Abstract
Mei4 is a key sporulation-specific transcription factor in fission yeast. Ectopic expression of Mei4 in vegetative cells caused formation of nucleated membranous compartments, which shared common features with normal forespore membranes, thereby perturbing nuclear division. These results suggest why expression of development-specific transcription factors must be strictly controlled.
SPORULATION is a major developmental phase accompanying meiosis in the fission yeast Schizosaccharomyces pombe. Spore formation initiates with assembly of double unit membranes, termed forespore membranes (FSMs) (Yoo et al. 1973), which develop into the spore plasma membrane (Hirata and Tanaka 1982). During meiosis II, membrane vesicles are recruited to the vicinity of spindle pole bodies (SPBs) and fuse there to generate FSMs. The FSM expands and eventually encapsulates each of the four nuclei generated by meiosis. Most sporulation-related genes are transcriptionally induced during sporulation (Mata et al. 2002), indicating that alteration of the transcriptional program underlies this dynamic cellular event. Mei4 is a sporulation-specific transcription factor possessing a forkhead domain (Horie et al. 1998). A mei4 mutant arrests at prophase I (Shimoda et al. 1985). More than 400 meiosis-upregulated genes are governed by Mei4 (Mata et al. 2007). Previous studies have shown that overproduction of Mei4 in vegetative cells induces its target genes (Horie et al. 1998; Abe and Shimoda 2000; Mata et al. 2007). Thus, these observations indicate that Mei4 is a key regulator of sporulation. To better understand the significance of this development-specific transcription factor, we report here the morphological consequences of ectopic overproduction of Mei4 in vegetative cells.
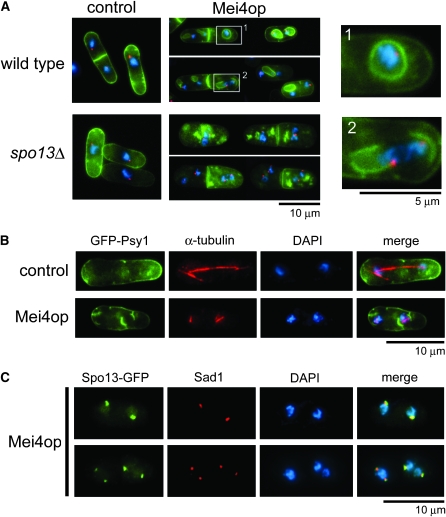
Mei4 was overproduced under the nmt1 promoter in a strain carrying GFP-tagged Psy1 to observe FSM-like membranous structures within the cytoplasm (Table 1). Psy1 is an S. pombe homolog of syntaxin, localizes to the plasma membrane during vegetative growth, and translocates to the FSM after meiosis I (Nakamura et al. 2001), and thus GFP-Psy1 was used here as an FSM marker. Overproduction of Mei4 was confirmed by Western analysis, and the level was much higher than that during meiosis (supporting information, Figure S1). In vegetative cells, the GFP-Psy1 signal was observed mainly on the plasma membrane and the septum (Figure 1A, control). About 70% of Mei4-overproducing cells contained cytoplasmic membranous structures that were visualized by GFP-Psy1 (Figure 1A). A significant proportion of these structures appeared to be either anucleate or nucleate membrane compartments (Figure 1A, wild-type Mei4op). Because most of these structures were in close contact with SPBs (Figure 1A), we then determined whether these membrane-like structures were formed from spindle poles. As shown in Figure 1B, four cup-shaped GFP-Psy1 signals were observed at both ends of two spindle microtubules in Mei4-overproducing cells. In contrast, a typical mitotic spindle formed in cells harboring the control plasmid (Figure 1B). Prior to FSM formation, a meiotic SPB component Spo13 is expressed in a Mei4-dependent manner and recruited to the SPB, which is essential for initiation of FSM formation (Nakase et al. 2008). Spo13-GFP fluorescence was not detected in vegetative cells (data not shown). In Mei4-overproducing vegetative cells, Spo13-GFP was observed as dots in the periphery of nuclei. Immunofluorescence microscopy with a Sad1 antibody showed that Spo13 colocalized with Sad1, an SPB-resident protein. These data suggest that ectopic expression of Mei4, in turn, results in production of Spo13 and activates the SPB to form the membranous structure.
TABLE 1.
Strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| MKW5 (FY7456)a | h90 | Nakamura-Kubo et al. (2003) |
| MM72-4C (FY6845)a | h− leu1-32 ura4-D18 | YGRC |
| YM24 (FY12104)a | h90 ade6≪GFP-psy1 leu1-32 | This study |
| TN427 | h90 ade6≪GFP-psy1 leu1≪nmt1-mei4 | This study |
| YN310 | h− leu1≪GFP-psy1 ade6-M210 | Nakase et al. (2008) |
| YN311 | h− leu1≪GFP-psy1 mes1-B44 ura4-D18 | This study |
| YN312 (FY12490)a | h90 spo13∷ura4+ leu1≪GFP-psy1 ade6-M210 ura4-D18 | Nakase et al. (2008) |
| HM4832 |
h+/h+ pat1-114/pat1-114 mei4-HA∷Kanr/mei4+ ade6-M210/ade6-M216 leu1-32/leu1+ |
Murakami-Tonami et al. (2007) |
These strains were obtained from the Yeast Genetic Resource Center of Japan, supported by the National BioResource Project (YGRC/NBRP) (http:/yeast.lab.nig.ac.jp/nig/). S. pombe strains constructed in this study will be deposited at the YGRC/NBRP.
Figure 1.—
Ectopic overproduction of Mei4 causes formation of membrane-like structures resembling FSMs. (A) Wild-type (YN310) and spo13Δ mutant cells (YN312) carrying either the control plasmid pREP1A or pREP1A(mei4) were incubated in MM + N (thiamine-free medium) at 30° for 16 hr. Cells were fixed with glutaraldehyde and paraformaldehyde as described (Hagan and Hyams 1988). The SPB was visualized by indirect immunofluorescence microscopy, using rabbit anti-Sad1 antibody (a generous gift from O. Niwa) and Alexa 546-conjugated goat anti-rabbit IgG (Molecular Probes, Eugene, OR). To visualize the nuclear chromatin region, cells were stained with 4′,6-diamidino-2-phenylindole (DAPI) at 1 μg/ml. Stained cells were observed by fluorescence microscopy, using a microscope (model BX50; Olympus, Tokyo, Japan) equipped with a charge-coupled device (CCD) camera (Cool-SNAP; Roper Scientific, San Diego). Blue, chromatin; red, SPB; green, GFP-Psy1. Magnified images (wild-type cells expressing Mei4) are also shown in the right panels. (B) Wild-type cells (YN310) carrying either pREP1A or pREP1A(mei4) were fixed and microtubules were visualized by mouse anti-α-tubulin antibody TAT-1 (Woods et al. 1989) and Cy3-conjugated secondary antibody (Sigma, St. Louis). Blue, chromatin; red, microtubules; green, GFP-Psy1. (C) Wild-type cells (MM72-4C) carrying pAL (spo13-GFP) and pREP2 (mei4) were incubated in MM + N at 30° for 16 hr. Fixed cells were examined by DAPI and GFP, as well as with an anti-Sad1 antibody.
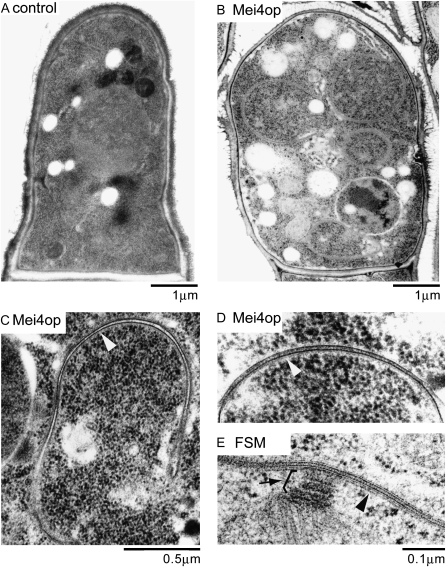
Thin-section electron microscopy confirmed membranous compartments in the cytoplasm of Mei4-overproducing cells (Figure 2B). The membranes were composed of double-unit membranes like the FSM. Furthermore, the interval between unit membranes was very similar to the FSM (Figure 2, C–E). In contrast, no such compartment was observed in control cells (Figure 2A). It is known that many ribosomes attach to the endoplasmic reticulum (ER) membrane (Nakamura-Kubo et al. 2003). However, no ribosomes were found on the membranous structure, indicating that the structure induced by Mei4 overproduction is not related to the ER. We conclude that the intracellular membranous compartments induced by Mei4 overproduction are an FSM-equivalent structure.
