Abstract
Acetylcholine (ACh), the first neurotransmitter discovered, participates in many CNS functions including sensory and motor processing, sleep, nociception, mood, stress response, attention, arousal, memory, motivation and reward. These diverse cholinergic effects are mediated by nicotinic (nAChR) and muscarinic (mAChR) type cholinergic receptors. The goal of this review is to synthesize a growing literature that supports the potential role of ACh as a treatment target for stimulant addiction. ACh interacts with the dopaminergic reward system in the ventral tegmental area (VTA), nucleus accumbens (NAc) and prefrontal cortex (PFC). In the VTA, both nAChR and mAChR stimulate the dopaminergic system. In the NAc, cholinergic interneurons integrate cortical and subcortical information related to reward. In the PFC, the cholinergic system contributes to the cognitive aspects of addiction. Preclinical studies support a facilitative role of nicotinic agonists in the development of stimulant addiction. Muscarinic agonists seem to have an inhibitory role depending on the subtype of mAChR. In human studies acetylcholine esterase (AChE) inhibitors, which increase synaptic ACh levels, have shown promise for the treatment of stimulant addiction. Further studies testing the efficacy of cholinergic medications for stimulant addiction are warranted.
Keywords: acetylcholine, dopamine, nicotinic, muscarinic, stimulant addiction, brain reward system
1. Introduction
Acetylcholine (ACh) is the founding member of the class of biochemicals known as neurotransmitters.[1] Expressed in both the peripheral (PNS) and central nervous systems (CNS), ACh is essential to diverse bodily functions and processes. In the PNS, cholinergic transmission is essential in muscle contraction and autonomic nervous system functions,[2, 3] while in the CNS, it plays a number of important roles, including sensory and motor processing, sleep, nociception, mood, stress response, attention, arousal, memory, motivation and reward.[4-6] A large body of evidence also supports the role of ACh in initiation and maintenance of addictive processes. The goal of this paper is to synthesize the growing literature that supports a prominent part of ACh in both the initiation and maintenance of addiction to stimulant drugs of abuse, specifically cocaine and amphetamines, as well as the potential role of ACh as a treatment target for stimulant addiction. Our review complements an excellent paper by Williams and Adinoff [7] which provided a comprehensive review of the cholinergic system and cocaine addiction, with a focus on neural circuits and neurobiology. In this paper, first we briefly overview the cholinergic system [for more extensive reviews see, 3, 5-7, 8, 9, 10]. Next, we review the nascent clinical literature that shows the emerging significance of the cholinergic system as a treatment target in stimulant addiction.
2. The Cholinergic System
2.1. Overview
In this section, we review ACh biosynthesis and metabolism, receptor types, and distribution in the CNS.
2.2. Cholinergic Biosynthesis and Metabolism
The neurotransmitter ACh is synthesized from acetyl-CoA and choline by choline acetyltransferase (ChAT), an enzyme primarily expressed by cholinergic neurons [6]. ChAT is a specific marker for identifying cholinergic neurons in the central and peripheral nervous systems.[11] Following its release to the synaptic cleft, ACh is hydrolyzed by the enzyme acetylcholinesterase (AChE). This enzyme is the target of a group of medications called AChE inhibitors, which increase levels of acetylcholine available at both nicotinic (nAChR) and muscarinic (mAChR) type cholinergic receptors.[12]
2.3 Cholinergic distribution
In the CNS, the cholinergic system includes cholinergic projection neurons and cholinergic interneurons (see Figure 1).[5, 9]
Figure 1.

This figure illustrates the cholinergic pathways into the ventral tegmental area (VTA) and prefrontal cortex. The main neurotransmitters connecting VTA, prefrontal cortex and nucleus accumbens (NAc) are also indicated next to arrows.
While the cholinergic projection neurons are located in the basal forebrain and the brainstem, cholinergic interneurons are located mainly in the striatum and nucleus accumbens (NAc). Basal forebrain cholinergic neurons are located in the medial septal nucleus, the vertical and horizontal limb nuclei of Broca, and the nucleus basalis of Meynert.[9] These neurons project mainly to the cerebral cortex, hippocampus and amygdala. The brainstem cholinergic neurons are located in pedunculopontine tegmental and the laterodorsal tegmental nuclei and project to the brainstem and midbrain including the ventral tegmental area (VTA), where dopaminergic neurons are found.[9]
2.4. Receptor types
In the synaptic cleft, ACh interacts with nAChR or mAChR, named based on their differential sensitivity to the exogenous ligands, respectively muscarine and nicotine. These receptors differ both in their structure and function.
2.4.1. Muscarinic receptors
mAChR are G-protein coupled, can be either excitatory or inhibitory, and are relatively slower acting compared to nAChR.[3, 13] mAChR represent approximately 90 percent of the cholinergic receptors. There are 5 different muscarinic receptors that are divided in two groups based on their G-protein coupling mechanisms: M1, M3 and M5 vs. M2 and M4.[2, 14] The M1, M3 and M5 receptors are coupled to the Gq/G11 family, whereas the M2 and M4 receptors prefer Gi/Go type of G-proteins.[14] The M1, M3, and M5 mAChR are found post-synaptically and they activate several effectors including phospholipase C, intracellular calcium, inositol triphosphatase, and MAPK (mitogen activated protein kinase) [15]. The M2 and M4 mAChR are found both pre- and post-synaptically and inhibit adenylyl cyclase and voltage-operated calcium channels and activate MAP kinases and G-protein-activated inwardly rectifying potassium channels (GIRK) channels [15]. These signaling cascades are essential for many neuronal functions including neuronal differentiation, survival, and synaptic plasticity, which also play an important role in addiction.[16-18] The M1, M4 and M5 subtype mAChR are predominantly expressed in the CNS, whereas the M2 and M3 mAChR subtypes are found both in the PNS and CNS.[19]
The M1 mAChR is widely expressed in the brain including the cerebral cortex, hippocampus and striatum.[20] The M1 receptors, the predominant mAChR in the CNS, are implicated in learning and memory processes. M1 agonists are potential treatment agents for Alzheimer's disease and other cognitive disorders.[21] The M3 receptors are less densely distributed in the CNS and its function is not well-characterized.[19] The M5 receptors are expressed on the dopaminergic neurons in the substantia nigra and the VTA (ventral tegmental area). Stimulation of M5 receptors facilitates dopamine (DA) release in the NAc. The M5 type mAchR are potential treatment targets for stimulant addiction, Parkinson's disease and schizophrenia.[21]
Pre-synaptically located M2 and M4 mAChR regulate release of acetylcholine and other neurotransmitters including DA [6, 22]. The M2 receptors are widely expressed in the CNS,[20] including the brainstem, thalamus, cortex, hippocampus and striatum, where they control ACh release. Blockage of M2 receptors, by inhibiting autoreceptor functioning, increases cholinergic activity. The M2 receptors are potential treatment targets for Alzheimer's disease and pain.[21] The M4 mAChR are found in the midbrain, cortex, hippocampus, and striatum.[20] The stimulation of M4 mAChR is thought to decrease the activity of dopaminergic VTA neurons, leading to reduced DA release in the NAc.[23] The M4 receptor is potential treatment targets for schizophrenia, Parkinson's disease and possibly drug addiction.[21]
2.4.2. Nicotinic Receptors
nAChR are ligand-gated ion channels, permeable to sodium, potassium, and calcium ions. These receptors show relatively fast responses, within milliseconds, and are excitatory.[8, 13] Most nAChR in the CNS are presynaptically located and they modulate the release of acetylcholine, DA, serotonin, glutamate, GABA, and norepinephrine.[8] nAChR can also be postsynaptically located as those on the dopaminergic neurons in the VTA. nAChR are pentameric combinations of 12 subunits (α2-α10 and β2-β4). Most nicotinic receptors contain α4, β2 or α7 subunits.[8] β2-containing receptors are critical for the addictive as well as cognitive performance-enhancing properties of nicotine.[24, 25] Nicotine withdrawal has been shown to reduce brain reward function in rats, which may also be mediated by α4, β2 or α7 subunits [26]
2.5. Summary
ACh acts on mAChR and nAChR. mAChR are G-protein coupled and are slower acting compared to nAChR. nAChR are ligand-gated ion channels and mediate excitatory, fast responses. Cholinergic neurons are either projection neurons or interneurons. While the cholinergic projection neurons are located in the basal forebrain and the brainstem, cholinergic interneurons are located mainly in the striatum and NAc.
