Abstract
The ubiquitin carboxyl-terminal esterase L1 gene, UCHL1, located on chromosome 4p14, has been studied as a potential candidate gene for Parkinson's disease risk. The authors conducted a Human Genome Epidemiology review and meta-analysis of published case-control studies of the UCHL1 S18Y variant and Parkinson's disease in Asian and Caucasian samples. The meta-analysis of studies in populations of Asian ancestry showed a statistically significant association between the Y allele and reduced risk of Parkinson's disease under a recessive model (odds ratio (OR) for YY vs. SY + SS = 0.79, 95% confidence interval (CI): 0.67, 0.94; P = 0.006). For a dominant model, the association was not significant in Asian populations (OR for YY + SY vs. SS = 0.88, 95% CI: 0.68, 1.14; P = 0.33). For populations of European ancestry, the meta-analysis showed a significant association between the Y allele and decreased risk of Parkinson's disease under a dominant model (OR = 0.89, 95% CI: 0.81, 0.98; P = 0.02) but not under a recessive model (OR = 0.92, 95% CI: 0.66, 1.30; P = 0.65). Using the Venice criteria, developed by the Human Genome Epidemiology Network Working Group on the assessment of cumulative evidence, the authors concluded that moderate evidence exists for an association between the S18Y variant and Parkinson's disease.
Keywords: case-control studies, epidemiology, genes, genetics, meta-analysis, Parkinson disease, review, UCHL1
GENE
The ubiquitin carboxyl-terminal esterase L1 gene, UCHL1, located on chromosome 4 (4p14), spans 11.5 kb and has 9 exons (Online Mendelian Inheritance in Man (OMIM); www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=OMIM)). UCHL1 protein (OMIM #191342) expression is specific to neurons, cells of the diffuse neuroendocrine system, testes, and ovaries (1, 2). UCHL1 protein is especially abundant in the brain, constituting 1%–2% of soluble brain protein (3), and has been localized to Lewy bodies and other inclusion bodies characteristic of human neurodegenerative diseases (4, 5).
The UCHL1 enzyme is involved in the ubiquitin proteasome system, a cellular pathway responsible for the degradation of misfolded and damaged proteins (6). The ubiquitin proteasome system has been postulated to play an important role in the etiology of Parkinson's disease (for a review, refer to Lim and Tan (7)). Specifically, UCHL1 is a member of the ubiquitin C-terminal hydrolase family of deubiquitinating enzymes, which catalyze the hydrolysis of polymeric ubiquitin chains (8). In addition to a deubiquitination function, in vitro studies have shown that UCHL1 has dimerization-dependent ubiquitin ligase activity (9). UCHL1-deficient mice, known as gracile axonal dystrophy mice, show neuronal loss in the spinal gracile tract and exhibit early development sensory ataxia and progressive motor ataxia (10). In vitro studies of such mice show that UCHL1 noncovalently bonds to ubiquitin, thereby preventing ubiquitin degradation and maintaining the cellular pool of free ubiquitin (11). Posttranslational regulation, such as monoubiquitination, is hypothesized to play an important role in UCHL1 protein function (12).
UCHL1 has also been studied as a candidate gene for Huntington's disease (1, 13, 14) and Alzheimer's disease (3, 15), but evidence is not sufficient to support or refute an association. Additional studies have suggested that epigenetic changes altering UCHL1 gene and protein expression may also serve as a marker of disease status or prognosis for several types of cancer (2, 16, 17).
GENE VARIANTS
Two variants in the UCHL1 gene have been examined in association with Parkinson's disease: I93M and S18Y (for a previous review, refer to Healy et al. (1)). The I93M missense variant results from a cytosine-to-guanine transversion in codon 93 of exon 4. This variant was originally identified in 2 German siblings with familial Parkinson's disease (18). The affected subjects had a form of disease that clinically resembled idiopathic Parkinson's disease; however, the father (a presumed carrier of the mutation) did not display the Parkinson's disease phenotype. In vitro studies indicate that this mutation results in partial loss of UCHL1 hydrolytic function (18, 19). The S18Y variant results from a cytosine-to-adenine transversion at codon 18 in exon 3 (rs5030732). This variant was originally identified in a study screening Parkinson's disease patients for UCHL1 polymorphisms (20). In vitro studies provide evidence that the S18Y mutation results in decreased ligase activity and in slightly increased hydrolase activity compared with wild-type UCHL1 (9, 19).
GENE VARIANT FREQUENCY
Several studies have specifically screened for the I93M mutation in Parkinson's disease subjects (21) and controls (20, 22–24), including one relatively large sample of 229 German Parkinson's disease patients (25). However, to date, there has been no documented occurrence of the I93M variant besides the 2 siblings in the original German Parkinson's disease family (1).
Our literature search did not identify any studies that have conducted population-based surveys of S18Y frequency. Therefore, we summarized allele frequency based on control samples from case-control studies. The frequency of the Y allele varies across geographic regions (Table 1). The Y allele frequency is higher among individuals of Asian descent (46%–61%) than among those of European descent (16%–24%). To date, no studies have been known to examine the frequency of the UCHL1 S18Y variant in other populations, such as Africans, Southeast Asians, Native Americans, Pacific Islanders, or Australian Aboriginals.
Table 1.
Worldwide Frequency of the UCHL1 Y Allele
| Geographic Region and Study (Reference No.) | Country | Ethnic Group | No. | Allele Frequency | 95% Confidence Intervala |
| Asia | |||||
| Wang et al., 2002 (98) | China | Asian | 160 | 0.47 | 0.41, 0.53 |
| Mizuta et al., 2006 (106) | Japan | Asian | 736 | 0.49 | 0.47, 0.50 |
| Momose et al., 2002 (107) | Japan | Asian | 248 | 0.49 | 0.45, 0.53 |
| Satoh and Kuroda, 2001 (23) | Japan | Asian | 155 | 0.54 | 0.49, 0.59 |
| Zhang et al., 2000 (108) | Japan | Asian | 160 | 0.61b | 0.56, 0.67 |
| Tan et al., 2006 (109) | Singapore | Asian | 341 | 0.46b | 0.42, 0.50 |
| Australia | |||||
| Mellick and Silburn, 2000 (100) | Australia | Caucasian | 142 | 0.16 | 0.11, 0.20 |
| Europe | |||||
| Elbaz et al., 2003 (97) | France | Caucasian | 488 | 0.19b | 0.17, 0.22 |
| Levecque et al., 2001 (101) | France | Caucasian | 93 | 0.18 | 0.13, 0.24 |
| Wintermeyer et al., 2000 (25) | Germany | Caucasian | 200 | 0.20 | 0.16, 0.24 |
| Savettieri et al., 2001 (102) | Italy | Caucasian | 165 | 0.17 | 0.13, 0.21 |
| Carmine Belin et al., 2007c (99) | Sweden | Caucasian | 285 | 0.17 | 0.14, 0.20 |
| Healy et al., 2006 (26) | United Kingdom | Caucasian | 1,482 | 0.17b | 0.15, 0.18 |
| North America | |||||
| Facheris et al., 2005 (92) | United States | Caucasian | 497 | 0.19b | 0.16, 0.21 |
| Hutter et al., 2008 (95) | United States | Caucasian | 2,016 | 0.19 | 0.18, 0.20 |
| Maraganore et al., 1999 (20) | United States | Caucasian | 110 | 0.23 | 0.18, 0.29 |
| Maraganore et al., 2004d (91) | United States | Caucasian | 80 | 0.24 | 0.17, 0.31 |
| Zhang et al., 2000 (108) | United States | Caucasian | 142 | 0.19b | 0.14, 0.25 |
Abbreviation: UCHL1, ubiquitin carboxyl-terminal esterase L1 gene.
Calculated by using exact confidence intervals.
Allele frequencies were calculated from genotype frequencies in controls.
Incorrect frequency was presented in the initial publication; corrected frequency was obtained courtesy of the study author.
Excluding cases included in genotype counts in Maraganore et al. (20).
Additional single nucleotide polymorphisms (SNPs) have been characterized through resequencing and the HapMap Project (http://hapmap.ncbi.nlm.nih.gov/abouthapmap.html). Healy et al. (26) resequenced the UCHL1 gene in 64 unrelated white individuals, identifying 5 SNPs with minor allele frequencies less than 5% and 23 SNPs with minor allele frequencies greater than 5%. They used information on linkage disequilibrium between these SNPs to determine that 3 SNPs were needed to tag common variation in the gene (r2 > 0.75) (26). We examined the current HapMap data and found 10 SNPs with minor allele frequencies greater than 5% in each of the 4 HapMap populations (Yoruba from Ibadan, Nigeria; Japanese from Tokyo, Japan; Han Chinese from Beijing, China; and Utah residents of northern and western European ancestry) (27). Using tagSNP methods based on pairwise linkage disequilibrium (r2 ≥ 0.80) (28), we determined that 4 tagSNPs are needed to capture common variants (minor allele frequencies greater than 5%) for Utah residents of northern and western European ancestry, Japanese from Tokyo, and Han Chinese from Beijing and 5 tagSNPs for the Yoruba population from Ibadan. Aside from the paper by Healy et al., none of the other published studies have examined these variants in relation to Parkinson's disease.
