Abstract
Using Drosophila spermatogenesis as a model, we show that function of the β-tubulin C-terminal tail (CTT) is not independent of the body of the molecule. For optimal microtubule function, the β-tubulin CTT and body must match. β2 is the only β-tubulin used in meiosis and spermatid differentiation. β1-tubulin is used in basal bodies, but β1 cannot replace β2. However, when β1 is co-expressed with β2, both β-tubulins are equally incorporated into all microtubules, and males exhibit near wild type fertility. In contrast, co-expression of β2β1C and β1β2C, two reciprocal chimeric molecules with bodies and tails swapped, results in defects in meiosis, cytoskeletal microtubules, and axonemes; males produce few functional sperm and few or no progeny. In these experiments, all the same β-tubulin parts are present, but unlike the co-assembled native β-tubulins, the “trans” configuration of the co-assembled chimeras is poorly functional. Our data thus reveal essential intra-molecular interactions between the CTT and other parts of the β-tubulin molecule, even though the CTT is a flexible surface feature of tubulin heterodimers and microtubules. In addition, we show that Drosophila sperm tail length depends on the total tubulin pool available for axoneme assembly and spermatid elongation. D. melanogaster and other Drosophila species have extraordinarily long sperm tails, the length of which is remarkably constant in wild type flies. We show that in males of experimental genotypes that express wild type tubulins but have half the amount of the normal tubulin pool size, sperm tails are substantially shorter than wild type.
Keywords: Drosophila, germ line, sperm, tubulin, microtubules, cytoskeleton, axoneme
INTRODUCTION
We have studied the relationship between β-tubulin primary structure and microtubule function, using Drosophila melanogaster spermatogenesis as a model system. In the post-mitotic germ cells, a single β-tubulin isoform, β2-tubulin, is used for all microtubule functions [Kemphues et al., 1982]: meiosis; several different sets of cytoskeletal microtubules; and assembly of the motile sperm flagellum, the fly’s only motile 9+2 axoneme. By examining mutations in the β2 gene and experimentally replacing β2 with other β-tubulins, we have shown that different aspects of microtubule function have different requirements for the sequence of the component β-tubulin [Kemphues et al., 1982; Fuller et al., 1987, 1988; Hoyle and Raff, 1990; Fackenthal et al., 1995; Hoyle et al., 1995, 2001; Popodi et al., 2005, 2008]. Although heterologous β-tubulins can provide some of β2’s functions, no other β-tubulin can fully replace β2. We have identified axoneme-specific requirements for the β-tubulin C-terminal tail (CTT), including a sequence motif common to all axonemal β-tubulins [Hoyle and Raff, 1990; Fackenthal et al., 1993; Hoyle et al., 1995, 2001; Raff et al., 1997, 2000; Nielsen et al., 2001; Nielsen and Raff, 2002; Popodi et al., 2005, 2008]. Not all axoneme-specific features are mediated via the CTT. For example, we have shown that the sequence in the internal variable domain at residues 55–57 constitutes an axoneme signature for addition of the outer dynein arms, independent of the CTT [Raff et al., 2008]. Both β1 and β2 have this axoneme signature. However, the β2 CTT is essential for axonemes [Fackenthal et al., 1993; Hoyle et al., 2001]. Nonetheless, Drosophila axoneme assembly and other spermatogenic microtubule functions can accommodate a mix of β-tubulins, as is the normal in vivo situation, for example, in mammalian cilia [Vent et al., 2005]. The functional ratio depends on the sequence of the heterologous β-tubulin. Thus Drosophila β1-tubulin, normally expressed only in earlier stages of spermatogenesis, can not replace β2, and expression of an excess of β1 relative to β2 in the post-mitotic germ cells disrupts axoneme assembly. However, spermatogenesis is nearly normal in males that co-express equal amounts of β1 and β2 [Raff et al., 2000; Neilsen et al., 2001; Neilsen and Raff, 2002].
The CTTs of both α- and β-tubulin lie on the surface of the tubulin heterodimer and of microtubules [Nogales et al., 1998, 1999; Amos, 2000]. The CTTs are unresolved in the three-dimensional crystallographic structure, suggesting that the CTT is a flexible feature. In Tetrahymena, exchanging the α-tubulin and β-tubulin CTTs results in no loss of microtubule function and supports robust growth, demonstrating independence between the CTT and the body of the tubulin molecule [Duan and Gorovsky, 2002]. The genome of this unicellular organism has limited tubulin gene diversity, with a single α-tubulin gene and two β-tubulin genes that differ in coding sequence only in two amino acids [Barahona et al., 1988; Eisen et al., 2006]. In this study we addressed the issue of the independence of the CTT domain in a multi-cellular organism with multiple tubulin genes by exchanging the CTTs of β-tubulins that have been shown to have different functional specializations [Raff et al., 2000; Neilsen et al., 2001; Neilsen and Raff, 2002; Popodi et al., 2008].
We compared microtubule function in spermatogenesis in males that co-express β1 and β2 with males that co-express the two reciprocal chimeric molecules β2β1C and β1β2C, in which the bodies and tails are swapped. Table I shows the body and CTT constitution of the β tubulins examined in this study. Neither β1β2C nor β2β1C can alone support spermatogenesis (Table II, below; Nielsen et al. [2001], Popodi et al. [2008]). In principle, however, co-expression of equal amounts of the two chimeras would provide microtubules with precisely the same functional β-tubulin domains as would co-expression of the two native β-tubulins from which they are derived. In contrast to the situation in Tetrahymena, our data show that in Drosophila, function of the β-tubulin CTT is not independent of the body of the molecule. As diagrammed in Fig. 1, we found that functionality was substantially decreased when the CTTs and bodies were provided in an inter-molecular configuration. Our data reveal a required intra-molecular relationship between the CTT and other parts of the molecule, essential for optimal function in both axonemes and other microtubules.
Table I. Native and chimeric β-tubulins tested in the post-mitotic male germ cells.
The carboxy termini of β2-tubulin, β1-tubulin and the reciprocal chimeric tubulins are shown. Sequences in the body of the chimeric β-tubulins are identical to the native sequences. The variable carboxyl-terminal tail (CTT) consists of the last 15 amino acids of β2 and the last 16 amino acids of β1. The β2 axoneme motif is underlined [Raff et al., 1997]. The β1 CTT does not contain an axoneme motif. Based on the 3-dimensional model for porcine brain tubulins [Nogales et al., 1998, 1999], the unresolved or ‘flexible’ region of the β-tubulin carboxyl terminus begins at position 428 in Drosophila β2 and β1.
| β-tubulin | “Body” of the molecule | Source of C-terminal tail | C-terminal tail sequence | Reference |
|---|---|---|---|---|
| β2 | β2 | β2 | EEGEFDEDEEGGGDE446 | Rudolph et al., 1987; Hoyle et al., 1995 |
| β1 | β1 | β1 | EDAEFEEEQEAEVDEN447 | Raff et al., 2000; Nielsen et al., 2001 |
| β1β2C | β1 | β2 | EEGEFDEDEEGGGDE446 | Nielsen et al., 2001 |
| β2β1C | β2 | β1 | EDAEFEEEQEAEVDEN447 | Popodi et al., 2008 |
Table II.
Axonemes and sperm production in males expressing β2 or variant β-tubulins
| β-tubulin in axonemesa | Axoneme morphologyb | Maximum spermatid cyst length (μ=m)c | Mature motile sperm producedd | Length of motile sperm (μ=m)e | Male Fertilityf |
|---|---|---|---|---|---|
| β2 Wild type | Normal 9+2 | 1952 (15) | Normal amounts (25/25) | 1754 (10) | Fertile (29/29) |
| One copy β2 (50% tubulin pool) | Normal 9+2 | 1656 (18) | Reduced amounts (20/22) | 1551 (13) | Fertile, reduced 37% (27/27) |
| β2ΔC | No axonemes made | 927 (11) | None | Sterile | |
| β1 | Proximal: 9+0; Distal: axonemes absent | 874 (19) | None | Sterile | |
| β2 and β1 | Normal 9+2 | 1917 (5) | Normal amounts (20/20) | 1792 (11) | Fertile, slightly reduced 68% (31/34) |
| β2β1C | Proximal: 9+2 (57%); 9+0 (43%); Distal: axonemes fragmented or absent | 1240 (5) | None | Sterile | |
| β1β2C | Proximal: 9+2 (47%); 9+0 (53%); Distal: axonemes absent | 445 (10) | None | Sterile | |
| β2β1C and β1β2C | 9+2 (95%); 9+0 (5%); Some axonemes defective or fragmented | 1674 (15) | Small amounts (31/125) | 1814 (6) | Sterile to very weakly fertile 5% (4/45) |
Except for males with only one copy of β2, data are for males with two β-tubulin genes and normal tubulin pool size. Males expressing β2 and β1, or β2β1C and β1β2C, had one gene copy of each; both β-tubulins were co-incorporated into testis microtubules, including sperm axonemes (Fig. 2). The transgenic constructs used were all previously characterized and are described in experimental procedures.
Axoneme morphology in males co-expressing β2β1C and β1β2C was determined in this study. Axoneme morphology in other experimental genotypes was described previously (see references for transgenic constructs in experimental procedures). Normal sperm axoneme morphology is 9+2 (Fig. 2). Proximal axoneme morphology indicates morphology in cross sections of intact axonemes as initiated at the basal body. Distal fragmentation or absence of axonemes reflects failure to maintain longitudinal stability [Nielsen et al., 2001; Nielsen and Raff, 2002; Popodi et al., 2008].
Drosophila spermatids develop in syncytial cysts that elongate during spermatogenesis. Cyst elongation requires microtubule function (see text), and membrane recruitment [Ghosh-Roy et al., 2004], which was normal in all genotypes in this study. Average of the maximum cyst length per male is shown; number of males scored in parentheses.
Numbers in parentheses show the number of males that had motile sperm of the total scored. Amounts indicate amount of sperm in seminal vesicles of males that had motile sperm relative to wild type.
Average sperm length is shown for 3–5 sperm per male; number of males shown in parentheses. Sperm length approximates axoneme length (sperm nucleus, 7 μm).
Numbers in parentheses show the number of males with progeny per total number of males tested. Average number of progeny for males that gave progeny is given as a percent of the progeny produced by control wild type males in the same experiment.
Fig. 1. Relative function of native and “trans” β-tubulin configurations in Drosophila spermatogenesis.
