Abstract
Background
In patients with sarcoidosis, sudden death is a leading cause of mortality, which may represent unrecognized cardiac involvement. Delayed-enhancement cardiovascular magnetic resonance (DE-CMR) can detect minute amounts of myocardial damage. We sought to compare DE-CMR with standard clinical evaluation for the identification of cardiac involvement.
Methods and Results
Eighty-one consecutive patients with biopsy proven extra-cardiac sarcoidosis were prospectively recruited for a parallel and masked comparison of cardiac involvement between: (1) DE-CMR, and (2) standard clinical evaluation using consensus criteria (modified Japanese Ministry of Health [JMH] guidelines). Standard evaluation included 12-lead electrocardiography and at least one dedicated non-CMR cardiac study (echocardiography, radionuclide scintigraphy, or cardiac catheterization). Patients were followed 21±8 months for major adverse events (death, defibrillator shock, or pacemaker requirement).
Patients were predominantly middle-aged (46±11 years), female (62%), African-American (73%), had chronic sarcoidosis (median, 7 years), and preserved LVEF (median, 56%). DE-CMR identified cardiac involvement in 21 patients (26%) and JMH criteria in 10 (12%, 8 overlapping), a more than two-fold higher rate for DE-CMR (p=0.005). All patients with myocardial damage on DE-CMR had coronary disease excluded by x-ray angiography. Pathology evaluation in 15 patients (19%) identified 4 with cardiac sarcoidosis; all 4 were positive by DE-CMR whereas 2 were JMH positive. On follow-up, 8 had adverse events including 5 cardiac deaths. Patients with myocardial damage on DE-CMR had a 9-fold higher rate of adverse events and a 11.5-fold higher rate of cardiac death than patients without damage.
Conclusion
In patients with sarcoidosis, DE-CMR is more than twice as sensitive for cardiac involvement than current consensus criteria. Myocardial damage detected by DE-CMR appears to be associated with future adverse events including cardiac death, but events were few and this needs confirmation in a larger cohort.
Keywords: cardiovascular magnetic resonance, delayed-enhancement imaging, cardiac sarcoidosis
INTRODUCTION
Cardiac involvement is clinically evident in only 5% of patients with sarcoidosis,1 yet myocardial lesions are found at autopsy in 20–60%.2–4 Importantly, sudden death is the leading cause of mortality in patients with sarcoidosis in Japan, and perhaps the second most common after pulmonary complications in the United States.4,5 Therefore, it has been postulated that cardiac involvement in patients with sarcoidosis is often clinically unrecognized and is a primary cause of death.6
Antemortem recognition of cardiac sarcoidosis may be difficult in part because of the lack of a sensitive diagnostic method. Although a positive endomyocardial biopsy may be specific for cardiac involvement, the diagnostic yield is low even in patients with signs and symptoms consistent with cardiac sarcoidosis, presumably because of the patchy nature of the disease.7
Consensus criteria for the diagnosis of cardiac sarcoidosis have been developed by the Japanese Ministry of Health and Welfare (JMH).8 These criteria involve multiple diagnostic tests, including an electrocardiogram (ECG) and a dedicated cardiac imaging study (echocardiogram, radionuclide scintigraphy, or cardiac catheterization), and provide guidance for identifying cardiac involvement when endomyocardial biopsy is either not performed or is non-diagnostic. In general, the imaging studies are used to identify ventricular dilatation, abnormal function, or perfusion defects. However, a large amount of myocardial damage may be required before these are evident.9,10
Delayed-enhancement cardiac magnetic resonance (DE-CMR) using gadolinium contrast is a relatively recent technique that allows visualization of even minute amounts of myocardial damage.11,12 We hypothesized that in patients with sarcoidosis, DE-CMR would be more sensitive at detecting cardiac involvement than standard clinical evaluation using consensus criteria. Additionally, in order to assess the prognostic significance of myocardial damage identified by DE-CMR, all patients were followed for adverse clinical events.
METHODS
Population and Protocol
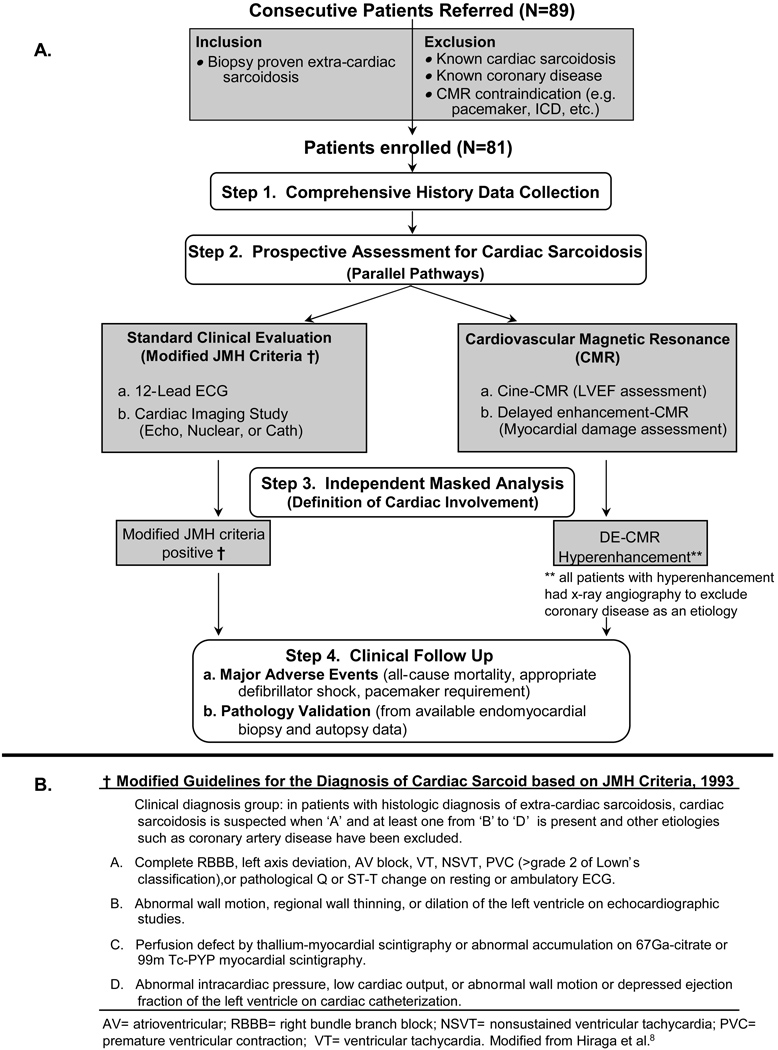
From September 2002 through December 2004 we approached pulmonary, rheumatology, and general medicine physicians whose clinical practices included patients with sarcoidosis. We evaluated consecutive patients referred from these practices for possible inclusion in the study. The primary criterion for enrollment was the presence of biopsy proven extra-cardiac sarcoidosis. We excluded patients in whom the diagnosis of cardiac sarcoidosis had already been given, since we wished to compare strategies for obtaining this diagnosis prospectively. Other exclusion criteria included prior myocardial infarction or known coronary disease, which could potentially confound both DE-CMR and consensus criteria interpretations, and standard CMR contraindications (Figure 1A). All patients gave informed written consent for the protocol, which was approved by the Duke Institutional Review Board.
