment and the neural fate is consolidated by expression of SOX1 after an additional week (Fig. 1A) (Li et al., 2005; Pankratz et al., 2007). In the absence of exogenous growth factors, hESC-differentiated NE cells at day 10 and day 17 uniformly exhibited a rostral phenotype by expressing anterior transcription factors including PAX6, LHX2 and OTX2 (see Fig. S2 in the supplementary material) (Pankratz et al., 2007). FOXG1, a telencephalic transcription factor, appeared in NE cells at day 17 and its expression was maintained at day 24 and even in postmitotic neurons 1 month after differentiation (Fig. 1B). Its mRNA was detected as early as day 10 (Fig. 1C). The predominant and persistent expression of anterior genes was accompanied by the low and unchanged expression of EN1 and HOXB4, transcription factors expressed by the mid/hind-brain and spinal cord cells, as assayed by qPCR (Fig. 1C). These results indicate that hESCs differentiate to forebrain progenitors in the absence of morphogens.
Fig. 1.
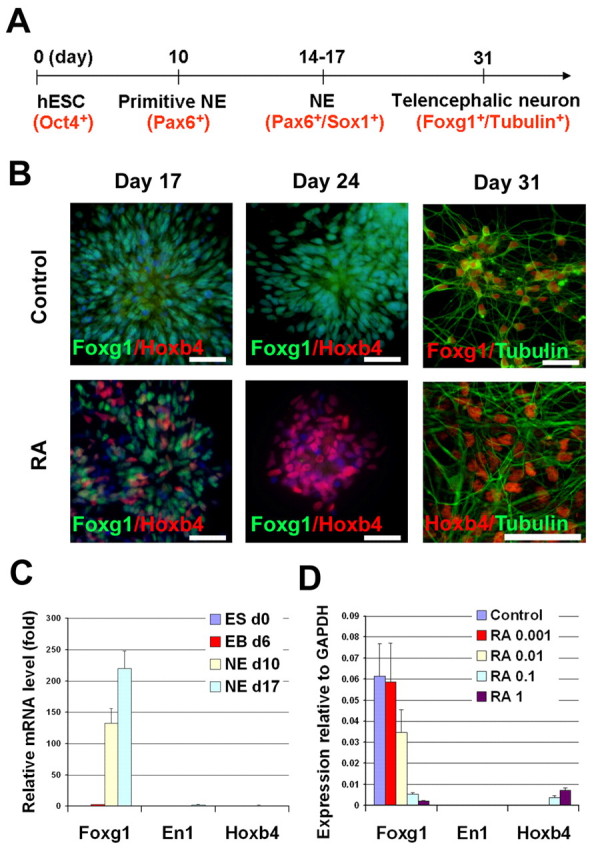
Generation of telencephalic neurons from hESCs. (A) A schematic of the procedure for generating telencephalic progenitors from hESCs. (B) In the control group, the expression of FOXG1 was maintained at 1 month after differentiation, whereas retinoic acid treatment (RA; 0.1 μM) resulted in the loss of FOXG1 expression and the generation of HOXB4+ cells. (C) The change in expression of anteroposterior homeodomain genes during neural differentiation using qPCR. ES, embryonic stem; EB, embryoid body; NE, neuroepithelium. (D) RA, added to the primitive neuroepithelial (NE) cells (at day 10) for 1 week, induced a dose-dependent repression of FOXG1 and expression of HOXB4 mRNA. Data are presented as mean±s.d., n=3. Blue, Hoechst-stained nuclei. Scale bars: 50 μm.
We and others have shown that retinoid acid (RA) can induce a caudal phenotype when applied at an early stage (Irioka et al., 2005; Li et al., 2005). Indeed, the addition of RA from day 10 induced the expression of HOXB4 and a concomitant lack of FOXG1+ cells at both the protein (Fig. 1B) and the mRNA level in a dose-dependent manner (Fig. 1D). However, RA treatment after day 17 had little effect on the induction of caudal genes and the inhibition of FOXG1 (Li et al., 2005). Thus, although the early primitive anterior NE cells can be readily patterned to regional progenitors (Pankratz et al., 2007), the FOXG1-expressing cells, after 2-3 weeks of hESC differentiation without exogenous morphogens, are likely to be committed to the telencephalic fate.
The dorsal phenotype of the telencephalic progenitors depends on Wnt proteins
Early NE cells in the chick exhibit a ventral phenotype by expressing Nkx2-1 (Gunhaga et al., 2003), as do the NE cells differentiated from mESCs in the absence of morphogens (Gaspard et al., 2008). We sought to determine the dorsal-ventral phenotype of the human telencephalic precursor. The expression of PAX6, a transcription factor of the dorsal telencephalic progenitor (Campbell, 2003), was present in nearly all of the FOXG1+ telencephalic progenitors (95% of all cells), at 1 month after differentiation in the absence of growth factors (Fig. 2A). After 1 month of differentiation, cells become either committed progenitors or postmitotic neurons (Fig. 1A) (Pankratz et al., 2007). This suggests that the telencephalic precursors exhibit a dorsal phenotype. This was further supported by the complete lack of expression of NKX2-1 (Fig. 2A), a transcription factor expressed by ventral progenitors (Campbell, 2003). Also, the relative amount of PAX6 mRNA as compared with NKX2-1 mRNA, quantified by RT-qPCR, substantiated the immunocytochemical observations (Fig. 2B). The expression of EMX1 (a dorsal telencephalic marker) and DLX2 (expressed by ventral telencephalic cells) mRNA showed the same trend, confirming the dorsal identity of these precursors (Fig. 2B). By contrast, NE cells differentiated from mESCs using the same culture medium showed predominant expression of NKX2-1 and little PAX6 (see Fig. S3 in the supplementary material), consistent with a recent report using a similar serum-free medium (Gaspard et al., 2008). Thus, the majority of human telencephalic precursors carry a dorsal phenotype in the absence of morphogens.