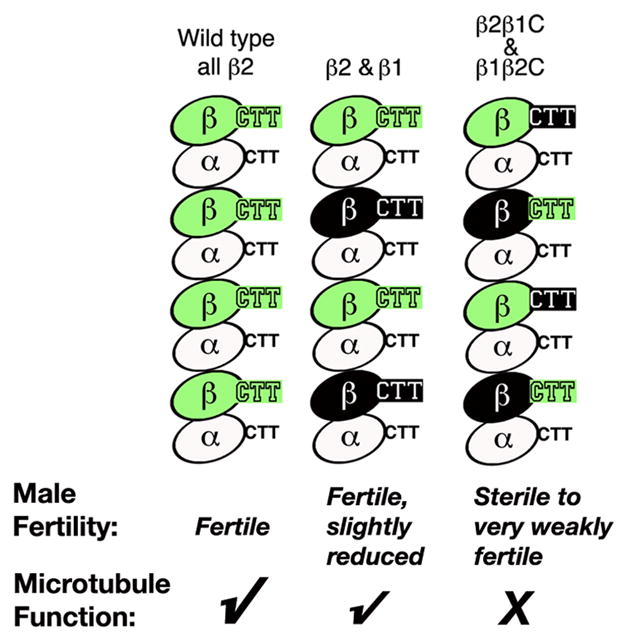
Portions of microtubule protofilaments are shown schematically. Co-assembly of β1 plus β2 supports nearly normal function of all categories of microtubules in spermatogenesis, whereas the combination of the paired reciprocal chimeric β-tubulins, β1β2C plus β2β1C, is poorly functional. Co-assembled heterodimers containing different β-tubulin species are shown uniformly distributed in a microtubule protofilament for simplicity. However, the in vivo situation would most likely represent random representation of co-assembled species in the microtubule polymer.
MATERIALS AND METHODS
Transgenic constructs encoding β-tubulins
The Drosophila transgenic strains used in this study express β-tubulins in the post-mitotic germ cells under control of β2 promoter elements, and all of these transgenes produce stable β-tubulin at levels similar to endogenous β2 [Hoyle et al., 1995; Nielsen et al., 2001; Popodi et al., 2008]. Constructs used in this study have been characterized previously: (1) β2ΔC: the entire CTT deleted (B2t.432, [Fackenthal et al., 1993]). (2) Transgenic β1: wild type β1 sequence driven in the post-mitotic germ cells [Raff et al., 2000; Nielsen et al., 2001; Nielsen and Raff, 2002]; in wild-type flies endogenous β1 is expressed only in pre-meiotic cells and is not used in axonemes. (3) β1β2C: the β1 CTT replaced by the β2 CTT (β1-β2i, [Nielsen et al., 2001; Nielsen and Raff, 2002]). (4) β2β1C: the β2 CTT replaced by the β1 CTT (β2-β1C16, [Popodi et al., 2008]). For all transgenes and for the paired chimeras, several different transgenic inserts were examined to ensure that all phenotypes reflect expression of the transgenic β-tubulins and were not due to site of insertion or other effects.
Analysis of Drosophila testis tubulins: 2D gels and antibodies
Two-dimensional gel electrophoresis of testis proteins was done as described previously [Hoyle et al., 2001; 2008; Popodi et al., 2005, 2008]. Tubulins were detected with monoclonal antibodies DM1A (anti α-tubulin, Sigma) and E7 (anti-β-tubulin, Developmental Studies Hybridoma Bank). Poly-glycylated tubulin was detected with R-polygly antiserum kindly provided by Dr. Martin Gorovsky [Duan and Gorovsky, 2002], which detects glycylated α- and β-tubulins. Primary antibodies were detected using a horseradish peroxidase-conjugated goat anti-mouse or goat anti-rabbit secondary antibody (Jackson ImmunoResearch) and displayed using 4-chloro 1-napthol and hydrogen peroxide. The gels shown in Fig. 2 show total staining for testis tubulins using all of the above antibodies; the signal from each antiserum was verified in preliminary experiments [see also Popodi et al., 2005, 2008].
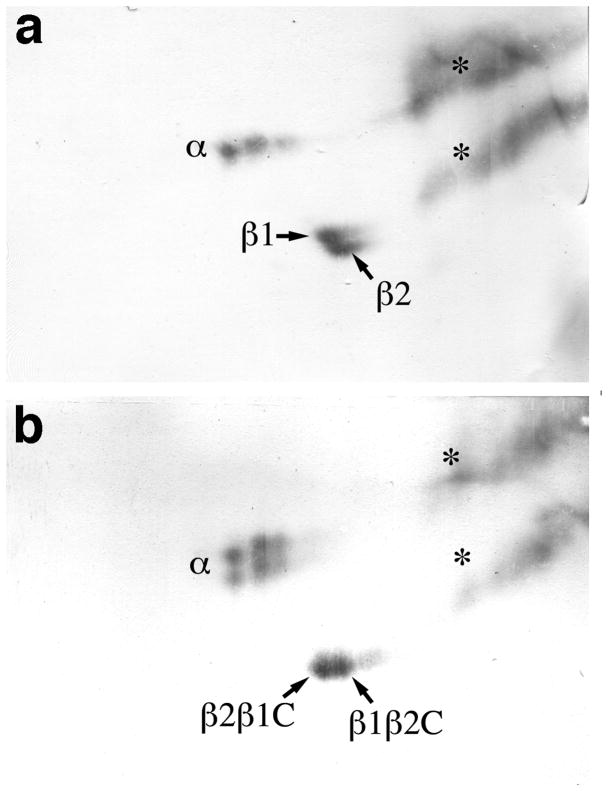
Fig. 2. Expression of chimeric and normal β-tubulins in Drosophila testes.