Figure 2.—
Fine structure of Mei4-overproducing cells. (A–D) Wild-type cells (YN310) carrying pREP1A or pREP1A(mei4) were cultured in MM + N at 30° for 16 hr. Samples for electron microscopy were prepared as described (Ye et al. 2007) and sections were viewed using an electron microscope (H-7600; Hitachi, Tokyo) at 100 kV. White arrowheads indicate the FSM-like membranous structure. (E) Fine structure of sporulating cell (MKW5). Arrowhead and arrow indicate the FSM and the SPB, respectively.
During vegetative growth, most cells are mononucleate because the cell cycle of S. pombe has a long G2 phase, and cell separation occurs by the end of S phase. However, multinucleate cells were often observed by Mei4 overproduction (Figure 1, A and C). Approximately 60% of the cells were binucleate, and ∼25% were tri- or tetranucleate, while only 17% of control cells were binucleate (Table 2). We examined nuclear division in the mes1 mutant, which is defective in the second meiotic division (Bresch et al. 1968). In Mei4-overproducing cells, the mes1 mutation significantly increased the number of mononucleate cells and, remarkably, diminished the number of tri- or tetranucleate cells (Table 2). A unique nuclear division pattern caused by Mei4 overproduction may require Mes1-mediated meiotic function. Interestingly, these abnormal nuclear divisions occurred with concomitant formation of abnormal FSM-like membranous structures. These membranous structures were observed in >80% of the binucleate and multinucleate cells. In contrast, only 8.6% of the mononucleate cells formed such structures. In mes1 cells overproducing mei4+, no membranous structures were observed (data not shown). Although the cytoplasmic membranous compartments were not formed in spo13Δ cells, abnormal nuclear division occurred to the same extent in wild-type cells (data not shown). These observations suggest that ectopic formation of FSM-like membranes per se does not cause the unusual nuclear division pattern.
TABLE 2.
Cell types observed during ectopic overproduction of Mei4
| No. of nuclei per cell |
||||
|---|---|---|---|---|
| 1 |
2 |
3 |
4 |
|
| Cell type (%) | ||||
| Wild type | ||||
| Control | 83.4 | 16.6 | 0 | 0 |
| Mei4op | 19.0 | 56.0 | 15.5 | 9.8 |
| mes1 | ||||
| Control | 83.7 | 16.3 | 0 | 0 |
| Mei4op |
55.5 |
41.1 |
2.4 |
1.3 |
Wild-type cells (YN310) carrying pREP1A (control) or pREP1A(mei4) (Mei4op) and mes1 cells (YN311) carrying pREP1A (control) or pREP1A(mei4) (Mei4op) were incubated in MM + N medium for 18 hr at 30°. Cells were fixed and stained with DAPI. For each sample, >200 cells were counted. The experiment was performed three times with reproducible results. The results presented are from a representative single experiment.
In this study, we provide evidence that untimely expression of a key sporulation-specific transcription factor Mei4 causes a striking morphological consequence, assembly of prespore-like membrane compartments. A genomewide DNA microarray analysis has shown that expression of few meiosis-upregulated genes is not reduced in mei4Δ but increased by Mei4 overproduction (Mata et al. 2007). S. pombe has four forkhead transcription factors other than Mei4. If the recognition sequences of these forkhead proteins resemble each other, overproduced Mei4 might enhance expression of genes that are normally governed by other forkhead transcription factors. As Fkh2 has been reported to be implicated in sporulation (Szilagyi et al. 2005), there is a possibility that some target genes of Fkh2 are induced by overproduced Mei4. Such artifactual expression might contribute partly to the observed morphological consequences.
Several spore wall biosynthetic enzymes such as β-glucan synthase Bgs2 are known to localize to the FSM (Liu et al. 2000; Martin et al. 2000). However, Bgs2-GFP did not localize to the membranous structure but dispersed in the cytoplasm in Mei4-overproducing cells (data not shown). Because no viable spores could be produced, Mei4 expression is insufficient to mediate differentiation of vegetative cells to asci.
In addition, Mei4 overproduction perturbed nuclear division, depending partly on meiotic function, including Mes1 activity. In addition to transcriptional control of mei4+, the transcript is selectively removed by the determinant of selective removal (DSR)-Mmi1 system, in which mRNAs containing a cis-acting region called the DSR are recognized by a YTH-family RNA-binding protein Mmi1 and are degraded in the exosome (Harigaya et al. 2006). In the present study, overproduction of Mei4 from the multicopy plasmid pREP1 or pREP1A may have compensated for normal elimination of mei4 mRNAs by the DSR-Mmi1 system. Indeed, a substantial amount of Mei4 was detected in these cells (Figure S1). In summary, our observations indicate that expression of development-specific transcription factors must be under strict control. We presume that fission yeast has the DSR-Mmi1 system to ensure this control.