To identify the molecular pathways that underlie the default dorsal phenotype of our hESC-derived NE cells, we performed microarray analyses and compared gene expression profiles between cells at the ESC stage (day 0), ESC aggregate stage (day 6), primitive NE stage (day10) and definitive NE stage (day 17). During differentiation (day 0 to day 17), SHH expression remained at a low level (Fig. 2C). The SHH receptors patched 1 and 2, and their downstream effectors GLI1 and GLI2, were present at a very low level and showed little change during differentiation (Fig. 2C). By contrast, several Wnt ligands, including WNT6, WNT5B and WNT8B, and Wnt receptors, including frizzled (FZD) homologs 1, 2 and 3, were expressed at a high level and increased immediately after the start of neural differentiation (day 6 to day 17; Fig. 2D). The expression of WNT3A, as analyzed by qPCR, showed a 2- to 3-fold increase in NE cells (day 10 and day 17) as compared with hESCs (see Fig. S4 in the supplementary material). This is similar to the expression of WNT3 or WNT1, which showed a mild increase by microarray analysis (Fig. 2D). These results suggest that a high level of endogenous Wnt signaling and/or a low level of SHH signaling are responsible for the default dorsal phenotype of the telencephalic precursors.
Regulation of GLI3 by Wnt proteins contributes to the dorsal telencephalic phenotype
Our microarray data showed that GLI3 was expressed at a high level in dorsal NE cells (Fig. 2C), closely correlating with high Wnt signaling (Fig. 2D). As GLI3 can be regulated by Wnt proteins (Alvarez-Medina et al., 2008; Yu et al., 2008), we hypothesized that Wnt proteins contribute to the dorsal telencephalic phenotype by regulating GLI3. Indeed, the addition of WNT3A-conditioned medium from HEK293FT cells for 1 week, from day 10 after NE differentiation, significantly increased the level of GLI3 (Fig. 2E). This resulted in the upregulation of PAX6 and EMX1 mRNA (Fig. 2F). By contrast, treatment of the cultures with the Wnt antagonist DKK1 (100 ng/ml, blocking the canonical Wnt pathway) for the same period exerted a small but significant decrease in GLI3 (Fig. 2E).
Different forms of the GLI3 protein can mediate opposite effects of hedgehog signaling; the full-length protein acts as an activator (GLI3-190, 190 kDa), whereas the proteolyzed truncated peptide acts as a repressor (GLI3-R) (Ahn and Joyner, 2004; Bai et al., 2004; Litingtung et al., 2002; Tyurina et al., 2005; Bok et al., 2007; Wang et al., 2000; Wang et al., 2007). We examined which forms of GLI3 were presented by the NE cells. Western blotting analysis indicated that under both control and Wnt-treated conditions, the truncated GLI3 was the dominant form and the full-length GLI3 was almost absent in the day 17 neuroepithelia (Fig. 3A). This suggests that Wnt proteins promote the formation of the repressive form of GLI3 to antagonize hedgehog signaling, thus contributing to the default dorsal telencephalic fate of NE cells.
Fig. 3.
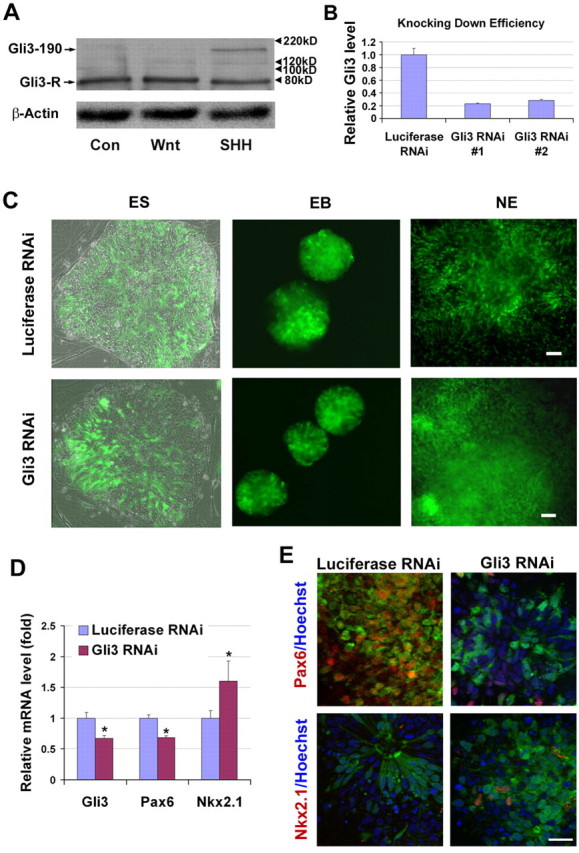
Role of GLI3 in dorsal-ventral pattering. (A) Western blotting showed the expression of GLI3 proteins by NE cells from control, WNT3A-treated and SHH (500 ng/ml)-treated groups. GLI3-190, full-length active form; GLI3-R, truncated repressive form. (B) HEK293 cells were transiently transfected with vectors containing luciferase shRNA or GLI3 shRNA. qRT-PCR analysis showed a significant decrease in GLI3 mRNA expression in the GLI3 RNAi, but not the luciferase RNAi, group. Data are presented as mean±s.d., n=3. Two sets of shRNAs possess similar knockdown efficiency. (C) Human ESCs infected with lentiviruses for luciferase RNAi and GLI3 RNAi showed a similar population of RNAi-expressing cells, as indicated by GFP expression in the differentiated NE cells. Note that not all cells were GFP+ as the transduction efficiency was not 100%. (D) qRT-PCR showing the expression of GLI3, PAX6, and NKX2-1 by NE cells at day 17. Data are presented as mean±s.d., n=3. *, P<0.05 between GLI3 RNAi and luciferase RNAi groups by two-sided t-test. (E) The expression of PAX6 and NKX2-1 proteins in cells from both luciferase RNAi and GLI3 RNAi groups 1 month after differentiation. Blue, Hoechst-stained nuclei. Scale bars: 50 μm in C; 20 μm in E.
