Abstract
The dorsolateral prefrontal cortex (DLPFC) has been implicated in the pathophysiology of mental disorders. Previous region-of-interest MRI studies that attempted to delineate this region adopted various landmarks and measurement techniques, with inconsistent results. We developed a new region-of-interest measurement method to obtain morphometric data of this region from structural MRI scans, taking into account knowledge from cytoarchitectonical postmortem studies and large inter-individual variability of this region. MRI scans of ten subjects were obtained, and DLPFC tracing was performed in the coronal plane by two independent raters using the semi-automated software Brains2. The intra-class correlation coefficients between two independent raters were 0.94 for the left DLPFC and 0.93 for the right DLPFC. The mean ± SD DLPFC volumes were 9.23 ± 2.35 ml for the left hemisphere and 8.20 ± 2.08 ml for the right hemisphere. Our proposed method has high inter-rater reliability and is easy to implement, permitting the standardized measurement of this region for clinical research applications.
Keywords: neuroimaging, schizophrenia, bipolar disorder, depression
1. Introduction
The dorsolateral prefrontal cortex (DLPFC) plays a key role in a variety of cognitive processes (Nathaniel-James and Frith, 2002). There is strong evidence that DLPFC dysfunctions are involved in the neuropsychological deficits commonly observed among schizophrenic patients (Bunney and Bunney, 2000). In addition, this region shares connections with several brain structures as part of frontal-subcortical neuronal circuits implicated in the processing of emotions (Tekin and Cummings, 2002). Thus, it is likely that the DLPFC is involved in the pathophysiology of mood disorders. Functional studies found decreased blood flow and metabolism in this region among unipolar patients (Drevets et al, 2000; Soares and Mann, 1997; Soares, 2003). Decreased levels of N-acetyl-aspartate (NAA), a marker of neuronal viability, have been demonstrated through MRI spectroscopy in the DLPFC of bipolar patients (Winsberg et al, 2000; Sassi et al, 2005). Postmortem cell counting studies demonstrated reduced cell density and/or size in the DLPFC of subjects diagnosed with major depressive disorder (Rajkowska et al, 1999; Cotter et al, 2002) and bipolar disorder (Rajkowska et al, 2001; Cotter et al, 2002).
The DLPFC is located on the lateral and dorsal part of the medial convexity of the frontal lobe and comprises Brodmann's areas 9 and 46 and a few transitional areas: 9-8, 9-45, 46-10, and 46-45 (Rajkowska and Goldman-Rakic, 1995a). Macroscopic delineation of the DLPFC faces the problem of high individual variability in the exact extent of its cytoarchitectonic boundaries (Rajkowska and Goldman-Rakic, 1995b). Consequently, there is no standardized procedure to obtain DLPFC measurements “in vivo”, which has led to reporting of inconsistent results in the few region-of-interest MRI studies that examined the DLPFC of humans; across the board, different landmarks and measurement techniques have been utilized (Schlaepter et al, 1994; Seidman et al, 1994; Crespo-Facorro et al., 1999; Tisserand et al, 2001, Zuffante et al, 2001). It is our goal to provide supporting evidence for standardization of such measurements.
We developed a region-of-interest method to obtain volumetric measurements of the DLPFC from MRI scans through manual tracing. In this technique, we utilized macroscopic anatomical landmarks to delimit this region, which were established based on the cytoarchitectonic features of areas 9 and 46, as well as the inter-individual variability in their extent and location, as previously ascertained in post-mortem studies (Rajkowska & Goldman-Rakic, 1995 a, b).
2. Methods
2.1 Participants
MRI images of ten healthy right-handed subjects, one male and nine females with a median age of 26.8 yrs ± 6.5 yrs were obtained.
2.2 MRI acquisition
MRI brain images were obtained using a 1.5T Philips Intera 8.1.1 scanner at the South Texas Veterans Health Care System, Audie Murphy Division. Images were acquired using T-1 weighted fast field echo sequence (3D T1-FFE) in the coronal plane (TR=25ms, TE=5 ms, FOV=240×220 cm, slice thickness=1.0 mm, gap= 0, NEX= 2, matrix size=256 × 256).