3. The Cholinergic System in Relation to Reward Circuitry
3.1. Overview
Next, we review the distribution of ACh neurons and receptors in the CNS, with emphasis on cholinergic modulation of essential brain reward circuitry.
3.2. Brain reward circuitry
The main components of the brain reward circuit are thought to include the mesocorticolimbic DA system originating from the VTA of the midbrain and targeting a number of limbic and cortical structures, including the NAc, amygdala, and PFC.[27] DA release, especially in the NAc, is a critical event mediating the rewarding effects of stimulant drugs. Stimulants enhance dopaminergic activity by either facilitating release (e.g., amphetamines) and/or blocking uptake (e.g., cocaine) of DA.[28] DA release in the NAc is modulated by GABA, glutamate and ACh. Glutamate, the main excitatory neurotransmitter in the brain, plays an important role in learning and memory.[29] GABA is the main inhibitory neuron in the brain and the output neurons in the NAc (medium spiny neurons) are GABAergic.[30]
3.3. Cholinergic modulation of the Brain Reward Circuit
Within the brain reward circuit, the cholinergic and mesolimbic dopaminergic systems interact closely in the VTA, NAc and PFC.
3.3.1. Ventral Tegmental Area
Cholinergic projections to VTA dopaminergic neurons derive from brainstem cholinergic neurons.[31, 32] In the VTA, β2 containing nAChR increase the activity of dopaminergic neurons, which leads to increased DA release in terminal regions including NAc and PFC.[24] In addition, activation of α7-nicotinic receptors on glutamate terminals arising from PFC increase glutamate release in the VTA, which excites DA neurons in the VTA to release DA in the NAc.[33] The resultant DA release in the NAc is critical for the rewarding and addictive effects of nicotine. The M5 mAChR are found on the dopaminergic neurons and similar to nAChR, their activation increases dopaminergic activity.[34] Thus, both types of cholinergic receptors, mAChR and nAChR, stimulate dopaminergic neurons in the VTA.
3.3.2. Nucleus Accumbens
DA release in the NAc is a critical event mediating the rewarding effects of stimulant drugs. In the NAc, ACh is released from the cholinergic interneurons, which have extensive dendritic and axonal connections with other cells in the in NAc.[30, 34] An early hypothesis posited that the cholinergic and dopaminergic neurotransmitters act antagonistically in the NAc,[35] however, more recent evidence supports a more complementary interaction between these neurotransmitters. While D1 dopaminergic receptor activation increases ACh release in the NAc, D2 dopaminergic activation reduces ACh release.[36] The activity of medium spiny neurons (the main output neurons of the NAc) is also modulated by both cholinergic and dopaminergic input, which may be excitatory or inhibitory depending on the receptor subtypes that are stimulated: the D1 dopaminergic and M1 mAChR are excitatory while D2 dopaminergic and M4 mAChR are inhibitory.[34] Cholinergic interneurons receive dopaminergic input from the VTA, and glutamatergic input mainly from the PFC, hippocampus, and amygdala.[37] This DA and glutamate convergence on cholinergic interneurons may provide a mechanism for DA-mediated reward to be associated with glutamate-mediated learning and contextual information.[37] Accordingly, cholinergic interneurons control the translation of reward signals into contextually appropriate behavior.[35]
3.2.3. Prefrontal Cortex
The functions attributed to the PFC include attention, working memory, response inhibition, affective processing, decision making and goal-directed behaviors.[38] Compulsive drug use is the hallmark of drug addiction; it is characterized by behavioral inflexibility and more specifically, decreased ability to disengage from the processing of drug-related stimuli [39], both functions that are thought to be governed by the PFC. Many studies have demonstrated that chronic stimulant use is associated with a range of PFC dysfunction including poor sustained attention, response inhibition, and decision making.[40-45] In neuroimaging studies, stimulant users show decreased PFC activity and this decrease is associated with craving for cocaine in cocaine-dependent individuals.[46] In line with these findings, many preclinical studies, using animal models of relapse, have demonstrated that the PFC controls stress- and cocaine-induced reinstatement of cocaine self-administration.[47-49] The contribution of the cholinergic system in these processes is not well understood.
Amphetamines and cocaine increase ACh release in the PFC.[50, 51] Increased ACh release in the PFC accompanies development of psychomotor sensitization to amphetamines, suggesting participation of the prefrontal cholinergic system in neuronal adaptations leading to stimulant addiction.[39] The contribution of nAChR and mAChR in these processes is not well characterized. In one study, local application of the muscarinic antagonist, scopolamine, to the PFC, enhanced response to intravenous cocaine self-administration in rats,[52] supporting the role of mAChR in stimulant reinforcement. Prefrontal cortical nicotinic receptors are thought to be involved in the cognitive enhancing properties of nicotine, although the exact mechanisms remain to be elucidated.[53]
3.3. Summary
To summarize, the cholinergic system interacts with the dopaminergic reward system at three levels: VTA, NAc, and PFC. In the VTA, both nAChR and mAChR stimulate the dopaminergic system. In the NAc, cholinergic interneurons integrate the cortical and subcortical information related to reward. In the PFC, the cholinergic system contributes to the cognitive control of addictive processes, although the neurobiological mechanisms remain to be elucidated.
4. Cholinergic System as a Target for Stimulant Addiction
4.1. Overview
The cholinergic system has been most extensively examined in connection to dementia and other cognitive disorders. However, preclinical and human studies examining the possible role of the cholinergic system in stimulant addiction have begun accumulate. These studies will be summarized below.
4.2. Preclinical Studies
Many preclinical studies have also demonstrated that ACh modulates the rewarding effects of stimulants. It has been demonstrated that ablation of cholinergic neurons in the NAc through administration of a cholinergic immune toxin results in mice showing greater sensitivity and preference to cocaine.[54] In contrast, enhancement of cholinergic transmission by treatment with the AChE inhibitor, physostigmine, decreased cocaine self-administration in Rhesus monkeys.[55] Another AChE inhibitor, galantamine, which also allosterically potentiates nAChR, inhibited cocaine-induced hyperlocomotion in mice.[56] Galantamine also inhibited the dextroamphetamine-induced unrest, arousal, and stereotypy in Cebus monkeys.[57] Similarly, donepezil, another AChE inhibitor, reduced locomotor sensitivity and preference to cocaine in mice.[58] In rats, donepezil, as well as nicotine, treatment also reduced methamphetamine-seeking behavior.[59] Donepezil's effects on methamphetamine-seeking behavior were blocked by systemic mecamylamine, an nAChR antagonist, but not by a mAChR antagonist, scopolamine, suggesting that the inhibitory effects of donepezil were mediated through nAChR.[59]
Further pharmacological studies using drugs selective for mAChR or nAChR support the conclusion that both receptor types participate in stimulant effects. In rats, treatment with repeated nicotine administration enhanced cocaine self-administration.[60] Interestingly, the nAChR antagonist, mecamylamine, reduced, but did not prevent escalation of cocaine self-administration by rats.[61] These findings suggest that nAChR play an important role in the transition to cocaine addiction.