To verify the involvement of GLI3 in the specification of the dorsal telencephalic fate, we knocked down GLI3 expression by infecting the hESCs with lentivirus containing shRNA that targeted GLI3 or luciferase (as a control) (see Fig. S1 in the supplementary material). Human ESCs were infected with lentiviruses containing GLI3 shRNA (shRNA 1), which showed a clear knockdown effect in HEK293 cells (Fig. 3B), or luciferase shRNA, and then differentiated into NE cells and forebrain progenitors using the same paradigm (Fig. 3C). Neural differentiation and transduction efficiency, as shown by columnar NE morphology and GFP expression, were similar between the luciferase and GLI3-knockdown groups (Fig. 3C). At day 17, NE cells differentiated from the GLI3 shRNA-infected group (GLI3 RNAi) showed a lower GLI3 mRNA expression as compared with those from the luciferase RNAi group. Moreover, the GLI3 RNAi group had reduced expression of PAX6 mRNA and increased expression of NKX2-1 mRNA (Fig. 3D). At the cellular level, the GLI3 shRNA-infected cells, indicated by GFP, were negative or only weakly stained for PAX6 (Fig. 3E). In addition, some GLI3 knockdown cells, although not all, were positive for NKX2-1 (Fig. 3E). Together, these data suggest that at least one mechanism underlying the default dorsal phenotype of telencephalic precursors is the upregulation of the hedgehog repressor GLI3 by Wnt proteins.
The activation of SHH and/or inhibition of Wnt permits the specification of ventral telencephalic progenitors
In differentiating mESCs, blocking SHH signaling converts the default ventral telencephalic precursors to dorsal progenitors (Gaspard et al., 2008). As the default dorsal human neural precursors are attributed to the inhibition of SHH signaling by Wnt proteins, we asked whether SHH could override the inhibitory effect of Wnt proteins to convert the dorsal precursors to ventral progenitors. NE cells were treated with SHH (0, 100 and 500 ng/ml) from day 10 and the expression of NKX2-1 and PAX6 was examined at 1 month after differentiation from hESCs. At the lower dosage (100 ng/ml), SHH reduced the PAX6-expressing cell population to 61% and increased the NKX2-1+ cell population to 30% (Fig. 4A,B). At the higher dosage (500 ng/ml), SHH almost completely eliminated the PAX6-expressing cells, while increasing the NKX2-1+ ventral progenitors to 84%. These cells retained the expression of FOXG1, indicating their forebrain identity. These results indicate that SHH can override the inhibitory effect of Wnt proteins and induce the primitive dorsal precursors to ventral progenitors.
We next asked whether inhibition of Wnt signaling is sufficient to specify ventral telencephalic progenitors. Primitive NE cells were treated with DKK1 (100 ng/ml) for 1 week from day 10 and the expression of NKX2-1 and PAX6 was examined at day 17. DKK1 alone had little effect on the induction of NKX2-1 or the repression of PAX6 (Fig. 4C,D). Treatment with a higher concentration of DKK1 (500 ng/ml) similarly did not increase the NKX2-1 cell population. We then asked whether Wnt inhibition has a synergistic effect with SHH in ventralization. The combination of DKK1 (100 ng/ml) and SHH (100 ng/ml) increased NKX2-1 mRNA and decreased PAX6 mRNA significantly as compared with SHH alone or the control group (Fig. 4C,D). A similar effect was observed when SHH was combined with DKK1-conditioned medium (data not shown). Further culturing of the cells that were treated with SHH and DKK1 resulted in a large proportion of NKX2-1+ cells and a loss of PAX6-expressing cells at 1 month following hESC differentiation, comparable to the cells that were treated with a high concentration of SHH (Fig. 4A,B). In these ventralized cells, nearly all the NKX2-1+ cells were also positive for FOXG1, confirming their telencephalic identity (see Fig. S5 in the supplementary material). These results suggest that the repression of Wnt proteins collaborates with SHH in efficiently inducing the ventral fate of the telencephalic precursors.