The remainder of this review focuses on the S18Y variant only, because it is the best studied of the 2 variants. Until recently, the I93M variant had been identified in only 2 individuals, and a comprehensive tagSNP assessment of this gene had been performed by only Healy et al. (26).
DISEASE
Clinical and pathologic characteristics
Parkinson's disease is a progressive, neurodegenerative disorder clinically defined by a combination of cardinal motor features: tremor, rigidity, bradykinesia (slowness of movement), and postural instability (29). The mainstay of treatment for motor symptoms is dopamine replacement therapy (e.g., levodopa), but current therapeutic strategies are only palliative and do not substantially alter the underlying degenerative process. A number of nonmotor features that are difficult to treat are common in Parkinson's disease, including cognitive dysfunction, sleep disorders, psychosis, depression, anosmia, and autonomic insufficiency (30).
Pathologically, Parkinson's disease is characterized by a profound loss of dopaminergic neurons within the substantia nigra pars compacta and the presence of intraneuronal, cytoplasmic, eosinophilic inclusions called Lewy bodies (31). Lewy bodies contain abnormal protein aggregates, and 2 of the most abundant constituents are α-synuclein and ubiquitin (32, 33). Parkinson's disease becomes clinically evident when approximately 80% of striatal dopamine and 50% of nigral dopaminergic neurons are lost (34).
Most clinicians and researchers consider neuropathologic diagnosis to be the “gold standard” for Parkinson's disease. Clinicopathologic series indicate that diagnostic accuracy based on clinical findings alone ranges from 75.6% to 98.6% (35–39). Accuracy is highest when the diagnosis is made by a movement disorder specialist and is based on longitudinal assessments (36). The clinical diagnostic criteria most commonly used in research are those of the United Kingdom Parkinson's Disease Society Brain Bank (40).
Parkinson's disease prevalence
Parkinson's disease is the second most common neurodegenerative disease worldwide, after Alzheimer's disease. Parkinson's disease prevalence increases with age, so its burden is expected to increase with the aging US population (41). Prevalence of Parkinson's disease is higher in men than in women (42–44), although this finding is not observed in all studies. This gender difference is hypothesized to result from hormonal differences or differences in exposure to environmental risk factors (45).
Studies of Parkinson's disease prevalence and incidence have been conducted in various populations worldwide (41, 45–48). Data suggest that prevalence and incidence are similar across European and Asian populations. Even after accounting for differences in age structure, prevalence is lower in Africa than in Europe and Asia (48, 49). However, studies have differed in terms of case definitions, methods of case ascertainment, and age categories examined. Therefore, these trends may simply reflect methodological differences between studies (45, 50).
Genetic risk factors
Parkinson's disease is hypothesized to be a complex disease, with genetic and environmental contributions to its etiology. Evidence for genetic factors in Parkinson's disease was first mentioned in 1902, when William Gowers, a British neurologist, noted that approximately 15% of his Parkinson's disease patients reported an affected family member (51). Although the genetic basis for Parkinson's disease is sometimes debated (52), and results from twin studies are inconsistent (53, 54), a large number of recent case-control studies have demonstrated that, compared with controls, Parkinson's disease subjects are 2–14 times more likely to report a family history of the disease (55).
Further evidence for the genetic basis of Parkinson's disease comes from the identification of causal genes in families in which Parkinson's disease is inherited as a simple Mendelian disorder, either in an autosomal dominant or an autosomal recessive pattern (for recent reviews, refer to Tan and Skipper (56), Klein and Schlossmacher (57), Belin and Westerlund (58), and Lesage and Brice (59)). Table 2 provides details of regions identified as causal for monogenetic forms of Parkinson's disease. Mutations in these genes typically follow Mendelian patterns of inheritance within families. In some cases, the mutations result in disorders with clinical and/or pathologic distinctions from classic Parkinson's disease, most notably several monogenetic forms of the disease with an earlier age at onset.
Table 2.
PARK Regions That Have Been Linked to Parkinson's Disease in Family-based Studies
| Locus | Gene in Region Linked to Parkinson's Disease | Chromosomal Location | Form of Parkinson's Disease |
| PARK1 | SNCA | 4q21 | Autosomal dominant |
| PARK2 | PRKN | 6q25.2–q27 | Autosomal recessive (juvenile onset) |
| PARK3 | —a | 2p13 | Autosomal dominant |
| PARK4 | SNCA | 4p21 | Autosomal dominant |
| PARK5 | — | 4p14 | Autosomal dominant and sporadic |
| PARK6 | PINK1 | 1p36 | Autosomal recessive |
| PARK7 | DJ-1 | 1p36 | Autosomal recessive and early onset |
| PARK8 | LRRK2 | 12q12 | Autosomal dominant and sporadic |
| PARK9 | ATP13A2 | 1p36 | Early onset |
| PARK10 | — | 1p32 | Idiopathic |
| PARK11 | — | 2q36–q37 | Autosomal dominant and sporadic |
| PARK12 | — | X | Familial (mode of inheritance not known) |
| PARK13 | HTRA2 | 2p13 | Idiopathic |
Abbreviations: ATP13A2, ATPase type 13A2 gene; DJ-1, oncogene DJ1; HTRA2, HtrA serine peptidase 2 gene; LRRK2, leucine-rich repeat kinase-2 gene; PARK, a region identified in linkage studies of Parkinson's disease; PINK1, PTEN-induced putative kinase 1 gene; PRKN, Parkinson's disease gene; SNCA, α-synuclein gene.
—, not known.
Traditionally, regions identified in linkage studies of Parkinson's disease are initially given the designation PARK# (e.g., PARK1, PARK2, PARK3). Autosomal dominant forms of Parkinson's disease have been associated with mutations in the α-synuclein gene (SNCA) (PARK1 and PARK4 locus, chromosomal region 4q21) (60) and the leucine-rich repeat kinase-2 gene (LRRK2) (PARK8 locus, 12p11.2–q13.1) (61, 62). Autosomal recessive forms have been associated with mutations in the parkin gene (PARK2, 6q25–27) (63), oncogene DJ1 (DJ-1) (PARK7, 1p36) (64), the PTEN-induced putative kinase 1 gene (PINK1) (PARK6, 1p36) (65), and the ATPase type 13A2 gene (ATP13A2) (PARK9, 1p36) (66). Additional regions have been identified through linkage studies, including the PARK3 (2p13) (67), PARK10 (1p32) (68, 69), and PARK11 (2q36–q37) (70) regions. Specific causal genes have yet to be identified in these latter regions, and characterization of candidate genes remains an active area of research. For example, recent studies have nominated the sepiapterin reductase gene (SPR) as the most likely candidate in the PARK3 region (71). The discovery of these causal mutations has provided important insights into biochemical pathways involved in the etiology of Parkinson's disease.
In addition, association studies have examined several other potential Parkinson's disease susceptibility genes identified on the basis of their function or by their role in other neurodegenerative diseases (72). The PDGene website (www.pdgene.org), funded primarily by The Michael J. Fox Foundation for Parkinson's Research and the Alzheimer Research Forum, provides summaries of results from genetic epidemiologic studies of Parkinson's disease. More than 250 proposed Parkinson's disease susceptibility genes have been evaluated, and a number of potential pathways underlying Parkinson's disease etiology have been implicated, including those involved in mitochondrial stress, the ubiquitin proteasome system, and axon guidance. Of the genes summarized on PDGene, the strongest, most consistently replicated associations are seen for GBA (glucosidase, beta, acid), LRRK2, SNCA, and MAPT (microtubule-associated protein tau). More recently, additional candidate genes have been proposed based on the results of 3 genome-wide association studies (73–75) and a genome-wide meta-analysis of 2 of these studies (76). The results from the initial 2 genome-wide association studies have not been consistently replicated (77–80). The most recent genome-wide association study focused on familial Parkinson's disease and supported associations for MAPT and SNCA as well as identifying a potential new susceptibility region near GAK/DGKQ (diacylglycerol kinase, theta/cyclin G-associated kinase) on chromosome 4 (4, 75).