In other studies, mAChR medications have been examined in various models of drug addiction. The mAChR agonist, oxotremorine, has been shown to reduce amphetamine-induced locomotor activity, DA release in NAc, and cocaine self-administration in rats,[50, 62, 63] and mice.[64] On the other hand, atropine, an mAChR antagonist, reduced cocaine self-administration in Rhesus monkeys trained to self-administer cocaine.[65] Similarly, scopolamine, another mAChR antagonist, enhanced cocaine induced locomotor activity,[66] and reduced cocaine self-administration in Rhesus monkeys.[67]
The individual role of mAChR subtypes in stimulant addiction was examined using selective gene targeting approach. Mice lacking M1 receptors showed elevated DA levels in the striatum and enhanced locomotor activity in response to dextroamphetamine.[68] In another study, compared to wild type, M1-deficient mice showed a reduced conditioned place preference for cocaine or morphine.[69] Similar findings were obtained with the M1 antagonist pirenzepine, further supporting the role of M1 receptors in stimulant addiction.[69]
M4 deficient mice displayed elevated DA basal values and enhanced DA response to dextroamphetamine in the NAc.[23] Consistent with these findings, in another study, two M4 positive allosteric modulators have been shown to reverse amphetamine-induced locomotion.[70] These findings support the possibility that M4 mAChR agonists may also be potential treatment agents for stimulant addiction.
The M5 deficient mice, compared to the wild-type, showed reduced cocaine self-administration and withdrawal-induced anxiety.[71] These findings suggest that M5 receptors might be a potential target for stimulant addiction. However, no ligands selective for M5 mAChR are available yet.
4.3. Human Studies
There have been few human studies examining cholinergic medications as potential treatments for stimulant addiction. Janowsky et al.[72] first examined whether AChE inhibitors modulate amphetamine response several decades ago. In that study, physostigmine, an AChE inhibitor, attenuated the subjective effects of a stimulant medication, methylphenidate, in bipolar and schizophrenic patients. These findings were extended in more recent studies. De La Garza et al.[73] examined the effects of rivastigmine (1.5 or 3 mg/day), an AChE inhibitor, on intravenous methamphetamine responses (30 mg/day) in 23 methamphetamine dependent humans. In that study, 3 mg of rivastigmine treatment for 11 days attenuated the methamphetamine-induced diastolic blood pressure increases as well as subjective ratings of “desire” and “anxious”. The same study also examined methamphetamine self-administration behavior, which was not affected by rivastigmine treatment.[74] These findings are promising, and warrant further studies examining AChE inhibitors as potential treatments for stimulant addiction.
We are aware of one clinical trial in which a AChE inhibitor was evaluated for cocaine addiction.[75] In that 10-week study, donepezil at 10 mg was well tolerated but did not change cocaine use behavior. The study had a small sample size (only 17 subjects assigned to donepezil) providing limited statistical power to test the efficacy of donepezil for cocaine addiction. Only one dose of donepezil dose was evaluated. However, those treated with donepezil did show significant reductions in craving and other indexes of severity of addiction to cocaine and other drugs. Other AChE inhibitors have not been evaluated as potential treatment agents for stimulant addiction.
Two human studies have evaluated whether nAChR agonists can change typical subjective and physiological responses to stimulants. In one study, 7 cigarettes smokers, who occasionally used cocaine, were treated with 14 mg nicotine or placebo patch followed by intranasal cocaine (0.9 mg/kg or placebo). Nicotine patch, compared to placebo, attenuated cocaine-induced “high” and “stimulated” and increased the latency to detect cocaine effects without affecting physiological responses or pharmacokinetics of cocaine.[76] However, in another study, the 21 mg nicotine patch, compared to placebo, did not affect the subjective or reinforcing effects of intravenous cocaine (15 or 30 mg/70 kg).[77] These inconsistent findings could be due to differences in subject population (dependent vs. occasional cocaine users) as well as route of cocaine administration (intranasal vs. intravenous). Since both studies used a single dose of active nicotine patch (14 or 21 mg), dose-dependent effects of nicotine on cocaine responses were not examined. Future studies addressing these limitations will provide a clearer picture on how nAChR agonists affect typical stimulant effects in humans.
The nAChR antagonist, mecamylamine, has also been examined for cocaine addiction. A single 2.5 mg mecamylamine treatment reduced cue-induced cocaine craving in 23 cocaine users.[78] A randomized, double-blind, placebo-controlled study examined the efficacy of mecamylamine (6 mg/day) or placebo transdermal patches for a 16-week as a treatment of cocaine addiction.[79] In that study, mecamylamine did not reduce cocaine use or craving for cocaine. However, the small sample size, a total of 35 subjects, offered very limited statistical power.[79] Further, only one dose of mecamylamine was tested, and only the transdermal route of administration was used.
In a human laboratory study, Penetar and colleagues[80] examined the effects of the conjoint administration of cocaine with a mAChR antagonist, benztropine, in occasional cocaine users. Benztropine, an antimuscarinic and antihistamine medication, is commonly used to alleviate the extrapyramidal side effects (stiffness) from typical antipsychotics. Benztropine at 1, 2 or 4 mg, given as a single acute dose did not affect subjective or physiological effects from 0.9 mg/kg intranasally administered cocaine. Given that benztropine has significant antihistamine effects, it remains to be determined whether selective mAChR antagonists, like scopolamine or atropine, would alter cocaine's effects in humans.
To summarize, preclinical studies support a facilitative role of nAChR in the development of stimulant addiction. Among mAChR, M1, M4 and M5 subtypes seem to be potential targets for stimulant addiction. As summarized in Table 1, there have been only a few studies to evaluate cholinergic medications for treatment of stimulant addiction
Table 1.
Effects of Cholinergic Agents on Stimulants in Humans
| N | Cholinergic Agent | Stimulant | Outcome | |||
|---|---|---|---|---|---|---|
| Cholinersterase Inhibitors | Agent | Dose(s) | Stimulant | Dose(s) | ||
| Janowsky et al. [72] 1973a | 36 | Physostigmine | Up to 2.5 mgd | Methylphenidate | .5 mg/kgd | Decreased subjective response |
| De La Garza et al., [73, 74]2008a,b a | 22 | Rivastigmine | 1.5 or 3 mg/dayd | Methamphetamine | 30 mg/dayd | Decreased subjective response and diastolic blood pressure, no change in self-administration. |
| Winhusen et al.[75] 2005b | 67 | Donepezil | 10 mgc | Cocaine | - b | No change in cocaine use |
| nAChR Agonists | ||||||
| Kouri et al.[76] 2001 a | 7 | Nicotine | 14 mgf | Cocaine | .9 mg/kge | Decreased subject response |
| Sobel et al.[77] 2004 a | 9 | Nicotine | 15, 30 mg/70 kgd | Cocaine | 15, 30 mg/70 kgd | No change in subjective response |
| nAChR Antagonists | ||||||
| Reid et al.[78] 1999 a | 23 | Mecamylamine | 2.5 mgc | Cocaine | - b | Reduced cue-induced craving |
| Reid et al.[79] 2005 b | 35 | Mecamylamine | 6 mgf | Cocaine | - b | No change in cocaine craving or use |
| mAChR Antagonists | ||||||
| Penetar et al.[80] 2006 a | 16 | Benztropine | 1, 2, 4 mgc | Cocaine | .9 mg/kge | No change in subjective response |
Human laboratory study.