Acknowledgments
We thank H. Murakami of Nagoya City University for strains and O. Niwa of Kazusa DNA Research Institute and K. Gull of the University of Manchester for antibodies. This study was supported by a Grant-in-Aid for Scientific Research on Priority Areas “Life of Proteins” to T.N. from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. Y.N. was supported by an exploratory research grant from the Japan Society for the Promotion of Science.
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.109.106906/DC1.
References
- Abe, H., and C. Shimoda, 2000. Autoregulated expression of Schizosaccharomyces pombe meiosis-specific transcription factor Mei4 and a genome-wide search for its target genes. Genetics 154 1497–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresch, C., G. Muller and R. Egel, 1968. Genes involved in meiosis and sporulation of a yeast. Mol. Gen. Genet. 102 301–306. [DOI] [PubMed] [Google Scholar]
- Hagan, I. M., and J. S. Hyams, 1988. The use of cell division cycle mutants to investigate the control of microtubule distribution in the fission yeast Schizosaccharomyces pombe. J. Cell Sci. 89 343–357. [DOI] [PubMed] [Google Scholar]
- Harigaya, Y., H. Tanaka, S. Yamanaka, K. Tanaka, Y. Watanabe et al., 2006. Selective elimination of messenger RNA prevents an incidence of untimely meiosis. Nature 442 45–50. [DOI] [PubMed] [Google Scholar]
- Hirata, A., and K. Tanaka, 1982. Nuclear behavior during conjugation and meiosis in the fission yeast Schizosaccharomyces pombe. J. Gen. Appl. Microbiol. 28 263–274. [Google Scholar]
- Horie, S., Y. Watanabe, K. Tanaka, S. Nishiwaki, H. Fujioka et al., 1998. The Schizosaccharomyces pombe mei4+ gene encodes a meiosis-specific transcription factor containing a forkhead DNA-binding domain. Mol. Cell. Biol. 18 2118–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J., X. Tang, H. Wang and M. Balasubramanian, 2000. Bgs2p, a 1,3-beta-glucan synthase subunit, is essential for maturation of ascospore wall in Schizosaccharomyces pombe. FEBS Lett 478 105–108. [DOI] [PubMed] [Google Scholar]
- Martin, V., J. C. Ribas, E. Carnero, A. Duran and Y. Sanchez, 2000. bgs2+, a sporulation-specific glucan synthase homologue is required for proper ascospore wall maturation in fission yeast. Mol. Microbiol. 38 308–321. [DOI] [PubMed] [Google Scholar]
- Mata, J., R. Lyne, G. Burns and J. Bahler, 2002. The transcriptional program of meiosis and sporulation in fission yeast. Nat. Genet. 32 143–147. [DOI] [PubMed] [Google Scholar]
- Mata, J., A. Wilbrey and J. Bahler, 2007. Transcriptional regulatory network for sexual differentiation in fission yeast. Genome Biol. 8 R217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami-Tonami, Y., C. Yamada-Namikawa, A. Tochigi, N. Hasegawa, H. Kojima et al., 2007. Mei4p coordinates the onset of meiosis I by regulating cdc25+ in fission yeast. Proc. Natl. Acad. Sci. USA 104 14688–14693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, T., M. Nakamura-Kubo, A. Hirata and C. Shimoda, 2001. The Schizosaccharomyces pombe spo3+ gene is required for assembly of the forespore membrane and genetically interacts with psy1(+)-encoding syntaxin-like protein. Mol. Biol. Cell 12 3955–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura-Kubo, M., T. Nakamura, A. Hirata and C. Shimoda, 2003. The fission yeast spo14+ gene encoding a functional homologue of budding yeast Sec12 is required for the development of forespore membranes. Mol. Biol. Cell 14 1109–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakase, Y., M. Nakamura-Kubo, Y. Ye, A. Hirata, C. Shimoda et al., 2008. Meiotic spindle pole bodies acquire the ability to assemble the spore plasma membrane by sequential recruitment of sporulation-specific components in fission yeast. Mol. Biol. Cell 19 2476–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda, C., A. Hirata, M. Kishida, T. Hashida and K. Tanaka, 1985. Characterization of meiosis-deficient mutants by electron microscopy and mapping of four essential genes in the fission yeast Schizosaccharomyces pombe. