Abstract
Background
Dementia is a leading cause of death in the United States but is underrecognized as a terminal illness. The clinical course of nursing home residents with advanced dementia has not been well described.
Methods
We followed 323 nursing home residents with advanced dementia and their health care proxies for 18 months in 22 nursing homes. Data were collected to characterize the residents’ survival, clinical complications, symptoms, and treatments and to determine the proxies’ understanding of the residents’ prognosis and the clinical complications expected in patients with advanced dementia.
Results
Over a period of 18 months, 54.8% of the residents died. The probability of pneumonia was 41.1%; a febrile episode, 52.6%; and an eating problem, 85.8%. After adjustment for age, sex, and disease duration, the 6-month mortality rate for residents who had pneumonia was 46.7%; a febrile episode, 44.5%; and an eating problem, 38.6%. Distressing symptoms, including dyspnea (46.0%) and pain (39.1%), were common. In the last 3 months of life, 40.7% of residents underwent at least one burdensome intervention (hospitalization, emergency room visit, parenteral therapy, or tube feeding). Residents whose proxies had an understanding of the poor prognosis and clinical complications expected in advanced dementia were much less likely to have burdensome interventions in the last 3 months of life than were residents whose proxies did not have this understanding (adjusted odds ratio, 0.12; 95% confidence interval, 0.04 to 0.37).
Conclusions
Pneumonia, febrile episodes, and eating problems are frequent complications in patients with advanced dementia, and these complications are associated with high 6-month mortality rates. Distressing symptoms and burdensome interventions are also common among such patients. Patients with health care proxies who have an understanding of the prognosis and clinical course are likely to receive less aggressive care near the end of life.
Agrowing number of americans are dying with dementia.1 Prior work suggests that patients with advanced dementia are under-recognized as being at high risk for death and receive suboptimal palliative care.2-4 The lack of information characterizing the final stage of dementia may impede the quality of care provided to these patients.
Our current understanding of end-stage dementia is based on findings from retrospective studies,3-7 cross-sectional studies,8 or investigations of hospitalized patients.9-12 The clinical course of advanced dementia has not been described in a rigorous, prospective manner. The incidence of clinical complications, the extent of physical suffering, and the use of burdensome interventions are not well understood.
A better understanding of the clinical trajectory of end-stage dementia is a critical step toward improving the care of patients with this condition. This knowledge would help to give health care providers, patients, and families more realistic expectations about what they will confront as the disease progresses and the end of life approaches. To this end, we conducted an 18-month, multicenter, prospective study of 323 nursing home residents with advanced dementia.
Methods
Study Design
Data were obtained from the Choices, Attitudes, and Strategies for Care of Advanced Dementia at the End-of-Life (CASCADE) study, a prospective cohort study of nursing home residents with advanced dementia and their families (health care proxies) that was funded by the National Institutes of Health. The study’s overriding goal was to address major gaps in knowledge concerning care for patients with advanced dementia. A detailed description of the study design is provided elsewhere.13 The institutional review board at Hebrew SeniorLife in Boston approved the conduct of the study. Health care proxies provided written informed consent for the residents’ participation in the study and for their own.
Selection of Study Subjects
Subjects were recruited between February 2003 and August 2007 from 22 nursing homes with more than 60 beds each, located within 60 miles of Boston. At baseline and quarterly thereafter, facility administrators identified residents who met the following requirements: age, 60 years or older; score on the Cognitive Performance Scale from the most recent Minimum Data Set assessment, 5 or 6; and length of stay, more than 30 days. The Cognitive Performance Scale is a validated measure that uses five variables from the Minimum Data Set to group residents into categories ranging from intact cognition (indicated by a score of 0) to very severe impairment (indicated by a score of 6). A score of 5 corresponds to a mean (±SD) score of 5.1±5.3 on the Mini-Mental State Examination.14 Residents meeting these requirements were evaluated for the following additional eligibility criteria: cognitive impairment due to dementia, as documented in the chart; stage 7 on the Global Deterioration Scale,15 as determined by the resident’s nurse (range of stages, 1 through 7); and the availability of an appointed health care proxy who could communicate in English. At stage 7 on the Global Deterioration Scale, patients have profound cognitive deficits (inability to recognize family members), minimal verbal communication, total functional dependence, incontinence of urine and stool, and inability to ambulate independently.
Data Collection
Data on nursing home residents were collected from chart reviews, interviews with nurses, and brief physical examinations at baseline and once per quarter for up to 18 months; for residents who died during the study period, data were also collected within 14 days after the death. These data included sociodemographic characteristics, health status, clinical complications, distressing symptoms, burdensome interventions (hospitalization, emergency room visit, parenteral therapy, or tube feeding), and use of hospice care.