2.3 Tracing procedures
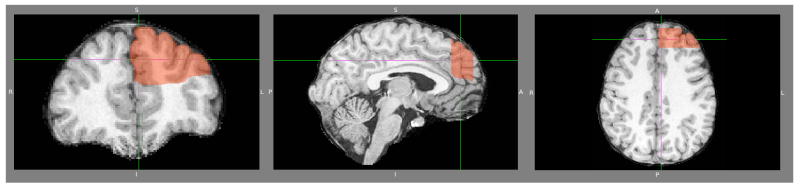
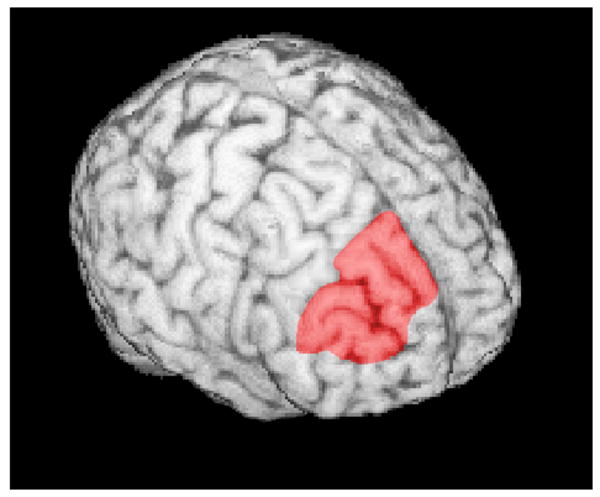
Tracing and measurements were performed manually (Figure 1) with the semi-automated software Brains2 (Magnotta et al, 2002). The left and right DLPFC were traced separately, due to the proximity of the medial borders within the left and right hemispheres. After the region-of-interest was delimited, gray matter volumes were automatically obtained. The software allowed for clear 2-D views from different vantage points along different planes of the region-of-interest, and that resource was utilized in cases where the anatomical landmarks were not clear in the coronal plane (Figure 3). It also allowed for 3D modeling of the lateral surface of the DPFC (Figure 4).
Figure 1. DLPFC tracing.
Tracing started on the slice immediately anterior to the most rostral one where corpus callosum could be seen as a bridge and was performed from posterior to anterior. Medial and lateral portions of area 9 were delineated through a line traced from the tip of the superior frontal gyrus to the bottom of the superior frontal sulcus.
Figure 3. A three plane view of the dorsal prefrontal cortex.
Figure 4. 3D rendering of the lateral surface of the DPFC.
2.4 Anatomical landmarks
The adopted anatomical landmarks for areas 9 and 46 (plus transitional areas 9-8, 9-45, 46-10, and 46-45) were based on postmortem studies by Rajkowska and Goldman-Rakic (1995a, b). Using a set of cytoarchitectonic criteria, they established that area 9 is consistently located in the superior frontal gyrus, whereas area 46 can be found in the middle frontal gyrus. Those conclusions were reached through the postmortem analysis of 17 human subjects (age range: 23-73 years) with no record of neurological disease (Rajkowska & Goldman-Rakic, 1995a).
Considering the variability among several subjects studied and the extent of the transitional areas described by the authors (Rajkowska & Goldman-Rakic, 1995a), we established that the tracing should start on the slice located immediately anterior to the most rostral one where corpus callosum could be seen (Figure 1). Tracing was performed from posterior to anterior and continued until the most anterior slice where the superior and middle frontal gyrus could be distinguished; that is, until the slice immediately posterior to the first one where the frontal pole could be visualized, since this region has been previously adopted as the anterior border of DLPFC (Tisserand et al, 2002). Interestingly, postmortem cytoarchitectonic studies (Rajkowska & Goldman-Rakic, 1995a, b) reveal that this landmark corresponds approximately to the cytoarchitectonic border between Brodmann's area 10 and 9.