Environmental risk factors
A number of environmental exposures have been evaluated as risk factors for the development of Parkinson's disease (45, 81), with the most commonly studied being smoking, caffeine or coffee consumption, alcohol consumption, and use of nonsteroidal antiinflammatory drugs. Each of these exposures has been shown to be associated with a decreased risk of Parkinson's disease, and the smoking association has been the most consistently replicated (45, 82, 83). To date, there is no evidence for 2-way or 3-way interactions between smoking, coffee consumption, and use of over-the-counter nonsteroidal antiinflammatory drugs (83). The effects are instead thought to be independent and cumulative, although evidence is limited (83, 84).
Case-control studies have also examined exposures related to living in a rural setting, including the use of well water, farming, and pesticide exposure. The limited evidence available suggests a more than 2-fold increased risk of Parkinson's disease associated with pesticide exposure and a weaker association with well-water exposure, farming as an occupation, and generally living in a rural setting (82, 85–88).
Other environmental risk factors possibly associated with Parkinson's disease include educational status, occupation, body mass index, dairy product consumption, and estrogen exposure, although results have been inconsistent. Higher levels of education have been shown to be associated with increased risk of Parkinson's disease, and physicians have been found to have higher rates of Parkinson's disease than nonphysicians (89). Construction workers, miners, production workers, metal workers, and engineers have lower rates of Parkinson's disease than people in other occupations (89). In addition, Parkinson's disease is typically less common in women than in men (42). Evidence from animal models, as well as from humans, suggests that estrogens may protect the nigrostriatal dopaminergic pathway affected in Parkinson's disease (90). Additional studies with large sample sizes must be completed to establish the role of these exposures in Parkinson's disease development.
ASSOCIATIONS
Studies were selected through a computerized search of PubMed and the Web of Science by using the keywords “Parkinson disease,” “Parkinson's disease,” “Parkinsons disease,” “PD,” “UCHL1,” “UCH-L1,” and “UCHL-1.” We included papers published through July 1, 2008. References in the papers found via the computerized search were examined to identify additional studies. Studies were included if they provided original data on the association of Parkinson's disease with UCHL1, were published in peer-reviewed journals, and used either a case-control or a family-based design. Two of the authors (M. R. and C. H.) independently performed this search, reviewed all studies, and abstracted data using a standardized form.
Some papers contained discrepancies between the sample sizes and the genotype/allele counts presented. When discrepancies were found, we either referred to updated data in existing meta-analyses (26, 91) or contacted the corresponding author to resolve the discrepancy. To date, studies of the S18Y variant and Parkinson's disease have been performed exclusively in populations of European or Asian descent. One study was primarily family based (92), whereas the remainder used case-control designs. For the study that was both family based and case-control, we present only the case-control results in our tables and meta-analysis. In addition to ethnic group differences, other important differences between studies were the control selection methods and the covariates used as adjustment factors in the analysis. Most studies adjusted for covariates, including age and sex, and covariates included in the model do not appear to account for differences in results.
We conducted a meta-analysis to evaluate the relation between UCHL1 S18Y and Parkinson's disease. Because of allele frequency differences between ethnic groups and potential population stratification, we performed the meta-analysis separately for studies of subjects of European ancestry (11 studies) and Asian ancestry (5 studies). Currently, there is no conclusive evidence for the genetic model underlying the potential relation between S18Y genotype and Parkinson's disease; therefore, we examined a dominant, recessive, and additive model of inheritance for the minor (Y) allele. The overall odds ratio, 95% confidence interval, and P value were calculated by using a random-effects model (93). The random-effects (DerSimonian-Laird) model accounts for between-study heterogeneity. Forest plots were used to display the results from individual studies, as well as summary results. The significance of between-study heterogeneity was evaluated by using Cochran's Q statistic. If the P value was less than 0.10, the heterogeneity was considered statistically significant. We also quantified heterogeneity using the I2 metric (94). I2 takes values between 0% and 100%; higher values indicate higher levels of heterogeneity.
Pearson's χ2 was used to evaluate Hardy-Weinberg equilibrium in the control group for all studies. Samples with P values below 0.05 were considered out of Hardy-Weinberg disequilibrium and were excluded from the meta-analysis. One study (Satoh and Kuroda (23)) was out of Hardy-Weinberg equilibrium in the control group (P = 0.01) and was excluded from the primary meta-analysis. For sensitivity analysis, we repeated the meta-analysis including the Satoh and Kuroda study. We also repeated the analysis excluding the subset of the Maraganore et al. study (20) included in their initial 1999 report and excluding the study by Facheris et al. (92) because it was primarily family based. All analyses were performed by using Stata software, version 9.0 (Stata Corporation, College Station, Texas).
RESULTS
Table 3 summarizes the evidence for an association between UCHL1 S18Y and Parkinson's disease from 6 case-control studies of subjects of Asian ancestry and 12 case-control studies of subjects of European ancestry. The table includes genotype counts, a summary of the primary finding as reported in the original publication, and a calculation of the crude odds ratios for the comparison of SY versus SS and YY versus SS. Although the results are not completely consistent across studies, the overall results show little evidence for an association with the S18Y variant. With the exception of the 2 recent studies by Healy et al. (26) (1,536 cases and 1,487 controls) and Hutter et al. (95) (1,757 cases and 2,016 controls), the sample sizes for these studies were relatively small (median sample size: 169 cases and 165 controls).
Table 3.
Genotype Counts From Case-Control Studies Evaluating the Association Between the UCHL1 S18Y Variant and Parkinson's Disease Published Through July 1, 2008
| Country and Study (Reference No.) | Genotype | No. of Cases | No. of Controls | Recalculated Odds Ratioa | 95% Confidence Interval | P Value |
| Asia | ||||||
| China | ||||||
| Wang et al., 2002 (98) | SS | 40 | 45 | 1.00 | 0.802 | |
| SY | 82 | 80 | 1.15 | 0.66, 2.02 | ||
| YY | 38 | 35 | 1.22 | 0.62, 2.4 | ||
| Japan | ||||||
| Mizuta et al., 2006b (106) | SS | 149 | 199 | 1.00 | 0.165 | |
| SY | 340 | 366 | 1.22 | 0.94, 1.59 | ||
| YY | 124 | 171 | 1.00 | 0.72, 1.37 | ||
| Momose et al., 2002 (107) | SS | 71 | 61 | 1.00 | 0.136 | |
| SY | 119 | 122 | 0.84 | 0.54, 1.31 | ||
| YY | 40 | 65 | 0.53 | 0.30, 0.92 | ||
| Satoh and Kuroda, 2001 (23) | SS | 28 | 41 | 1.00 | 0.011 | |
| SY | 35 | 62 | 0.83 | 0.42, 1.64 | ||
| YY | 11 | 52 | 0.31 | 0.12, 0.74 | ||
| Zhang et al., 2000 (108) | SS | 52 | 35 | 1.00 | 0.095 | |
| SY | 77 | 86 | 0.60 | 0.34, 1.05 | ||
| YY | 31 | 39 | 0.54 | 0.27, 1.06 | ||
| Singapore | ||||||
| Tan et al., 2006 (109) | SS | 93 | 71 | 1.00 | 0.202 | |
| SY | 194 | 172 | 0.86 | 0.58, 1.27 | ||
| YY | 88 | 98 | 0.69 | 0.44, 1.07 | ||
| Australia | ||||||
| Australia | ||||||
| Mellick and Silburn, 2000 (100) | SS | 100 | 101 | 1.00 | 0.188 | |
| SY | 33 | 38 | 0.88 | 0.49, 1.56 | ||
| YY | 9 | 3 | 3.03 | 0.72, 17.81 | ||
| Europe | ||||||
| France | ||||||
| Elbaz et al., 2003 (97) | SS | 139 | 323 | 1.00 | 0.182 | |
| SY | 67 | 145 | 1.07 | 0.74, 1.55 | ||
| YY | 3 | 20 | 0.35 | 0.07, 1.21 | ||
| Levecque et al., 2001 (101) | SS | 76 | 64 | 1.00 | 0.852 | |
| SY | 33 | 24 | 1.16 | 0.59, 2.27 | ||
| YY | 5c | 5 | 0.84 | 0.19, 3.84 | ||
| Germany | ||||||
| Wintermeyer et al., 2000 (25) | SS | 169 | 128 | 1.00 | 0.059 | |
| SY | 51 | 65 | 0.59 | 0.38, 0.94 | ||
| YY | 9 | 7 | 0.97 | 0.31, 3.17 | ||
| Italy | ||||||
| Savettieri et al., 2001 (102) | SS | 118 | 115 | 1.00 | 0.999 | |
| SY | 46 | 45 | 1.00 | 0.60, 1.67 | ||
| YY | 5 | 5 | 0.97 | 0.22, 4.36 | ||
| Sweden | ||||||
| Carmine Belin et al., 2007d (99) | SS | 218 | 191 | 1.00 | 0.216 | |
| SY | 74 | 89 | 0.73 | 0.50, 1.07 | ||
| YY | 4 | 5 | 0.70 | 0.14, 3.31 | ||
| United Kingdom | ||||||
| Healy et al., 2006 (26) | SS | 1,074 | 1,028 | 1.00 | 0.54 | |
| SY | 409 | 418 | 0.94 | 0.79, 1.1 | ||
| YY | 44 | 36 | 1.17 | 0.73, 1.89 | ||
| North America | ||||||
| United States | ||||||
| Facheris et al., 2005 (92) | SS | 44 | 41 | 1.00 | 0.044 | |
| SY | 26 | 23 | 1.05 | 0.49, 2.26 | ||
| YY | 0 | 6 | 0 | 0, 0.62 | ||
| Hutter et al., 2008 (95) | SS | 1,191 | 1,324 | 1.00 | 0.388 | |
| SY | 509 | 621 | 0.91 | 0.79, 1.95 | ||
| YY | 57 | 71 | 0.89 | 0.61, 1.29 | ||
| Maraganore et al., 1999c (20) | SS | 95 | 64 | 1.00 | 0.068 | |
| SY | 35 | 42 | 0.56 | 0.31, 1.01 | ||
| YY | 2 | 4 | 0.34 | 0.03, 2.45 | ||
| Maraganore et al., 2004e (91) | SS | 120 | 48 | 1.00 | ||
| SY | 48 | 25 | 0.77 | 0.41, 1.45 | ||
| YY | 7 | 7 | 0.4 | 0.11, 1.42 | ||
| Zhang et al., 2000 (108) | SS | 108 | 105 | 1.00 | 0.285 | |
| SY | 40 | 36 | 1.08 | 0.62, 1.89 | ||
| YY | 5 | 1 | 4.86 | 0.53, 232.03 | ||
Abbreviation: UCHL1, ubiquitin carboxyl-terminal esterase L1 gene.