The sociodemographic data (from the baseline chart review) included information on age, sex, length of nursing home stay, race or ethnic group, marital status, and whether the resident lived in a special care unit for dementia. Data on health status included the underlying cause of dementia documented in the chart (Alzheimer’s disease, vascular dementia, or another cause), functional and cognitive status, and coexisting conditions. Functional status was quantified by nurses with the use of the Bedford Alzheimer’s Nursing Severity Subscale (on which scores range from 7 to 28, with higher scores indicating greater functional disability).16 A brief cognitive examination included the Test for Severe Impairment (on which scores range from 0 to 24, with lower scores indicating greater impairment).17
All clinical complications occurring between assessments were determined from a chart review, including suspected pneumonia, febrile episodes, eating problems, and other sentinel events. If pneumonia was suspected, documentation by a physician, nurse practitioner, or physician assistant was required. Febrile episodes (exclusive of suspected pneumonia episodes) were defined according to temperature (oral, ≥37.8°C [100°F]; rectal, ≥38.3°C [101°F]; or axillary, ≥37.2°C [99°F]) and timing (at least once within a 7-day period, with more than one occurrence of fever recorded within 7 days considered to be a single episode). Eating problems included documentation of weight loss, swallowing or chewing problems, refusal to eat or drink, suspected dehydration, and persistently reduced oral intake. Other sentinel events were defined as acute medical conditions that had the potential to lead to a clinically significant change in health status (e.g., hip fracture).
Signs of pain and dyspnea as observed and documented by the residents’ care providers were quantified as follows: “none,” “rarely” (<5 days per month), “sometimes” (5 to 10 days per month), “often” (11 to 20 days per month), and “almost daily” (more than 20 days per month). These variables were dichotomized as none or rarely versus sometimes, often, or almost daily. Aspiration, agitated behavior, and pressure ulcers were documented on the basis of interviews with nurses. (For pressure ulcers, the number and stage — I through IV — were recorded18 and categorized as “any pressure ulcer stage II or greater” or “none or stage I only.”)
The exact dates on which residents underwent the following interventions were ascertained from charts: parenteral therapy (defined as intravenous or subcutaneous hydration or administration of intravenous or intramuscular antimicrobial agents), hospitalizations, emergency room visits, and tube feeding. Referral to hospice care was also determined as recorded on patient charts.
Data on health care proxies were collected at baseline and included information on age, sex, relationship to the resident, whether the proxy understood the type of clinical complications expected in advanced dementia, and whether a nursing home physician had informed the proxy of the prognosis or the clinical complications expected in advanced dementia. At each quarterly assessment, the health care proxy was asked whether he or she thought the resident had less than 6 months to live. Health care proxies were also asked to estimate the number of years since the diagnosis of dementia had been made.
Statistical Analysis
Characteristics of nursing home residents and their health care proxies were described with the use of means for continuous variables and frequencies for categorical variables. Survival analysis was used to describe mortality. Survival time was defined as the number of days between the baseline assessment and the date of death. Data were censored at 18 months, for residents who remained alive at that time, or at the time of loss to follow-up.
We calculated the cumulative incidences of the first pneumonia episode, first febrile episode, and the onset of an eating problem, accounting for the competing risk of death.19 Survival analysis was used to determine the risk of death after these complications developed. Survival time was defined as the number of days between the date the complication occurred and the date of death. Exact dates were available for pneumonia and febrile episodes. For residents who entered the study with eating problems, the baseline date was considered to be the date of onset. For residents in whom an eating problem developed during the study, the date of onset was estimated to be midway between the date of the assessment that was conducted when the problem was first recorded and the date of the preceding assessment. For comparison purposes, survival curves were generated to describe the mortality of residents without the complication. These curves included data from residents who never had the complication as well as data from other residents before the complication developed; thereafter these residents contributed data to the group with complications. Survival curves for overall mortality and survival after clinical complications were constructed with the use of median age, median time since the diagnosis of dementia, and distribution of residents according to sex (percentage of female residents).
The proportions of residents who had distressing symptoms, underwent burdensome interventions, or were referred to hospice care during follow-up were determined. Among residents who died, the proportions with these symptoms and interventions at specified intervals before death were calculated.
Finally, the associations between the health care proxy’s perceptions of the resident’s prognosis, understanding of the clinical course, and receipt or nonreceipt of such information from a physician (independent variables) were compared with the likelihood that a burdensome intervention had occurred during the last 3 months of the resident’s life (outcome). The health care proxy’s perception of the prognosis was ascertained from the last interview before the resident’s death. These analyses were conducted with the use of generalized estimating equations to account for clustering at the facility level and were adjusted for the presence or absence of pneumonia, a febrile episode, or another sentinel event during the last 3 months of life. All analyses were conducted with the use of SAS software, version 9.1 (SAS Institute).