Figure 1. Enrollment and Protocol.
Panel A outlines the enrollment criteria and the four separate steps of the study protocol. Panel B outlines the modified Japanese Ministry of Health (JMH) guidelines for the diagnosis of cardiac sarcoidosis in those with biopsy proven extra-cardiac sarcoidosis. See text for further details.
The study protocol involved four distinct steps after enrollment (Figure 1A). The first was the collection of a complete medical history from each patient, which included demographic information, site of tissue diagnosis for sarcoidosis, organs involved, years since diagnosis, and medication treatment history. The second was a parallel assessment for cardiac involvement. Patients underwent both a standard clinical evaluation applying modified JMH guidelines (Figure 1B) and had a CMR study. The third was masked determination of cardiac involvement by modified JMH criteria and the analysis of DE-CMR. The fourth step involved clinical follow-up to track major adverse events and the availability of cardiac tissue from either endomyocardial biopsy or autopsy to validate imaging findings.
Standard Clinical Evaluation
In the absence of histological evidence of cardiac sarcoidosis, the JMH criteria require a combination of an electrocardiographic abnormality and evidence of ventricular dilatation, dysfunction, or perfusion defects on either echocardiography, radionuclide scintigraphy, or cardiac catheterization (Figure 1B). Therefore, all patients underwent 12-lead electocardiography and at least one dedicated cardiac imaging study (non-CMR). All ECG and imaging data were reviewed blinded to clinical history and CMR findings.
Endomyocardial biopsy was performed at the discretion of the treating physician and patient after enrollment, and was not mandated. Similar to previous studies,13 JMH criteria were modified by excluding endomyocardial biopsy as a diagnostic parameter since not all patients underwent biopsy. However, all cardiac tissue obtained during follow-up from endomyocardial biopsy or autopsy was evaluated in a systematic manner (see Pathology Validation section). X-ray coronary angiography was also performed at the discretion of the treating physician, but in general, patients with abnormalities on noninvasive cardiac imaging (non-CMR or CMR) underwent angiography to exclude obstructive coronary disease (defined as >50% narrowing of the luminal diameter of at least one major epicardial artery14).
Cardiovascular Magnetic Resonance
Acquisition
Images were acquired on a 1.5-T scanner (Siemens Sonata) using a phased-array coil during repeated breath-holds. Steady-state free-precession cine images were acquired in multiple short-axis (every 10 mm throughout the entire LV) and 3 long-axis planes for assessment of left-ventricular function. Typical parameters were: TR 3.0 ms, TE 1.5 ms; flip angle 60°; temporal resolution, 35 ms; in-plane resolution 1.7×1.4 mm. Standard delayed-enhancement images were acquired using a segmented inversion-recovery gradient-echo sequence (in-plane resolution 1.8×1.4 mm; temporal resolution, 160–200 ms) 10 minutes after contrast administration (gadoversetamide, 0.15 mmol/kg) in the identical planes as cine imaging (both, slice thickness 6 mm, gap 4 mm).15 Inversion delay times were typically 280–360 ms, and total time for the CMR evaluation was approximately 30 minutes.
Analysis
Scans were placed in random order and analyzed masked to all clinical information including JMH status. Cine and DE-CMR images were evaluated separately. Left-ventricular ejection fraction (LVEF) and LV volumes were quantitatively measured on the basis of end-diastolic and end-systolic endocardial contours from the stack of short-axis cine images.16 The presence, location, and extent of hyperenhanced tissue, which was assumed to represent scarred, damaged myocardium,16 was determined by the consensus of two observers using a standard 17-segment LV model and a 5-point scale for each segment (0=no hyperenhancement; 1=1–25% area hyperenhanced; 2=26–50%; 3=51–75%; and 4=76–100%).17,18 Global hyperenhancement volume as a percentage of LV myocardium was calculated by summing the segments with hyperenhancement (each weighted by the midpoint of the range of hyperenhancement for the given segmental score, ie. 1=13%; 2=38%; 3=63%; 4=88%) and dividing by 17.17,18 Additionally, the pattern of hyperenhancement was classified as either CAD-type or non-CAD-type as described previously.19,20 In brief, since ischemic injury progresses as a “wavefront” from the subendocardium to the epicardium,21 hyperenhancement involving the LV subendocardium was considered CAD-type. Conversely, hyperenhancement patterns that spared the subendocardium and instead were limited to the middle or epicardial portion of the LV wall were considered non-CAD-type. With this classification, non-transmural hyperenhancement of the RV side of the interventricular septum was considered non-CAD-type. The RV free wall was also evaluated for hyperenhancement (classified as non-CAD-type), however, given the limited spatial resolution of DE-CMR in comparison to normal RV wall thickness, only the presence or absence of RV hyperenhancement was scored.
Clinical Follow Up
Clinical information concerning adverse events was obtained via: (1) telephone interview with the patient, or, if deceased, with family members, (2) contact with the patient’s physician(s), (3) hospital records, and (4) death certificates. The prespecified primary endpoint was a composite of all-cause mortality or symptomatic arrhythmia, defined as ventricular tachyarrhythmia leading to appropriate cardioverter-defibrillator discharge (based on stored electrograms) or symptomatic bradyarrhythmia leading to pacemaker implantation. No patient was lost to follow-up. For deceased patients the cause of death was identified in all cases and classified in accordance with standard criteria.22 The secondary endpoint was death from cardiac causes alone. All event information was obtained and classified without knowledge of JMH status or CMR findings.
All patients were enrolled before the recent FDA alerts regarding the rare occurrence of nephrogenic systemic fibrosis associated with gadolinium administration.23 The majority had normal renal function (all had GFR >45 mL/min/1.73m2), and none developed nephrogenic systemic fibrosis during the follow-up period.
Pathology Validation
During follow up, all patients that underwent endomyocardial biopsy and/or post-mortem autopsy were identified. A cardiovascular pathologist unaware of JMH status and CMR findings examined all cardiac tissue samples. Histological samples were considered positive for cardiac sarcoidosis based on the presence of noncaseating epithelioid granulomas in the absence of organisms or particles that could lead to granuloma formation.1 The presence and location of myocardial fibrosis/scarring was also noted.
Statistical Analysis
Continuous, normally distributed data were expressed as mean±SD and between group comparisons were made using two sample t tests. The median and first and third quartiles (Q1, Q3) were used to summarize non-normally distributed, continuous data, and between group comparisons were made using Wilcoxon tests. Comparisons of discrete variables between groups were made using chi square tests; Fisher’s exact test was used in those instances where the expected cell count was <5. The comparison of cardiac involvement by JMH versus DE-CMR was made using McNemar’s test. Logistic regression analysis was used to assess the relationship between clinical characteristics (listed in Table 1) and the presence of cardiac involvement on DE-CMR. Event-free and cardiac survival (time to first event) were plotted as Kaplan-Meier curves. All statistical tests were two-tailed and p<0.05 was regarded as significant. S-PLUS (version 8.0, Insightful Software, Seattle, WA) was used to perform the statistical analyses. All authors have read and agree to the manuscript as written. Raymond Kim and Manesh Patel had full access to and take responsibility for the integrity of the data.
Table 1.