Unadjusted odds ratios were calculated by the authors of the present review using raw genotype data presented in published studies.
Excluding cases included in genotype counts in the Momose et al. study (107).
Incorrect count presented in the initial publication; corrected genotype count obtained from Maraganore et al. (91).
Incorrect counts presented in the initial publication; corrected genotype counts obtained courtesy of the author.
Excluding cases included in genotype counts in the Maraganore et al. study (20).
Figure 1 shows the results of the meta-analysis for populations reported to be of Asian ancestry. The overall odds ratio under a dominant model was not significantly different from 1 (odds ratio (OR) = 0.88, 95% confidence interval (CI): 0.68, 1.14; P = 0.33); however, there was evidence for a significant association under a recessive model (OR = 0.79, 95% CI: 0.67, 0.94; P = 0.01). Including the one study in which allele frequencies were not in Hardy-Weinberg equilibrium (23) did not substantively change the results. There was marginal evidence for between-study heterogeneity under the dominant model (P = 0.044; I2 = 56.0%) but not under the recessive model (P = 0.463; I2 = 0.0%). The odds ratio for meta-analysis under a random-effects, additive model (coding genotypes 0, 1, 2 based on numbers of copies of the Y allele) was significant for populations of Asian descent (OR = 0.83, 95% CI: 0.71, 0.99; P = 0.029; I2 = 59.9%; heterogeneity P = 0.029). When we excluded the study out of Hardy-Weinberg equilibrium (Satoh and Kuroda (23)), the odds ratio for meta-analysis under a random-effects, additive model suggested a trend toward reduced risk for populations of Asian descent, although it was not statistically significant (OR = 0.87, 95% CI: 0.75, 1.02; P = 0.080; I2= 50.2%; heterogeneity P = 0.090).
Figure 1.
Meta-analysis of published Parkinson's disease–UCHL1 S18Y case-control association studies of individuals of Asian ancestry: A) dominant and B) recessive model of inheritance. For each study (ordered by publication year), the odds ratio (OR) and 95% confidence interval (CI) for Parkinson's disease comparing the referent group to the risk group are shown. The overall odds ratio (dotted line and diamond) and 95% confidence interval (calculated under a random-effects model) are also shown. The referent group under the dominant model comprises the SS homozygotes, and the referent group under the recessive model comprises the SS homozygotes and SY heterozygotes. The size of each box, representing each odds ratio estimate, reflects the sample size of the study relative to the other studies. UCHL1, ubiquitin carboxyl-terminal esterase L1 gene.
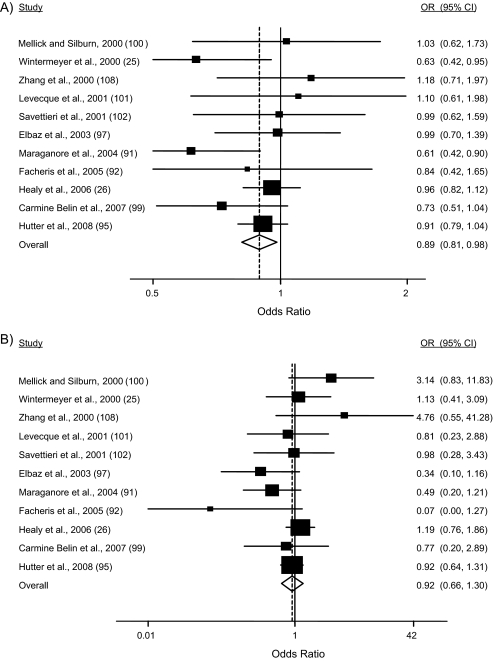
Figure 2 shows the results of the meta-analysis for subjects of European ancestry. In contrast to the results in Asian samples, the overall odds ratio under a dominant model was significantly different from 1 (OR = 0.89, 95% CI: 0.81, 0.98; P = 0.02); however, the odds ratio was not significant under a recessive model (OR = 0.92, 95% CI: 0.66, 1.30; P = 0.65). Excluding data from the first study published (20) or from the family-based study (92) did not substantively change the results of the analysis. There was no significant evidence for heterogeneity between studies (P = 0.355, I2 = 11.0% for the dominant model; P = 0.154, I2 = 25.1% for the recessive model). The odds ratio for meta-analysis under a random-effects, additive model (coding genotypes 0, 1, 2 based on numbers of copies of the Y allele) was significant in populations of European descent (OR = 0.90, 95% CI: 0.82, 0.99; P = 0.035; I2= 21.9%; heterogeneity P = 0.23).
Figure 2.
Meta-analysis of published Parkinson's disease–UCHL1 S18Y case-control association studies of individuals of European ancestry: A) dominant and B) recessive model of inheritance. For each study (ordered by publication year), the odds ratio (OR) and 95% confidence interval (CI) for Parkinson's disease comparing the referent group to the risk group are shown. The overall odds ratio (dotted line and diamond) and 95% confidence interval (calculated under a random-effects model) are also shown. The referent group under the dominant model comprises the SS homozygotes, and the referent group under the recessive model comprises the SS homozygotes and SY heterozygotes. The size of each box, representing each odds ratio estimate, reflects the sample size of the study relative to the other studies. Complete data from Maraganore et al. (91) are presented (including data from Maraganore et al. (20) and additional data included in Maraganore et al. (91)). UCHL1, ubiquitin carboxyl-terminal esterase L1 gene.
Two studies previously performed analyses to summarize the association between the UCHL1 S18Y variant and Parkinson's disease across studies. The first was a pooled analysis by Maraganore et al. (91), combining data from 11 studies with subjects of both European and Asian descent, including an unpublished abstract not used in our review (96). The authors of this pooled analysis concluded that UCHL1 is a susceptibility gene, with carriers of the Y allele at reduced risk of Parkinson's disease (meta-analysis OR under a dominant model comparing Y/Y + Y/S vs. S/S = 0.84, 95% CI: 0.73, 0.95). The second was a meta-analysis by Healy et al. (26) that examined 8 studies of white subjects and concluded that UCHL1 is not a susceptibility gene (meta-analysis under a dominant model OR = 0.96, 95% CI: 0.86, 1.08).
The major difference between the 2 meta-analyses was the ethnic groups studied. Maraganore et al. (91) included all studies, whereas Healy et al. (26) restricted their meta-analysis to white populations. Indeed, in the Maraganore et al. study, the meta-analysis results were not significant when restricted to white populations. Our results are consistent with these findings, in that we found slight differences in the meta-analysis results for populations of Asian descent compared with populations of European descent. Notably, the association was significant under a recessive model in Asian populations but under a dominant model in populations of European descent. This finding may reflect underlying biologic differences or simply the differences in allele frequencies. There is more power to detect a recessive association in populations of Asian descent, because the minor allele frequency is higher.