As endogenous Wnt proteins determine the dorsal identity of the precursors by inducing GLI3, we examined whether ventralization of the precursors by SHH or SHH and DKK1 is achieved by regulating GLI3. GLI3 mRNA was not altered by a 1-week treatment with SHH (100 ng/ml; Fig. 4E). DKK1 alone showed a mild, but significant, decrease in GLI3 expression (Fig. 2E). However, the combination of DKK1 and SHH significantly decreased GLI3 expression as compared with both the SHH and control groups. This suggests that DKK1 facilitates SHH activity by repressing the expression of GLI3, which mainly exhibits a truncated repressive form in the absence of morphogen treatment. Nevertheless, questions remain as to how SHH alone (at high concentrations) can override the Wnt inhibition in specifying the ventral fate. Interestingly, high concentrations of SHH significantly inhibited GLI3 expression compared with low concentrations of SHH (Fig. 4E). Western blotting revealed that high SHH treatment decreased the truncated (repressive) GLI3 and induced the full-length (active) GLI3 (Fig. 3A). This suggests that one possible mechanism by which SHH induces the ventral fate is to regulate GLI3 expression, especially the ratio of repressive to active forms of GLI3. Thus, GLI3 is likely to be the convergent point for Wnt and SHH signaling in regulating the dorsal versus ventral fate of telencephalic progenitors.
Dorsal progenitors differentiate into glutamatergic neurons
Glutamatergic neurons in the forebrain develop from dorsal progenitors and their transmitter phenotype is tightly linked to the expression of the pan-neuronal factor NGN2 and, more specifically, TBR1 and CTIP2 (Parras et al., 2002; Hevner et al., 2006). Neurons that differentiated from the dorsal progenitors in the absence of exogenous morphogens for 6 weeks exhibited a pyramidal morphology with extensive neurite outgrowth (Fig. 5A). A large population (79%) of βIII-tubulin+ neurons expressed TBR1 (Fig. 5A,B), the glutamatergic transcription factor (Hevner et al., 2006). TBR1 expression was maintained after the neurons became more mature by expressing MAP2 (Fig. 5C). By contrast, few TBR1-expressing neurons were observed in cultures that were induced to the ventral fate by SHH and DKK1 or a high concentration of SHH alone (Fig. 4A,B). This expression pattern was also observed by western blot analysis (Fig. 5I). We thus conclude that forebrain glutamatergic neurons are differentiated from the dorsal telencephalic progenitors.
Fig. 5.
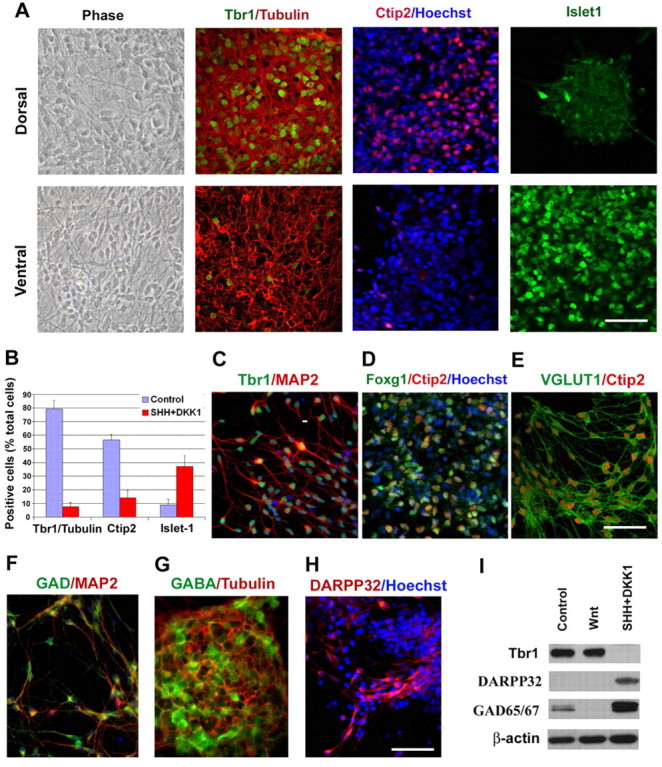
Differentiation of glutamatergic and GABAergic neurons from dorsal and ventral progenitors. (A,B) Comparison of the expression of TBR1, CTIP2 and ISLET1 between the neurons that were developed from dorsal (control group) and ventral (DKK1 plus SHH group) progenitors. (C-E) Dorsal progenitors differentiated into cells that were positive for TBR1 (C), CTIP2 (D) and VGLUT1 (E). (F-H) Ventral progenitors differentiated into neurons that expressed GAD (F), GABA (G) and DARPP32 (H). (I) Western blot analysis showing differential expression of TBR1, DARPP32 and GAD65/67 between cultures patterned for dorsal and ventral identities. Blue, Hoechst-stained nuclei. Scale bars: 50 μm.
Glutamatergic neurons in the telencephalon include different sub-populations of cortical projection neurons. CTIP2 is a transcription factor that is expressed by subcerebral projection neurons (Arlotta et al., 2005). Over half (57%) of the neurons that were differentiated from dorsal progenitors expressed CTIP2, whereas only 14% of the cells generated from ventral progenitors were labeled with CTIP2 (Fig. 5A,B). These CTIP2 cells retained expression of FOXG1 after 8 weeks of differentiation, confirming their forebrain identity (Fig. 5D). Almost all of the CTIP2+ cells then became positive for vesicular glutamate transporter 1 (VGLUT1), a marker expressed by mature glutamatergic neurons (Fig. 5E). These results indicate that cortical projection glutamatergic neurons can be efficiently generated following patterning of the neuroepithelia to the dorsal telencephalic fate.