Baseline Characteristics
| Characteristic | Overall (n = 81) |
DE-CMR + (n = 21) |
DE-CMR − (n = 60) |
P value |
|---|---|---|---|---|
| Age (years) | 46±11 | 49±9 | 45±11 | 0.11 |
| Female gender | 50 (62%) | 11 (52%) | 39 (65%) | 0.31 |
| Race | ||||
| Caucasian | 21 (26%) | 6 (29%) | 15 (25%) | 0.80 |
| African-American | 59 (73%) | 15 (71%) | 44 (73%) | |
| Other | 1 (1%) | 0 (0%) | 1 (2%) | |
| Extracardiac Sarcoidosis | ||||
| Positive Biopsy Site (may be more than one) | ||||
| Lung | 59 (73%) | 15 (71%) | 44 (73%) | 1.00 |
| Lymph Node | 15 (19%) | 5 (24%) | 10 (17%) | 0.52 |
| Skin | 10 (12%) | 5 (24%) | 5 (8%) | 0.12 |
| Liver | 6 (7%) | 0 (0%) | 6 (10%) | 0.19 |
| Other | 8 (10%) | 1 (5%) | 7 (12%) | 0.45 |
| Clinical Organ Involvement (may be more than one) | ||||
| Lung | 77 (95%) | 20 (95%) | 57 (95%) | 1.00 |
| Skin | 17 (21%) | 7 (33%) | 10 (17%) | 0.13 |
| Liver | 13 (16%) | 2 (10%) | 11 (18%) | 0.50 |
| Eye | 8 (10%) | 1 (5%) | 7 (12%) | 0.45 |
| Other | 20 (25%) | 5 (24%) | 15 (25%) | 1.00 |
| Number of Organs Involved | ||||
| 1 site | 54 (67%) | 13 (62%) | 41 (68%) | 0.26 |
| 2 sites | 18 (22%) | 7 (33%) | 11 (18%) | |
| 3 or more sites | 9 (11%) | 1 (5%) | 8 (13%) | |
| Years Since Diagnosis (median [Q1,Q3]) | 7.0 [3,12] | 10.0 [3,18] | 6.5 [3,11] | 0.46 |
| Medications | ||||
| Use at any time since diagnosis | ||||
| No medications | 7 (9%) | 2 (10%) | 5 (8%) | 1.00 |
| Steroid | 74 (91%) | 19 (90%) | 55 (92%) | 1.00 |
| Methotrexate | 2 (2%) | 0(0%) | 2 (3%) | 0.61 |
| Hydroxychloroquine | 2 (2%) | 1 (5%) | 1 (2%) | 0.45 |
| Azathioprine | 2 (2%) | 0 (0%) | 2 (3%) | 0.61 |
| Infliximab | 1 (1%) | 0 (0%) | 1 (2%) | 1.00 |
| Current Steroid Use | 53 (65%) | 17 (81%) | 36 (60%) | 0.11 |
| Cardiac Symptoms | ||||
| None | 64 (79%) | 14 (67%) | 50 (83%) | 0.11 |
| Syncope | 2 (2%) | 2 (10%) | 0 (0%) | 0.06 |
| Palpitations | 6 (7%) | 1 (5%) | 5 (8%) | 0.68 |
| Chest Pain | 5 (6%) | 1 (5%) | 4 (7%) | 1.00 |
| Functional Class | ||||
| No limitations | 77 (95%) | 18 (86%) | 59 (98%) | 0.06 |
| NYHA Class I-II | 3 (4%) | 2 (10%) | 1 (2%) | |
| NYHA Class III | 1 (1%) | 1 (5%) | 0 (5%) | |
| 12-lead Electrocardiogram | ||||
| Grade I atrioventricular block | 3 (4%) | 1 (5%) | 2 (3%) | 0.15 |
| Grade II or III atrioventricular block | 0 (0%) | - | - | - |
| Left bundle-branch block | 1 (1%) | 1 (5%) | 0 (0%) | 1.00 |
| Right bundle-branch block | 4 (5%) | 2 (10%) | 2 (3%) | 0.26 |
| Q-wave | 4 (5%) | 3 (14%) | 1 (2%) | 0.053 |
| JMH Criteria Positive for Cardiac Sarcoidosis | 10 (12%) | 8 (38%) | 2 (3%) | 0.002 |
JMH = Japanese Ministry of Health; NYHA = New York Heart Association.
RESULTS
Population
Among 89 consecutive patients referred for possible enrollment into the study, 8 were excluded. Four did not have documentation of biopsy proven extra-cardiac sarcoidosis, two were found to have a clinical history of prior myocardial infarction, and two decided not to participate prior to CMR. The remaining 81 patients met criteria and were enrolled. The baseline features of the patients are presented in Table 1. Patients were predominantly middle-aged (46±11 years), female (62%), and African-American (73%). The most common extracardiac site with clinical involvement was lung (95%), and more than one site was identified in 33% of patients. In general, patents had chronic sarcoidosis (median of 7 years since diagnosis), 65% were being treated with steroids at the time of enrollment, and few had any cardiac symptoms. At the time of CMR, the majority of patients (95%) had no NYHA class functional limitations.
Cardiac Involvement by DE-CMR and JMH Criteria
CMR examinations were completed in all 81 patients, and all scans were included in the analysis. For evaluation of JMH status, a total of 133 non-CMR cardiac imaging studies were completed, representing an average of 1.6 studies per patient (echocardiography, n=81; radionuclide scintigraphy, n=18; cardiac catheterization, n=34). Ambulatory electrocardiography was performed in few patients (n=8) and did not result in change in JMH status compared with 12-lead ECG alone. Hyperenhancement consistent with myocardial damage was identified in 21 patients (26%) by DE-CMR. Typical images are shown in Figure 2. JMH criteria identified cardiac involvement in 10 patients (12%), of whom 8 were also positive by DE-CMR. The rate of cardiac involvement by DE-CMR was more than two-fold higher than by JMH status (p=0.005). Of the baseline clinical features in Table 1, only JMH status was predictive of the presence of hyperenhancement in either univariable or multivariable logistic regression analysis (p=0.002).
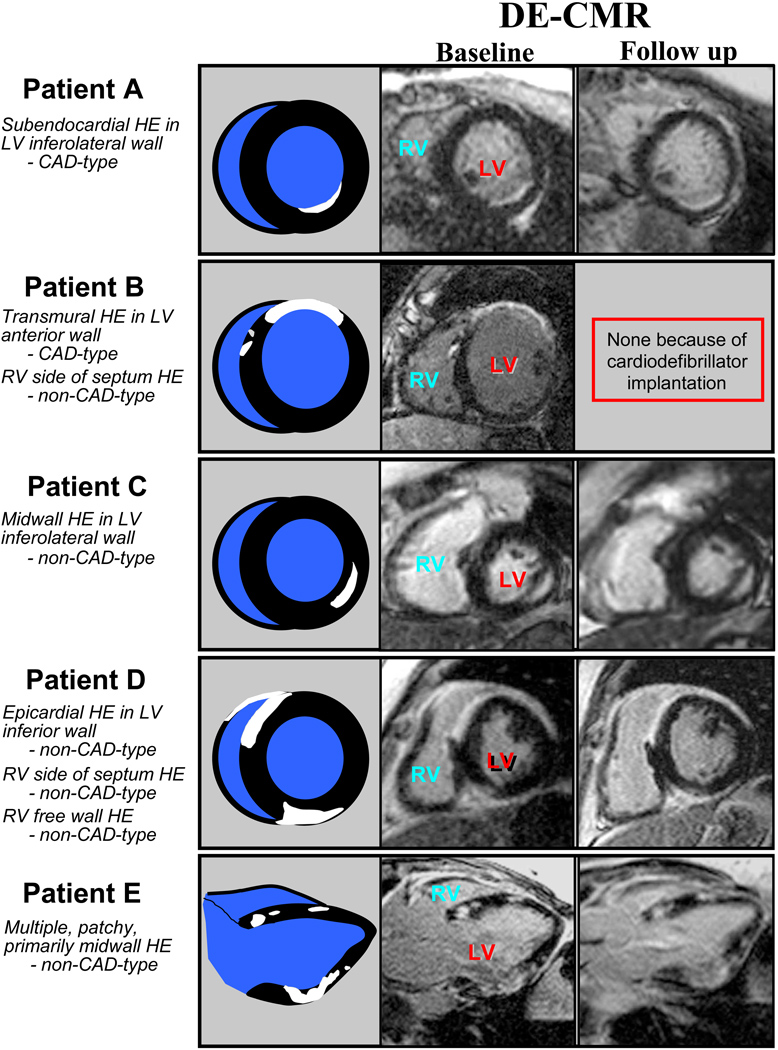
Figure 2. Patterns of Hyperenhancement in DE-CMR Positive Patients.
Images from five patients positive for cardiac involvement by DE-CMR are shown. A variety of hyperenhancement patterns are demonstrated, and these were classified as CAD-type or non-CAD-type depending on whether the left ventricular subendocardium was involved (see text for further details). Cartoon representations of the DE-CMR images are shown immediately adjacent. White (hyperenhanced) regions depict areas of cardiac involvement. The right-most column shows images from repeat DE-CMR scans performed during the follow-up period. These demonstrate the persistence of hyperenhancement.
Hyperenhancement Characteristics
In patients with hyperenhancement, a median of 6.1% of the LV myocardium (2.3%, 19.0%) was involved. On a regional basis, 70% (79/113) of affected segments had nontransmural involvement (<50% hyperenhanced). The location of hyperenhancement was variable (collectively, none of the 17 myocardial segments were spared from involvement), however there was a predilection for the basal and/or mid-ventricular septum. This region was involved in 76% of affected patients (16/21). Concerning the pattern of involvement, 48% of hyperenhanced regions were classified as CAD-type and 52% as non-CAD-type. On a patient basis, 86% of affected patients (18/21) had at least one region with hyperenhancement in a non-CAD-type pattern. Examples of patients with various hyperenhancement patterns are shown in Figure 2. A relatively common non-CAD-type pattern occurring in 67% of DE-CMR positive patients was subendocardial hyperenhancement of the RV side of the interventricular septum; however, only rarely was this widespread, and usually only 1 or 2 of the 5 septal segments were involved. In 4 patients (all of whom also had LV hyperenhancement), hyperenhancement of the RV free wall was observed. In all 4, the RV outflow tract and/or anterobasal region of the RV was involved. All 21 patients with hyperenhancement underwent x-ray coronary angiography; none had evidence of obstructive coronary disease.
During the follow-up period, patients with hyperenhancement were contacted for a repeat DE-CMR scan to evaluate the persistence of hyperenhancement. However, 7 had interval placement of a cardiac pacemaker or defibrillator, 4 died, and 4 declined. The remaining 6 patients had a follow-up scan 8.3±5 months after the initial scan. Analysis of follow-up images revealed: (1) all hyperenhanced regions identified on the baseline scan were visible on the repeat scan in the same myocardial locations (Figure 2), (2) no new areas of hyperenhancement were observed, and (3) planimetry demonstrated that the spatial extent of hyperenhancement was unchanged (baseline, 8.7±2.7% versus follow-up, 8.4±2.4% of LV myocardium, p=0.84).
Left Ventricular Function and Volume
Overall, the median LVEF by cine-CMR was 56% (48%, 61%). LVEF was lower in DE-CMR positive compared with DE-CMR negative patients (median, 45% vs. 57%, p<0.0001), however, 29% of DE-CMR positive patients had LVEF >50%. The median LV end-diastolic volume was 101 ml (89ml, 137 ml), and was similar in DE-CMR positive compared with DE-CMR negative patients (p=0.41). Likewise, the extent of hyperenhancement by DE-CMR was correlated with LVEF (r = −0.54, p<0.001) but only weakly with LV end-diastolic volume (r = 0.20, p=0.08).
Cardiac Pathology
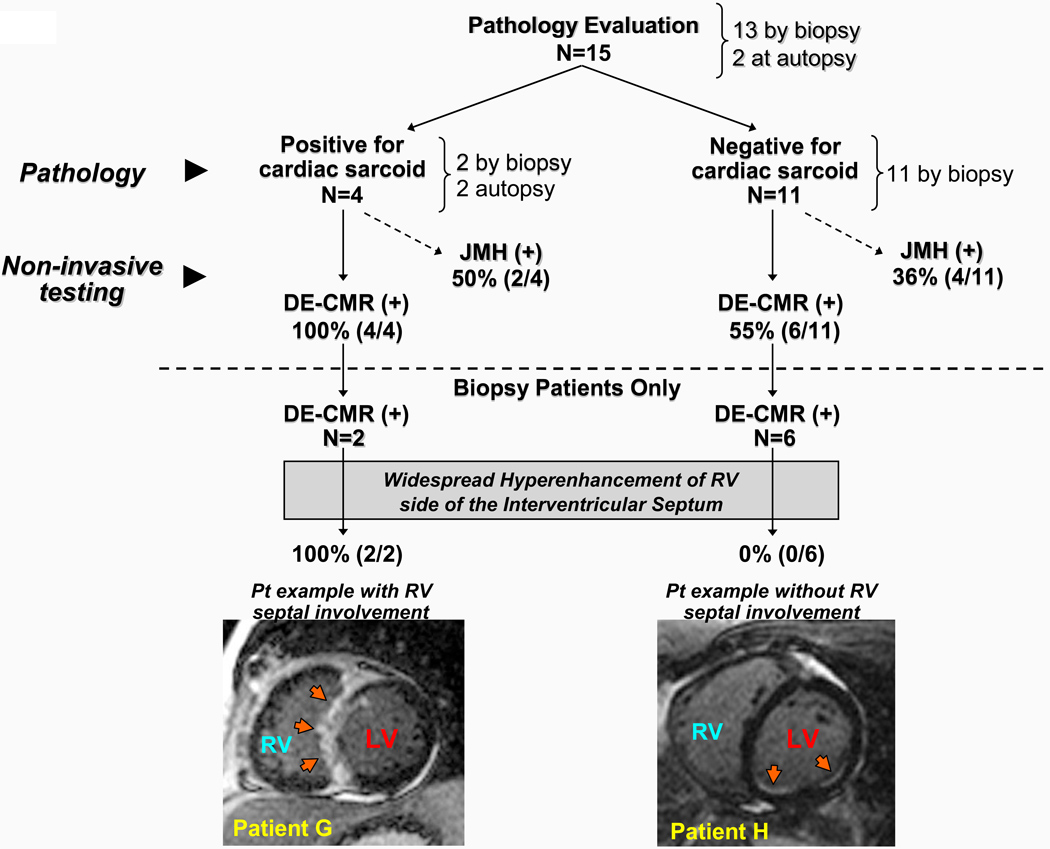
Tissue was obtained for pathology evaluation in 15 patients (19%), 13 by endomyocardial biopsy and 2 at autopsy. Figure 3 demonstrates the concordance between antemortem findings by DE-CMR with postmortem pathology in one of the autopsy patients. Figure 4 outlines the results in all 15 patients with cardiac pathology in comparison with DE-CMR findings. Of the 4 patients who were positive for cardiac sarcoidosis by pathology, DE-CMR demonstrated hyperenhancement in all 4 (100%), whereas JMH status was positive in 2 (50%). Of the 11 patients who were negative for cardiac sarcoidosis by pathology, DE-CMR was positive for cardiac involvement in 6 (55%), and JMH status was positive in 4 (36%). Importantly, in all 6 patients with discordant findings between pathology and DE-CMR, tissue was obtained only by endomyocardial biopsy of the RV side of the interventricular septum, and none had widespread hyperenhancement of the RV side of the interventricular septum (involvement of more than 3 of the 5 septal segments). Specifically, in 2 patients the interventricular septum was completely free of hyperenhancement; in 1 patient a single segment was involved; in 2 patients, 2 segments were involved; and in 1 patient, 3 segments were involved.