INTERACTIONS
Most association studies of the UCHL1 S18Y variant and Parkinson's disease have focused on main effects between S18Y and case-control status. However, a number of studies have also examined potential effect modification by age. More specifically, 4 studies found evidence for an association when restricting the analysis to early-onset cases (20, 97–99), but 3 studies did not (1, 95, 100). A major difficulty when comparing results across studies is the difference in the age cutoff used to define early onset. The most common was 50 years (1, 95, 98–101), but cutoff points of 59 years (102) and 67 years (91) were also used. Further studies differ as to whether cases were stratified by age at onset, age at diagnosis, or age at study entry and whether controls were also stratified by age. These inconsistencies limit our ability to perform a rigorous meta-analysis stratified by age.
To date, 3 studies have examined gene-gene and/or gene-environment interactions involving UCHL1 and Parkinson's disease. Elbaz et al. (97) examined potential interactions of the UCHL1 S18Y variant with smoking and pesticide use; they did not observe a main effect for the S18Y variant, and there was no evidence for an interaction with either of these environmental exposures. Maraganore et al. (103) reported evidence for gene-gene interaction between UCHL1 and α-synuclein genotypes in women, although they did not find an interaction in men. A third study by McCulloch et al. (84) examined UCHL1 S18Y in conjunction with 3 other candidate gene polymorphisms (SNCA Rep1, MAPT H1/H2 haplotypes, and apolipoprotein E (APOE) e2/e3/e4) and 2 environmental factors (smoking and caffeinated coffee consumption). The authors did not observe evidence for a main effect with UCHL1 S18Y or for interactions with smoking or caffeinated coffee consumption. However, they did report evidence for gene-environment interactions for polymorphisms in APOE and coffee as well as for SNCA and smoking (84).
LABORATORY AND POPULATION TESTS
UCHL1 genotyping is performed routinely in research settings; however, a UCHL1 laboratory test is not commercially available at this time. Given the lack of conclusive evidence supporting a strong association between UCHL1 polymorphisms and Parkinson's disease, it seems unlikely that UCHL1 population testing will be undertaken in the near future.
OTHER PUBLIC HEALTH APPLICATIONS
The Venice criteria were developed by the Human Genome Epidemiology Network (HuGENet) Working Group to provide guidance in assessing the cumulative epidemiologic evidence of genetic association studies (104). Briefly, Ioannidis et al. (104) suggest that 3 areas be considered when assessing the published literature: amount of evidence, replication, and protection from bias. The evidence in each of these areas is scored as either A, B, or C and is used in combination to provide an overall assessment of the credibility of the association between the genetic variant and phenotype in question. Evidence meeting the highest standards are given a score of A (more than 1,000 total subjects in the least common genotype group; extensive replication with good consistency between studies; little to no obvious bias in estimates for amount of evidence, replication, and protection from bias), whereas meeting moderate criteria receives a score of B (e.g., 100–1,000 total subjects in the least common genotype group; replication, but moderate inconsistency between studies; missing information to assess bias), and those of lowest quality receive a score of C. These criteria were applied as follows to evaluate the overall evidence for an association between the UCHL1 S18Y variant and Parkinson's disease.
First, we considered the amount of evidence. In Asian samples, our meta-analysis showed a significantly reduced risk of Parkinson's disease for the Y variant under a recessive model but not under a dominant model. In populations of European descent, our meta-analysis found a significantly reduced risk of Parkinson's disease under a dominant model but not a recessive model. The frequency of the Y allele ranges from 47% to 61% in samples from Asian countries and from 16% to 22% in populations of European origin, and the total sample size in our meta-analysis was 13,398 cases and controls (3,412 Asian, 9,986 European). Therefore, the amount of evidence supporting the association exceeds the n (minor allele) = 1,000 needed to qualify as “large-scale evidence” (category A). Second, we considered the level of replication. We observed only minimal between-study heterogeneity in our meta-analysis; however, the total number of studies examining the association of UCHL1 S18Y with Parkinson's disease in each ethnic group was relatively small. Therefore, we consider the credibility based on replication to be part of category B.
Finally, we considered the potential for bias. There was no obvious bias that may have affected the UCHL1–Parkinson's disease association, but, as is true for many candidate gene studies, most studies did not publish sufficient information to fully evaluate the potential for bias (16). Bias is a particularly important issue regarding the association between UCHL1 S18Y and Parkinson's disease given the relatively small magnitude of the effect. A weak amount of bias could drive the entire association. Thus, we assign a score of B for protection from bias. The overall score using the Venice criteria is therefore “ABB,” consistent with moderate evidence supporting the association between UCHL1 S18Y and Parkinson's disease.
Given the moderate degree of evidence for UCHL1 as a Parkinson's disease susceptibility gene, and the lack of effective prevention measures for Parkinson's disease, there is no current public health application for this association. This situation might change in the future if neuroprotective therapies are developed that, if started earlier, could delay disease onset. Finally, it is also possible that interactions between genetic variants and pharmacologic agents will be identified such that genotypes for UCHL1 or other susceptibility genes might prove useful in tailoring a patient's medication regimen.
CONCLUSIONS AND RECOMMENDATIONS FOR FUTURE RESEARCH
In summary, the evidence for the S18Y variant of UCHL1 as a Parkinson's disease susceptibility factor is moderate and suggests an effect size of 0.79–0.92. This effect size is similar to the magnitude of effect sizes reported by other genome-wide association studies of complex disease (76) and for replication of candidate genes (103). Two previous meta-analyses have been published on this subject. We added 2 studies in Asian populations and 2 studies in populations of European descent not included in the previous meta-analyses. Consistent with Maraganore et al. (91), we found a significant result under a recessive model but not a dominant model in Asian populations. Our paper is the first known to show a significant association in a meta-analysis of populations of European descent, although both previous meta-analyses found a nonsignificant trend toward an association in a dominant model (26, 91). It is possible that our study is the first adequately powered to detect a significant association given the moderate effect size and the lower allele frequency in European populations.
Additional large, well-designed studies in Asian populations and in other ethnic groups are needed to determine whether these effects are consistent across groups. Ideally, these studies would have adequate sample sizes and detailed/standardized phenotypic information to comprehensively evaluate gene-gene and gene-environment interactions. Such studies would benefit from using a tagSNP approach to comprehensively examine variation across the UCHL1 gene rather than focusing solely on the S18Y variant.
Additional research focusing on the molecular biology of UCHL1 function and Parkinson's disease is also needed. In particular, studies should attempt to better elucidate the role of UCHL1 in neuronal function and the role of the ubiquitin proteasome system in Parkinson's disease pathogenesis (105). Combining human genetic studies and functional analyses may provide new insights that could ultimately translate into more effective prevention and treatment options for Parkinson's disease.
Acknowledgments
Author affiliations: Department of Epidemiology, University of Washington School of Public Health, Seattle, Washington (Margaret Ragland, Carolyn Hutter, Karen Edwards); Institute for Public Health Genetics, University of Washington School of Public Health, Seattle, Washington (Margaret Ragland, Karen Edwards); Geriatric Research Education and Clinical Center, Veterans Affairs Puget Sound Health Care System, Seattle, Washington (Cyrus Zabetian); and Department of Neurology, University of Washington School of Medicine, Seattle, Washington (Cyrus Zabetian).
This project was financially supported under a cooperative agreement from the Centers for Disease Control and Prevention through the Association of Schools of Public Health, grant U36/CCU300430-23 and U10/CCU025038 (Karen L. Edwards, Principal Investigator).
The authors thank Saee Hamine for her assistance in preparing the manuscript for publication.
Conflict of interest: none declared.