Ventralized telencephalic progenitors generate GABAergic neurons
GABAergic neurons in the telencephalon, including those in the cerebral cortex, originate from the ventral progenitors (Marin and Rubenstein, 2001). Progenitors in the ventral telencephalon differentiate into GABAergic projection neurons, such as medium spiny projection GABAergic neurons, as well as some GABAergic interneurons. In the present study, continued culture of the ventralized telencephalic progenitors for 6 weeks resulted in ~40% cells that were positive for ISLET1, a marker for ventral progenitors and postmitotic neurons (Fig. 5A,B). By contrast, cultures from dorsal progenitors only generated 9% ISLET1+ cells. Continued differentiation of the ventral progenitors for an additional month resulted in maturation of neurons, as evidenced by expression of βIII-tubulin and MAP2, some of which became positive for glutamate decarboxylase 65/67 (GAD 65/67; Fig. 5F), an enzyme involved in the synthesis of GABA, and GABA (Fig. 5G). Some cells became positive for dopamine- and cAMP-regulated phosphoprotein-32 (DARPP32; Fig. 5H), a marker for projection GABAergic neurons (Anderson and Reiner, 1991). Western blot analysis confirmed the expression of GAD65/67 and DARPP32 in the ventralized cell population (Fig. 5I). Together, these results indicate that ventralized neural progenitor cultures generate forebrain GABAergic neurons, including the projection striatal GABA neurons.
Human ESC-derived forebrain glutamatergic and GABAergic neurons are electrophysiologically active
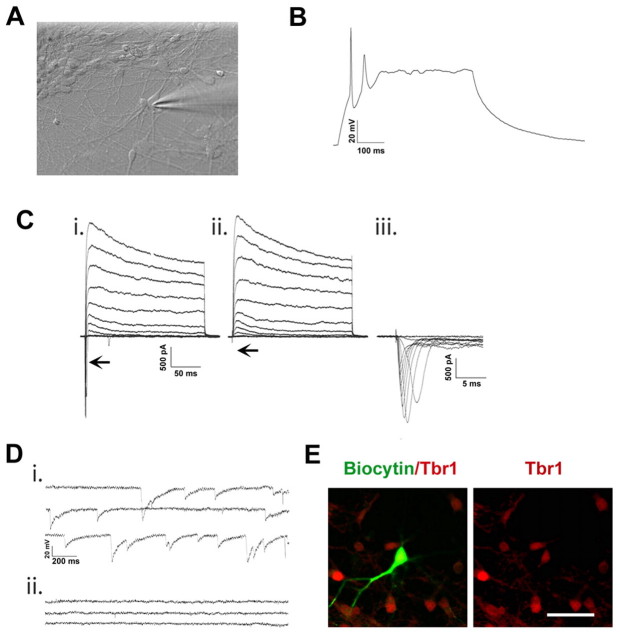
The functional characteristics of the hESC-generated neurons were analyzed by patch clamp recordings 10 to 12 weeks after differentiation, a time-point when hESC-derived neurons become electrophysiologically active (Johnson et al., 2007). We have shown previously that hESC-differentiated neurons in the absence of morphogens, and primarily the glutamatergic neurons as suggested by the present study, can fire mature action potentials and receive excitatory synaptic inputs (Johnson et al., 2007). Here we examined both the dorsally and ventrally patterned neuronal populations (Fig. 6A). In both populations, cells were able to fire action potentials with a large amplitude and a short duration upon current injection and occasionally a second action potential was observed at the tenth week of differentiation (Fig. 6B). In voltage clamp, both inward and outward currents were recorded (Fig. 6Ci). Inward currents could be blocked by 1 μM tetrodotoxin (Fig. 6Cii), indicative of the sodium currents that are responsible for the depolarization phase of the action potential (Fig. 6Ciii). Both a transient and sustained outward current were recorded from the majority of cells tested, similar to potassium currents previously reported from hESC-derived neurons (Fig. 6Ci,ii) (Johnson et al., 2007). In both cell cultures, spontaneous synaptic activity was recorded in a subset of cells, confirming that these cells formed functional synapses within a neuronal network. In the ventrally patterned neuronal population, the synaptic activity was blocked by bicuculin, indicating GABAergic inputs (Fig. 6D). The identity of the recorded cells was determined by the inclusion of biocytin in the pipette solution and subsequent staining after patch clamp recordings, as exemplified by the expression of TBR1, confirming the glutamatergic identity of the patched neurons in the dorsally patterned group (Fig. 6E).
Fig. 6.
Human ESC-derived neurons functionally mature in vitro. (A) A phase-contrast image of a neuronal culture during patch clamp recordings. (B) Action potentials are elicited upon a 20 pA current injection in neurons differentiated for 12 weeks in vitro. (C) Inward and outward currents are elicited upon voltage steps (−50 mV to 50 mV) in a 12-week-old neuron (i). Application of 1 μM tetrodotoxin (ii) completely eliminated inward currents (i,ii, arrows) that are characteristic of Na+ currents (iii). (D) In the ventrally patterned neuronal population, spontaneous postsynaptic currents were recorded from 10-week-old neurons (i) and application of 50 μM bicucculine completely inhibited postsynaptic currents (ii). (E) Immunostaining showed the expression of TBR1 by a biocytin-labeled neuron after patch clamping. Scale bar: 50 μm.
DISCUSSION
Our results indicate that the hESC-differentiated neural progenitors exhibit a predominantly cortical (dorsal) identity, contrary to the ventral phenotype of mESC-differentiated neural progenitors under similar conditions. Nevertheless, the molecular signals that pattern the dorsal versus ventral telencephalic fate are very similar to those of mouse neural progenitors, namely Wnt proteins for the dorsal fate and SHH for the ventral identity. Furthermore, we found that Wnt proteins and SHH control the dorsal versus ventral fate of the telencephalic progenitors by modulating GLI3 at both mRNA and protein levels. With these findings, we show that cortical glutamatergic neurons and ventral GABAergic neurons can be efficiently differentiated from hESCs by coordinating Wnt and SHH signaling.