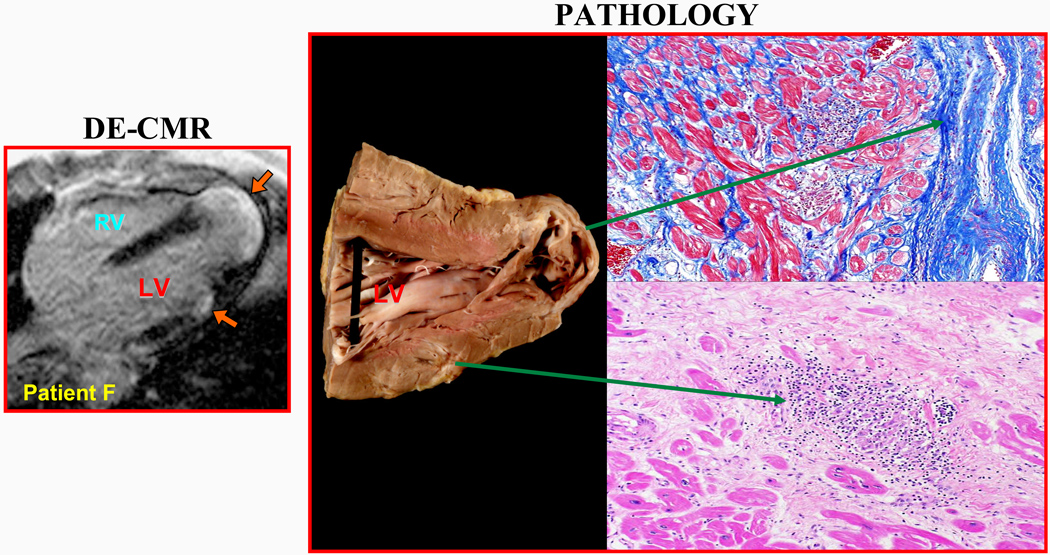
Figure 3. Comparison of DE-CMR to Autopsy Findings in One Patient.
Antemortem DE-CMR study showed two regions of hyperenhancement (orange arrows). The patient died 6 months after DE-CMR. Gross examination of the heart demonstrated aneurysmal dilatation of the LV apex with wall thinning and macroscopically visible scarring. A smaller region of scar tissue was also observed in the lateral wall. These regions matched the areas of involvement seen on DE-CMR. Histological sections prepared from these 2 regions demonstrated dense fibrosis (top, masson trichrome stain) and granulomatous inflammation within patchy fibrosis (bottom, hematoxylin-eosin stain). Examination of the coronary arteries demonstrated the absence of obstructive atherosclerotic disease.
Figure 4. Summary of Cardiac Pathology Evaluation.
Cardiac sarcoidosis was diagnosed by pathology evaluation in 4 patients. All 4 had cardiac involvement by DE-CMR. Of the 2 patients with positive endomyocardial biopsy, both had widespread hyperenhancement of the RV side of the interventricular septum (involvement of all 5 septal segments; example, Patient G). Conversely, of 11 patients with negative endomyocardial biopsy, 6 had cardiac involvement by DE-CMR. These 6 had no or limited involvement of the RV side of the interventricular septum (example, Patient H). See text for further details.
Major Adverse Events
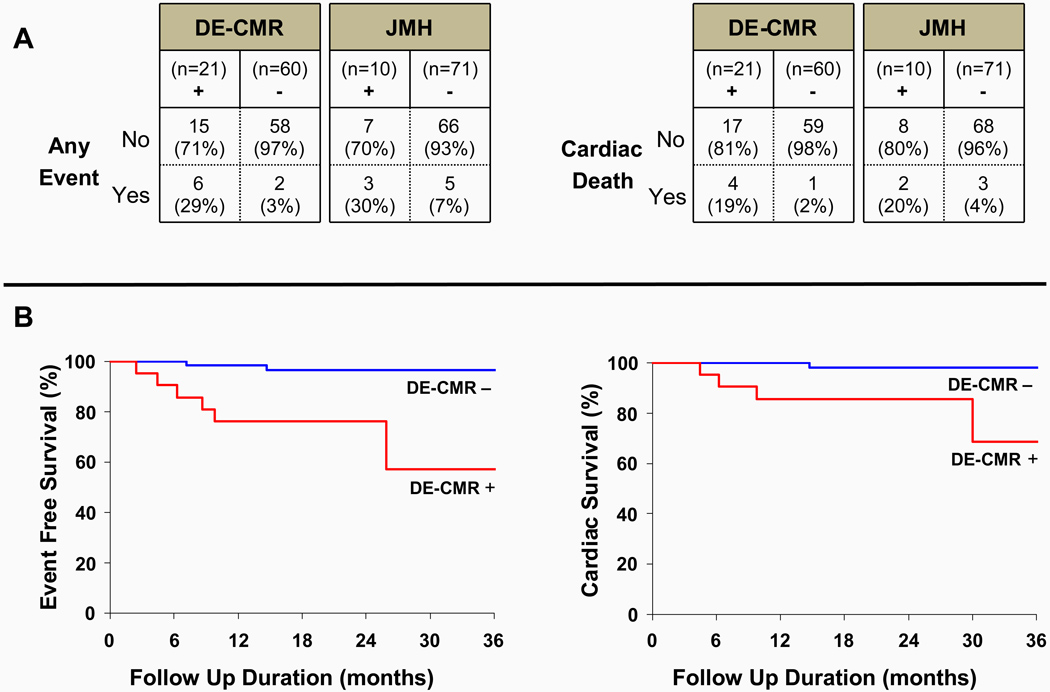
During a mean follow-up duration of 21±8 months, 8 patients had major adverse events: 6 died, 2 had ventricular tachyarrhythmia leading to defibrillator discharge (confirmed by stored electrograms; 1 patient also died later), and 1 developed atrioventricular block leading to pacemaker placement. Of the 6 deaths, 5 were cardiac and 1 was from pulmonary complications. The events according to DE-CMR and JMH status are shown in Figure 5A. Of the two patients without cardiac involvement by DE-CMR who had adverse events (1 cardiac and 1 non-cardiac death), both were also JMH negative. Kaplan-Meier survival curves are shown in Figure 5B. In DE-CMR positive patients, the event rate and cardiac death rate were 9-fold and 11.5-fold higher, respectively, than in DE-CMR negative patients (adverse events: 17.2% versus 1.9% per year; cardiac death: 11.5% versus 1.0% per year). In JMH positive patients, the event rate and cardiac death rate were 3.5-fold and 3.9-fold higher, respectively, than in JMH negative patients (adverse events: 14.5% versus 4.1% per year; cardiac death: 9.7% versus 2.5% per year). Given the few events, statistical comparisons between survival curves were not performed.