Glossary
Abbreviations
- CI
confidence interval
- OR
odds ratio
- PARK
a region identified in linkage studies of Parkinson's disease
- SNP
single nucleotide polymorphism
- UCHL1
ubiquitin carboxyl-terminal esterase L1
References
- 1.Healy DG, Abou-Sleiman PM, Wood NW. Genetic causes of Parkinson's disease: UCHL-1. Cell Tissue Res. 2004;318(1):189–194. doi: 10.1007/s00441-004-0917-3. [DOI] [PubMed] [Google Scholar]
- 2.Yu J, Tao Q, Cheung KF, et al. Epigenetic identification of ubiquitin carboxyl-terminal hydrolase L1 as a functional tumor suppressor and biomarker for hepatocellular carcinoma and other digestive tumors. Hepatology. 2008;48(2):508–518. doi: 10.1002/hep.22343. [DOI] [PubMed] [Google Scholar]
- 3.Wilkinson KD, Lee KM, Deshpande S, et al. The neuron-specific protein PGP 9.5 is a ubiquitin carboxyl-terminal hydrolase. Science. 1989;246(4930):670–673. doi: 10.1126/science.2530630. [DOI] [PubMed] [Google Scholar]
- 4.Lowe J, McDermott H, Landon M, et al. Ubiquitin carboxyl-terminal hydrolase (PGP 9.5) is selectively present in ubiquitinated inclusion bodies characteristic of human neurodegenerative diseases. J Pathol. 1990;161(2):153–160. doi: 10.1002/path.1711610210. [DOI] [PubMed] [Google Scholar]
- 5.Moore DJ, West AB, Dawson VL, et al. Molecular pathophysiology of Parkinson's disease. Annu Rev Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- 6.Ciechanover A, Brundin P. The ubiquitin proteasome system in neurodegenerative diseases: sometimes the chicken, sometimes the egg. Neuron. 2003;40(2):427–446. doi: 10.1016/s0896-6273(03)00606-8. [DOI] [PubMed] [Google Scholar]
- 7.Lim KL, Tan JM. Role of the ubiquitin proteasome system in Parkinson's disease. BMC Biochem. 2007;8(suppl 1):S13. doi: 10.1186/1471-2091-8-S1-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wing SS. Deubiquitinating enzymes—the importance of driving in reverse along the ubiquitin-proteasome pathway. Int J Biochem Cell Biol. 2003;35(5):590–605. doi: 10.1016/s1357-2725(02)00392-8. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Fallon L, Lashuel HA, et al. The UCH-L1 gene encodes two opposing enzymatic activities that affect alpha-synuclein degradation and Parkinson's disease susceptibility. Cell. 2002;111(2):209–218. doi: 10.1016/s0092-8674(02)01012-7. [DOI] [PubMed] [Google Scholar]
- 10.Saigoh K, Wang YL, Suh JG, et al. Intragenic deletion in the gene encoding ubiquitin carboxy-terminal hydrolase in gad mice. Nat Genet. 1999;23(1):47–51. doi: 10.1038/12647. [DOI] [PubMed] [Google Scholar]
- 11.Osaka H, Wang YL, Takada K, et al. Ubiquitin carboxy-terminal hydrolase L1 binds to and stabilizes monoubiquitin in neuron. Hum Mol Genet. 2003;12(16):1945–1958. doi: 10.1093/hmg/ddg211. [DOI] [PubMed] [Google Scholar]
- 12.Meray RK, Lansbury PT., Jr Reversible monoubiquitination regulates the Parkinson disease-associated ubiquitin hydrolase UCH-L1. J Biol Chem. 2007;282(14):10567–10575. doi: 10.1074/jbc.M611153200. [DOI] [PubMed] [Google Scholar]
- 13.Nazé P, Vuillaume I, Destée A, et al. Mutation analysis and association studies of the ubiquitin carboxy-terminal hydrolase L1 gene in Huntington's disease. Neurosci Lett. 2002;328(1):1–4. doi: 10.1016/s0304-3940(02)00231-8. [DOI] [PubMed] [Google Scholar]
- 14.Ross CA, Pickart CM. The ubiquitin-proteasome pathway in Parkinson's disease and other neurodegenerative diseases. Trends Cell Biol. 2004;14(12):703–711. doi: 10.1016/j.tcb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Xue S, Jia J. Genetic association between ubiquitin carboxy-terminal hydrolase-L1 gene S18Y polymorphism and sporadic Alzheimer's disease in a Chinese Han population. Brain Res. 2006;1087(1):28–32. doi: 10.1016/j.brainres.2006.02.121. [DOI] [PubMed] [Google Scholar]
- 16.Kagara I, Enokida H, Kawakami K, et al. CpG hypermethylation of the UCHL1 gene promoter is associated with pathogenesis and poor prognosis in renal cell carcinoma. J Urol. 2008;180(1):343–351. doi: 10.1016/j.juro.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 17.Okochi-Takada E, Nakazawa K, Wakabayashi M, et al. Silencing of the UCHL1 gene in human colorectal and ovarian cancers. Int J Cancer. 2006;119(6):1338–1344. doi: 10.1002/ijc.22025. [DOI] [PubMed] [Google Scholar]
- 18.Leroy E, Boyer R, Auburger G, et al. The ubiquitin pathway in Parkinson's disease. Nature. 1998;395(6701):451–452. doi: 10.1038/26652. [DOI] [PubMed] [Google Scholar]
- 19.Nishikawa K, Li H, Kawamura R, et al. Alterations of structure and hydrolase activity of parkinsonism-associated human ubiquitin carboxyl-terminal hydrolase L1 variants. Biochem Biophys Res Commun. 2003;304(1):176–183. doi: 10.1016/s0006-291x(03)00555-2. [DOI] [PubMed] [Google Scholar]
- 20.Maraganore DM, Farrer MJ, Hardy JA, et al. Case-control study of the ubiquitin carboxy-terminal hydrolase L1 gene in Parkinson's disease. Neurology. 1999;53(8):1858–1860. doi: 10.1212/wnl.53.8.1858. [DOI] [PubMed] [Google Scholar]
- 21.Harhangi BS, Farrer MJ, Lincoln S, et al. The Ile93Met mutation in the ubiquitin carboxy-terminal-hydrolase-L1 gene is not observed in European cases with familial Parkinson's disease. Neurosci Lett. 1999;270(1):1–4. doi: 10.1016/s0304-3940(99)00465-6. [DOI] [PubMed] [Google Scholar]
- 22.Lincoln S, Vaughan J, Wood N, et al. Low frequency of pathogenic mutations in the ubiquitin carboxy-terminal hydrolase gene in familial Parkinson's disease. Neuroreport. 1999;10(2):427–429. doi: 10.1097/00001756-199902050-00040. [DOI] [PubMed] [Google Scholar]
- 23.Satoh J, Kuroda Y. A polymorphic variation of serine to tyrosine at codon 18 in the ubiquitin C-terminal hydrolase-L1 gene is associated with a reduced risk of sporadic Parkinson's disease in a Japanese population. J Neurol Sci. 2001;189(1-2):113–117. doi: 10.1016/s0022-510x(01)00555-x. [DOI] [PubMed] [Google Scholar]
- 24.Shi Q, Tao E. An Ile93Met substitution in the UCH-L1 gene is not a disease-causing mutation for idiopathic Parkinson's disease. Chin Med J (Engl) 2003;116(2):312–313. [PubMed] [Google Scholar]
- 25.Wintermeyer P, Krüger R, Kuhn W, et al. Mutation analysis and association studies of the UCHL1 gene in German Parkinson's disease patients. Neuroreport. 2000;11(10):2079–2082. doi: 10.1097/00001756-200007140-00004. [DOI] [PubMed] [Google Scholar]
- 26.Healy DG, Abou-Sleiman PM, Casas JP, et al. UCHL-1 is not a Parkinson's disease susceptibility gene. Ann Neurol. 2006;59(4):627–633. doi: 10.1002/ana.20757. [DOI] [PubMed] [Google Scholar]
- 27.International HapMap Consortium. The International HapMap Project. Nature. 2003;426(6968):789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 28.Carlson CS, Eberle MA, Rieder MJ, et al. Selecting a maximally informative set of single-nucleotide polymorphisms for association analyses using linkage disequilibrium. Am J Hum Genet. 2004;74(1):106–120. doi: 10.1086/381000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samii A, Nutt JG, Ransom BR. Parkinson's disease. Lancet. 2004;363(9423):1783–1793. doi: 10.1016/S0140-6736(04)16305-8. [DOI] [PubMed] [Google Scholar]
- 30.Jankovic J. Parkinson's disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79(4):368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 31.Forno LS. Neuropathology of Parkinson's disease. J Neuropathol Exp Neurol. 1996;55(3):259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Leverenz JB, Umar I, Wang Q, et al. Proteomic identification of novel proteins in cortical Lewy bodies. Brain Pathol. 2007;17(2):139–145. doi: 10.1111/j.1750-3639.2007.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spillantini MG, Crowther RA, Jakes R, et al. Alpha-synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with Lewy bodies. Proc Natl Acad Sci U S A. 1998;95(11):6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. 1991;114(pt 5):2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- 35.Hughes AJ, Ben-Shlomo Y, Daniel SE, et al. What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study. Neurology. 1992;42(6):1142–1146. doi: 10.1212/wnl.42.6.1142. [DOI] [PubMed] [Google Scholar]