Neuroepithelial cells first acquire a rostral phenotype during early development (Stern, 2001). Consistent with this, NE cells that are differentiated from mouse and human ESCs first exhibit an anterior phenotype by expressing anterior transcription factors (Watanabe et al., 2005; Li et al., 2005; Pankratz et al., 2007; Watanabe et al., 2007; Elkabetz et al., 2008; Gaspard et al., 2008). With culturing, a large proportion of hESC-derived neural progenitors become caudalized, and by applying LEFTYA, DKK1 and BMPR1A-FC to block Wnt, Nodal and BMP, 30% of the progenitors can be maintained as telencephalic (Watanabe et al., 2007). Neural progenitors differentiated from hESCs using a stroma cell co-culture method exhibit a similar proportion of telencephalic progenitors (Elkabetz et al., 2008). We have previously shown that hESC-differentiated neural progenitors in a serum-free culture condition uniformly exhibit an anterior phenotype (Li et al., 2005; Pankratz et al., 2008). In the present study, we further demonstrated that the majority (80%) of these anterior progenitors become cortical neurons in the absence of morphogens without being caudalized. This indicates the intrinsic tendency of hESCs to generate cortical neural cells.
Cultures of chick explants (Gunhaga et al., 2003) and neural progenitors from mESCs (Gaspard et al., 2008) indicate that the vast majority of neural progenitors carry a ventral identity and this default ventral fate is due to intrinsic SHH signaling. Interestingly, and somewhat surprisingly, hESC-derived neural progenitors, differentiated under similar serum-free culture conditions, exhibit a predominantly dorsal phenotype. Dorsal-ventral patterning of the telencephalic progenitors is regulated by Wnt proteins and SHH signaling. Our previous (Li et al., 2005; Pankratz et al., 2007) and present studies show that there is little expression of SHH in the cultures of neuroepithelia in the first 3 weeks of culture, whereas Wnt proteins and their downstream molecules are highly expressed right after the generation of neuroepithelia. Thus, the predominantly dorsal identity of the hESC-derived neural progenitors is probably due to the endogenous Wnt signaling and/or lack of SHH signaling. Indeed, application of SHH and/or inhibition of Wnt proteins can efficiently pattern the dorsal NE cells to acquire a ventral identity (also see below). Sasai and colleagues reported the generation of dorsal telencephalic progenitors from mESCs by using a three-dimensional aggregation culture system, although how it was achieved was not examined (Eiraku et al., 2008). We have now shown that at least one mechanism is the repression of SHH signaling through upregulation of the repressive GLI3 by Wnt proteins. This suggests that human neural progenitors in culture employ a similar molecular mechanism to that used in other vertebrates to regulate the dorsal-ventral fate, despite the opposite default dorsal-ventral phenotype.
The predominant Wnt signaling underlying the default dorsal identity in human NE cells predicts that cortical neurons will be generated without the need for exogenous morphogens, whereas the differentiation of ventral telencephalic neurons requires activation of SHH and/or inhibition of Wnt proteins, a strategy that is opposite of that for mESCs (Gaspard et al., 2008). This hypothesis is confirmed by the results shown in the present study in which cortical glutamatergic neurons are efficiently generated in the absence of morphogens, whereas ventral telencephalic GABA neurons, including DARPP32+ projection striatal GABA neurons, are induced with a high concentration of SHH or a low dose of SHH together with Wnt inhibitors. Moreover, we revealed that graded ventralization can be achieved by regulating these two signaling pathways. Dosing of a low concentration of SHH alone resulted in the differentiation of both lateral (ISLET1+, panDLX+) and medial (NKX2-1+) ganglionic eminence progenitors, whereas additional Wnt inhibition (by DKK1) further ventralized the human neural progenitors, resulting in a predominant population of NKX2-1+ MGE progenitors. Furthermore, we revealed that specification of ventral progenitors by Wnt inhibitors functions to release the inhibition of SHH signaling via downregulation of the repressive GLI3. A high dose of SHH also significantly inhibited the repressive GLI3 and induced a weak GLI3 activator, thus more efficiently ventralizing the telencephalic progenitors. This coincides with a very recent study that revealed that Gli transcription factors, including GLI3, are involved in the patterning of the ventral telencephalon by SHH in mice (Yu et al., 2009). Therefore, the coordination of Wnt proteins and SHH via GLI3 determines the dorsal versus ventral fate of the telencephalic progenitors.
Progenitors in the dorsal and ventral telencephalon develop into glutamatergic and GABAergic neurons during development (Marin and Rubenstein, 2001). Our present work demonstrates the generation of functional GABAergic and glutamatergic neurons from the ventral and dorsal progenitors, respectively. Not only does the development of these neurons from hESCs in vitro follow the in vivo principle, but these functional GABAergic or glutamatergic neurons also maintain the expression of the telencephalic marker FOXG1 in long-term culture. These in vitro-generated functional telencephalic neurons provide both a unique paradigm to study human forebrain development and a potential source for application in degenerative diseases involving telencephalic neurons, such as Huntington's disease (Lee and Kim, 2006; Cowan and Raymond, 2006).
Supplementary Material
Acknowledgements
We thank Y. Sasai for generously providing the FOXG1 antibody. This study was supported by the National Institutes of Health (R01 NS045926 to S.-C.Z. and R21 NS055261 to X.-J.L.), the ALS Association and partly by a core grant to the Waisman Center from the National Institute of Child Health and Human Development (P30 HD03352). Deposited in PMC for release after 12 months.