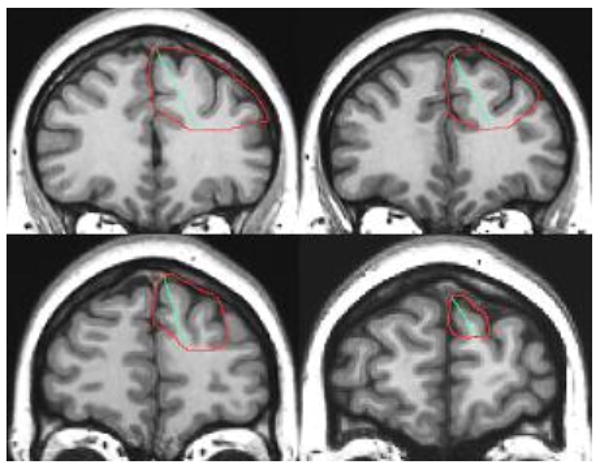
In most of the slices, the cingulate sulcus was adopted as the medial border of the superior frontal gyrus, and the inferior frontal sulcus was adopted as the inferior border of the middle frontal gyrus (Figure 2). In the most rostral slices, the limits adopted were the superior rostral sulcus and the frontomarginal sulcus, respectively. Following this procedure, a total measure of the “dorsal prefrontal cortex” (DPFC) was obtained.
Figure 2. Anatomical landmarks for the DPFC tracing.
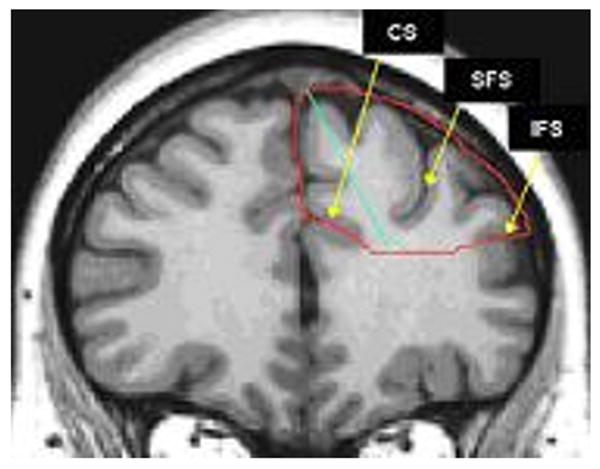
The DPFC was delimited medially by the cingulate sulcus (CS) and inferiorly by the inferior frontal sulcus (IFS). Medial and lateral portions of DPFC were delineated through a line traced from the tip of the superior frontal gyrus to the bottom of the superior frontal sulcus (SFS).
Afterwards, a line was traced from the tip of the superior frontal gyrus to the bottom of the superior frontal sulcus, in order to delineate the medial and lateral portions of area 9. The medial and lateral parts of area 9 are located on the medial and lateral hemisphere surfaces, respectively, and they differ by their fine cytoarchitectonic features (Rajkowska & Goldman-Rakic, 1995a, b). The volume of the medial portion was subtracted from the total to calculate the DLPFC volume. These limits were based on an MRI-based parcellation method for the frontal lobe proposed by Crespo-Facorro et al. (1999) and cytoarchitectonic observations on the rostral border of area 9 (Rajkowska & Goldman-Rakic, 1995a, b).
2.5 Reliability of the method
MRI images of ten healthy right-handed subjects (1 male/ 9 females, age=26.8 ± 6.5 years) were obtained and traced by two independent trained evaluators in order to assess the inter-rater reliability of the method. Raters underwent approximately two weeks of training before they independently traced the MRI images. Appropriate measures were taken to safeguard the subjects' identities. The study was approved by the UTHSCSA Institutional Review Board, and informed consent was obtained from all subjects. Inter-rater reliability was measured with the intra-class correlation coefficient (ICC).
3. Results
Following the above procedures, it took around 30 minutes to complete the tracing and measurement of the DLPFC in one scan bilaterally. On average, 17 to 20 slices were traced on each scan.
The mean ± SD volumes of the DPFC in the sample were 13.45 ± 3.48 ml for the left hemisphere and 12.35 ± 2.72 ml for the right hemisphere. The between raters ICCs were 0.94 for the right hemisphere and 0.96 for the left.
The mean ± SD DLPFC volumes were 9.23 ± 2.35 ml for the left hemisphere and 8.20 ± 2.08 ml for the right. A high inter-rater reliability was obtained on these measurements (ICC = 0.93 for the right DLPFC and ICC = 0.94 for the left DLPFC). The DLPFC volumes and other data for all subjects are listed in Table 1.
Table 1.