Figure 5. Events According to DE-CMR and JMH Status.
Panel A outlines adverse events according to DE-CMR and JMH status. Kaplan-Meier curves in Panel B demonstrate event-free and cardiac survival was reduced in patients positive for cardiac involvement by DE-CMR. See text for further details.
DISCUSSION
In this study of consecutive patients with biopsy proven extra-cardiac sarcoidosis who were prospectively screened for cardiac involvement, the principal finding was that DE-CMR identified myocardial abnormalities in significantly more patients than a standard clinical evaluation based on the consensus guidelines from the Japanese Ministry of Health (26% vs. 12%). An additional finding was that DE-CMR positive patients had a higher rate of adverse events including cardiac death during follow-up compared with DE-CMR negative patients, however the cohort was small and there were few events.
The prevalence of cardiac sarcoidosis of 26% found by DE-CMR is consistent with autopsy series in the United States, which have found myocardial lesions in 20–27% of patients with sarcoidosis.2,3 For many reasons, the lower detection rate from the standard assessment is not surprising. As a first step in the diagnosis of cardiac sarcoidosis, JMH guidelines require an abnormal electrocardiogram, yet even in patients with findings of extensive myocardial infiltration on autopsy, the premortem electrocardiogram may be normal in 25%.3 More importantly, necropsy studies have demonstrated that many sarcoid patients suffering sudden death have only limited cardiac involvement, with lesions that are small and patchy.3,4,24 Premortem, it is unlikely that these lesions would have been associated with gross changes in ventricular cavity size or function, or detected by traditional cardiac imaging techniques. In the present study, the median extent of damage detected by DE-CMR was only 6.1% of myocardial mass. Additionally, on a regional basis, the majority of affected segments (70%) had non-transmural involvement (<50% hyperenhanced). The latter is important since non-transmural scarring—even if overall scar size is considerable—is frequently associated with normal ventricular wall motion9 and normal myocardial perfusion by nuclear scintigraphy.10 Thus, the ability of DE-CMR to detect small and/or nontransmural myocardial damage is likely the reason why a more than two-fold higher rate of cardiac disease was identified compared with the standard assessment.
Improved diagnostic sensitivity may come at the cost of lower specificity. On this point we note that none of the abnormalities detected by cardiac imaging, either as part of the JMH guidelines or from DE-CMR, are specific for cardiac sarcoidosis. For instance, any cardiomyopathic process may meet the JMH criteria of abnormal wall motion. Similarly, hyperenhancement on DE-CMR representing scar tissue and/or necrosis,12,19,20 may result from a variety of etiologies including ischemic heart disease. To account for this, as part of the study design, we excluded patients with prior myocardial infarction or known coronary disease. Furthermore, all patients with hyperenhancement underwent coronary angiography and none had evidence of obstructive coronary disease. As a consequence, in our study population of patients with biopsy proven sarcoidosis, it is highly likely that hyperenhancement represents cardiac sarcoidosis rather than another nonischemic process (e.g. cardiac amyloidosis, etc.), since it is improbable that patients would be afflicted concurrently with two rare disorders.
Our pathology data are also consistent with the interpretation that hyperenhancement in the study population represents cardiac sarcoidosis. Of the 4 patients with pathology confirmed cardiac sarcoid (2 at autopsy, 2 by endomyocardial biopsy), all 4 were identified by DE-CMR whereas the standard assessment identified only 2. Moreover, in the 2 with autopsy, gross examination of the heart demonstrated a nearly 1-to-1 concordance between the location, shape, and extent of myocardial lesions and in vivo hyperenhancement (Figure 3). Interestingly, of the 13 patients undergoing endomyocardial biopsy, only 2 were positive for cardiac sarcoidosis. This diagnostic yield of 15% is similar to that found by Uemura et al.7 who reported a positive biopsy rate of only 19% in a study cohort in whom the clinical diagnosis of cardiac sarcoidosis had already been made. Importantly, Uemura et al.7 concluded that patients with clinical evidence of cardiac involvement be treated for cardiac sarcoidosis despite negative myocardial biopsies, since inhomogeneous myocardial involvement and sampling error will frequently lead to false negative biopsies. Our results suggest that 6 of 11 patients had false negative biopsies (Figure 3), and the DE-CMR images indicate a mechanism: none of the 6 had widespread hyperenhancement of the right ventricular side of the interventricular septum, which was the source of tissue for endomyocardial biopsy.
These findings suggest that DE-CMR provides additional value to standard assessment for the diagnosis of cardiac sarcoidosis, and bolster similar conclusions from prior investigations.13,25 However, from a clinical care standpoint, arguably the prognostic implications are most important. In this regard, the present study suggests that damage detected by DE-CMR may be associated with future adverse clinical events in patients with sarcoidosis. Even if small, regions of myocardial damage identified by DE-CMR may provide substrate for ventricular arrhythmias and conduction disturbances,26 and DE-CMR has shown prognostic utility independent of common clinical and functional predictors in other cardiac disorders.27–30 Nonetheless, in the current study events were few and caution should be used in interpreting these results until confirmed in a larger cohort.
The current report appears to be the first to prospectively demonstrate a link between a noninvasive index of cardiac involvement and clinical outcome in patients with sarcoidosis. Yazaki et al,31 retrospectively identified 75 Japanese patients with the clinical diagnosis of cardiac sarcoidosis. Multivariable analysis demonstrated that left-ventricular enlargement by echocardiography, sustained ventricular tachycardia, and NYHA functional class were independent predictors of all-cause mortality. Notably, in this cohort many patients had heart failure symptoms and NYHA functional class was the most powerful prognostic predictor. Smedema et al.32 reviewed data in 101 consecutive patients with pulmonary sarcoidosis assessed at two university medical centers in the Netherlands. Although a battery of tests, including CMR, was performed in many patients, and during a mean follow-up of 1.7 years, there were 4 deaths and 9 received a pacemaker and/or implantable cardioverter/defibrillator, the study was primarily descriptive and there was no data relating results of noninvasive testing with clinical outcome. Additionally, it appears that only a minority of patients underwent inversion-recovery DE-CMR, based on another report the same year involving many of the same patients.13 In most patients, CMR images were acquired using an older spin-echo sequence, and this is important since there are considerable differences in image quality between these techniques.33 Recently, Mehta et al.34 reported on a prospective evaluation of 62 ambulatory patients with biopsy-proven extra-cardiac sarcoidosis who were screened for cardiac involvement. Using a structured algorithm, 26 patients underwent CMR and 25 had PET. Based on abnormalities found on either CMR or PET, 24 patients (39%) were given the diagnosis of cardiac sarcoidosis. Over a mean follow-up of 1.8 years, no patient died or had ventricular arrhythmias, and the authors concluded that sarcoidal lesions seen on CMR or PET do not predict arrhythmias in patients with preserved cardiac function. At first glance, this report appears to contrast with the current investigation. However, we note that hyperenhancement on DE-CMR was found only in 8 patients, and the chance of a type II error seems high. Conversely, in patients with negative DE-CMR scans, the results of this report appear to corroborate our finding of a low event rate (< 2% per year) and a benign course.