Results
Characteristics of the Subjects
Among the 1763 nursing home residents who met the study’s screening criteria, 572 (32.4%) met all the eligibility criteria. Among those who were eligible, 323 residents with advanced dementia (56.5%) and their health care proxies were recruited. Eligible residents excluded from the study because their health care proxies declined participation did not differ significantly with respect to age or sex from those who were enrolled. Only three residents were lost to follow-up, all because of relocation to nonparticipating facilities.
The residents’ mean age was 85.3 years (median, 86.0); 85.4% of the residents were women, 89.5% were white (10.2% were black, and 0.3% were Asian), and 19.8% were married (61.0% were widowed, and 19.2% were divorced or never married) (Table 1). The median length of the nursing home stay was 3.0 years, and the median interval since the diagnosis of dementia was 6.0 years. Alzheimer’s disease was the most common cause of dementia. Residents had severe functional disability (mean score on the Bedford Alzheimer’s Nursing Severity Subscale, 21.0) and cognitive disability (72.7% had a score of 0 on the Test for Severe Impairment). The health care proxies’ mean age was 59.9±11.6 years; 63.8% of the proxies were women, and the relationship to the resident was categorized as child (67.5%), spouse (10.2%), other family member (17.7%), guardian (3.1%), or friend (1.5%).
Table 1. Baseline Characteristics of Nursing Home Residents with Advanced Dementia*.
| Characteristic | All Residents (N = 323) |
|---|---|
| Age — yr | 85.3±7.5 |
| Female sex — no. (%) | 276 (85.4) |
| White race — no. (%)† | 289 (89.5) |
| Married — no. (%) | 64 (19.8) |
| Median length of stay — yr | 3.0 |
| Median time from dementia diagnosis to study entry — yr | 6.0 |
| Lived in special care unit for dementia — no. (%) | 141 (43.7) |
| Cause of dementia — no. (%) | |
| Alzheimer’s disease | 234 (72.4) |
| Vascular disease | 55 (17.0) |
| Other | 41 (12.7) |
| Coexisting conditions — no. (%) | |
| Active cancer | 4 (1.2) |
| Chronic obstructive lung disease | 36 (11.1) |
| Congestive heart failure | 57 (17.6) |
| Score on Bedford Alzheimer’s Nursing Severity Subscale‡ | 21.0±2.3 |
| Score of 0 on Test for Severe Impairment — no. (%)§ | 233 (72.1) |
Plus-minus values are means ±SD.
Race was determined from baseline charts and confirmed by proxies.
Scores on the Bedford Alzheimer’s Nursing Severity Subscale range from 7 to 28; higher scores indicate greater functional disability.
Scores on the Test for Severe Impairment range from 0 to 24; lower scores indicate greater cognitive impairment. For the analysis of baseline characteristics, the score was classified as 0 or higher than 0.
Survival and Clinical Complications
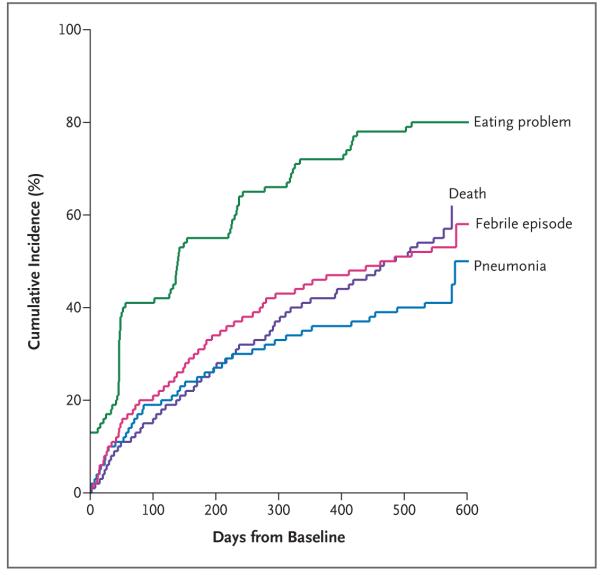
More than half the 323 residents (177, or 54.8%) died over the 18-month course of the study. The adjusted median survival was 478 days (Fig. 1), and the probability of death within 6 months was 24.7%. Most of the deaths occurred in the nursing home (93.8%).
Figure 1. Overall Mortality and the Cumulative Incidences of Pneumonia, Febrile Episodes, and Eating Problems among Nursing Home Residents with Advanced Dementia.