DPFC and DLPFC volumes obtained through MRI among 10 subjects
| Case | Age (years) | Sex | Left DPFC (ml) | Left DLPFC (ml) | Right DPFC (ml) | Right DLPFC (ml) |
|---|---|---|---|---|---|---|
| 20004 | 32 | female | 11.93 | 8.97 | 8.36 | 5.44 |
| 20011 | 28 | female | 7.52 | 5.38 | 8.49 | 5.46 |
| 20012 | 24 | female | 8.62 | 5.98 | 9.99 | 5.96 |
| 20028 | 35 | female | 15.82 | 11.78 | 11.66 | 8.04 |
| 20088 | 20 | female | 13.19 | 8.76 | 12.58 | 7.32 |
| 20104 | 28 | male | 16.63 | 12.33 | 13.66 | 9.69 |
| 20135 | 38 | female | 13.83 | 8.40 | 14.38 | 9.89 |
| 20145 | 19 | female | 12.26 | 8.70 | 13.13 | 9.22 |
| 20158 | 21 | female | 17.21 | 10.28 | 14.64 | 9.74 |
| 20162 | 23 | female | 17.52 | 11.69 | 16.59 | 11.28 |
4. Discussion
Our findings suggest that the proposed method for DLPFC measurement has high inter-rater reliability and is easy to implement. Raters could be trained to achieve high reliability in a short period of time; the simplicity of the method makes its implementation feasible in different research settings.
Even though some structural MRI studies have previously examined the DLPFC of patients with mental disorders and healthy controls, the different tracing and measurement methods utilized have lacked homogeneity. This fact probably accounts for the large variability in their results. Tisserand et al (2002) utilized specific software (DISPLAY, Montreal Neurologic Institute) that enabled them to perform tracing on the surface rendered image. This study targeted age-related changes in the frontal lobes, and included 57 healthy subjects. The DLPFC was defined as Brodman areas 8, 9 and 46, and was delimited by the inferior frontal sulcus (lateral ventral border), the paracingulate sulcus (medial border), the precentral sulcus (posterior border) and the frontal pole (anterior border). The inter-rater reliability regarding the DLPFC was 0.97 (left side) and 0.96 (right side). Due to some methodological issues, results were reported as a measure of the “lateral frontal cortex”, which included the DLPFC as described above plus the inferior frontal gyrus. Thus, the comparison between their results and ours is difficult. The same applies to another study (Wible et al, 1997), which is based in the division of the prefrontal cortex into seven different regions, measuring separately the superior and middle gyrus.
On the other hand, our mean volumes are similar to the ones found by Seidman et al (1994), who examined the DLPFC of schizophrenic patients. In that study, tracing was performed in the coronal plane, and the DLPFC was delimited by the superior frontal sulcus and the anterior ramus of the lateral sulcus. Therefore, the DLPFC included the middle and the inferior frontal gyrus. The most posterior slice was the first one where the corpus callosum could be seen, and the tracing was performed until the most anterior slice where the frontal lobe was present.
Another study (Schlaepfer et al, 1994) examined the DLPFC of schizophrenic patients, bipolar patients and healthy controls. Segmentation was carried out in the axial plane, according to “approximate Broadman cortical areas”. Results were described as a single measure representing the “heteromodal association cortex”, which included the DLPFC, the superior temporal lobe and the inferior parietal cortex.
Finally, some automated methods have been developed to allow the measurement of cortical structures, including the prefrontal cortex (Riffkin et al, 2005; Lopez-Garcia et al, 2006). Whereas automatic parcellation of the brain represents a promising resource in the study of brain structures among psychiatric patients, it does not take into account the individual variability of anatomical landmarks, which is very high and therefore more problematic for highly variable structures such as the DLPFC. Our method aims at overcoming that limitation, which is critical in the case of the DLPFC.
Our method has some limitations that are worth discussing. Tracing was performed manually, in the coronal plane (while we also had concurrent axial and sagital views to aid in the tracing), and the correct delimitation of our region-of-interest had to take into consideration the inter-individual and inter-hemispheric anatomical variation, a common limitation of topographic methods that utilize sulcal landmarks (Crespo-Facorro et al, 1999). One of our key landmarks, the inferior frontal sulcus, cannot be located in about 14% of the cases and varies to some degree in its location, size and shape (Zernov, cited by Rajkowska, 1995b). Whereas the most caudal slice could be clearly identified, the most rostral one was subject to differences among the different subjects. Thereby, the present tracing method demands a certain degree of familiarity with the gross anatomy of the frontal lobes.