Clinical trial.
Oral route.
Intravenous route.
Intranasal route.
Transdermal route.
5. Future Directions for Medications Development
Based on the preclinical and clinical studies summarized above, the cholinergic medications that have potential for the treatment of stimulant addiction include AChE inhibitors, as well as medications targeting nAChR and mAChR.
5.1 AChE Inhibitors
As mentioned above, AChE inhibitors increase the synaptic concentrations of ACh, which leads to increased stimulation of both nAChR and mAChR. Several AChE inhibitors, including tacrine, rivastigmine, donepezil, and galantamine are available for clinical use for the treatment of dementia.[81-83] Other disorders characterized with cognitive impairment, including Parkinson's disease, traumatic brain injury and schizophrenia are also potential treatment targets [84-86] for AChE inhibitors. AChE inhibitors differ in their pharmacological as well as side effect profiles. The most common adverse effects of AChE inhibitors include diarrhea, nausea, vomiting, loss of appetite and dizziness. Tacrine, the first AChE inhibitor that received FDA approval in the US, has limited use due to hepatotoxicity and short half-life.[12] Donepezil and rivastigmine are more potent AChE inhibitors than galantamine.[87] Galantamine binds to nicotinic receptors, especially α7 and α4β2 subtypes, and enhances responses to acetylcholine, effectively resulting in DA release in the mesocorticolimbic pathway.[88] To date, only one study has evaluated galantamine as treatment for addiction. In a sample of 114 alcohol-dependent individuals, galantamine was associated with significant reductions in smoking compared with placebo.[89] Interestingly, the galantamine effect occurred even though smoking was not specifically targeted. In addition, given the promising human laboratory findings with physostigmine and rivastigmine in combination with amphetamines, [72, 73] further studies evaluating the efficacy of different AChE inhibitors for stimulant addiction are warranted.
Another potential treatment target for AChE inhibitors is cognitive impairment associated with long-term stimulant use. Cocaine and methamphetamine users have well-documented impairment in attention, working memory, decision-making and response inhibition. [40-44, 45]. Studies also suggest that cognitive impairments seen in stimulant users were not reversible with short-term abstinence.[40] Importantly, these cognitive impairments seems to predict poor treatment outcome in stimulant users who are in treatment.[90-92] Whether AChE inhibitors improve cognitive performance in stimulant users has not been examined. Thus, AChE inhibitors, by improving cognitive impairments found in stimulant users, may prove to be useful in treating stimulant addicted individuals.
5.2 Medications Targeting nAChR
An important development in nicotinic pharmacotherapies is the availability of partial nAChR agonists for clinical use. Cytisine, a plant alkaloid has been used for smoking cessation in Eastern Europe, is a partial α4β2 nAChR agonist.[93] A synthetic variation of cytisine is varenicline, a partial agonist at the α4β2 nAChR, has recently been marketed for smoking cessation. Varenicline inhibits nicotine's ability to activate nAChR and release DA and may not cause rapid desensitization at the nAChR, which limits the therapeutic utility of nAChR agonists.[94] Several other partial nAChR agonists, including dianicline and ispronicline, are undergoing human studies for smoking cessation and treatment of dementia.[95] Partial nAChR agonists may also have value for stimulant addiction pharmacotherapy, given the role of nicotinic receptors in stimulant effects. Varenicline and other partial nAChR agonists remain to be evaluated for stimulant addiction. The most common adverse effects of varenicline include nausea, vomiting, difficulty sleeping, headache and vivid dreams. Less common but more serious adverse effects include abnormal behavior and suicidal thoughts [96].
Another plant alkaloid, lobeline, has antagonistic effects on the α3β2 and α4β2 nAChR but has nicotinic agonistic effects as well.[97] Lobeline has been evaluated for smoking cessation, with no evidence of efficacy observed.[98] Lobeline also increases DA release from dopaminergic synapses, and blocks the DA transporter, similar to stimulants.[99] In preclinical studies, lobeline blocks the behavioral and neurochemical effects of stimulants.[100] Based on these preclinical findings, lobeline has been suggested to be a potential treatment for stimulant addiction.[97] Lobeline is currently being tested in humans as a treatment of methamphetamine addiction.[101]
5.3 Medications targeting mAChR
Development of medications targeting the M1 mAChR has been an important goal, especially for the treatment of Alzheimer's disease and other cognitive disorders. Muscarinic agonists that have reached human clinical studies included arecoline, carbachol, cevimeline, xanomeline, milameline and sabcomeline.[21] Unfortunately, none of these medications moved into clinical practice for the treatment of Alzheimers' disease or other cognitive disorders primarily due to their affinity to other mAChR subtypes, especially to peripheral M2 and M3 mAChR, which mediates adverse effects like diarrhea and nausea which limits their clinical use.
The difficulty in developing M1 selective compounds has been attributed to high sequence similarity across subtypes, especially in their ACh binding sites.[21] An alternative strategy has been to develop compounds that selectively bind to their target mAChR subtypes through allosteric mechanisms.[15] Compounds selective for M1, M2 and M4 mAChR, with more favorable side effect profiles than nonselective mAChR have been developed.[102-105]. The availability of such compounds will also have greatly facilitate the development of novel treatments for stimulant addiction.
6. Conclusions
As summarized above, preclinical and clinical studies support the potential utility of cholinergic system as a target to develop medications for stimulant addiction. Further studies are warranted to test the efficacy of AChE inhibitors as well as nicotinic and muscarinic medications of the treatment of stimulant addiction.
Figure 2.
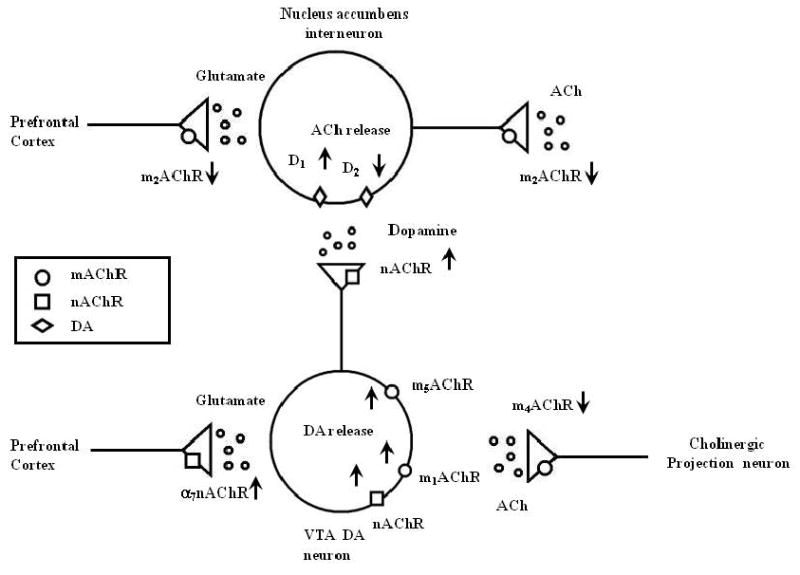
This figure illustrates the effects of muscarinic (mAChR) and nicotinic (nAChR) cholinergic receptor subtypes on glutamate, dopamine (DA) and acetylcholine (Ach) release in the ventral tegmental area (VTA) dopaminergic and the nucleus accumbens cholinergic interneuron. See text for details.