Our study has a number of limitations. If patients systematically had ambulatory electrocardiography in addition to the resting 12-lead and had undergone every non-CMR cardiac imaging procedure, the number of patients with cardiac involvement by JMH criteria would likely have been higher. However, we attempted to compare DE-CMR with a clinically plausible approach (an average of 1.6 non-CMR imaging studies were performed per patient), and we note that an earlier investigation utilizing both resting and ambulatory ECG and multiple non-CMR imaging modalities demonstrated similar findings to the current study—namely that DE-CMR was more sensitive for cardiac involvement than standard clinical approaches.13 T2-weighted sequences aimed at detecting acute edema/inflammation were not performed, and there may be some uncertainty regarding the precise pathophysiological interpretation of myocardial hyperenhancement. However, in our study cohort, nearly all patients had chronic sarcoidosis (median of 7 years since diagnosis) and none had acute cardiac symptoms. Moreover, repeat DE-CMR in a subset of patients demonstrated that the location, shape, and extent of hyperenhancement were unchanged (Figure 2). Along with the pathology findings, these suggest that hyperenhancement represents scar and/or chronic granulomatous tissue rather than acute necrosis with inflammation in our population. There appears to be racial differences in both the prevalence of cardiac sarcoidosis and its morbidity and mortality.5 Most of our patients were African-American, and caution should be used in extrapolating our findings to other patient populations. While we believe our study represents the largest experience with contemporary DE-CMR in patients with sarcoidosis, to date, the cohort was relatively small, from a single-center, and clinical events were few. Our findings will need to be replicated in a larger, preferably multi-center investigation.
The presence of severe left-ventricular dysfunction (LVEF ≤ 30–40%) is often a key indication for clinical management decisions including implantable cardioverter-defibrillator therapy.35 Yet in our study only 2 of the 8 patients with adverse events during follow-up had an LVEF less than 40%. These data suggest that in patients with sarcoidosis—as in other populations36—there may be a paradox in that although low LVEF identifies a group with relatively high risk, the majority of adverse events occur in patients with preserved LVEF. Since DE-CMR detected 6 of 8 patients with adverse events in the current study, future investigations could focus on whether DE-CMR can improve the management of patients with sarcoidosis, including the early diagnosis of cardiac involvement and the potential for prophylactic defibrillator use.
Acknowledgments
Funding Sources
Funding for the research was provided in part by National Institutes of Health grants RO1-HL64726 (RJK), RO1-HL63268 (RMJ), and the Joseph C. Greenfield Scholar Fund (MRP).
Footnotes
Conflicts of Interests Disclosures
Drs Kim and Judd are inventors of a US patent on Delayed Enhancement MRI, which is owned by Northwestern University. There are no other conflicts of interest or financial relationships to disclose.
REFERENCES
- 1.Hunninghake GW, Costabel U, Ando M, Baughman R, Cordier JF, du Bois R, Eklund A, Kitaichi M, Lynch J, Rizzato G, Rose C, Selroos O, Semenzato G, Sharma OP. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:149–173. [PubMed] [Google Scholar]
- 2.Longcope WT, Freiman DG. A study of sarcoidosis; based on a combined investigation of 160 cases including 30 autopsies from The Johns Hopkins Hospital and Massachusetts General Hospital. Medicine. 1952;31:1–132. [PubMed] [Google Scholar]
- 3.Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation. 1978;58:1204–1211. doi: 10.1161/01.cir.58.6.1204. [DOI] [PubMed] [Google Scholar]
- 4.Matsui Y, Iwai K, Tachibana T, Fruie T, Shigematsu N, Izumi T, Homma AH, Mikami R, Hongo O, Hiraga Y, Yamamoto M. Clinicopathological study of fatal myocardial sarcoidosis. Ann N Y Acad Sci. 1976;278:455–469. doi: 10.1111/j.1749-6632.1976.tb47058.x. [DOI] [PubMed] [Google Scholar]
- 5.Iwai K, Sekiguti M, Hosoda Y, DeRemee RA, Tazelaar HD, Sharma OP, Maheshwari A, Noguchi TI. Racial difference in cardiac sarcoidosis incidence observed at autopsy. Sarcoidosis. 1994;11:26–31. [PubMed] [Google Scholar]
- 6.Sharma OP, Maheshwari A, Thaker K. Myocardial sarcoidosis. Chest. 1993;103:253–258. doi: 10.1378/chest.103.1.253. [DOI] [PubMed] [Google Scholar]
- 7.Uemura A, Morimoto S, Hiramitsu S, Kato Y, Ito T, Hishida H. Histologic diagnostic rate of cardiac sarcoidosis: evaluation of endomyocardial biopsies. Am Heart J. 1999;138:299–302. doi: 10.1016/s0002-8703(99)70115-8. [DOI] [PubMed] [Google Scholar]
- 8.Hiraga H, Yuwa K, Hiroe M. Guideline for the diagnosis of cardiac sarcoidosis: study report on diffuse pulmonary disease [in Japanese] The Japanese Ministry of Health and Welfare. 1993:23–24. [Google Scholar]
- 9.Mahrholdt H, Wagner A, Parker M, Regenfus M, Fieno DS, Bonow RO, Kim RJ, Judd RM. Relationship of contractile function to transmural extent of infarction in patients with chronic coronary artery disease. J Am Coll Cardiol. 2003;42:505–512. doi: 10.1016/s0735-1097(03)00714-9. [DOI] [PubMed] [Google Scholar]
- 10.Wagner A, Mahrholdt H, Holly TA, Elliott MD, Regenfus M, Parker M, Klocke FJ, Bonow RO, Kim RJ, Judd RM. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study.[see comment] Lancet. 2003;361:374–379. doi: 10.1016/S0140-6736(03)12389-6. [DOI] [PubMed] [Google Scholar]
- 11.Ricciardi MJ, Wu E, Davidson CJ, Choi KM, Klocke FJ, Bonow RO, Judd RM, Kim RJ. Visualization of discrete microinfarction after percutaneous coronary intervention associated with mild creatine kinase-MB elevation. Circulation. 2001;103:2780–2783. doi: 10.1161/hc2301.092121. [DOI] [PubMed] [Google Scholar]