Overall mortality for the nursing home residents during the 18-month course of the study is shown. The residents’ median age was 86 years, and the median duration of dementia was 6 years; 85.4% of residents were women.
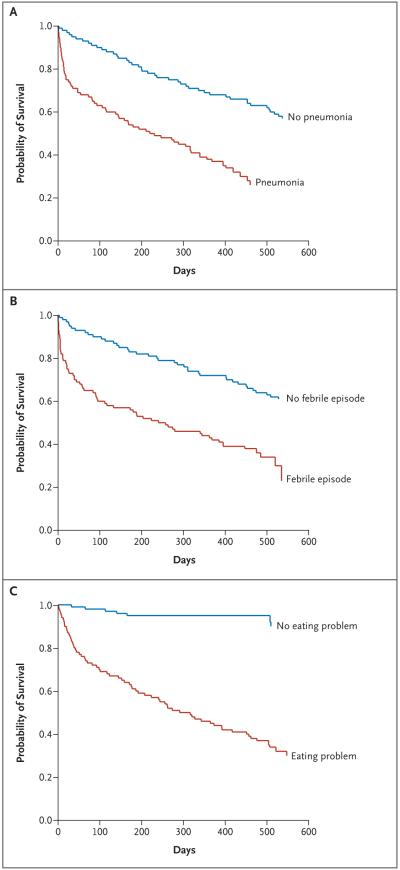
During the study period, the probability of at least one episode of pneumonia was 41.1%; a febrile episode, 52.6%; and an eating problem, 85.8% (Fig. 1). The adjusted 6-month mortality rates after the development of pneumonia, a febrile episode, and eating problems were 46.7%, 44.5%, and 38.6%, respectively (Fig. 2). In each case, these rates were substantially higher than the mortality rates for residents in whom such complications had not developed at the time of the assessment and for those in whom they never developed. Among the 177 decedents, the proportions who had these complications in the last 3 months of life were as follows: pneumonia, 37.3%; febrile episodes, 32.2%; and eating problems, 90.4%.
Figure 2. Survival after the First Episode of Pneumonia, the First Febrile Episode, and the Development of an Eating Problem.
Panel A shows the results of the survival analysis for pneumonia, Panel B the results for a febrile episode, and Panel C the results for an eating problem. The red line in each panel shows survival after development of these complications. The blue line in each panel shows the estimated survival before the complication developed or in its absence (for residents in whom the complication never developed). All curves are presented for the median age (86 years), median duration of dementia (6 years), and distribution according to sex (85.4% women).
A total of 42 sentinel events occurred in 31 of the 323 residents (9.6%) over the 18-month study period. Seizures accounted for 14 of the 42 events (33.3%), gastrointestinal bleeding for 11 (26.2%), hip fractures for 3 (7.1.0%), other bone fractures for 4 (9.5%), stroke for 3 (7.1%), pulmonary embolus for 1 (2.3%), myocardial infarction for 1 (2.3%), and other events for 5 (11.9%). Sentinel events rarely precipitated death — only seven events occurred during the last 3 months of life among residents who died: two strokes, two seizures, one hip fracture, one episode of gastroin-testinal bleeding, and one myocardial infarction.
Distressing Symptoms
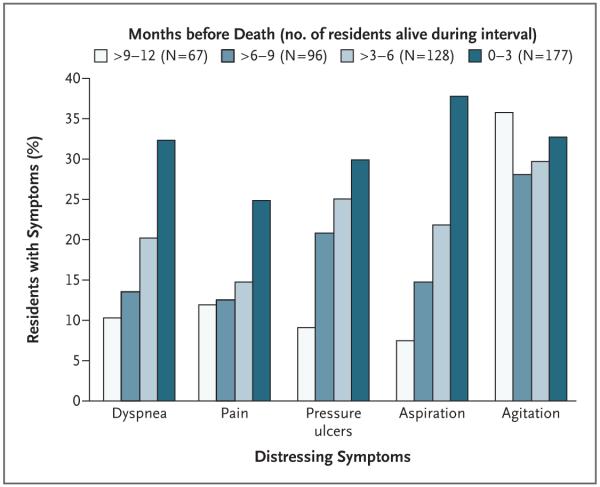
The proportions of residents who had distressing symptoms at some point during the 18-month follow-up period were as follows: dyspnea (≥5 days per month), 46.0%; pain (≥5 days per month), 39.1%; pressure ulcers (stage II or higher), 38.7%; agitation, 53.6%; and aspiration, 40.6%. Among residents who died, the proportion who had dyspnea, pain, pressure ulcers, and aspiration increased as the end of life approached (Fig. 3).
Figure 3. Proportion of Nursing Home Residents Who Had Distressing Symptoms at Various Intervals before Death.