Acknowledgments
The authors were supported by career development awards, K02-DA021304 (MS) and K01-DA-019446 (MM), by RO1DA019885, RO1DA020752, RO1DA014537 and the Veterans Administration Mental Illness Research, Education and Clinical Center (MIRECC).
References
- 1.Cooper JR, Bloom FE, Roth RH. The biochemical basis of neuropharmacology. 8th. Oxford; New York: Oxford University Press; 2003. [Google Scholar]
- 2.Eglen RM. Muscarinic receptor subtypes in neuronal and non-neuronal cholinergic function. Auton Autacoid Pharmacol. 2006 Jul;26(3):219–33. doi: 10.1111/j.1474-8673.2006.00368.x. [DOI] [PubMed] [Google Scholar]
- 3.Gu Q. Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience. 2002;111(4):815–35. doi: 10.1016/s0306-4522(02)00026-x. [DOI] [PubMed] [Google Scholar]
- 4.Lucas-Meunier E, Fossier P, Baux G, Amar M. Cholinergic modulation of the cortical neuronal network. Pflugers Arch. 2003 Apr;446(1):17–29. doi: 10.1007/s00424-002-0999-2. [DOI] [PubMed] [Google Scholar]
- 5.Perry E, Walker M, Grace J, Perry R. Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends Neurosci. 1999 Jun;22(6):273–80. doi: 10.1016/s0166-2236(98)01361-7. [DOI] [PubMed] [Google Scholar]
- 6.Smythies J. Section I. The cholinergic system. Int Rev Neurobiol. 2005;64:1–122. doi: 10.1016/S0074-7742(05)64001-9. [DOI] [PubMed] [Google Scholar]
- 7.Williams MJ, Adinoff B. The Role of Acetylcholine in Cocaine Addiction. Neuropsychopharmacology. 2007 Oct 10; doi: 10.1038/sj.npp.1301585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- 9.Mesulam MM. The cholinergic innervation of the human cerebral cortex. Prog Brain Res. 2004;145:67–78. doi: 10.1016/S0079-6123(03)45004-8. [DOI] [PubMed] [Google Scholar]
- 10.Raedler TJ, Bymaster FP, Tandon R, Copolov D, Dean B. Towards a muscarinic hypothesis of schizophrenia. Mol Psychiatry. 2007 Mar;12(3):232–46. doi: 10.1038/sj.mp.4001924. [DOI] [PubMed] [Google Scholar]
- 11.Oda Y. Choline acetyltransferase: the structure, distribution and pathologic changes in the central nervous system. Pathol Int. 1999 Nov;49(11):921–37. doi: 10.1046/j.1440-1827.1999.00977.x. [DOI] [PubMed] [Google Scholar]
- 12.Lane RM, Potkin SG, Enz A. Targeting acetylcholinesterase and butyrylcholinesterase in dementia. Int J Neuropsychopharmacol. 2006 Feb;9(1):101–24. doi: 10.1017/S1461145705005833. [DOI] [PubMed] [Google Scholar]
- 13.Clader JW, Wang Y. Muscarinic receptor agonists and antagonists in the treatment of Alzheimer's disease. Curr Pharm Des. 2005;11(26):3353–61. doi: 10.2174/138161205774370762. [DOI] [PubMed] [Google Scholar]
- 14.Caulfield MP, Birdsall NJ. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998 Jun;50(2):279–90. [PubMed] [Google Scholar]
- 15.Wess J, Eglen RM, Gautam D. Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat Rev Drug Discov. 2007 Sep;6(9):721–33. doi: 10.1038/nrd2379. [DOI] [PubMed] [Google Scholar]
- 16.Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol. 2008 May;154(2):327–42. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007 Nov;8(11):844–58. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 18.Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of ERK in cocaine addiction. Trends Neurosci. 2006 Dec;29(12):695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Wess J. Muscarinic acetylcholine receptor knockout mice: novel phenotypes and clinical implications. Annu Rev Pharmacol Toxicol. 2004;44:423–50. doi: 10.1146/annurev.pharmtox.44.101802.121622. [DOI] [PubMed] [Google Scholar]
- 20.Oki T, Takagi Y, Inagaki S, Taketo MM, Manabe T, Matsui M, et al. Quantitative analysis of binding parameters of [3H]N-methylscopolamine in central nervous system of muscarinic acetylcholine receptor knockout mice. Brain Res Mol Brain Res. 2005 Jan 5;133(1):6–11. doi: 10.1016/j.molbrainres.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Langmead CJ, Watson J, Reavill C. Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol Ther. 2008 Feb;117(2):232–43. doi: 10.1016/j.pharmthera.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Felder CC, Bymaster FP, Ward J, DeLapp N. Therapeutic opportunities for muscarinic receptors in the central nervous system. J Med Chem. 2000 Nov 16;43(23):4333–53. doi: 10.1021/jm990607u. [DOI] [PubMed] [Google Scholar]
- 23.Tzavara ET, Bymaster FP, Davis RJ, Wade MR, Perry KW, Wess J, et al. M4 muscarinic receptors regulate the dynamics of cholinergic and dopaminergic neurotransmission: relevance to the pathophysiology and treatment of related CNS pathologies. FASEB J. 2004 Sep;18(12):1410–2. doi: 10.1096/fj.04-1575fje. [DOI] [PubMed] [Google Scholar]
- 24.Picciotto MR, Corrigall WA. Neuronal systems underlying behaviors related to nicotine addiction: neural circuits and molecular genetics. J Neurosci. 2002;22(9):3338–41. doi: 10.1523/JNEUROSCI.22-09-03338.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Picciotto MR, Zoli M, Lena C, Bessis A, Lallemand Y, Le Novere N, et al. Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Nature. 1995 Mar 2;374(6517):65–7. doi: 10.1038/374065a0. [DOI] [PubMed] [Google Scholar]
- 26.Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998 May 7;393(6680):76–9. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- 27.Koob GF. Neural mechanisms of drug reinforcement. Ann N Y Acad Sci. 1992 Jun 28;654:171–91. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- 28.Kahlig KM, Binda F, Khoshbouei H, Blakely RD, McMahon DG, Javitch JA, et al. Amphetamine induces dopamine efflux through a dopamine transporter channel. Proc Natl Acad Sci U S A. 2005 Mar 1;102(9):3495–500. doi: 10.1073/pnas.0407737102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tzschentke TM, Schmidt WJ. Glutamatergic mechanisms in addiction. Mol Psychiatry. 2003 Apr;8(4):373–82. doi: 10.1038/sj.mp.4001269. [DOI] [PubMed] [Google Scholar]
- 30.Di Chiara G, Morelli M, Consolo S. Modulatory functions of neurotransmitters in the striatum: ACh/dopamine/NMDA interactions. Trends Neurosci. 1994 Jun;17(6):228–33. doi: 10.1016/0166-2236(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 31.Forster GL, Blaha CD. Laterodorsal tegmental stimulation elicits dopamine efflux in the rat nucleus accumbens by activation of acetylcholine and glutamate receptors in the ventral tegmental area. Eur J Neurosci. 2000 Oct;12(10):3596–604. doi: 10.1046/j.1460-9568.2000.00250.x. [DOI] [PubMed] [Google Scholar]