Testis proteins separated by two-dimensional gel electrophoresis were immuno-stained for α-tubulin (α), β-tubulin (β) and the CTT-specific post-tranlational modification, polyglycylation (*) as described in Materials and Methods (acid side of the isoelectric focusing dimension of the 2D gel is to the right). Immunoblots show the make up of the tubulins present in the stable testis tubulin pool [Hoyle et al., 2001]. (a) Testis proteins from males expressing one gene copy of endogenous wild type β2 and one copy of transgenic β1expressed under the control of the β2 regulatory sequences. (b) Testis proteins from males expressing one gene copy each of the two chimeric β-tubulins that are the subject of this study, β2β1C and β1β2C, in the absence of any wild-type β2-tubulin. In both genotypes the two β-tubulins expressed are present in equal amounts, equivalent to the amount of wild type β2 (panel a). Transgenic β1 expressed in the post-mitotic male germ cells co-migrates with the small amount of endogenous β1 expressed in earlier stages of spermatogenesis [Hoyle et al., 2001, 2008; Nielsen et al., 2001; Raff et al., 2000]. β2β1C and β1β2C resolve into closely spaced spots, as do the native β-tubulins, β1 and β2. The spots representing β2β1C and β1β2C are distinguishable because β2β1C stains with an anti-β1-specific antiserum, whereas β1β2C does not [Popodi et al., 2008]. All males were wild type for α-tubulin; that is, expressing two copies of a single isotype, α84B-tubulin, which resolves into two to four spots (in the isoelectrofocusing dimension reflecting α-tubulin acetylation in axonemal structures and in the SDS dimension reflecting slight variations in electrophoretic conditions (see Methods; [Matthews et al., 1989; Hutchens et al., 1997]). The amount of tubulin expressed by each individual construct used in this study has been reported previously (see Methods).
Sperm production and fertility
The outcome of spermatogenesis in males expressing different germ line tubulins was assessed by two parameters. Sperm production was scored by the presence of mature motile sperm in seminal vesicles dissected from males held away from females for 5 days or more. Fertility was scored as relative fecundity, determined as the number of progeny produced compared to progeny produced by wild type males in standardized mating tests, as described in Popodi et al. [2005, 2008].
Microscopy
Light microsopy was done on a Zeiss Axioplan microscope equipped with a Diagnostic Instruments, Inc. Spot CCD camera and software package. Live or orcein-stained spreads of testes were prepared as described in Hutchens et al. [1997] and viewed under phase or bright field optics, respectively. Length of spermatid cysts was measured in live spreads of testis contents under phase optics. Testes contain cysts of spermatids at all stages of development; therefore the longest cysts in each male were measured, i.e., those containing the most elongated spermatids present in each genotype (see spermatid bundles in elongating cysts in Fig. 3d–f). The length of sperm produced by fertile males was measured in spreads of motile sperm dissected from the seminal vesicles.
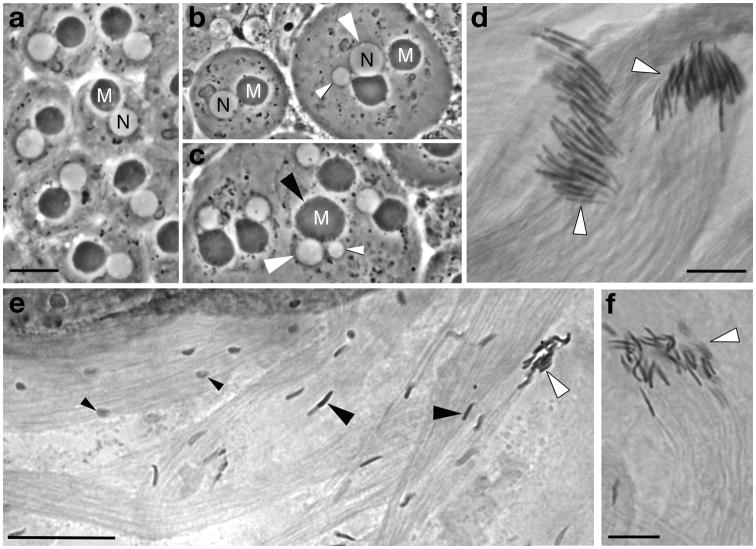
Fig. 3. Meiosis and cytoskeletal microtubule-mediated processes in spermatogenesis in males expressing β1 plus β2 or the paired chimeric β-tubulins with bodies and C-terminal tails swapped.
(a–c) Live young post-meiotic “onion-stage” spermatids with round white nuclei (N) and round (pre-elongation) dark mitochondrial derivatives (M). (a) Spermatids from a fertile male expressing equal amounts of β1 and β2. Spermatids are normal with uniform one to one arrangement of haploid nuclei and normal mitochondrial derivatives. Morphology of spermatids directly reflects meiosis: Spermatid nuclear size indicates nuclear ploidy [Hardy, 1975], and the one-to-one arrangement of nuclei and mitochondrial derivatives of nearly equal size indicates successful cytokinesis [Tokuyasu, 1975]. (b, c) Spermatids in males expressing equal amounts of β1β2C and β2β1C. Most spermatids in most cysts are normal, but some meiotic defects occur in nearly all males of this genotype. (b) The spermatid on the left is normal (compare nuclear and mitochondrial size and arrangement to a). Spermatids on the right have abnormal large and small nuclei (white arrowheads) indicating aneuploidy resulting from defective karyokinesis in meiosis. (c) Two aneuploid nuclei (white arrowheads) associated with a single abnormally large MD (black arrowhead), indicating defective karyokinesis and also failure of cytokinesis at one of the meiotic divisions. (d–f) Elongating spermatid bundles stained with orcein to display sperm nuclei. (d) Spermatid bundles in a male expressing equal amounts of β1 and β2. Spermatids have normal needle-like shaped nuclei that are well aligned at the tips of developing bundles (white arrowheads). The spermatid bundle on the right is slightly more mature than the one on the left, with heads more tightly packed at the tip of the bundle. (e) Spermatids in a male expressing equal amounts of β1β2C and β2β1C. Many nuclei in developing cysts in males of this genotype are poorly shaped and not aligned (small black arrowheads); in addition, many spermatids with shaped nuclei also fail to be correctly aligned (large black arrowheads). Maturing bundles with aligned spermatids with shaped nuclei (white arrowhead) have many fewer spermatids than normal (compare to d). (f) Spermatid bundle (white arrowhead), showing the reduced number of sperm heads resulting from the disruption of alignment (compare to d). Scale bars: 10 μm for (a–c), 10 μm for (d, f), 25 μm for (e).