Burdensome Interventions
During the 18-month follow-up period, 34.4% of all residents in the study were treated with parenteral therapy, 16.7% were hospitalized, 9.6% were taken to the emergency room, and 8.0% were tubefed. Among the 177 residents who died, the numbers receiving these interventions during the last 3 months of life were as follows: 52 (29.4%) received parenteral therapy, 22 (12.4%) were hospitalized, 5 (2.8%) were taken to the emergency room, 13 (7.3%) underwent tube feeding, and 72 (40.7%) underwent any one of these interventions. The most common reason for the 22 hospitalizations was pneumonia, which accounted for 15 of them (68.2%), followed by other infections, accounting for 3 (13.6%); heart failure, 2 (9.1%); hip fracture, 1 (4.5%); and dehydration, 1 (4.5%).
Hospice Referral
(29.9%) received hospice referrals, which occurred at the following intervals before death: 0 to 7 days, 26.4%; 8 to 90 days, 30.2%; 91 to 180 days, 17.0%; and more than 181 days, 26.4%.
Health Care Proxies’ perceptions
Among all 323 health care proxies, 96.0% believed comfort was the primary goal of care, and at the last assessment, 20.0% of health care proxies believed that the resident for whom they were responsible had less than 6 months to live. Only 18.0% of health care proxies stated that they had received prognostic information from a physician. Whereas 81.4% of the proxies felt they understood which clinical complications to expect in advanced dementia, only 32.5% stated that a physician had counseled them about these complications.
In the subgroup of health care proxies for residents who died, the distribution of variables characterizing perceptions of the prognosis and expected complications was similar to the distribution in the overall group (Table 2). After adjustment for clustering at the facility level and for the presence or absence of pneumonia, febrile episodes, and other sentinel events, residents whose health care proxies believed that the resident had less than 6 months to live and understood the clinical complications expected in advanced dementia were less likely to undergo a burdensome intervention during the final 3 months of life than were residents whose health care proxies did not have this understanding of the prognosis and expected complications (adjusted odds ratio, 0.12; 95% confidence interval, 0.04 to 0.37). Receipt or nonreceipt of physician counseling was not associated with the likelihood of interventions (data not shown).
Table 2. Burdensome Interventions in Nursing Home Residents during Their Last 3 Months of Life According to Health Care Proxies’ Understanding of Prognosis and Expected Clinical Complications*.
| Proxy’s Understanding of Prognosis and Expected Complications | Residents Who Died during 18-Mo Study Period (N = 177) |
Residents Who Underwent Any Burdensome Intervention during Last 3 Mo of Life |
Odds Ratio for Burdensome Intervention during Last 3 Mo of Life (95% CI)† |
|
|---|---|---|---|---|
| Unadjusted | Adjusted | |||
| no. (%) | no./total no. (%) | |||
| Believed resident had <6 mo to live | ||||
| Yes | 46 (26.0) | 14/46 (30.4) | 0.45 (0.19–1.04) | 0.34 (0.14–0.81) |
| No | 131 (74.0) | 58/131 (44.3) | Reference category | Reference category |
| Understood expected clinical complications | ||||
| Yes | 146 (82.5) | 52/146 (35.6) | 0.30 (0.15–0.62) | 0.33 (0.17–0.63) |
| No | 31 (17.5) | 20/31 (64.5) | Reference category | Reference category |
| Believed resident had <6 mo to live and understood expected clinical complications |
37 (20.9) | 10/37 (27.0) | 0.13 (0.04–0.44) | 0.12 (0.04–0.37) |
| Either believed resident had <6 mo to live or understood expected clinical complications, but not both |
118 (66.7) | 46/118 (39.0) | 0.23 (0.10–0.57) | 0.25 (0.13–0.49) |
| Neither believed resident had <6 mo to live nor understood expected clinical complications |
22 (12.4) | 16/22 (72.7) | Reference category | Reference category |
Burdensome interventions included any hospitalization or emergency room visit, parenteral therapy (administration of intravenous or subcutaneous hydration, intravenous antimicrobial agents, or intramuscular antimicrobial agents), and tube feeding. Of the 177 residents who died during the 18-month study period, 72 (40.7%) underwent at least one burdensome intervention in the last 3 months of life. CI denotes confidence interval.
Both the unadjusted and adjusted odds ratios were calculated with the use of generalized estimating equations to account for clustering at the facility level. The adjusted odds ratios were also adjusted for pneumonia (in 66 of the 177 residents [37.3%]), febrile episode (57 [32.2%]), and other sentinel events, such as hip fracture (8 [4.5%]) in the last 3 months of life.
