Abstract
It has been determined previously that polymorphonuclear leukocytes, or PMNs, can facilitate melanoma cell extravasation through the endothelium under shear conditions [1,2]. The interactions between melanoma cells and PMNs are mediated by the β2-integrins expressed by PMNs and intercellular adhesion molecules (ICAM-1) expressed on melanoma cells. In this study, the kinetics of these interactions was studied using a parallel plate flow chamber. The dissociation rates were calculated under low force conditions for ICAM-1 interactions with both β2-integrins, LFA-1 (CD11a/CD18) and Mac-1 (CD11b/CD18), together and separately by using functional blocking antibodies on PMNs. The kinetics of PMNs stimulated with IL-8 was also determined. It was concluded that the small number of constitutively expressed active β2-integrins on PMNs are sufficient to bind to ICAM-1 expressed on melanoma cells and that the intrinsic dissociation rate for these adhesion molecules appear to be more dependent on what method is used to determine them than on what cells express them.
Keywords: Adhesion, ICAM-1, β2 integrins, Dissociation rates, Shear flow
1 Introduction
Cancer is the second leading cause of death in the United States, causing almost one quarter of the total deaths each year [3]. Skin cancer is the most common form, accounting for approximately half of all occurrences. Although melanoma comprises only 4% of the skin cancer cases, it accounts for approximately 73% of skin cancer fatalities. Significantly higher mortality is seen in patients diagnosed with metastatic melanomas (16% survival rate), which are melanomas that have spread from the original tumor, over those that are localized to an original tumor (93% survival rate) [3].
Cancer metastasis is a complex process in which tumor cells detach from an original tumor site and are carried to a new site by the bloodstream. This process includes the attachment to and migration across a blood vessel wall after a tumor cell enters the bloodstream. Neutrophils, or PMNs, are a subpopulation of white blood cells that are also transported through the body by the flow of blood and have the capacity to migrate through blood vessel walls. Activated PMNs go through a process called extravasation, in which they first adhere to a vessel wall then migrate through the cell layers of the blood vessel and into the surrounding tissue to reach a site of inflammation.
The multi-step process of PMN extravasation has been extensively researched and many of the mechanisms have been documented. PMN tethering to an endothelial wall is initially mediated by selectins expressed on the endothelium binding to ligands on the PMN surface. These bonds form and break quickly, which results in PMNs rolling along the blood vessel wall. For shear-resistant adhesion to occur, the β2-integrins on PMNs bind to intercellular adhesion molecule-1 (ICAM-1) expressed by the endothelial cells.
In contrast, previous investigation has determined melanoma cells do not express selectin ligands or β2-integrins in sufficient concentrations to mediate these cells rolling on or adhering to the endothelium. Melanoma cells, however, do express ICAM-1, which can possibly bind to the integrins expressed by PMNs [1]. Under static conditions, melanoma cells can migrate through the endothelium, however, when a shear flow of 4 dyn/cm2 is introduced the migration is significantly decreased. When PMNs are introduced under the same shear stress conditions, 85% of the melanoma cell migration is recovered [1, 2, 4]. This evidence led to the suggestion that PMNs may facilitate melanoma cell adhesion to and migration through the endothelium.
Further studies revealed the frequency that PMNs capture melanoma cells from the free stream to adhere to the endothelium is significantly decreased when the shear rate of the flow is increased or the β2-integrins or ICAM-1 are blocked and is significantly increased when PMNs are stimulated with stimulatory factors such as interleukin-8 (IL-8) or PMA [1,2,4]. This suggests β2-integrin/ICAM-1 binding is responsible for the adhesion between PMNs and melanoma cells, and thus the migration, observed under flow conditions.
Cellular adhesion is mediated by the formation of receptor-ligand bonds. The combination of a receptor and a ligand that results in cellular adhesion can be considered as a chemical reaction. Chemical kinetics is the study of the rates of chemical reactions and has been used to study cellular adhesion. The rate of receptor-ligand binding depends on both the time of receptor-ligand interaction (governed by the hydrodynamics of the fluid flow) and the intrinsic kinetic parameters of the molecules. The adhesion of PMNs to the endothelium has been widely studied and these intrinsic kinetic parameters of the molecules involved have been documented. Experimentally determined intrinsic association and dissociation rates of the selectins and integrins have been published by several authors [5,6,7] and a review paper [8] tabulated those previously determined rates for PMN adhesion molecules. These rates were determined for not only selectin molecules, but also integrins binding with ICAM-1 on the endothelium.