The involvement of the DLPFC in psychiatric disorders has received special attention in recent years, but the nature of this involvement is still to be clarified. Further, it is not well established whether the role of this structure in the pathophysiology of mental disorders is disease-specific or represents a feature common to different types of psychosis (Dolan et al, 1993). The finding of hypoperfusion in the DLPFC of depressed subjects, as demonstrated by PET studies, was regarded as a marker of state, which would revert during remission periods (Bench et al, 1995; for a review, see Bearden et al, 2001).
On the other hand, postmortem studies have demonstrated distinct DLPFC abnormalities in patients with schizophrenia and mood disorders, with increased neuronal density among the former ones and decreased neuronal density, glial density and glial hypertrophy among the latter ones (Selemon et al, 1995, 1998; Rajkowska et al, 1999; Rajkowska et al, 2001; Selemon and Rajkowska, 2003). These findings suggest the existence of abnormalities in this region that are not necessarily restricted to specific mood states, but may be related to progressive developmental and/or degenerative processes. Therefore, an adequate approach to obtain accurate morphometric measurements of the DLPFC “in vivo” is of great relevance for studies that may further the understanding of the pathophysiology of mood disorders.
In conclusion, our proposed method has good validity, as it is based on cytoarchitectonic anatomical criteria from previous postmortem studies, has high inter-rater reliability and is easy to implement. Future studies should assess its usefulness in different research settings and specific patient populations.
Acknowledgments
This study was supported in part by MH 68766, MH 69774, MH61578, MH60451, RR17701, NARSAD, the Veterans Administration (VA Merit Review) and CAPES Foundation (Brazil).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bearden CE, Hoffman KM, Cannon TD. The neuropsychology and neuroanatomy of bipolar affective disorder: a critical review. Bipolar Disorders. 2001;3:106–50. doi: 10.1034/j.1399-5618.2001.030302.x. discussion 151-3. [DOI] [PubMed] [Google Scholar]
- Bench CJ, Frackowiak RS, Dolan RJ. Changes in regional cerebral blood flow on recovery from depression. Psychological Medicine. 1995;25:247–61. doi: 10.1017/s0033291700036151. [DOI] [PubMed] [Google Scholar]
- Bunney WE, Bunney BG. Evidence for a compromised dorsolateral prefrontal cortical parallel circuit in schizophrenia. Brain Research Brain Research Reviews. 2000;31:138–46. doi: 10.1016/s0165-0173(99)00031-4. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex. 2002;12(4):386–94. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- Crespo-Facorro B, Kim JJ, Andreasen NC, O'Leary DS, Wiser AK, Bailey JM, Harris G, Magnotta VA. Human frontal cortex: an MRI-based parcellation method. Neuroimage. 1999;10:500–19. doi: 10.1006/nimg.1999.0489. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Bench CJ, Liddle PF, Friston KJ, Frith CD, Grasby PM, Frackowiak RS. Dorsolateral prefrontal cortex dysfunction in the major psychoses; symptom or disease specificity? Journal of Neurology, Neurosurgery and Psychiatry. 1993;56:1290–4. doi: 10.1136/jnnp.56.12.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging studies of mood disorders. Biological Psychiatry. 2000;48:813–29. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- Lopez-Garcia P, Aizenstein HJ, Snitz BE, Walter RP, Carter CS. Automated ROI-based brain parcellation analysis of frontal and temporal brain volumes in schizophrenia. Psychiatry Research: Neuroimaging. 2006;147:153–161. doi: 10.1016/j.pscychresns.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Magnotta VA, Harris G, Andreasen NC, O'Leary DS, Yuh WT, Heckel D. Structural MR image processing using the BRAINS2 toolbox. Computerized Medical Imaging and Graphics. 2002;26:251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- Nathaniel-James DA, Frith CD. The role of the dorsolateral prefrontal cortex: evidence from the effects of contextual constraint in a sentence completion task. Neuroimage. 2002;16:1094–1102. doi: 10.1006/nimg.2002.1167. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: I. Remapping of areas 9 and 46 using quantitative criteria. Cerebral Cortex. 