- 36.Hughes AJ, Daniel SE, Ben-Shlomo Y, et al. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain. 2002;125(pt 4):861–870. doi: 10.1093/brain/awf080. [DOI] [PubMed] [Google Scholar]
- 37.Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hughes AJ, Daniel SE, Lees AJ. Improved accuracy of clinical diagnosis of Lewy body Parkinson's disease. Neurology. 2001;57(8):1497–1499. doi: 10.1212/wnl.57.8.1497. [DOI] [PubMed] [Google Scholar]
- 39.Rajput AH, Rozdilsky B, Rajput A. Accuracy of clinical diagnosis in parkinsonism—a prospective study. Can J Neurol Sci. 1991;18(3):275–278. doi: 10.1017/s0317167100031814. [DOI] [PubMed] [Google Scholar]
- 40.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1988;51(6):745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dorsey ER, Constantinescu R, Thompson JP, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68(5):384–386. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- 42.Shulman LM, Bhat V. Gender disparities in Parkinson's disease. Expert Rev Neurother. 2006;6(3):407–416. doi: 10.1586/14737175.6.3.407. [DOI] [PubMed] [Google Scholar]
- 43.Elbaz A, Bower JH, Maraganore DM, et al. Risk tables for parkinsonism and Parkinson's disease. J Clin Epidemiol. 2002;55(1):25–31. doi: 10.1016/s0895-4356(01)00425-5. [DOI] [PubMed] [Google Scholar]
- 44.Hirtz D, Thurman DJ, Gwinn-Hardy K, et al. How common are the “common” neurologic disorders? Neurology. 2007;68(5):326–337. doi: 10.1212/01.wnl.0000252807.38124.a3. [DOI] [PubMed] [Google Scholar]
- 45.Elbaz A, Tranchant C. Epidemiologic studies of environmental exposures in Parkinson's disease. J Neurol Sci. 2007;262(1–2):37–44. doi: 10.1016/j.jns.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 46.von Campenhausen S, Bornschein B, Wick R, et al. Prevalence and incidence of Parkinson's disease in Europe. Eur Neuropsychopharmacol. 2005;15(4):473–490. doi: 10.1016/j.euroneuro.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 47.Zhang ZX, Roman GC, Hong Z, et al. Parkinson's disease in China: prevalence in Beijing, Xian, and Shanghai. Lancet. 2005;365(9459):595–597. doi: 10.1016/S0140-6736(05)17909-4. [DOI] [PubMed] [Google Scholar]
- 48.Okubadejo NU, Bower JH, Rocca WA, et al. Parkinson's disease in Africa: a systematic review of epidemiologic and genetic studies. Mov Disord. 2006;21(12):2150–2156. doi: 10.1002/mds.21153. [DOI] [PubMed] [Google Scholar]
- 49.Schoenberg BS, Osuntokun BO, Adeuja AO, et al. Comparison of the prevalence of Parkinson's disease in black populations in the rural United States and in rural Nigeria: door-to-door community studies. Neurology. 1988;38(4):645–646. doi: 10.1212/wnl.38.4.645. [DOI] [PubMed] [Google Scholar]
- 50.Albert SM. Projecting neurologic disease burden: difficult but critical. Neurology. 2007;68(5):322–323. doi: 10.1212/01.wnl.0000254509.76719.01. [DOI] [PubMed] [Google Scholar]
- 51.Gowers WR. A Manual of the Diseases of the Nervous System. 2nd ed. Philadelphia, PA: Blakiston; 1902. [Google Scholar]
- 52.Cummings SR. Understanding Parkinson disease. JAMA. 1999;281(4):276–278. doi: 10.1001/jama.281.4.376. [DOI] [PubMed] [Google Scholar]
- 53.Tanner CM, Ottman R, Goldman SM, et al. Parkinson disease in twins: an etiologic study. JAMA. 1999;281(4):341–346. doi: 10.1001/jama.281.4.341. [DOI] [PubMed] [Google Scholar]
- 54.Payami H, Zareparsi S, James D, et al. Familial aggregation of Parkinson disease: a comparative study of early-onset and late-onset disease. Arch Neurol. 2002;59(5):848–850. doi: 10.1001/archneur.59.5.848. [DOI] [PubMed] [Google Scholar]
- 55.Foltynie T, Sawcer S, Brayne C, et al. The genetic basis of Parkinson's disease. J Neurol Neurosurg Psychiatry. 2002;73(4):363–370. doi: 10.1136/jnnp.73.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tan EK, Skipper LM. Pathogenic mutations in Parkinson disease. Hum Mutat. 2007;28(7):641–653. doi: 10.1002/humu.20507. [DOI] [PubMed] [Google Scholar]
- 57.Klein C, Schlossmacher MG. Parkinson disease, 10 years after its genetic revolution: multiple clues to a complex disorder. Neurology. 2007;69(22):2093–2104. doi: 10.1212/01.wnl.0000271880.27321.a7. [DOI] [PubMed] [Google Scholar]
- 58.Belin AC, Westerlund M. Parkinson's disease: a genetic perspective. FEBS J. 2008;275(7):1377–1383. doi: 10.1111/j.1742-4658.2008.06301.x. [DOI] [PubMed] [Google Scholar]
- 59.Lesage S, Brice A. Parkinson's disease: from monogenic forms to genetic susceptibility factors. Hum Mol Genet. 2009;18(R1):R48–R59. doi: 10.1093/hmg/ddp012. [DOI] [PubMed] [Google Scholar]
- 60.Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276(5321):2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 61.Paisán-Ruíz C, Lang AE, Kawarai T, et al. LRRK2 gene in Parkinson disease: mutation analysis and case control association study. Neurology. 2005;65(5):696–700. doi: 10.1212/01.wnl.0000167552.79769.b3. [DOI] [PubMed] [Google Scholar]
- 62.Zimprich A, Biskup S, Leitner P, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44(4):601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 63.Kitada T, Asakawa S, Hattori N, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392(6676):605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 64.Bonifati V, Rizzu P, van Baren MJ, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299(5604):256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 65.Valente EM, Abou-Sleiman PM, Caputo V, et al. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304(5674):1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 66.Ramirez A, Heimbach A, Gründemann J, et al. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet. 2006;38(10):1184–1191. doi: 10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- 67.Gasser T, Müller-Myhsok B, Wszolek ZK, et al. A susceptibility locus for Parkinson's disease maps to chromosome 2p13. Nat Genet. 1998;18(3):262–265. doi: 10.1038/ng0398-262. [DOI] [PubMed] [Google Scholar]
- 68.Hicks AA, Pétursson H, Jónsson T, et al. A susceptibility gene for late-onset idiopathic Parkinson's disease. Ann Neurol. 2002;52(5):549–555. doi: 10.1002/ana.10324. [DOI] [PubMed] [Google Scholar]
- 69.Li YJ, Scott WK, Hedges DJ, et al. Age at onset in two common neurodegenerative diseases is genetically controlled. Am J Hum Genet. 2002;70(4):985–993. doi: 10.1086/339815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pankratz N, Nichols WC, Uniacke SK, et al. Genome-wide linkage analysis and evidence of gene-by-gene interactions in a sample of 362 multiplex Parkinson disease families. Hum Mol Genet. 2003;12(20):2599–2608. doi: 10.1093/hmg/ddg270. [DOI] [PubMed] [Google Scholar]
- 71.Sharma M, Mueller JC, Zimprich A, et al. The sepiapterin reductase gene region reveals association in the PARK3 locus: analysis of familial and sporadic Parkinson's disease in European populations. J Med Genet. 2006;43(7):557–562. doi: 10.1136/jmg.2005.039149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klein C, Lohmann-Hedrich K. Impact of recent genetic findings in Parkinson's disease. Curr Opin Neurol. 2007;20(4):453–464. doi: 10.1097/WCO.0b013e3281e6692b. [DOI] [PubMed] [Google Scholar]
- 73.Maraganore DM, de Andrade M, Lesnick TG, et al. High-resolution whole-genome association study of Parkinson disease. Am J Hum Genet. 2005;77(5):685–693. doi: 10.1086/496902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Freedman ML, Reich D, Penney KL, et al. Assessing the impact of population stratification on genetic association studies. Nat Genet. 2004;36(4):388–393. doi: 10.1038/ng1333. [DOI] [PubMed] [Google Scholar]