Resting PMNs express very few β2-integrins in their high affinity states. Approximately 1,000 of the 15,000 expressed Mac-1 molecules and 9,000 of the 50,000 LFA-1 molecules expressed per cell are in a high affinity state before activation [9]. Previous adhesion studies have shown that LFA-1 is more important for the initial tethering step of PMN adhesion to ICAM-1, whereas Mac-1 serves to stabilize already formed adhesions [10,11 for example]. For the interactions of LFA-1 with ICAM-1, a range of intrinsic dissociation rates have been determined using different methods. Zhang et al [12] calculated a dissociation rate of 0.17 s−1 using an LFA-1 expressing T cell hybridoma line and immobilized ICAM-1 to perform atomic force microscopy (AFM) and apply Bell’s model [13]. A dissociation rate of 0.3 s−1 was estimated by Vitte et al [14] using a parallel plate flow chamber experiment utilizing Jurkat cells and immobilized ICAM-1, and Tominaga et al estimated a rate of 0.1 s−1 using a surface plasmon resonance (SPR) assay using soluble forms of both ICAM-1 and LFA-1 [15]. There is a higher variability in the association rates estimated using the different assays and cell types. Association rates were estimated from the SPR assay as 200,000 M−1s−1 [15] and from the parallel plate flow chamber assay as 82 M−1s−1 [14].
The interactions of PMNs with melanoma cells have not been studied as extensively and it is unknown whether the ICAM-1 expressed by melanoma cells has consistent binding properties with the ICAM-1 expressed by the endothelium. Estimating the kinetic parameters that describe the interactions of ICAM-1 expressed by melanoma cells with β2-integrins expressed by PMNs is the focus of the current study. Comparing these estimates to previously calculated kinetic parameters for the same molecules expressed on different cells will give an indication of whether the cell type or the molecular expression is more important in determining the binding parameters.
2 Materials and Methods
2.1 Cell culture
C8161.c9 cells were prepared and maintained as previously described [1,16]. The surface expression of ICAM-1 on C8161 cells was approximately 1625 molecules per cell, corresponding to approximately 2 molecules per μm2 (unpublished data). For some experiments, the C8161 cells were treated with 12 μg ICAM-1 antibody (R&D Systems, Minneapolis, MN) per 35mm petri dish for a minimum of 60 minutes before use. This concentration was determined to be functionally blocking by flow cytometry.
2.2 PMN isolation and preparation
Fresh blood was obtained from healthy adults following protocol approved by the Pennsylvania State University Institutional Review Board. Isolation and enriching of the PMN population was completed using histopaque gradient (Sigma, St. Louis, MO). The PMN layer was suspended in Dulbecco’s phosphate-buffered saline (DPBS) with 0.1% human serum albumin (HSA, Sigma) and washed. ACK lysis buffer (0.15 M NH4Cl, 10.0mM KHCO3, 0.1 mM Na2EDTA in distilled H2O) was added to lyse the erythrocytes. PMNs were then washed with DPBS/0.1% HSA, and rocked at 4 ° C until used, no longer than 4 hours. Before use in or treatment for an experiment, the cells were resuspended in RPMI 1640 medium with 25 mM HEPES (Biosource, Camarillo, CA) supplemented with 0.1% bovine serum albumin (BSA) at a concentration of 106 cells/ml.
In blocking assays, CD11a (from LFA-1) or CD11b (from Mac-1) on PMNs were functionally blocked by adding 1 μg/ml or 8 μg/ml of the respective antibody (CalTag Laboratories) to the PMN suspension for at least 30 minutes prior to use. Antibody was present during all experiments. To stimulate PMNs, IL-8 (R&D Systems, Minneapolis, MN; 1ng/ml, 1 hour) was added to the suspension of PMNs. To block CD11a and CD11b when PMNs were stimulated with IL-8, 15 μg/ml and 40 μg/ml blocking antibody were added, respectively. Saturating concentrations of all antibodies were determined by flow cytometric analysis and IgG isotype controls were completed for each blocking antibody to verify the specificity (data not shown).
2.3 Parallel plate flow assay
The flow experiments were performed in a parallel plate flow chamber (Glycotech; Gaithersburg, MD) mounted on a microscope with a 10X objective with an additional 1.5X tube factor magnification, giving a total magnification of 15X. A CCD camera (pco.1600; Cooke Corp, Romulus, MI) recorded each experiment to the on-board camera memory which was subsequently downloaded to a PC. A confluent monolayer of C8161 cells was cultured in 35mm petri dishes (Corning, Acton, MA) and used as the bottom surface of the flow chamber.