- 32.Woolf NJ, Butcher LL. Cholinergic systems in the rat brain: III. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain. Brain Res Bull. 1986 May;16(5):603–37. doi: 10.1016/0361-9230(86)90134-6. [DOI] [PubMed] [Google Scholar]
- 33.Barazangi N, Role LW. Nicotine-induced enhancement of glutamatergic and GABAergic synaptic transmission in the mouse amygdala. J Neurophysiol. 2001 Jul;86(1):463–74. doi: 10.1152/jn.2001.86.1.463. [DOI] [PubMed] [Google Scholar]
- 34.Calabresi P, Picconi B, Parnetti L, Di Filippo M. A convergent model for cognitive dysfunctions in Parkinson's disease: the critical dopamine-acetylcholine synaptic balance. Lancet Neurol. 2006 Nov;5(11):974–83. doi: 10.1016/S1474-4422(06)70600-7. [DOI] [PubMed] [Google Scholar]
- 35.Cragg SJ. Meaningful silences: how dopamine listens to the ACh pause. Trends Neurosci. 2006 Mar;29(3):125–31. doi: 10.1016/j.tins.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Bertorelli R, Consolo S. D1 and D2 dopaminergic regulation of acetylcholine release from striata of freely moving rats. J Neurochem. 1990 Jun;54(6):2145–8. doi: 10.1111/j.1471-4159.1990.tb04922.x. [DOI] [PubMed] [Google Scholar]
- 37.Berlanga ML, Olsen CM, Chen V, Ikegami A, Herring BE, Duvauchelle CL, et al. Cholinergic interneurons of the nucleus accumbens and dorsal striatum are activated by the self-administration of cocaine. Neuroscience. 2003;120(4):1149–56. doi: 10.1016/s0306-4522(03)00378-6. [DOI] [PubMed] [Google Scholar]
- 38.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 39.Sarter M, Bruno JP, Parikh V, Martinez V, Kozak R, Richards JB. Forebrain dopaminergic-cholinergic interactions, attentional effort, psychostimulant addiction and schizophrenia. Exs. 2006;98:65–86. doi: 10.1007/978-3-7643-7772-4_4. [DOI] [PubMed] [Google Scholar]
- 40.Ersche KD, Clark L, London M, Robbins TW, Sahakian BJ. Profile of executive and memory function associated with amphetamine and opiate dependence. Neuropsychopharmacology. 2006;31(5):1036–47. doi: 10.1038/sj.npp.1300889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verdejo-Garcia A, Benbrook A, Funderburk F, David P, Cadet JL, Bolla KI. The differential relationship between cocaine use and marijuana use on decision-making performance over repeat testing with the Iowa Gambling Task. Drug Alcohol Depend. 2007 Sep 6;90(1):2–11. doi: 10.1016/j.drugalcdep.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verdejo-Garcia AJ, Perales JC, Perez-Garcia M. Cognitive impulsivity in cocaine and heroin polysubstance abusers. Addict Behav. 2007 May;32(5):950–66. doi: 10.1016/j.addbeh.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 43.Rogers RD, Robbins TW. Investigating the neurocognitive deficits associated with chronic drug misuse. Curr Opin Neurobiol. 2001;11(2):250–7. doi: 10.1016/s0959-4388(00)00204-x. [DOI] [PubMed] [Google Scholar]
- 44.Jovanovski D, Erb S, Zakzanis KK. Neurocognitive deficits in cocaine users: a quantitative review of the evidence. J Clin Exp Neuropsychol. 2005;27(2):189–204. doi: 10.1080/13803390490515694. [DOI] [PubMed] [Google Scholar]
- 45.Bolla KI, Rothman R, Cadet JL. Dose-related neurobehavioral effects of chronic cocaine use. J Neuropsychiatry Clin Neurosci. 1999;11(3):361–9. doi: 10.1176/jnp.11.3.361. [DOI] [PubMed] [Google Scholar]
- 46.Volkow ND, Wang GJ, Fowler JS, Logan J, Franceschi D, Maynard L, et al. Relationship between blockade of dopamine transporters by oral methylphenidate and the increases in extracellular dopamine: therapeutic implications. Synapse. 2002 Mar 1;43(3):181–7. doi: 10.1002/syn.10038. [DOI] [PubMed] [Google Scholar]
- 47.Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2003 Jul;168(12):66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- 48.Di Pietro NC, Black YD, Kantak KM. Context-dependent prefrontal cortex regulation of cocaine self-administration and reinstatement behaviors in rats. Eur J Neurosci. 2006 Dec;24(11):3285–98. doi: 10.1111/j.1460-9568.2006.05193.x. [DOI] [PubMed] [Google Scholar]
- 49.Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, et al. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005 Feb;30(2):296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- 50.Ichikawa J, Chung YC, Li Z, Dai J, Meltzer HY. Cholinergic modulation of basal and amphetamine-induced dopamine release in rat medial prefrontal cortex and nucleus accumbens. Brain Res. 2002 Dec 20;958(1):176–84. doi: 10.1016/s0006-8993(02)03692-2. [DOI] [PubMed] [Google Scholar]
- 51.Zmarowski A, Sarter M, Bruno JP. Glutamate receptors in nucleus accumbens mediate regionally selective increases in cortical acetylcholine release. Synapse. 2007 Mar;61(3):115–23. doi: 10.1002/syn.20354. [DOI] [PubMed] [Google Scholar]
- 52.Ikemoto S, Goeders NE. Intra-medial prefrontal cortex injections of scopolamine increase instrumental responses for cocaine: an intravenous self-administration study in rats. Brain Res Bull. 2000 Jan 15;51(2):151–8. doi: 10.1016/s0361-9230(99)00214-2. [DOI] [PubMed] [Google Scholar]
- 53.Mansvelder HD, van Aerde KI, Couey JJ, Brussaard AB. Nicotinic modulation of neuronal networks: from receptors to cognition. Psychopharmacology (Berl) 2006;184(34):292–305. doi: 10.1007/s00213-005-0070-z. [DOI] [PubMed] [Google Scholar]
- 54.Hikida T, Kaneko S, Isobe T, Kitabatake Y, Watanabe D, Pastan I, et al. Increased sensitivity to cocaine by cholinergic cell ablation in nucleus accumbens. Proc Natl Acad Sci U S A. 2001 Nov 6;98(23):13351–4. doi: 10.1073/pnas.231488998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de la Garza R, Johanson CE. Effects of haloperidol and physostigmine on self-administration of local anesthetics. Pharmacol Biochem Behav. 1982 Dec;17(6):1295–9. doi: 10.1016/0091-3057(82)90138-1. [DOI] [PubMed] [Google Scholar]
- 56.Hikida T, Kitabatake Y, Pastan I, Nakanishi S. Acetylcholine enhancement in the nucleus accumbens prevents addictive behaviors of cocaine and morphine. Proc Natl Acad Sci U S A. 2003 May 13;100(10):6169–73. doi: 10.1073/pnas.0631749100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andersen MB, Werge T, Fink-Jensen A. The acetylcholinesterase inhibitor galantamine inhibits d-amphetamine-induced psychotic-like behavior in Cebus monkeys. J Pharmacol Exp Ther. 2007 Jun;321(3):1179–82. doi: 10.1124/jpet.107.119677. [DOI] [PubMed] [Google Scholar]