Discussion
This prospective cohort study of nursing home residents shows that patients with advanced dementia have a high mortality rate, that infections and eating problems are likely to develop in the terminal stage of dementia, and that distressing symptoms are common and increase as death approaches. Within 3 months before death, many of the residents in our study underwent burdensome interventions of questionable benefit. However, when health care proxies were aware of the poor prognosis and the expected clinical complications, residents were less likely to undergo these interventions in the final days of life.
Our study corroborates and extends prior research showing high mortality rates among patients with advanced dementia.10,11,20-22 With a 6-month mortality rate of 25% and a median survival of 1.3 years, advanced dementia is associated with a life expectancy similar to that for more commonly recognized end-of-life conditions, such as metastatic breast cancer23 and stage IV congestive heart failure.24 The idea that dementia is a terminal illness is further supported by our finding that most of the deaths were not precipitated by devastating acute events (e.g., myocardial infarction), other terminal diseases (e.g., cancer), or the decompensation of chronic conditions (e.g., congestive heart failure).
Although it is widely held that infections and eating difficulties are hallmarks of advanced dementia,25 there are few prospective data on the incidence of these complications. Over the course of 18 months, more than half of the residents in our study had infectious episodes, and 86% had eating problems. Survival was poor after the onset of these complications.7,11,12,26 These findings can be used to inform families and care providers that infections and eating problems should be expected and that their occurrence often indicates that the end of life is near. Families and providers should also understand that although these complications may be harbingers or even precipitants of death, as they are in other terminal diseases (e.g., the acquired immunodeficiency syndrome, cancer, and emphysema), it is the major illness, in this case dementia, that is the underlying cause of death.
Although the health care proxies for the nursing home residents in our study overwhelmingly felt that the primary goal of care was comfort, physical suffering was common among the residents. Our study extends prior work describing discomfort in advanced dementia,3-5,8,12,27-29 showing that as the end of life approaches, there is an increase in distressing symptoms, the frequency and pattern of which are similar to those in patients with terminal cancer.30 Moreover, patients with dementia who are dying often receive aggressive treatments, such as tube feeding or hospitalization for pneumonia, that may be of limited benefit and that are inconsistent with a palliative approach to care.3,10,11,31-33 Although some potentially burdensome interventions may be necessary to reduce physical suffering (e.g., hospitalization for fracture), such circumstances were infrequent in this study.
Patients who believe the end of life is near34 and who have a realistic understanding of the clinical problems characterizing terminal disease35 are more likely to receive care directed toward comfort. Our findings show that these observations extend to health care proxies for nursing home residents with advanced dementia. In a recent study, patients with cancer who had end-of-life discussions with their physicians were less likely to receive aggressive care in the final week of life than were patients who did not have such discussions with their physicians.36 However, we found that the mere fact that health care proxies received counseling was not correlated with a reduced rate of burdensome interventions. Rather, our findings suggest that it is the perceptions of the health care proxies, which may be a consequence of the quality of counseling, that are associated with the aggressiveness of end-of-life care.
Several limitations of this study deserve comment. First, because all 22 of the facilities in the CASCADE study are located in the Boston area, the extent to which our findings can be generalized to other geographic areas is uncertain. However, the characteristics of the nursing homes and residents in our sample are similar to those found nationwide.13 Second, data obtained from chart reviews and nurses’ reports may be inaccurate. For example, prior work suggests that pain is underdocumented by nurses, particularly in patients with dementia.8 Third, we can report only the associations between the health care proxies’ perceptions of prognosis and of the complications expected and the use or nonuse of aggressive interventions — we cannot draw conclusions about cause and effect. Finally, even though all nursing home residents were put through a rigorous process of examination to determine whether they met the criteria for advanced dementia, it was not feasible to determine the time at which they first met these criteria. Therefore, our sample does not represent an inception cohort, and survival times do not reflect survival from the onset of advanced dementia.
As the mortality rates for many leading causes of death have declined over the past decade, deaths from dementia have steadily increased.1 Patients, families, and health care providers must understand and be prepared to confront the end stage of this disease, which is estimated to afflict more than 5 million Americans currently and is expected to afflict more than 13 million by 2050.37 Our prospective study shows that dementia is a terminal illness and furthers our knowledge of the clinical complications characterizing its final stage. We have shown that an understanding of the prognosis and expected complications on the part of health care proxies reduces the likelihood that nursing home residents with advanced dementia who are nearing the end of life will undergo potentially burdensome interventions of unclear benefit. In addition, this study underscores the need to improve the quality of palliative care in nursing homes in order to reduce the physical suffering of residents with advanced dementia who are dying.