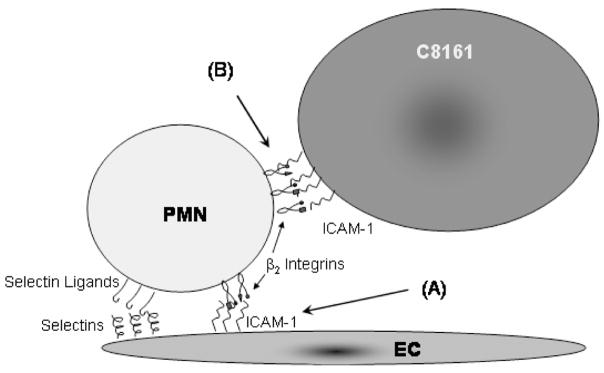
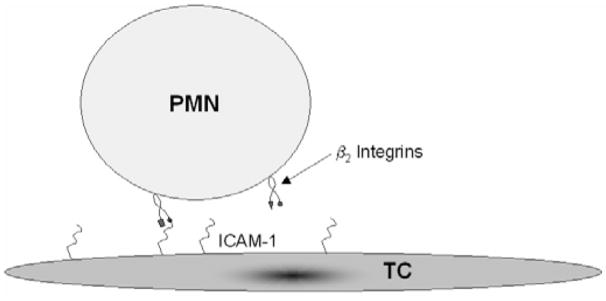
It has been suggested that PMNs may facilitate melanoma cells adhering to the endothelium. The interactions that may lead to this phenomenon are presented in Figure 1, in which a tumor cell adheres to a PMN that has already tethered to the endothelium. In order to determine the kinetics parameters that govern the binding of melanoma cells to PMNs, an experimental protocol was developed based on that described in Vitte et al [14] and the interactions involved in that setup are represented in Figure 2.
Figure 1.
PMN adhesion with the EC is mediated by both the selectins on the EC binding to selectin ligands expressed by PMNs and the ICAM-1 on the EC binding to β2-integrins on the PMN. Tumor cells may use ICAM-1 on their surface to bind to the β2-integrins on PMNs, thus achieving access to the EC.
Figure 2.
Illustration of the kinetics experiment methodology. Melanoma cells are cultured as a monolayer and PMNs are perfused over the surface at a very slow flow rate. PMNs adhere to the melanoma substrate when the β2-integrins expressed on PMNs binds to the ICAM-1 expressed on the tumor cells.
Media alone was preinfused into the chamber to allow the monolayer to reach equilibrium under no flow conditions for approximately 1–2 minutes before the cell suspension was perfused through the chamber. Using a syringe pump (Harvard Apparatus; Holliston, MA), a suspension of PMNs (106 cells/mL) was initially infused at a higher flow rate (125/s) for approximately 40 seconds to get the PMNs into the view screen. After the initial 40 seconds, the flow rate was decreased to a wall shear rate of 4.96 s−1(0.05 dyn/cm2) and kept constant for the extent of the experiment, approximately 3 minutes. The field of view was 418 μm long (direction of flow) and 400 μm wide (resolution of approximately 0.87 μm/pixel) and the frame rate was approximately 40 frames per second. Each experimental condition was repeated three to six times and the digital videos were analyzed offline.
2.4 Data analysis
Adhesion of the PMNs to the C8161 monolayer was determined manually from the digital video recordings. Adhesions were detectable down to approximately 0.1 seconds. The number of PMN adhesions, the length of each adhesion, the number of cells tracked, and the total distance the tracked cells traveled were recorded for each video and compiled with the remaining results for each condition. Following the method of Vitte et al [14], the intrinsic dissociation rate, , was calculated. In brief, each adhesion was assumed to be due to the formation of an individual bond. Using elementary chemical kinetics theory, the rate of dissociation of a bond can be determined using Eq. 1.