1995a;5:307–22. doi: 10.1093/cercor/5.4.307. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the Talairach Coordinate System. Cerebral Cortex. 1995b;5:323–37. doi: 10.1093/cercor/5.4.323. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Pittman SD, Dilley G, Overholser J, Meltzer H, Stockmeier C. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression, Biological Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Halaris A, Selemon LD. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biological Psychiatry. 2001;49:741–52. doi: 10.1016/s0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- Riffkin J, Yücel M, Maruff P, Wood SJ, Soulsby B, Olver J, Kyrios M, Velakoulis D, Pantelis C. A manual and automated MRI study of anterior cingulate and orbito-frontal cortices, and caudate nucleus in obsessive-compulsive disorder: comparison with healthy controls and patients with schizophrenia. Psychiatry Research. 2005;138:99–113. doi: 10.1016/j.pscychresns.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Sassi RB, Stanley JA, Axelson D, Brambilla P, Nicoletti MA, Keshavan MS, Ramos RT, Ryan N, Birmaher B, Soares JC. Reduced NAA levels in the dorsolateral prefrontal cortex of young bipolar patients. American Journal of Psychiatry. 2005;162:2109–15. doi: 10.1176/appi.ajp.162.11.2109. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Harris GJ, Tien AY, Peng LW, Lee S, Federman EB, Chase GA, Barta PE, Pearlson GD. Decreased regional cortical gray matter volume in schizophrenia. American Journal of Psychiatry. 1994;151:842–8. doi: 10.1176/ajp.151.6.842. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Yurgelun-Todd D, Kremen WS, Woods BT, Goldstein JM, Faraone SV, Tsuang MT. Relationship of prefrontal and temporal lobe MRI measures to neuropsychological performance in chronic schizophrenia. Biological Psychiatry. 1994;35:235–46. doi: 10.1016/0006-3223(94)91254-8. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex: a morphometric analysis of prefrontal area 9 and occipital area 17. Archives of General Psychiatry. 1995;52:805–818. doi: 10.1001/archpsyc.1995.03950220015005. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Elevated neuronal density in prefrontal area 46 in brains from schizophrenic patients: Application of a 3-Dimensional, stereologic counting method. Journal of Comparative Neurology. 1998;392:402–412. [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G. Cellular pathology in the dorsolateral prefrontal cortex distinguishes schizophrenia from bipolar disorder. Current Molecular Medicine. 2003;3:427–436. doi: 10.2174/1566524033479663. [DOI] [PubMed] [Google Scholar]
- Soares JC. Contributions from brain imaging to the elucidation of pathophysiology of bipolar disorder. International Journal of Neuropsychopharmacology. 2003;6:171–80. doi: 10.1017/S1461145703003390. [DOI] [PubMed] [Google Scholar]
- Soares JC, Mann JJ. The functional neuroanatomy of mood disorders. Journal of Psychiatry Research. 1997;31:393–432. doi: 10.1016/s0022-3956(97)00016-2. [DOI] [PubMed] [Google Scholar]
- Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. Journal of Psychosomatic Research. 2002;53:647–54. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- Tisserand DJ, Pruessner JC, Sanz Arigita EJ, van Boxtel MP, Evans AC, Jolles J, Uylings HB. Regional frontal cortical volumes decrease differentially in aging: an MRI study to compare volumetric approaches and voxel-based morphometry. Neuroimage. 2002;17:657–69. [PubMed] [Google Scholar]
- Wible CG, Shenton ME, Fischer IA, Allard JE, Kikinis R, Jolesz FA, Iosifescu DV, McCarley RW. Parcellation of the human prefrontal cortex using MRI. Psychiatry Research. 1997;7:629–40. doi: 10.1016/s0925-4927(97)00060-7. [DOI] [PubMed] [Google Scholar]
- Winsberg ME, Sachs N, Tate DL, Adalsteinsson E, Spielman D, Ketter TA. Decreased dorsolateral prefrontal N-acetyl aspartate in bipolar disorder. Biological Psychiatry. 2000;47:475–81. doi: 10.1016/s0006-3223(99)00183-3. [DOI] [PubMed] [Google Scholar]
- Zuffante P, Leonard CM, Kuldau JM, Bauer RM, Doty EG, Bilder RM. Working memory deficits in schizophrenia are not necessarily specific or associated with MRI-based estimates of area 46 volumes. Psychiatry Research. 2001;108:187–209. doi: 10.1016/s0925-4927(01)00124-x. [DOI] [PubMed] [Google Scholar]