Acknowledgments
We thank the CASCADE study data-collection and management team (Ruth Carroll, Sara Hooley, Shirley Morris, Ellen Gornstein, Nina Shikhanov, Cherie Swift, and Margaret Bryan), all the staff at the participating nursing homes, and the residents and families who have generously given their time to this study.
Supported by a grant from the National Institute on Aging (R01 AG024091) and by a Midcareer Award in Patient-Oriented Research from the National Institute on Aging (K24 AG033640, to Dr. Mitchell).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Hoyert DL, Kung HC, Smith BL. Deaths: preliminary data for 2003. Natl Vital Stat Rep. 2005;53:1–48. [PubMed] [Google Scholar]
- 2.Sachs GA, Shega JW, Cox-Hayley D. Barriers to excellent end-of-life care for patients with dementia. J Gen Intern Med. 2004;19:1057–63. doi: 10.1111/j.1525-1497.2004.30329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell SL, Kiely DK, Hamel MB. Dying with advanced dementia in the nursing home. Arch Intern Med. 2004;164:321–6. doi: 10.1001/archinte.164.3.321. [DOI] [PubMed] [Google Scholar]
- 4.Di Giulio P, Toscani F, Villani D, Brunelli C, Gentile S, Spadin P. Dying with advanced dementia in long-term care geriatric institutions: a retrospective study. J Palliat Med. 2008;11:1023–8. doi: 10.1089/jpm.2008.0020. [DOI] [PubMed] [Google Scholar]
- 5.McCarthy M, Addington-Hall J, Altmann D. The experience of dying with dementia: a retrospective study. Int J Geriatr Psychiatry. 1997;12:404–9. [PubMed] [Google Scholar]
- 6.Chen JH, Chan DC, Kiely DK, Morris JN, Mitchell SL. Terminal trajectories of functional decline in the long-term care setting. J Gerontol A Biol Sci Med Sci. 2007;62:531–6. doi: 10.1093/gerona/62.5.531. [DOI] [PubMed] [Google Scholar]
- 7.Chen JH, Lamberg JL, Chen YC, et al. Occurrence and treatment of suspected pneumonia in long-term care residents dying with advanced dementia. J Am Geriatr Soc. 2006;54:290–5. doi: 10.1111/j.1532-5415.2005.00524.x. [DOI] [PubMed] [Google Scholar]
- 8.Black BS, Finucane T, Baker A, et al. Health problems and correlates of pain in nursing home residents with advanced dementia. Alzheimer Dis Assoc Disord. 2006;20:283–90. doi: 10.1097/01.wad.0000213854.04861.cc. [DOI] [PubMed] [Google Scholar]
- 9.Ahronheim JC, Morrison RS, Baskin SA, Morris J, Meier DE. Treatment of the dying in the acute care hospital: advanced dementia and metastatic cancer. Arch Intern Med. 1996;156:2094–100. [PubMed] [Google Scholar]
- 10.Meier DE, Ahronheim JC, Morris J, Baskin-Lyons S, Morrison RS. High shortterm mortality in hospitalized patients with advanced dementia: lack of benefit of tube feeding. Arch Intern Med. 2001;161:594–9. doi: 10.1001/archinte.161.4.594. [DOI] [PubMed] [Google Scholar]
- 11.Morrison RS, Siu AL. Survival in endstage dementia following acute illness. JAMA. 2000;284:47–52. doi: 10.1001/jama.284.1.47. [DOI] [PubMed] [Google Scholar]
- 12.Aminoff BZ, Adunsky A. Dying dementia patients: too much suffering, too little palliation. Am J Hosp Palliat Care. 2005;22:344–8. doi: 10.1177/104990910502200507. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell SL, Kiely DK, Jones RN, Prigerson H, Volicer L, Teno JM. Advanced dementia research in the nursing home: the CASCADE study. Alzheimer Dis Assoc Disord. 2006;20:166–75. doi: 10.1097/00002093-200607000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris JN, Fries BE, Mehr DR, et al. MDS Cognitive Performance Scale. J Gerontol. 1994;49:M174–M182. doi: 10.1093/geronj/49.4.m174. [DOI] [PubMed] [Google Scholar]
- 15.Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139:1136–9. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 16.Volicer L, Hurley AC, Lathi DC, Kowall NW. Measurement of severity in advanced Alzheimer’s disease. J Gerontol. 1994;49:M223–M226. doi: 10.1093/geronj/49.5.m223. [DOI] [PubMed] [Google Scholar]
- 17.Albert M, Cohen C. The Test for Severe Impairment: an instrument for the assessment of patients with severe cognitive dysfunction. J Am Geriatr Soc. 1992;40:449–53. doi: 10.1111/j.1532-5415.1992.tb02009.x. [DOI] [PubMed] [Google Scholar]