- 12.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 13.Smedema JP, Snoep G, van Kroonenburgh MP, van Geuns RJ, Dassen WR, Gorgels AP, Crijns HJ. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol. 2005;45:1683–1690. doi: 10.1016/j.jacc.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 14.Klocke FJ, Baird MG, Lorell BH, Bateman TM, Messer JV, Berman DS, O'Gara PT, Carabello BA, Russell RO, Jr, Cerqueira MD, St John Sutton MG, DeMaria AN, Udelson JE, Kennedy JW, Verani MS, Williams KA, Antman EM, Smith SC, Jr, Alpert JS, Gregoratos G, Anderson JL, Hiratzka LF, Faxon DP, Hunt SA, Fuster V, Jacobs AK, Gibbons RJ, Russell RO. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging) J Am Coll Cardiol. 2003;42:1318–1333. doi: 10.1016/j.jacc.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Kim RJ, Shah DJ, Judd RM. How we perform delayed enhancement imaging. J Cardiovasc Magn Reson. 2003;5:505–514. doi: 10.1081/jcmr-120022267. [DOI] [PubMed] [Google Scholar]
- 16.Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 17.Sievers B, Elliott MD, Hurwitz LM, Albert TSE, Klem I, Rehwald WG, Parker MA, Judd RM, Kim RJ. Rapid detection of myocardial infarction by subsecond, free-breathing delayed contrast-enhancement cardiovascular magnetic resonance. Circulation. 2007;115:236–244. doi: 10.1161/CIRCULATIONAHA.106.635409. [DOI] [PubMed] [Google Scholar]
- 18.Kim RJ, Albert TS, Wible JH, Elliott MD, Allen JC, Lee JC, Parker M, Napoli A, Judd RM. Performance of delayed-enhancement magnetic resonance imaging with gadoversetamide contrast for the detection and assessment of myocardial infarction: an international, multicenter, double-blinded, randomized trial. Circulation. 2008;117:629–637. doi: 10.1161/CIRCULATIONAHA.107.723262. [DOI] [PubMed] [Google Scholar]
- 19.McCrohon JA, Moon JC, Prasad SK, McKenna WJ, Lorenz CH, Coats AJ, Pennell DJ. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54–59. doi: 10.1161/01.CIR.0000078641.19365.4C. [DOI] [PubMed] [Google Scholar]
- 20.Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J. 2005;26:1461–1474. doi: 10.1093/eurheartj/ehi258. [DOI] [PubMed] [Google Scholar]
- 21.Reimer KA, Lowe JE, Rasmussen MM, Jennings RB. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977;56:786–794. doi: 10.1161/01.cir.56.5.786. [DOI] [PubMed] [Google Scholar]
- 22.Epstein AE, Carlson MD, Fogoros RN, Higgins SL, Venditti FJ., Jr Classification of death in antiarrhythmia trials. J Am Coll Cardiol. 1996;27:433–442. doi: 10.1016/0735-1097(95)00482-3. [DOI] [PubMed] [Google Scholar]
- 23.Marckmann P, Skov L, Rossen K, Dupont A, Damholt MB, Heaf JG, Thomsen HS. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17:2359–2362. doi: 10.1681/ASN.2006060601. [DOI] [PubMed] [Google Scholar]
- 24.Perry A, Vuitch F. Causes of death in patients with sarcoidosis. A morphologic study of 38 autopsies with clinicopathologic correlations. Arch Pathol Lab Med. 1995;119:167–172. [PubMed] [Google Scholar]
- 25.Tadamura E, Yamamuro M, Kubo S, Kanao S, Saga T, Harada M, Ohba M, Hosokawa R, Kimura T, Kita T, Togashi K. Effectiveness of delayed enhanced MRI for identification of cardiac sarcoidosis: comparison with radionuclide imaging. Am J Roentgenol. 2005;185:110–115. doi: 10.2214/ajr.185.1.01850110. [DOI] [PubMed] [Google Scholar]
- 26.Kim RJ, Judd RM. Gadolinium-enhanced magnetic resonance imaging in hypertrophic cardiomyopathy: in vivo imaging of the pathologic substrate for premature cardiac death? J Am Coll Cardiol. 2003;41:1568–1572. doi: 10.1016/s0735-1097(03)00190-6. [DOI] [PubMed] [Google Scholar]
- 27.Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, Sheppard MN, Poole-Wilson PA, Pennell DJ. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977–1985. doi: 10.1016/j.jacc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 28.Kwong RY, Chan AK, Brown KA, Chan CW, Reynolds HG, Tsang S, Davis RB. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113:2733–2743. doi: 10.1161/CIRCULATIONAHA.105.570648. [DOI] [PubMed] [Google Scholar]
- 29.Rochitte CE, Oliveira PF, Andrade JM, Ianni BM, Parga JR, Avila LF, Kalil-Filho R, Mady C, Meneghetti JC, Lima JA, Ramires JA. Myocardial delayed enhancement by magnetic resonance imaging in patients with Chagas' disease: a marker of disease severity. J Am Coll Cardiol. 2005;46:1553–1558. doi: 10.1016/j.jacc.2005.06.067. [DOI] [PubMed] [Google Scholar]
- 30.Kim HW, Klem I, Shah DJ, Wu E, Meyers SN, Parker MA, Crowley AL, Bonow RO, Judd RM, Kim RJ. Unrecognized non-Q-wave myocardial infarction: prevalence and prognostic significance indisease. PLoS Med. 2009;6:e1000057. doi: 10.1371/journal.pmed.1000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yazaki Y, Isobe M, Hiroe M, Morimoto S, Hiramitsu S, Nakano T, Izumi T, Sekiguchi M. The Central Japan Heart Study G. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol. 2001;88:1006–1010. doi: 10.1016/s0002-9149(01)01978-6. [DOI] [PubMed] [Google Scholar]
- 32.Smedema J-P, Snoep G, van Kroonenburgh MPG, van Geuns R-J, Dassen WRM, Gorgels AP, Crijns HJGM. Cardiac involvement in patients with pulmonary sarcoidosis assessed at two university medical centers in the Netherlands.[see comment] Chest. 2005;128:30–35. doi: 10.1378/chest.128.1.30. [DOI] [PubMed] [Google Scholar]
- 33.Simonetti OP, Kim RJ, Fieno DS, Hillenbrand HB, Wu E, Bundy JM, Finn JP, Judd RM. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218:215–223. doi: 10.1148/radiology.218.1.r01ja50215. [DOI] [PubMed] [Google Scholar]
- 34.Mehta D, Lubitz SA, Frankel Z, Wisnivesky JP, Einstein AJ, Goldman M, Machac J, Teirstein A. Cardiac involvement in patients with sarcoidosis: diagnostic and prognostic value of outpatient testing. Chest. 2008;133:1426–1435. doi: 10.1378/chest.07-2784. [DOI] [PubMed] [Google Scholar]
- 35.Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Smith SC, Jr, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B, Blanc JJ, Budaj A, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL. ACC/AHA/ESC 2006 Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e385–e-484. doi: 10.1161/CIRCULATIONAHA.106.178233. [DOI] [PubMed] [Google Scholar]
- 36.Stecker EC, Vickers C, Waltz J, Socoteanu C, John BT, Mariani R, McAnulty JH, Gunson K, Jui J, Chugh SS. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol. 2006;47:1161–1166. doi: 10.1016/j.jacc.2005.11.045. [DOI] [PubMed] [Google Scholar]