- 75.Pankratz N, Wilk JB, Latourelle JC, et al. Genomewide association study for susceptibility genes contributing to familial Parkinson disease. Hum Genet. 2009;124(6):593–605. doi: 10.1007/s00439-008-0582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Evangelou E, Maraganore DM, Ioannidis JP. Meta-analysis in genome-wide association datasets: strategies and application in Parkinson disease [electronic article] PLoS One. 2007;2(2):e196. doi: 10.1371/journal.pone.0000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Clarimon J, Scholz S, Fung HC, et al. Conflicting results regarding the semaphorin gene (SEMA5A) and the risk for Parkinson disease. Am J Hum Genet. 2006;78(6):1082–1084. doi: 10.1086/504727. author reply 1092–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Farrer MJ, Haugarvoll K, Ross OA, et al. Genomewide association, Parkinson disease, and PARK10. Am J Hum Genet. 2006;78(6):1084–1088. doi: 10.1086/504728. author reply 1092–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Goris A, Williams-Gray CH, Foltynie T, et al. No evidence for association with Parkinson disease for 13 single-nucleotide polymorphisms identified by whole-genome association screening. Am J Hum Genet. 2006;78(6):1088–1090. doi: 10.1086/504726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Elbaz A, Nelson LM, Payami H, et al. Lack of replication of thirteen single-nucleotide polymorphisms implicated in Parkinson's disease: a large-scale international study. Lancet Neurol. 2006;5(11):917–923. doi: 10.1016/S1474-4422(06)70579-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.de Lau LM, Breteler MM. Epidemiology of Parkinson's disease. Lancet Neurol. 2006;5(6):525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 82.Hancock DB, Martin ER, Mayhew GM, et al. Pesticide exposure and risk of Parkinson's disease: a family-based case-control study [electronic article] BMC Neurol. 2008;8:6. doi: 10.1186/1471-2377-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Powers KM, Kay DM, Factor SA, et al. Combined effects of smoking, coffee, and NSAIDs on Parkinson's disease risk. Mov Disord. 2008;23(1):88–95. doi: 10.1002/mds.21782. [DOI] [PubMed] [Google Scholar]
- 84.McCulloch CC, Kay DM, Factor SA, et al. Exploring gene-environment interactions in Parkinson's disease. Hum Genet. 2008;123(3):257–265. doi: 10.1007/s00439-008-0466-z. [DOI] [PubMed] [Google Scholar]
- 85.Gorell JM, Johnson CC, Rybicki BA, et al. The risk of Parkinson's disease with exposure to pesticides, farming, well water, and rural living. Neurology. 1998;50(5):1346–1350. doi: 10.1212/wnl.50.5.1346. [DOI] [PubMed] [Google Scholar]
- 86.Priyadarshi A, Khuder SA, Schaub EA, et al. Environmental risk factors and Parkinson's disease: a metaanalysis. Environ Res. 2001;86(2):122–127. doi: 10.1006/enrs.2001.4264. [DOI] [PubMed] [Google Scholar]
- 87.Ascherio A, Chen H, Weisskopf MG, et al. Pesticide exposure and risk for Parkinson's disease. Ann Neurol. 2006;60(2):197–203. doi: 10.1002/ana.20904. [DOI] [PubMed] [Google Scholar]
- 88.Kamel F, Tanner CM, Umbach DM, et al. Pesticide exposure and self-reported Parkinson's disease in the Agricultural Health Study. Am J Epidemiol. 2007;165(4):364–374. doi: 10.1093/aje/kwk024. [DOI] [PubMed] [Google Scholar]
- 89.Frigerio R, Elbaz A, Sanft KR, et al. Education and occupations preceding Parkinson disease: a population-based case-control study. Neurology. 2005;65(10):1575–1583. doi: 10.1212/01.wnl.0000184520.21744.a2. [DOI] [PubMed] [Google Scholar]
- 90.Shulman LM. Is there a connection between estrogen and Parkinson's disease? Parkinsonism Relat Disord. 2002;8(5):289–295. doi: 10.1016/s1353-8020(02)00014-7. [DOI] [PubMed] [Google Scholar]
- 91.Maraganore DM, Lesnick TG, Elbaz A, et al. UCHL1 is a Parkinson's disease susceptibility gene. Ann Neurol. 2004;55(4):512–521. doi: 10.1002/ana.20017. [DOI] [PubMed] [Google Scholar]
- 92.Facheris M, Strain KJ, Lesnick TG, et al. UCHL1 is associated with Parkinson's disease: a case-unaffected sibling and case-unrelated control study. Neurosci Lett. 2005;381(1-2):131–134. doi: 10.1016/j.neulet.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 93.Petitti D. Meta-analysis, Decision Analysis, and Cost-effectiveness Analysis: Methods for Quantitative Synthesis in Medicine. New York, NY: Oxford University Press; 1994. [Google Scholar]
- 94.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hutter CM, Samii A, Factor SA, et al. Lack of evidence for an association between UCHL1 S18Y and Parkinson's disease. Eur J Neurol. 2008;15(2):134–139. doi: 10.1111/j.1468-1331.2007.02012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gasser T, Pilz P, Omasmeier D, et al. Ubiquitin C-terminal hydrolase L1 in sporadic Parkinson's disease [abstract] Mov Disord. 2000:A201. [Google Scholar]
- 97.Elbaz A, Levecque C, Clavel J, et al. S18Y polymorphism in the UCH-L1 gene and Parkinson's disease: evidence for an age-dependent relationship. Mov Disord. 2003;18(2):130–137. doi: 10.1002/mds.10326. [DOI] [PubMed] [Google Scholar]
- 98.Wang J, Zhao CY, Si YM, et al. ACT and UCH-L1 polymorphisms in Parkinson's disease and age of onset. Mov Disord. 2002;17(4):767–771. doi: 10.1002/mds.10179. [DOI] [PubMed] [Google Scholar]
- 99.Carmine Belin A, Westerlund M, Bergman O, et al. S18Y in ubiquitin carboxy-terminal hydrolase L1 (UCH-L1) associated with decreased risk of Parkinson's disease in Sweden. Parkinsonism Relat Disord. 2007;13(5):295–298. doi: 10.1016/j.parkreldis.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 100.Mellick GD, Silburn PA. The ubiquitin carboxy-terminal hydrolase-L1 gene S18Y polymorphism does not confer protection against idiopathic Parkinson's disease. Neurosci Lett. 2000;293(2):127–130. doi: 10.1016/s0304-3940(00)01510-x. [DOI] [PubMed] [Google Scholar]
- 101.Levecque C, Destée A, Mouroux V, et al. No genetic association of the ubiquitin carboxy-terminal hydrolase-L1 gene S18Y polymorphism with familial Parkinson's disease. J Neural Transm. 2001;108(8-9):979–984. doi: 10.1007/s007020170017. [DOI] [PubMed] [Google Scholar]
- 102.Savettieri G, De Marco EV, Civitelli D, et al. Lack of association between ubiquitin carboxy-terminal hydrolase L1 gene polymorphism and PD. Neurology. 2001;57(3):560–561. doi: 10.1212/wnl.57.3.560. [DOI] [PubMed] [Google Scholar]
- 103.Maraganore DM, de Andrade M, Lesnick TG, et al. Complex interactions in Parkinson's disease: a two-phased approach. Mov Disord. 2003;18(6):631–636. doi: 10.1002/mds.10431. [DOI] [PubMed] [Google Scholar]
- 104.Ioannidis JP, Boffetta P, Little J, et al. Assessment of cumulative evidence on genetic associations: interim guidelines. Int J Epidemiol. 2008;37(1):120–132. doi: 10.1093/ije/dym159. [DOI] [PubMed] [Google Scholar]
- 105.Setsuie R, Wada K. The functions of UCH-L1 and its relation to neurodegenerative diseases. Neurochem Int. 2007;51(2-4):105–111. doi: 10.1016/j.neuint.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 106.Mizuta I, Satake W, Nakabayashi Y, et al. Multiple candidate gene analysis identifies alpha-synuclein as a susceptibility gene for sporadic Parkinson's disease. Hum Mol Genet. 2006;15(7):1151–1158. doi: 10.1093/hmg/ddl030. [DOI] [PubMed] [Google Scholar]
- 107.Momose Y, Murata M, Kobayashi K, et al. Association studies of multiple candidate genes for Parkinson's disease using single nucleotide polymorphisms. Ann Neurol. 2002;51(1):133–136. doi: 10.1002/ana.10079. [DOI] [PubMed] [Google Scholar]
- 108.Zhang J, Hattori N, Leroy E, et al. Association between a polymorphism of ubiquitin carboxy-terminal hydrolase L1 (UCH-L1) gene and sporadic Parkinson's disease. Parkinsonism Relat Disord. 2000;6(4):195–197. doi: 10.1016/s1353-8020(00)00015-8. [DOI] [PubMed] [Google Scholar]
- 109.Tan EK, Puong KY, Fook-Chong S, et al. Case-control study of UCHL1 S18Y variant in Parkinson's disease. Mov Disord. 2006;21(10):1765–1768. doi: 10.1002/mds.21064. [DOI] [PubMed] [Google Scholar]