- 58.Takamatsu Y, Yamanishi Y, Hagino Y, Yamamoto H, Ikeda K. Differential effects of donepezil on methamphetamine and cocaine dependencies. Ann N Y Acad Sci. 2006 Aug;1074:418–26. doi: 10.1196/annals.1369.042. [DOI] [PubMed] [Google Scholar]
- 59.Hiranita T, Nawata Y, Sakimura K, Anggadiredja K, Yamamoto T. Suppression of methamphetamine-seeking behavior by nicotinic agonists. Proc Natl Acad Sci U S A. 2006 May 30;103(22):8523–7. doi: 10.1073/pnas.0600347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bechtholt AJ, Mark GP. Enhancement of cocaine-seeking behavior by repeated nicotine exposure in rats. Psychopharmacology. 2002 Jul;162(2):178–85. doi: 10.1007/s00213-002-1079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hansen ST, Mark GP. The nicotinic acetylcholine receptor antagonist mecamylamine prevents escalation of cocaine self-administration in rats with extended daily access. Psychopharmacology. 2007 May 27; doi: 10.1007/s00213-007-0822-z. [DOI] [PubMed] [Google Scholar]
- 62.Kelly PH, Miller RJ. The interaction of neuroleptic and muscarinic agents with central dopaminergic systems. Br J Pharmacol. 1975 May;54(1):115–21. doi: 10.1111/j.1476-5381.1975.tb07417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mark GP, Kinney AE, Grubb MC, Zhu X, Finn DA, Mader SL, et al. Injection of oxotremorine in nucleus accumbens shell reduces cocaine but not food self-administration in rats. Brain Res. 2006 Dec 6;1123(1):51–9. doi: 10.1016/j.brainres.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rasmussen T, Sauerberg P, Nielsen EB, Swedberg MD, Thomsen C, Sheardown MJ, et al. Muscarinic receptor agonists decrease cocaine self-administration rates in drug-naive mice. Eur J Pharmacol. 2000 Aug 25;402(3):241–6. doi: 10.1016/s0014-2999(00)00442-8. [DOI] [PubMed] [Google Scholar]
- 65.Wilson MC, Schuster CR. Cholinergic influence on intravenous cocaine self-administration by rhesus monkeys. Pharmacol Biochem Behav. 1973 Nov-Dec;1(6):643–9. doi: 10.1016/0091-3057(73)90027-0. [DOI] [PubMed] [Google Scholar]
- 66.Wang JQ, McGinty JF. Muscarinic receptors regulate striatal neuropeptide gene expression in normal and amphetamine-treated rats. Neuroscience. 1996 Nov;75(1):43–56. doi: 10.1016/0306-4522(96)00277-1. [DOI] [PubMed] [Google Scholar]
- 67.Ranaldi R, Woolverton WL. Self-administration of cocaine: scopolamine combinations by rhesus monkeys. Psychopharmacology. 2002 Jun;161(4):442–8. doi: 10.1007/s00213-002-1069-3. [DOI] [PubMed] [Google Scholar]
- 68.Gerber DJ, Sotnikova TD, Gainetdinov RR, Huang SY, Caron MG, Tonegawa S. Hyperactivity, elevated dopaminergic transmission, and response to amphetamine in M1 muscarinic acetylcholine receptor-deficient mice. Proc Natl Acad Sci U S A. 2001 Dec 18;98(26):15312–7. doi: 10.1073/pnas.261583798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carrigan KA, Dykstra LA. Behavioral effects of morphine and cocaine in M1 muscarinic acetylcholine receptor-deficient mice. Psychopharmacology. 2007 May;191(4):985–93. doi: 10.1007/s00213-006-0671-1. [DOI] [PubMed] [Google Scholar]
- 70.Brady AE, Jones CK, Bridges TM, Kennedy JP, Thompson AD, Heiman JU, et al. Centrally active allosteric potentiators of the M4 muscarinic acetylcholine receptor reverse amphetamine-induced hyperlocomotor activity in rats. J Pharmacol Exp Ther. 2008 Sep 4; doi: 10.1124/jpet.108.140350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thomsen M, Woldbye DP, Wortwein G, Fink-Jensen A, Wess J, Caine SB. Reduced cocaine self-administration in muscarinic M5 acetylcholine receptor-deficient mice. J Neurosci. 2005 Sep 7;25(36):8141–9. doi: 10.1523/JNEUROSCI.2077-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Janowsky DS, el-Yousef MK, Davis JM, Sekerke HJ. Antagonistic effects of physostigmine and methylphenidate in man. Am J Psychiatry. 1973 Dec;130(12):1370–6. doi: 10.1176/ajp.130.12.1370. [DOI] [PubMed] [Google Scholar]
- 73.De La Garza R, Shoptaw S, Newton TF. Evaluation of the cardiovascular and subjective effects of rivastigmine in combination with methamphetamine in methamphetamine-dependent human volunteers. Int J Neuropsychopharmacol. 2008 Feb 4;:1–13. doi: 10.1017/S1461145708008456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De La Garza R, 2nd, Mahoney JJ, 3rd, Culbertson C, Shoptaw S, Newton TF. The acetylcholinesterase inhibitor rivastigmine does not alter total choices for methamphetamine, but may reduce positive subjective effects, in a laboratory model of intravenous self-administration in human volunteers. Pharmacol Biochem Behav. 2008 Apr;89(2):200–8. doi: 10.1016/j.pbb.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Winhusen TM, Somoza EC, Harrer JM, Mezinskis JP, Montgomery MA, Goldsmith RJ, et al. A placebo-controlled screening trial of tiagabine, sertraline and donepezil as cocaine dependence treatments. Addiction. 2005 Mar;100 1:68–77. doi: 10.1111/j.1360-0443.2005.00992.x. [DOI] [PubMed] [Google Scholar]
- 76.Kouri EM, Stull M, Lukas SE. Nicotine alters some of cocaine's subjective effects in the absence of physiological or pharmacokinetic changes. Pharmacol Biochem Behav. 2001 May-Jun;69(12):209–17. doi: 10.1016/s0091-3057(01)00529-9. [DOI] [PubMed] [Google Scholar]
- 77.Sobel BF, Sigmon SC, Griffiths RR. Transdermal nicotine maintenance attenuates the subjective and reinforcing effects of intravenous nicotine, but not cocaine or caffeine, in cigarette-smoking stimulant abusers. Neuropsychopharmacology. 2004 May;29(5):991–1003. doi: 10.1038/sj.npp.1300415. [DOI] [PubMed] [Google Scholar]
- 78.Reid MS, Mickalian JD, Delucchi KL, Berger SP. A nicotine antagonist, mecamylamine, reduces cue-induced cocaine craving in cocaine-dependent subjects. Neuropsychopharmacology. 1999 Mar;20(3):297–307. doi: 10.1016/S0893-133X(98)00076-1. [DOI] [PubMed] [Google Scholar]
- 79.Reid MS, Angrist B, Baker SA, O'Leary S, Stone J, Schwartz M, et al. A placebo controlled, double-blind study of mecamylamine treatment for cocaine dependence in patients enrolled in an opiate replacement program. Subst Abus. 2005 Jun;26(2):5–14. doi: 10.1300/j465v26n02_02. [DOI] [PubMed] [Google Scholar]