Electron microscopy was done as described previously [Hoyle et al. 2001; Nielsen and Raff, 2002; Popodi et al., 2005, 2008]. Proximal (near the basal body] and distal (nearer to the end of the sperm tail) axoneme morphology was examined in proximal and distal sections separated by approximately 1 mm.
RESULTS
Expression of paired chimeric β-tubulins: β2β1C and β1β2C contribute equally to the stable germ line tubulin pool
Figure 2 shows testis tubulins in males expressing one copy each β1 and β2 (Fig. 2a) and males expressing one copy each of the reciprocal chimeric proteins β2β1C and β1β2C (Fig. 2b). In both genotypes, each of the two β-tubulins expressed contributed equally to the stable testis tubulin pool. The high molecular weight acidic stripes recognized by the anti-Gly antiserum (*)are due to post-translation modifications of both α- and β-tubulins. As we have shown, the molecular weight and charge of the ‘stripe’ of antibody-stained modified tubulins are due to both glycylation and glutamylation [Hoyle et al., 2008; Popodi et al., 2005]. We have previously demonstrated that in Drosophila testes, only axoneme tubulins carry post-translational CTT modifications, and that axoneme architecture is required for the modifications to occur [Hoyle et al., 2008; Popodi et al., 2005], thus the presence of modifications shows that the tubulin species present in testes are incorporated into sperm axonemes.
The β-tubulins produced by the β1 and β2 pair and the β2β1C and β1β2C pair thus behaved the same in the testis tubulin pool. Therefore the deficits in function in males expressing the paired chimeras did not reflect differences in the relative size of the tubulin pool or axoneme-specific post-translational modifications. The 64 haploid spermatids formed by meiosis undergo several microtubule-mediated processes of differentiation that take place concurrently. These include processes mediated by several different sets of cytoskeletal microtubules, including sperm nuclear shaping, spermatid alignment, elongation of the sperm mitochondrial derivatives, as well as assembly of the motile sperm tail axoneme. We examined all of these processes to determine the reason for the substantial differences in the outcome of spermatogenesis in males expressing β1 and β2 compared to males expressing β2β1C and β1β2C.
A matched β-tubulin C-terminal tail and body are essential for meiosis and cytoskeletal microtubule function
We used light microscopy of testis spreads to compare the microtubule-mediated processes of meiosis, nuclear shaping, and spermatid alignment. Figure 3 shows that for all of these microtubule processes, a matched CTT and body are important. All of these processes were nearly wild type in males co-expressing β1 and β2 (Figs. 3a, d), but had significant defects in males co-expressing β1β2C and β2β1C (Figs. 3b, c and e, f). β1β2C/β2β1C males exhibited mild but consistent meiotic defects, reflected as occasional aneuploid nuclei resulting from meiotic nondisjunctional events, and less often, failed cytokinesis (Fig. 3b, c). β1β2C/β2β1C males exhibited severe defects in sperm nuclear shaping and spermatid alignment (Figs. 3e, f). In Drosophila, only mature motile sperm enter the seminal vesicles; misaligned or defective spermatids are not individualized, and are discarded during the individualization process [Lindsley and Tokuyasu, 1980]. Thus the defects we observed in microtubule-mediated processes in β1β2C/β2β1C males would have substantial consequences for the outcome of spermatogenesis, substantially reducing the amount of sperm that could be produced [see Fuller et al., 1988; Hoyle and Raff, 1990; Hutchens et al., 1995; Popodi et al., 2005, 2008]. As shown in Table II, males expressing β1and β2 produced normal amounts of sperm and had near normal fertility. In marked contrast, few of the males expressing equal amounts of β1β2C and β2β1C produced functional sperm; most of these males were sterile, while a few males were marginally fertile, producing only a few progeny.
Generation of functional axonemes requires intra-molecular interactions between the C-terminal tail and body of the β-tubulin molecule
Another measure of the outcome of spermatogenesis is elongation of the syncytial cysts in which the spermatids undergo differentiation to form sperm. Two microtubule-mediated events contribute to cyst elongation: axoneme assembly and elongation of the mitochondrial derivatives [Kemphues et al., 1982]. As shown in Table II and Figure 4, we found that in addition to the defects in other microtubule-mediated processes, β1β2C/β2β1C males also exhibit many errors in axonemes. The majority of axonemes in males expressing β1β2C and β2β1C have normal cross-sectional morphology (Fig. 4d), but a significant subset of axonemes exhibit defects similar to those seen in males expressing either of the two chimeras alone, including lack of central pairs and defective longitudinal stability (Fig. 4f). Thus intra-molecular interactions between body and CTT are also required for axonemes.