| (1) |
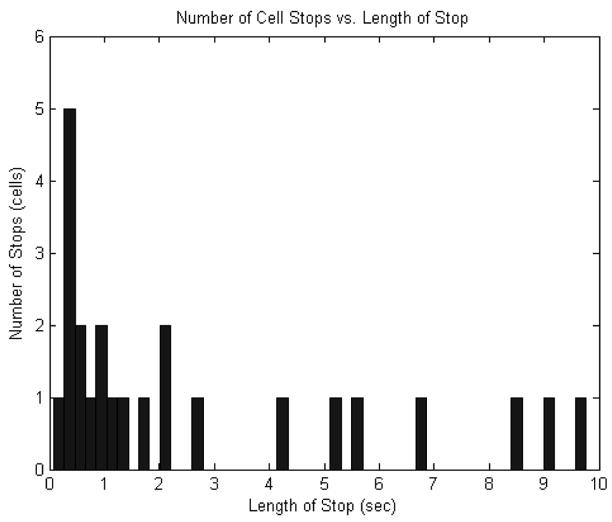
In this equation, is the dissociation rate constant for a bond breakage, t is time, and B(t)is the number of bonds at time, t. Since the number of bonds at zero time is known and the number of bonds at some later time can be determined, the rate constant can be calculated. Zero time was defined for each adhesion as the time of formation, so B(t = 0) is the total number of PMNs that adhered, or 100%. The number of PMN adhesions at any later time, B(t), is then the number of adhesions that lasted longer than that time. An unbinding plot can be formed by plotting ln[B(t)] versus time to visualize the trend of PMN dissociation. The data can alternatively be illustrated by the histogram, shown in Figure 3, of the length of PMN adhesions. The unbinding plot is in essence the result of starting with one hundred percent at zero time and at each subsequent time point subtracting the histogram value at that point.
Figure 3.
Histogram of the length of PMN adhesions to the C8161 monolayer.
For these studies, was determined directly from Eq. 2, achieved by rearranging Eq. 1.
| (2) |
The standard deviation of the can be calculated using the following statistically derived equation: SDk = [(B(t1) − B(t2))/B(t2)/B(t1)]0.5/(t2 − t1) [14].
The tethering frequency can be determined by dividing the total number of adhesions counted by the total distance all the tracked cells traversed. The standard deviation of this calculation can be estimated using N0.5/L, which was derived using known Poisson relations [14].
The estimated standard deviations were used in the student’s t-test in order to determine statistical significance of the results.
3 Results
3.1 Calculation of off-rates
Under the flow conditions of the parallel plate chamber experiments discussed here, PMNs were seen to arrest on the melanoma cell monolayer. ICAM-1 bonds with β2-integrins could withstand the small fluid forces applied to them in the system for varying timeframes. When ICAM-1 was blocked on the monolayer, the tethering frequency decreased by approximately 50%, illustrating the specificity of the binding (203 cells).
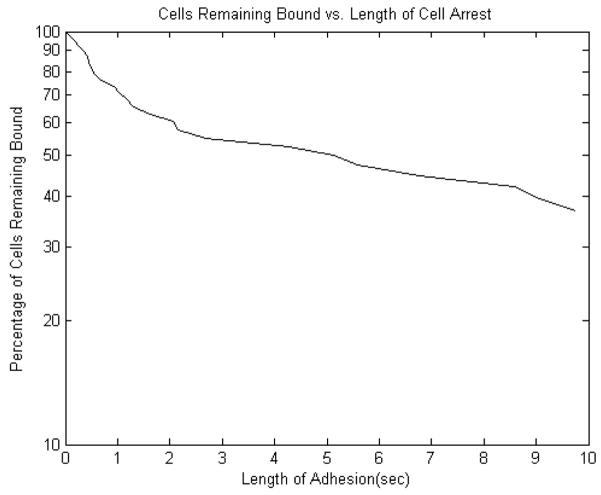
A sample unbinding plot is shown in Figure 4, which illustrates the trend of the lifetimes of individual bonds. The unbinding plots for all cases appear to have three regions that are approximately linear. From zero to one second and from one to two seconds appear linear, and then the remaining eight seconds also appear to show a linear trend. The off-rates for each condition were calculated for those three ranges. A steep unbinding plot results in a high off-rate, meaning cells detach quickly due to weak binding. A flatter unbinding plot results in a lower off-rate, which corresponds to slower cell detachment due to either stronger binding or the formation of more than one bond.
Figure 4.
Unbinding plot of interactions between unstimulated PMNs and a C8161 cell monolayer.
3.2 Kinetic off-rates of untreated PMNs binding to C8161 cells
The intrinsic dissociation rates for the binding between untreated PMNs and C8161 melanoma cells were estimated. CD11a and CD11b were blocked on the PMNs in order to isolate the kinetics of each integrin with the ICAM-1 expressed by melanoma cells. Table 1 compares the estimated dissociation rate constants, , calculated for the three linear ranges for these cases. The off-rates for these cases fall at the lower end of the spectrum of previously calculated off-rates, consistent with a low-affinity form of the β2-integrins [8].