Footnotes
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/136/23/4055/DC1
References
- Ahn S., Joyner A. L. (2004). Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell 118, 505-516 [DOI] [PubMed] [Google Scholar]
- Alvarez-Medina R., Cayuso J., Okubo T., Takada S., Marti E. (2008). Wnt canonical pathway restricts graded Shh/Gli patterning activity through the regulation of Gli3 expression. Development 135, 237-247 [DOI] [PubMed] [Google Scholar]
- Anderson K. D., Reiner A. (1991). Immunohistochemical localization of DARPP-32 in striatal projection neurons and striatal interneurons: implications for the localization of D1-like dopamine receptors on different types of striatal neurons. Brain Res. 568, 235-243 [DOI] [PubMed] [Google Scholar]
- Arlotta P., Molyneaux B. J., Chen J., Inoue J., Kominami R., Macklis J. D. (2005). Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron 45, 207-221 [DOI] [PubMed] [Google Scholar]
- Bai C. B., Stephen D., Joyner A. L. (2004). All mouse ventral spinal cord patterning by hedgehog is Gli dependent and involves an activator function of Gli3. Dev. Cell 6, 103-115 [DOI] [PubMed] [Google Scholar]
- Bok J., Dolson D. K., Hill P., Ruther U., Epstein D. J., Wu D. K. (2007). Opposing gradients of Gli repressor and activators mediate Shh signaling along the dorsoventral axis of the inner ear. Development 134, 1713-1722 [DOI] [PubMed] [Google Scholar]
- Campbell K. (2003). Dorsal-ventral patterning in the mammalian telencephalon. Curr. Opin. Neurobiol. 13, 50-56 [DOI] [PubMed] [Google Scholar]
- Chiang C., Litingtung Y., Lee E., Young K. E., Corden J. L., Westphal H., Beachy P. A. (1996). Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 383, 407-413 [DOI] [PubMed] [Google Scholar]
- Cowan C. M., Raymond L. A. (2006). Selective neuronal degeneration in Huntington's disease. Curr. Top. Dev. Biol. 75, 25-71 [DOI] [PubMed] [Google Scholar]
- Eiraku M., Watanabe K., Matsuo-Takasaki M., Kawada M., Yonemura S., Matsumura M., Wataya T., Nishiyama A., Muguruma K., Sasai Y. (2008). Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3, 519-532 [DOI] [PubMed] [Google Scholar]
- Elkabetz Y., Panagiotakos G., Al Shamy G., Socci N. D., Tabar V., Studer L. (2008). Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage. Genes Dev. 22, 152-165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspard N., Bouschet T., Hourez R., Dimidschstein J., Naeije G., van den Ameele J., Espuny-Camacho I., Herpoel A., Passante L., Schiffmann S. N., et al. (2008). An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature 455, 351-357 [DOI] [PubMed] [Google Scholar]
- Grove E. A., Fukuchi-Shimogori T. (2003). Generating the cerebral cortical area map. Annu. Rev. Neurosci. 26, 355-380 [DOI] [PubMed] [Google Scholar]
- Gunhaga L., Marklund M., Sjodal M., Hsieh J. C., Jessell T. M., Edlund T. (2003). Specification of dorsal telencephalic character by sequential Wnt and FGF signaling. Nat. Neurosci. 6, 701-707 [DOI] [PubMed] [Google Scholar]
- Hebert J. M., Mishina Y., McConnell S. K. (2002). BMP signaling is required locally to pattern the dorsal telencephalic midline. Neuron 35, 1029-1041 [DOI] [PubMed] [Google Scholar]
- Hevner R. F., Hodge R. D., Daza R. A., Englund C. (2006). Transcription factors in glutamatergic neurogenesis: conserved programs in neocortex, cerebellum, and adult hippocampus. Neurosci. Res. 55, 223-233 [DOI] [PubMed] [Google Scholar]
- Irioka T., Watanabe K., Mizusawa H., Mizuseki K., Sasai Y. (2005). Distinct effects of caudalizing factors on regional specification of embryonic stem cell-derived neural precursors. Brain Res. Dev. Brain Res. 154, 63-70 [DOI] [PubMed] [Google Scholar]
- Johnson M. A., Weick J. P., Pearce R. A., Zhang S. C. (2007). Functional neural development from human embryonic stem cells: accelerated synaptic activity via astrocyte coculture. J. Neurosci. 27, 3069-3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Shamy G. A., Elkabetz Y., Schofield C. M., Harrsion N. L., Panagiotakos G., Socci N. D., Tabar V., Studer L. (2007). Directed differentiation and transplantation of human embryonic stem cell-derived motoneurons. Stem Cells 25, 1931-1939 [DOI] [PubMed] [Google Scholar]
- Lee S. T., Kim M. (2006). Aging and neurodegeneration. Molecular mechanisms of neuronal loss in Huntington's disease. Mech. Ageing Dev. 127, 432-435 [DOI] [PubMed] [Google Scholar]
- Li X. J., Du Z. W., Zarnowska E. D., Pankratz M., Hansen L. O., Pearce R. A., Zhang S. C. (2005). Specification of motoneurons from human embryonic stem cells. Nat. Biotechnol. 23, 215-221 [DOI] [PubMed] [Google Scholar]
- Litingtung Y., Dahn R. D., Li Y., Fallon J. F., Chiang C. (2002). Shh and Gli3 are dispensable for limb skeleton formation but regulate digit number and identity. Nature 418, 979-983 [DOI] [PubMed] [Google Scholar]