- 80.Penetar DM, Looby AR, Su Z, Lundahl LH, Eros-Sarnyai M, McNeil JF, et al. Benztropine pretreatment does not affect responses to acute cocaine administration in human volunteers. Hum Psychopharmacol. 2006 Dec;21(8):549–59. doi: 10.1002/hup.810. [DOI] [PubMed] [Google Scholar]
- 81.Birks J. Cholinesterase inhibitors for Alzheimer's disease. Cochrane Database Syst Rev. 2006;(1):CD005593. doi: 10.1002/14651858.CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Farlow M. A clinical overview of cholinesterase inhibitors in Alzheimer's disease. Int Psychogeriatr. 2002;14 1:93–126. doi: 10.1017/s1041610203008688. [DOI] [PubMed] [Google Scholar]
- 83.Giacobini E. Cholinesterase inhibitors: new roles and therapeutic alternatives. Pharmacol Res. 2004 Oct;50(4):433–40. doi: 10.1016/j.phrs.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 84.Camicioli R, Gauthier S. Clinical trials in Parkinson's disease dementia and dementia with Lewy bodies. Can J Neurol Sci. 2007 Mar;34 1:S109–17. doi: 10.1017/s0317167100005679. [DOI] [PubMed] [Google Scholar]
- 85.Ochoa EL, Clark E. Galantamine may improve attention and speech in schizophrenia. Hum Psychopharmacol. 2006 Mar;21(2):127–8. doi: 10.1002/hup.751. [DOI] [PubMed] [Google Scholar]
- 86.Khateb A, Ammann J, Annoni JM, Diserens K. Cognition-enhancing effects of donepezil in traumatic brain injury. Eur Neurol. 2005;54(1):39–45. doi: 10.1159/000087718. [DOI] [PubMed] [Google Scholar]
- 87.Marco-Contelles J, do Carmo Carreiras M, Rodriguez C, Villarroya M, Garcia AG. Synthesis and pharmacology of galantamine. Chem Rev. 2006;106(1):116–33. doi: 10.1021/cr040415t. [DOI] [PubMed] [Google Scholar]
- 88.Schilstrom B, Ivanov VB, Wiker C, Svensson TH. Galantamine enhances dopaminergic neurotransmission in vivo via allosteric potentiation of nicotinic acetylcholine receptors. Neuropsychopharmacology. 2007;32(1):43–53. doi: 10.1038/sj.npp.1301087. [DOI] [PubMed] [Google Scholar]
- 89.Diehl A, Nakovics H, Croissant B, Smolka MN, Batra A, Mann K. Galantamine reduces smoking in alcohol-dependent patients: a randomized, placebo-controlled trial. Int J Clin Pharmacol Ther. 2006 Dec;44(12):614–22. doi: 10.5414/cpp44614. [DOI] [PubMed] [Google Scholar]
- 90.Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. 2006;81(3):313–22. doi: 10.1016/j.drugalcdep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 91.Aharonovich E, Nunes E, Hasin D. Cognitive impairment, retention and abstinence among cocaine abusers in cognitive-behavioral treatment. Drug Alcohol Depend. 2003;71(2):207–11. doi: 10.1016/s0376-8716(03)00092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Streeter CC, Terhune DB, Whitfield TH, Gruber S, Sarid-Segal O, Silveri MM, et al. Performance on the stroop predicts treatment compliance in cocaine-dependent individuals. Neuropsychopharmacology. 2008 Mar;33(4):827–36. doi: 10.1038/sj.npp.1301465. [DOI] [PubMed] [Google Scholar]
- 93.Etter JF, Lukas RJ, Benowitz NL, West R, Dresler CM. Cytisine for smoking cessation: a research agenda. Drug Alcohol Depend. 2008 Jan 1;92(13):3–8. doi: 10.1016/j.drugalcdep.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 94.Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, et al. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48(10):3474–7. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- 95.Dunbar G, Boeijinga PH, Demazieres A, Cisterni C, Kuchibhatla R, Wesnes K, et al. Effects of TC-1734 (AZD3480), a selective neuronal nicotinic receptor agonist, on cognitive performance and the EEG of young healthy male volunteers. Psychopharmacology. 2007;16:16. doi: 10.1007/s00213-006-0675-x. [DOI] [PubMed] [Google Scholar]
- 96.Product Information: CHANTIX(TM) oral tablets vot. New York, NY: Pfizer Labs; 2008. [Google Scholar]
- 97.Dwoskin LP, Crooks PA. A novel mechanism of action and potential use for lobeline as a treatment for psychostimulant abuse. Biochem Pharmacol. 2002 Jan 15;63(2):89–98. doi: 10.1016/s0006-2952(01)00899-1. [DOI] [PubMed] [Google Scholar]
- 98.Stead LF, Hughes JR. Lobeline for smoking cessation. Cochrane Database Syst Rev. 2000;(2):CD000124. doi: 10.1002/14651858.CD000124. [DOI] [PubMed] [Google Scholar]
- 99.Wilhelm CJ, Johnson RA, Eshleman AJ, Janowsky A. Lobeline effects on tonic and methamphetamine-induced dopamine release. Biochem Pharmacol. 2008 Mar 15;75(6):1411–5. doi: 10.1016/j.bcp.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Eyerman DJ, Yamamoto BK. Lobeline attenuates methamphetamine-induced changes in vesicular monoamine transporter 2 immunoreactivity and monoamine depletions in the striatum. J Pharmacol Exp Ther. 2005 Jan;312(1):160–9. doi: 10.1124/jpet.104.072264. [DOI] [PubMed] [Google Scholar]
- 101.Vocci FJ, Appel NM. Approaches to the development of medications for the treatment of methamphetamine dependence. Addiction. 2007 Apr;102 1:96–106. doi: 10.1111/j.1360-0443.2007.01772.x. [DOI] [PubMed] [Google Scholar]
- 102.Chan WY, McKinzie DL, Bose S, Mitchell SN, Witkin JM, Thompson RC, et al. Allosteric modulation of the muscarinic M4 receptor as an approach to treating schizophrenia. Proc Natl Acad Sci U S A. 2008 Aug 5;105(31):10978–83. doi: 10.1073/pnas.0800567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.May LT, Avlani VA, Langmead CJ, Herdon HJ, Wood MD, Sexton PM, et al. Structure-function studies of allosteric agonism at M2 muscarinic acetylcholine receptors. Mol Pharmacol. 2007 Aug;72(2):463–76. doi: 10.1124/mol.107.037630. [DOI] [PubMed] [Google Scholar]
- 104.Surig U, Gaal K, Kostenis E, Trankle C, Mohr K, Holzgrabe U. Muscarinic allosteric modulators: atypical structure-activity-relationships in bispyridinium-type compounds. Arch Pharm (Weinheim) 2006 Apr;339(4):207–12. doi: 10.1002/ardp.200600005. [DOI] [PubMed] [Google Scholar]
- 105.Valant C, Gregory KJ, Hall NE, Scammells PJ, Lew MJ, Sexton PM, et al. A novel mechanism of G protein-coupled receptor functional selectivity: Muscarinic partial agonist McN-A-343 as a bitopic orthosteric/allosteric ligand. J Biol Chem. 2008 Aug 22; doi: 10.1074/jbc.M803801200. [DOI] [PMC free article] [PubMed] [Google Scholar]