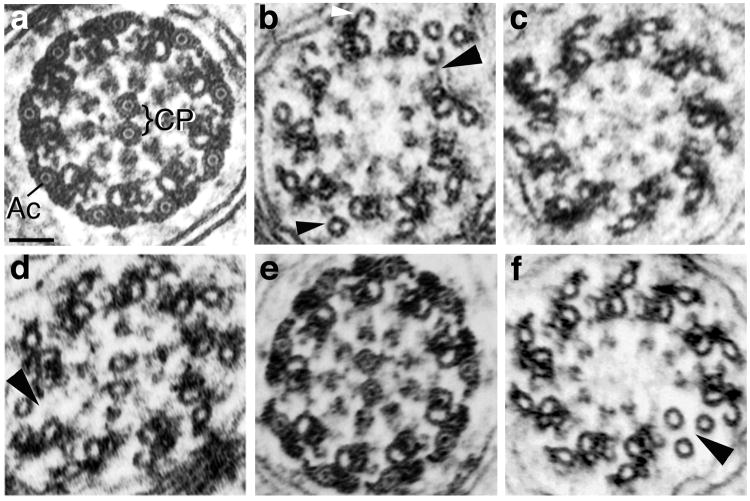
Fig. 4. Axoneme morphology in Drosophila males expressing native and chimeric β-tubulins.
Electron micrographs of axoneme cross-sections. (a) Wild type axoneme morphology in a fertile male expressing equal amounts of β1 and β2. In Drosophila and other insects, in addition to the 9+2 configuration of a ring of nine doublets surrounding a central pair of microtubules (CP), each doublet microtubule has an associated outer accessory microtubule (Ac). In mature axonemes, as shown here, the lumens of the central pair and accessory microtubules contain a luminal filament that appears as a dot in cross-section. (b–d) Typical morphology of intermediate stage axonemes from sterile males expressing transgenic β-tubulins in the absence of β2. (b) Axoneme from a male expressing β1. Central pair microtubules are absent, as is always the case in β1-expressing males. In addition, the ring of doublets is broken (large arrowhead) and some accessory microtubules are not associated with electron dense material (small arrowhead). Incomplete hook-shaped accessory microtubules (white arrowhead) are in the process of assembly [see Raff et al., 1997]. (c) Axoneme lacking central pair microtubules from a sterile male expressing β2β1C. About half of axonemes have central pairs in this genotype (Table II). (d) Axoneme from a sterile male expressing β1β2C. The central pair is present, but the axoneme’s doublet ring is disrupted (large arrowhead). About half of axonemes have central pairs in this genotype (Table II). (e, f) Examples of the range of axoneme morphology in sterile or weakly fertile males expressing one copy β1β2C and one copy β2β1C, in the absence of β2. (e) Nearly mature axoneme with wild type morphology (compare with a). (f) Intermediate stage axoneme lacking central pair microtubules. The doublet ring is also broken (arrowhead). Scale bar in (a) for all panels: 50 nm.
For wild type flies the length of mature spermatid cysts approximates the length of the sperm tail axoneme (Table II; Pitnick et al. [1995]). However, we and others [Hoyle and Raff, 1990; Hoyle et al., 1995; Ghosh-Roy et al., 2004] have shown that cyst length is not a reliable proxy for axoneme length in mutant flies. In this study we found that robust mitochondrial elongation can produce cysts up to half the length of wild-type in genotypes with no axonemes at all (β2ΔC, Table II). Comparison of cyst elongation in males of several sterile genotypes suggests that the body of β2-tubulin is best suited for the mitochondrial elongation microtubules: β2β1C produced longer cysts than either β1 or β1β2C (Table II). However, intact β1 is better for mitochondrial elongation (as inferred from cyst length) than the β1β2C chimera, another indicator of better function when the β-tubulin body and CTT are matched.
Cyst elongation shows that the combined chimeric β-tubulins can support reasonably good function of the cytoplasmic microtubules required for mitochondrial elongation, consistent with our observation in this and previous studies [Hoyle and Raff, 1990; Hoyle et al., 1995] that mitochondrial elongation is the least stringent of the microtubule processes mediated by β2. However, we found that the average cyst length for β1β2C/β2β1C males was slightly less than the length of sperm produced by these males. This apparent discrepancy likely reflects the very few mature sperm produced in this genotype. The relatively few wild type-length cysts that produced mature sperm would be under-represented in the sample of cysts that were measured. Overall, the paucity of functional sperm produced by males co-expressing the paired chimeras reflects the cumulative effect of defects in many of the microtubule processes that take place in spermatogenesis.
Drosophila sperm length depends on the size of the tubulin pool during sperm assembly
Males with only one functional copy of the β2 gene have half the normal tubulin pool size in the post-mitotic germ cells [Hoyle et al., 1995, 2001; Hutchens et al., 1997]. Axoneme morphology, meiosis and sperm nuclear shaping are normal, but spermatids are frequently misaligned, reducing the amount of sperm individualized [Fuller et al., 1988; Hoyle and Raff, 1990; Hutchens et al., 1995; Popodi et al., 2005, 2008]. Thus the size of the tubulin pool affects sperm production and male fertility even when the tubulins expressed are wild type.
A novel and unexpected finding that emerged from this study is that functional mature motile sperm are approximately 15% shorter when the tubulin pool is half the normal level (Table II). We did not anticipate this given the constancy of wild type sperm tail lengths in most Drosophila species [Pitnick et al., 1995]. All genotypes shown in the table were wild type for α-tubulin (two copies of the α84B gene). Males with one functional gene copy of α84B and one or two β2 genes have the same decreased tubulin pool size as males with only one copy of β2 [Hutchens et al., 1997; Hoyle et al., 2001]. We found that such males had the same decreased sperm length (average 1514 μm, for 15 males), confirming the interpretation that sperm length dependence is on the tubulin pool size and is not a function of β-tubulin copy number per se.