- Marin O., Rubenstein J. L. (2001). A long, remarkable journey: tangential migration in the telencephalon. Nat. Rev. Neurosci. 2, 780-790 [DOI] [PubMed] [Google Scholar]
- Olsson M., Campbell K., Turnbull D. H. (1997). Specification of mouse telencephalic and mid-hindbrain progenitors following heterotopic ultrasound-guided embryonic transplantation. Neuron 19, 761-772 [DOI] [PubMed] [Google Scholar]
- Olsson M., Bjorklund A., Campbell K. (1998). Early specification of striatal projection neurons and interneuronal subtypes in the lateral and medial ganglionic eminence. Neuroscience 84, 867-876 [DOI] [PubMed] [Google Scholar]
- Pankratz M. T., Li X. J., Lavaute T. M., Lyons E. A., Chen X., Zhang S. C. (2007). Directed neural differentiation of human embryonic stem cells via an obligated primitive anterior stage. Stem Cells 25, 1511-1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parras C. M., Schuurmans C., Scardigli R., Kim J., Anderson D. J., Guillemot F. (2002). Divergent functions of the proneural genes Mash1 and Ngn2 in the specification of neuronal subtype identity. Genes Dev. 16, 324-338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier A. L., Tabar V., Barberi T., Rubio M. E., Bruses J., Topf N., Harrison N. L., Studer L. (2004). Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc. Natl. Acad. Sci. USA 101, 12543-12548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy N. S., Cleren C., Singh S. K., Yang L., Beal M. F., Goldman S. A. (2006). Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat. Med. 12, 1259-1268 [DOI] [PubMed] [Google Scholar]
- Singh R. N., Nakano T., Xuing L., Kang J., Nedergaard M., Goldman S. A. (2005). Enhancer-specified GFP-based FACS purification of human spinal motor neurons from embryonic stem cells. Exp. Neurol. 196, 224-234 [DOI] [PubMed] [Google Scholar]
- Stern C. D. (2001). Initial patterning of the central nervous system: how many organizers? Nat. Rev. Neurosci. 2, 92-98 [DOI] [PubMed] [Google Scholar]
- Stuhmer T., Puelles L., Ekker M., Rubenstein J. L. (2002). Expression from a Dlx gene enhancer marks adult mouse cortical GABAergic neurons. Cereb. Cortex 12, 75-85 [DOI] [PubMed] [Google Scholar]
- Sussel L., Marin O., Kimura S., Rubenstein J. L. (1999). Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development 126, 3359-3370 [DOI] [PubMed] [Google Scholar]
- Thomson J. A., Itskovitz-Eldor J., Shapiro S. S., Waknitz M. A., Swiergiel J. J., Marshall V. S., Jones J. M. (1998). Embryonic stem cell lines derived from human blastocysts. Science 282, 1145-1147 [DOI] [PubMed] [Google Scholar]
- Tyurina O. V., Guner B., Popova E., Feng J., Schier A. F., Kohtz J. D., Karlstrom R. O. (2005). Zebrafish Gli3 functions as both an activator and a repressor in Hedgehog signaling. Dev. Biol. 277, 537-556 [DOI] [PubMed] [Google Scholar]
- Wang B., Fallon J. F., Beachy P. A. (2000). Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell 100, 423-434 [DOI] [PubMed] [Google Scholar]
- Wang C., Ruther U., Wang B. (2007). The Shh-independent activator function of the full-length Gli3 protein and its role in vertebrate limb digit patterning. Dev. Biol. 305, 460-469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Kamiya D., Nishiyama A., Katayama T., Nozaki S., Kawasaki H., Watanabe Y., Mizuseki K., Sasai Y. (2005). Directed differentiation of telencephalic precursors from embryonic stem cells. Nat. Neurosci. 8, 288-296 [DOI] [PubMed] [Google Scholar]
- Watanabe K., Ueno M., Kamiya D., Nishiyama A., Matsumura M., Wataya T., Takahashi J. B., Nishikawa S., Nishikawa S., Muguruma K., Sasai Y. (2007). A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 25, 681-686 [DOI] [PubMed] [Google Scholar]
- Wichterle H., Lieberam I., Porter J. A., Jessell T. M. (2002). Directed differentiation of embryonic stem cells into motor neurons. Cell 110, 385-397 [DOI] [PubMed] [Google Scholar]
- Yan Y., Yang D., Zarnowska E. D., Du Z., Werbel B., Valliere C., Pearce R. A., Thomson J. A., Zhang S. C. (2005). Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells 23, 781-790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Q. L., Stavridis M., Griffiths D., Li M., Smith A. (2003). Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat. Biotechnol. 21, 183-186 [DOI] [PubMed] [Google Scholar]
- Yu W., McDonnell K., Taketo M. M., Bai C. B. (2008). Wnt signaling determines ventral spinal cord cell fates in a time-dependent manner. Development 135, 3687-3696 [DOI] [PubMed] [Google Scholar]
- Yu W., Wang Y., McDonnell K., Stephen D., Bai C. B. (2009). Patterning of ventral telencephalon requires positive function of Gli transcription factors, Dev. Biol. 334, 264-275 [DOI] [PubMed] [Google Scholar]
- Zhang S. C., Wernig M., Duncan I. D., Brustle O., Thomson J. A. (2001). In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat. Biotechnol. 19, 1129-1133 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.