Table 1.
Detachment rates for three time ranges (shown +/− standard error).
| Cell Treatment | (0, 1)s | (1, 2)s | (2, 10)s |
|---|---|---|---|
| Untreated | 0.306 +/− 0.097 | 0.154 +/− 0.077 | 0.067 +/− 0.022 |
| Anti-CD11a (PMN) | 0.315 +/− 0.10 | 0.16 +/− 0.08 | 0.031 +/− 0.014 |
| Anti-CD11b (PMN) | 0.288 +/− 0.096 | 0.3 +/− 0.12 | 0.064 +/− 0.023 |
| IL-8 stimulated (PMN) | 0.495 +/− 0.13 | 0.128 +/− 0.074 | 0.099 +/− 0.29 |
| IL-8 + Anti-CD11a&b (PMN) | 0.651 +/− 0.14 | 0.406 +/− 0.14 | 0.087 +/− 0.031 |
Previous studies have shown that when LFA-1 is blocked on PMNs a larger decrease in adhesion is observed than when Mac-1 is blocked [10,17]. Therefore it was expected that the CD11b blocked case would be little different than the untreated case. When CD11b was blocked, the off-rate remained constant during the first 2 seconds then dropped for the remaining 8 seconds to match the unstimulated case. When CD11a was blocked, the off-rate decreased after 1 second, indicating the binding was stronger in this case over the remaining nine seconds than in either the unstimulated or anti-CD11b cases.
3.3 Effect of IL-8 stimulation on off-rates of PMNs binding to C8161 cells
Interleukin-8, or IL-8, is an inflammatory cytokine that has been detected after melanoma cells and PMNs have been in contact [4]. Endothelial cells near a site of inflammation secrete the cytokine into the vasculature in vivo to attract PMNs to that location. When PMNs are exposed to IL-8, the initial result is an upregulation of high-affinity β2-integrins on the PMN surface to mediate firm adhesion to the endothelium. After the initial upregulation of integrins, the number of receptors returns to the base expression level. However, the binding ability of the PMNs remains increased. It has been previously reported that, in the concentration and time-frame of IL-8 stimulation used for this study, the surface density of neither β2-integrin is increased, however, the binding activity of PMNs increases regardless of the molecular expression [18,19]. Detmers et al [19] suggested this was due to colocalization of molecules or an increase in affinity. Lum et al [20] imaged CD11a, CD11b, and active CD18 using fluorescently labeled antibodies and determined after stimulation, approximately 75% of the CD11a and about 35% of the CD11b colocalizes with active CD18, however this effect was only investigated after a short stimulation time. Seo et al [21] also found both LFA-1 and Mac-1 localized to different regions of activated PMNs when stimulated. Either the receptor aggregation or the affinity change may explain the increased binding activity of PMNs due to stimulation. In migration chamber experiments, a significant increase in melanoma extravasation through the endothelium was observed when PMNs were stimulated with IL-8 and the extravasation significantly decreased when soluble IL-8 was neutralized [2,4].
In order to determine what affect stimulation with IL-8 has on the kinetics of integrins binding to ICAM-1, IL-8 was added to the PMN suspension as in the migration experiments. Table 1 compares the dissociation rates calculated for PMNs stimulated with IL-8 to those on which both CD11a and CD11b were blocked. Both CD11a and CD11b were blocked on the IL-8 stimulated cells in order to determine the amount of non-specific binding that was present. Since the result is a significantly higher dissociation rate than the untreated case (p<0.1), there is very little non-specific binding present.
The unbinding trends for the initial one second of the IL-8 stimulated cases are consistent with each other and different from the unstimulated cases. After that time, the IL-8 case and unstimulated cases are again similar (data not shown). The IL-8 stimulation appears to increase the dissociation rate over the unstimulated case, which is inconsistent with previous studies indicating that IL-8 increases tumor cell binding to PMNs. This suggests that a mechanism other than the molecular kinetics is responsible for the increased binding.
3.4 PMNs are stimulated by melanoma cells
To investigate what affect the close contact of PMNs and melanoma cells has on PMNs at short exposure times, a flow cytometry study was completed. A slight increase in Mac-1 expression was observed after PMNs were in contact with a melanoma cell monolayer for short time periods, as evidenced by the over 1.8 times increase in mean fluorescence after one minute of exposure (data not shown). No change was seen in the LFA-1 expression for up to 5 minutes of contact. This suggests that the untreated PMNs were stimulated by the melanoma cell monolayer during the experiments, possibly to a greater extent than were the PMNs that were stimulated with IL-8 before the start of the experiment. The faster dissociation rate for the first one second of adhesion for IL-8 stimulated PMNs than the unstimulated PMNs suggests contact with the melanoma monolayer stimulates a higher affinity form of β2-integrins to be expressed by PMNs than the recombinant IL-8 alone.