DISCUSSION AND CONCLUSIONS
Whereas the β1 plus β2 combination provides a high degree of fidelity for all microtubule-mediated processes, the β1β2C plus β2β1C combination does not (Fig. 1). The paired chimeras supported spermatid cyst elongation and meiosis better than sperm nuclear shaping, spermatid alignment, or axoneme assembly. However, the inter-molecular arrangement of the β-tubulin CTTs and bodies provided by co-expression of the reciprocal chimeras was not fully sufficient for any of the classes of microtubules we examined. Thus the requirement for an intra-molecular relationship between the CTT and other parts of the molecule is a general requirement for all microtubules, not specific for any one class of microtubules.
Because the β-tubulin CTT is an apparently flexible surface feature, one might predict that its sequence would have little influence on the behavior of the rest of the molecule in the heterodimer. This in fact appears to be the case in Tetrahymena [Duan and Gorovsky, 2002]. However, our data show that in Drosophila, and by implication other multi-cellular organisms with complex tubulin families, normal microtubule function is possible only if the β-tubulin CTT matches the body of the molecule.
The simplest hypothesis to explain our finding is that for most microtubule functions, the β-tubulin CTT must interact with a region or regions elsewhere on the surface of the β-tubulin molecule. Given that the CTT is not resolved in the crystallographic structure, the association may perhaps be transient or itself flexible. Molecular and genetic dissection in Drosophila spermatogenesis of these β-tubulin intra-molecular interactions provides a powerful entrée to probing the three-dimensional interactions of the flexible CTT that are not visible in the crystallographic structure.
Acknowledgments
We thank Dr. Martin Gorovsky for the kind gift of the R-polygly antisera. The monoclonal antibody E7 developed by Michael Klymkowsky was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the Department of Biological Sciences, University of Iowa, Iowa City, IA 52242. This work was supported by NIH grant R01GM56493 to E.C.R.
References
- Amos LA. Focusing-in on microtubules. Curr Opin Struct Biol. 2000;10(2):236–41. doi: 10.1016/s0959-440x(00)00070-1. [DOI] [PubMed] [Google Scholar]
- Barahona I, Soares H, Cyrne L, Penque D, Denoulet P, Rodrigues-Pousada C. Sequence of one alpha- and two beta-tubulin genes of Tetrahymena pyriformis. Structural and functional relationships with other eukaryotic tubulin genes. J Mol Biol. 1988;202(3):365–82. doi: 10.1016/0022-2836(88)90271-9. [DOI] [PubMed] [Google Scholar]
- Duan J, Gorovsky MA. Both carboxy-terminal tails of alpha- and beta-tubulin are essential, but either one will suffice. Curr Biol. 2002;12(4):313–6. doi: 10.1016/s0960-9822(02)00651-6. [DOI] [PubMed] [Google Scholar]
- Eisen JA, Coyne RS, Wu M, Wu D, Thiagarajan M, Wortman JR, Badger JH, Ren Q, Amedeo P, Jones KM, et al. Macronuclear genome sequence of the ciliate Tetrahymena thermophila, a model eukaryote. PLoS Biol. 2006;4(9):e286. doi: 10.1371/journal.pbio.0040286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fackenthal JD, Hutchens JA, Turner FR, Raff EC. Structural analysis of mutations in the Drosophila beta 2-tubulin isoform reveals regions in the beta-tubulin molecular required for general and for tissue-specific microtubule functions. Genetics. 1995;139(1):267–86. doi: 10.1093/genetics/139.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fackenthal JD, Turner FR, Raff EC. Tissue-specific microtubule functions in Drosophila spermatogenesis require the β2-tubulin isotype-specific carboxy terminus. Dev Biol. 1993;158(1):213–27. doi: 10.1006/dbio.1993.1180. [DOI] [PubMed] [Google Scholar]
- Fuller MT, Caulton JH, Hutchens JA, Kaufman TC, Raff EC. Genetic analysis of microtubule structure: a beta-tubulin mutation causes the formation of aberrant microtubules in vivo and in vitro. J Cell Biol. 1987;104(3):385–94. doi: 10.1083/jcb.104.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller MT, Caulton JH, Hutchens JA, Kaufman TC, Raff EC. Mutations that encode partially functional beta 2 tubulin subunits have different effects on structurally different microtubule arrays. J Cell Biol. 1988;107(1):141–52. doi: 10.1083/jcb.107.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh-Roy A, Kulkarni M, Kumar V, Shirolikar S, Ray K. Cytoplasmic dynein-dynactin complex is required for spermatid growth but not axoneme assembly in Drosophila. Mol Biol Cell. 2004;15(5):2470–83. doi: 10.1091/mbc.E03-11-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy RW. The influence of chromosome content on the size and shape of sperm heads in Drosophila melanogaster and the demonstration of chromosome loss during spermiogenesis. Genetics. 1975;79(2):231–64. doi: 10.1093/genetics/79.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle HD, Hutchens JA, Turner FR, Raff EC. Regulation of beta-tubulin function and expression in Drosophila spermatogenesis. Dev Genet. 1995;16(2):148–70. doi: 10.1002/dvg.1020160208. [DOI] [PubMed] [Google Scholar]