- 18.National Pressure Ulcer Advisory Panel Pressure ulcers prevalence, cost and risk assessment: Consensus Development Conference Statement. Decubitus. 1989;2:24–8. [PubMed] [Google Scholar]
- 19.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 20.Luchins DJ, Hanrahan P, Murphy K. Criteria for enrolling dementia patients in hospice. J Am Geriatr Soc. 1997;45:1054–9. doi: 10.1111/j.1532-5415.1997.tb05966.x. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell SL, Kiely DK, Hamel MB, Park PS, Morris JN, Fries BE. Estimating prognosis for nursing home residents with advanced dementia. JAMA. 2004;291:2734–40. doi: 10.1001/jama.291.22.2734. [DOI] [PubMed] [Google Scholar]
- 22.Marsh GW, Prochoda KP, Pritchett E, Vojir CP. Predicting hospice appropriateness for patients with dementia of the Alzheimer’s type. Appl Nurs Res. 2000;13:187–96. doi: 10.1053/apnr.2000.7654. [DOI] [PubMed] [Google Scholar]
- 23.Fabio E, Biganzoli L. Breast cancer. In: Glare PC, Christakis NA, editors. Prognosis in advanced cancer. Oxford University Press; Oxford, England: 2008. pp. 123–32. [Google Scholar]
- 24.Nohria A, Lewis E, Stevenson LW. Medical management of advanced heart failure. JAMA. 2002;287:628–40. doi: 10.1001/jama.287.5.628. [DOI] [PubMed] [Google Scholar]
- 25.Volicer L. Management of severe Alzheimer’s disease and end-of-life issues. Clin Geriatr Med. 2001;17:377–91. doi: 10.1016/s0749-0690(05)70074-4. [DOI] [PubMed] [Google Scholar]
- 26.Volicer BJ, Hurley A, Fabiszewski KJ, Montgomery P, Volicer L. Predicting short-term survival for patients with advanced Alzheimer’s disease. J Am Geriatr Soc. 1993;41:535–40. doi: 10.1111/j.1532-5415.1993.tb01891.x. [DOI] [PubMed] [Google Scholar]
- 27.Kverno KS, Black BS, Blass DM, Geiger-Brown J, Rabins PV. Neuropsychiatric symptom patterns in hospice-eligible nursing home residents with advanced dementia. J Am Med Dir Assoc. 2008;9:509–15. doi: 10.1016/j.jamda.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caprio AJ, Hanson LC, Munn JC, et al. Pain, dyspnea, and the quality of dying in long-term care. J Am Geriatr Soc. 2008;56:683–8. doi: 10.1111/j.1532-5415.2007.01613.x. [DOI] [PubMed] [Google Scholar]
- 29.Shega JW, Hougham GW, Stocking CB, Cox-Hayley D, Sachs GA. Management of noncancer pain in community-dwelling persons with dementia. J Am Geriatr Soc. 2006;54:1892–7. doi: 10.1111/j.1532-5415.2006.00986.x. [DOI] [PubMed] [Google Scholar]
- 30.McCarthy EP, Phillips RS, Zhong Z, Drews RE, Lynn J. Dying with cancer: patients’ function, symptoms, and care preferences as death approaches. J Am Geriatr Soc. 2000;48(Suppl):S110–S121. doi: 10.1111/j.1532-5415.2000.tb03120.x. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell SL. A 93-year-old man with advanced dementia and eating problems. JAMA. 2007;298:2527–36. doi: 10.1001/jama.298.17.jrr70001. [DOI] [PubMed] [Google Scholar]
- 32.Finucane TE, Christmas C, Travis K. Tube feeding in patients with advanced dementia: a review of the evidence. JAMA. 1999;282:1365–70. doi: 10.1001/jama.282.14.1365. [DOI] [PubMed] [Google Scholar]
- 33.D’Agata EM, Mitchell SL. Patterns of antimicrobial use among nursing home residents with advanced dementia. Arch Intern Med. 2008;168:357–62. doi: 10.1001/archinternmed.2007.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weeks JC, Cook EF, O’Day SJ, et al. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA. 1998;279:1709–14. doi: 10.1001/jama.279.21.1709. Erratum, JAMA 2000;283:203. [DOI] [PubMed] [Google Scholar]
- 35.Volandes AE, Lehmann LS, Cook EF, Shaykevich S, Abbo ED, Gillick MR. Using video images of dementia in advance care planning. Arch Intern Med. 2007;167:828–33. doi: 10.1001/archinte.167.8.828. [DOI] [PubMed] [Google Scholar]
- 36.Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300:1665–73. doi: 10.1001/jama.300.14.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–22. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]