3.5 Tethering Frequencies
The tethering frequencies were also calculated from the experimental data, as discussed in Materials and Methods, and are compared in Table 2. All bond formations were considered tethers for this calculation, regardless of the length of time the cells were bound. In this case, a higher tethering frequency corresponds to cells adhering more often per distance and lower tethering frequencies correspond to cells adhering less frequently.
Table 2.
Attachment rate for PMN interactions with C8161 cells (shown +/− standard error).
| Cell Treatment | Cell number | Arrest Number | Attachment rate (mm−1) |
|---|---|---|---|
| Untreated | 756 | 38 | 0.135 +/− 0.022 |
| Anti-CD11a (PMN) | 766 | 37 | 0.125 +/− 0.021 |
| Anti-CD11b (PMN) | 777 | 36 | 0.137 +/− 0.023 |
| Anti-ICAM-1 (c8161) | 203 | 6 | 0.079 +/− 0.032 |
| IL-8 stimulated (PMN) | 848 | 49 | 0.144 +/− 0.023 |
| IL-8 + Anti-CD11a&b (PMN) | 769 | 46 | 0.164 +/− 0.024 |
All the cases shown here except the ICAM-1 blocked cases were not significantly different from the unstimulated case (p>0.2). However, the portion of those adhesions that remain longer than 2 seconds ranges between cases. For example, 35% are longer than 2 seconds in the IL-8 stimulated plus CD11a and CD11b blocked case whereas 63% of the adhesions in the unstimulated/unblocked case lasted longer than 2 seconds.
4 Discussion
The purpose of this study was to determine the intrinsic association and dissociation rates for melanoma cells binding to PMNs under a zero force condition, and . These experimentally determined rates can be used to calculate the probability that PMNs and melanoma cells will bind when an external flow exists using the equations developed by Bell [13] and Dembo [22]. The main conclusions of this study are that melanoma cells and PMNs adhere via a β2-integrin/ICAM-1 binding mechanism with an intrinsic dissociation rate of approximately 0.3 s−1, that under all conditions the binding exhibits a time-dependent strengthening, that close contact with melanoma cells may stimulate PMNs, and that the intrinsic dissociation rates of the involved molecules appear to be more dependent on the method used to calculate them than on the cell type that expresses them.
In general, the kinetics analysis presented here assumes each adhesion of a PMN to the monolayer is mediated by a single bond. The alternate possibility would be that each adhesion is mediated by the minimum number of bonds necessary to adhere the PMN to a melanoma cell. Under physiologic flow conditions, this minimum number will govern the probability of the two cell types adhering to each other, so the kinetic constants derived if this were the case would also be useful. However, following the arguments of Vitte et al [14], this is highly unlikely. In the unstimulated, as well as CD11a and CD11b blocked PMN cases reported here, the initial binding has a consistent of approximately 0.3 s−1, suggesting the availability of Mac-1 or LFA-1 does not affect this initial detachment rate. The approximate force on a formed bond would also be less than 1.5 pN, which is less than the unbinding force previously reported for single LFA-1/ICAM-1 interactions. These observations together with the low surface expression of adhesion molecules on both cell types indicate single bonds are indeed detected using this method.
The results show if a melanoma cell adheres to an unstimulated PMN for 2 seconds under the low force conditions, it is likely that it will remain bound for at least 10 seconds. The same trend is seen when PMNs are stimulated with IL-8, regardless of the faster dissociation rate exhibited in first 2 seconds.
The dissociation rates for β2-integrin/ICAM-1 interactions appear to be more dependent on the method used to calculate them than on the cells that express the molecule. The dissociation rate calculated in this study for the anti-CD11b case (~0.29) match almost exactly those determined by Vitte et al [14], who perfused Jurkat cells over an endothelial monolayer to determine the intrinsic dissociatin rate of LFA-1/ICAM-1 binding (~0.32). The dissociation rates presented here are within the range of those previously reported using different methods; however each method used to calculate the rate under zero force resulted in a different value.