- Hoyle HD, Raff EC. Two Drosophila beta tubulin isoforms are not functionally equivalent. J Cell Biol. 1990;111(3):1009–26. doi: 10.1083/jcb.111.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle HD, Turner FR, Brunick L, Raff EC. Tubulin sorting during dimerization in vivo. Mol Biol Cell. 2001;12(7):2185–94. doi: 10.1091/mbc.12.7.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle HD, Turner FR, Raff EC. Axoneme-dependent tubulin modifications in singlet microtubules of the Drosophila sperm tail. Cell Motil Cytoskeleton. 2008;65(4):295–313. doi: 10.1002/cm.20261. [DOI] [PubMed] [Google Scholar]
- Hutchens JA, Hoyle HD, Turner FR, Raff EC. Structurally similar Drosophila alpha-tubulins are functionally distinct in vivo. Mol Biol Cell. 1997;8(3):481–500. doi: 10.1091/mbc.8.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemphues KJ, Kaufman TC, Raff RA, Raff EC. The testis-specific beta-tubulin subunit in Drosophila melanogaster has multiple functions in spermatogenesis. Cell. 1982;31(3 Pt 2):655–70. doi: 10.1016/0092-8674(82)90321-x. [DOI] [PubMed] [Google Scholar]
- Lindsley DL, Tokuyasu KT. Spermatogenesis. In: Ashburner M, Wright TRF, editors. The Genetics and Biology of Drosophila. New York: Academic Press; 1980. pp. 225–294. [Google Scholar]
- Matthews KA, Miller DF, Kaufman TC. Developmental distribution of RNA and protein products of the Drosophila alpha-tubulin gene family. Dev Biol. 1989;132(1):45–61. doi: 10.1016/0012-1606(89)90203-0. [DOI] [PubMed] [Google Scholar]
- Nielsen MG, Raff EC. The best of all worlds or the best possible world? Developmental constraint in the evolution of beta-tubulin and the sperm tail axoneme. Evol Dev. 2002;4(4):303–15. doi: 10.1046/j.1525-142x.2002.02015.x. [DOI] [PubMed] [Google Scholar]
- Nielsen MG, Turner FR, Hutchens JA, Raff EC. Axoneme-specific beta-tubulin specialization: a conserved C-terminal motif specifies the central pair. Curr Biol. 2001;11(7):529–33. doi: 10.1016/s0960-9822(01)00150-6. [DOI] [PubMed] [Google Scholar]
- Nogales E, Whittaker M, Milligan RA, Downing KH. High-resolution model of the microtubule. Cell. 1999;96(1):79–88. doi: 10.1016/s0092-8674(00)80961-7. [DOI] [PubMed] [Google Scholar]
- Nogales E, Wolf SG, Downing KH. Structure of the alpha beta tubulin dimer by electron crystallography. Nature. 1998;391(6663):199–203. doi: 10.1038/34465. [DOI] [PubMed] [Google Scholar]
- Pitnick S, Markow TA, Spicer GS. Delayed male maturity is a cost of producing large sperm in Drosophila. Proc Natl Acad Sci U S A. 1995;92(23):10614–8. doi: 10.1073/pnas.92.23.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popodi EM, Hoyle HD, Turner FR, Raff EC. The proximal region of the beta-tubulin C-terminal tail is sufficient for axoneme assembly. Cell Motil Cytoskeleton. 2005;62(1):48–64. doi: 10.1002/cm.20085. [DOI] [PubMed] [Google Scholar]
- Popodi EM, Hoyle HD, Turner FR, Xu K, Kruse S, Raff EC. Axoneme specialization embedded in a “Generalist” beta-tubulin. Cell Motil Cytoskeleton. 2008;65(3):216–37. doi: 10.1002/cm.20256. [DOI] [PubMed] [Google Scholar]
- Raff EC, Fackenthal JD, Hutchens JA, Hoyle HD, Turner FR. Microtubule architecture specified by a beta-tubulin isoform. Science. 1997;275(5296):70–3. doi: 10.1126/science.275.5296.70. [DOI] [PubMed] [Google Scholar]
- Raff EC, Hoyle HD, Popodi EM, Turner FR. Axoneme beta-Tubulin Sequence Determines Attachment of Outer Dynein Arms. Curr Biol. 2008;18(12):911–4. doi: 10.1016/j.cub.2008.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff EC, Hutchens JA, Hoyle HD, Nielsen MG, Turner FR. Conserved axoneme symmetry altered by a component beta-tubulin. Curr Biol. 2000;10(21):1391–4. doi: 10.1016/s0960-9822(00)00784-3. [DOI] [PubMed] [Google Scholar]
- Rudolph JE, Kimble M, Hoyle HD, Subler MA, Raff EC. Three Drosophila beta-tubulin sequences: a developmentally regulated isoform (beta 3), the testis-specific isoform (beta 2), and an assembly-defective mutation of the testis-specific isoform (B2t8) reveal both an ancient divergence in metazoan isotypes and structural constraints for beta-tubulin function. Mol Cell Biol. 1987;7(6):2231–42. doi: 10.1128/mcb.7.6.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu KT. Dynamics of spermiogenesis in Drosophila melanogaster. VI. Significance of “onion” nebenkern formation. J Ultrastruct Res. 1975;53(1):93–112. doi: 10.1016/s0022-5320(75)80089-x. [DOI] [PubMed] [Google Scholar]
- Vent J, Wyatt TA, Smith DD, Banerjee A, Luduena RF, Sisson JH, Hallworth R. Direct involvement of the isotype-specific C-terminus of beta tubulin in ciliary beating. J Cell Sci. 2005;118(Pt 19):4333–41. doi: 10.1242/jcs.02550. [DOI] [PMC free article] [PubMed] [Google Scholar]