The kinetics theory governing association is more complicated than that governing dissociation and it is also more difficult to determine experimentally. Several approximation methods using data from experiments similar to those completed and described above have been reported in the literature. For example, a confinement region analysis was completed by Vitte et al [14] to calculate the intrinsic association rate by converting the surface density of ligands to a volume-density. A method was also suggested by Zhang and Neelamegham [23] for selectin binding which lumped several parameters together that are not explicitly known, such as number of ligands bound, to estimate the effective association rate. Both of these methods were attempted and intrinsic association rates of 3000 M−1s−1 and 200 s−1 were calculated.
A similar study of β2-integrin/ICAM-1 adhesion was completed by Lomakina and Waugh [24], who studied adhesion of neutrophils to immobilized ICAM-1 using a micropipette technique. They determined the adhesion probability after neutrophils were in contact with the ICAM-1 molecules for 2 seconds, which can be compared to the probability of PMNs to remain adhered for longer than 10 seconds if they initially tethered for 2 seconds in the current experiments. Lomakina and Waugh [24] estimated an adhesion probability of about 66% for the 2 second case and in the present study the comparable adhesion probability for unstimulated PMNs was approximately 58%. The lower percentage in the current study may be attributed to the density of ICAM-1 molecules on the surfaces used. In the micropipette study, the ICAM-1 was immobilized in a much higher concentration (~360 sites/μm2) than is estimated to be expressed by C8161 cells (~2 sites/μm2). Regardless, the similarity is notable and the zero force condition association rates calculated here are in the low range of values previously reported by other researchers [8].
Compared to association and dissociation rates calculated using alternative methods, the rates estimated in this study suggest the interactions between PMNs and melanoma cells under the experimental conditions used here are mediated by low-affinity binding of β2-integrins to ICAM-1. This could be due to both the location of the expressed molecules and the media used. Integrins are expressed on the body of PMNs, not on microvilli, which limits the availability of the molecules to ICAM-1. In contrast, experimental methods that use soluble molecules, such as SPR or AFM, may show a higher affinity due to the molecules being more likely to interact with a surface expressed molecule or with another soluble molecule than are two surface expressed molecules. The media in which the PMNs were suspended was not supplemented with additional ions such as Mg2+ or Mn2+, which have been shown to stimulate β2-integrin adhesion activity by either locking them in an active state or increasing the affinity for their ligand [25]. The roles of LFA-1 and Mac-1 in adhesion of PMNs to ICAM-1 were investigated by using CD11a and CD11b monoclonal antibodies. Ding et al [17] studied the overlapping roles of CD18, LFA-1 (CD11a/CD18) and Mac-1 (CD11b/CD18) in the immune response by developing CD-18-deficient mice as well as LFA-1 and Mac-1 deficient mice. Their results suggest that the contribution of Mac-1 in adhesion is overshadowed by LFA-1 if both are expressed. Lub et al [26] also reported LFA-1 and Mac-1 compete for binding with ICAM-1 if both molecules are present in similar concentrations. This may partially explain the lack of significant differences between the association and dissociation rates calculated for untreated, anti-CD11a, and anti-CD11b cases that we observed in the present study.
In conclusion, the method was successfully used to estimate the intrinsic association and dissociation rates under a zero force condition for melanoma cells binding to PMNs in a parallel plate flow chamber. It has been previously suggested that β2-integrin/ICAM-1 binding cannot support rolling interactions between PMNs and the endothelium under physiologic flow conditions. However, melanoma cell adhesion to PMNs in parallel plate and migration chamber experiments has been suggested to be mediated by these molecules. Therefore, the results presented here will be integrated into a computational fluid dynamics model of the interactions shown in Fig. 2, which is currently under development, to determine how these bonds mediate PMN-melanoma cell adhesion while exposed to external fluid forces.
Acknowledgments
This work was supported by the National Institutes of Health (CA-97306) and National Science Foundation (BES-0138474). The Authors are grateful to Frank Meeuwissen for data collection help and for many helpful discussions with Dr. Pierre Bongrand (Laboratoire d’Immunologie, France), as well as the support of the Penn State General Clinical Research Center (GCRC) staff that provided nursing care. The GCRC is supported by NIH grants M01-RR-010732 and C06-RR-016499.
References
- 1.Slattery M, Dong Cheng. Int J Cancer. 2003;106:713–722. doi: 10.1002/ijc.11297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liang S, Slattery M, Dong C. Exp Cell Res. 2005;310:282–292. doi: 10.1016/j.yexcr.2005.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Cancer Society, Inc. 2005 No. 3002.10. www.cancer.org.
- 4.Slattery MJ, Liang S, Dong C. Am J of Cell Phys. 2005;288:C831–C839. doi: 10.1152/ajpcell.00439.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alon R, Chen S, Puri KD, Finger EB, Springer T. J Cell Biol. 1997;138:1169–1180. doi: 10.1083/jcb.138.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith MJ, Berg EL, Lawrence MB. Biop J. 1999;77:3371–3383. doi: 10.1016/S0006-3495(99)77169-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wild MK, Huang M, Schulze-Horsel U, van der Merwe PA, Vetweber D. J of Biol Chem. 2001;276:31602–31612. doi: 10.1074/jbc.M104844200. [DOI] [PubMed] [Google Scholar]
- 8.Neelamegham S. Cell Commun Adhes. 2004;1:35–50. doi: 10.1080/15419060490471793. [DOI] [PubMed] [Google Scholar]
- 9.Simon SI, Green CE. Annu Rev Biomed Eng. 2005;7:151–185. doi: 10.1146/annurev.bioeng.7.060804.100423. [DOI] [PubMed] [Google Scholar]
- 10.Hentzen ER, Neelamegham S, Kansas GS, Benanti JA, McIntire LV, Smith CW, Simon SI. Blood. 2000;95:911–920. [PubMed] [Google Scholar]
- 11.Jadhav S, Konstantopoulos K. Am J Physiol Cell Physiol. 2002;283:C1133–C1143. doi: 10.1152/ajpcell.00104.2002. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Wojcikiewicz E, Moy V. Force Spectroscopy of the Leukocyte Function-Associated Antigen-1/Intercellular Adhesion Molecule-1 Interaction. Biop J. 2002;83:2270–2279. doi: 10.1016/S0006-3495(02)73987-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell GI. Science. 1978;200:618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- 14.Vitte J, Pierres A, Benoliel A, Bongrand P. J of Leuk Biol. 2004;76:1–9. doi: 10.1189/jlb.0204077. [DOI] [PubMed] [Google Scholar]
- 15.Tominaga Y, Kita Y, Satoh A, Asai S, Kato K, Ishikawa K, Horiuchi T, Takashi T. J of Immun. 1998;161:4016–4022. [PubMed] [Google Scholar]
- 16.Welch DR, Bisi JE, Miller BE, Conaway D, Seftor EA, Yohem KH, Gilmore LB, Seftor REB, Nakajima M, Hendrix MJC. Int J Cancer. 1991;47:227–237. doi: 10.1002/ijc.2910470211. [DOI] [PubMed] [Google Scholar]
- 17.Ding Z, Babensee JE, Simon SI, Lu H, Perrard JL, Bullard DC, Dai XY, Bromley SK, Dustin ML, Entman ML, et al. J of Immun. 1999;163:5029–5038. [PubMed] [Google Scholar]
- 18.Videm V, Strand E. Scand J of Immun. 2004;59:25–33. doi: 10.1111/j.0300-9475.2004.01351.x. [DOI] [PubMed] [Google Scholar]
- 19.Detmers PA, Siu L, Siu K, Olsen-Egbert E, Walz A, Baggiolini M, Cohn ZA. J Exp Med. 1990;171:1155–1162. doi: 10.1084/jem.171.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lum AFH, Green CE, Lee GR, Staunton DE, Simon SI. Jour of Biol Chem. 2002;277:20660–20670. doi: 10.1074/jbc.M202223200. [DOI] [PubMed] [Google Scholar]
- 21.Seo SM, McIntire LV, Smith CW. Am J Physiol Cell Physiol. 2001;281:C1568–C1578. doi: 10.1152/ajpcell.2001.281.5.C1568. [DOI] [PubMed] [Google Scholar]
- 22.Dembo M, Torney DC, Saxaman K, Hammer D. Proc R Soc London. 1988;234:55–83. doi: 10.1098/rspb.1988.0038. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Neelamegham S. Biop J. 2002;83:1934–1952. doi: 10.1016/S0006-3495(02)73956-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lomakina EB, Waugh RE. Biop J. 2004;86:1223–1233. doi: 10.1016/S0006-3495(04)74196-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diamond MS, Springer TA. Current Biol. 1994;4:506–517. doi: 10.1016/s0960-9822(00)00111-1. [DOI] [PubMed] [Google Scholar]
- 26.Lub M, van Kooyk Y, Figdor CG. Jour of Leuk Biol. 1996;59:648–655. doi: 10.1002/jlb.59.5.648. [DOI] [PubMed] [Google Scholar]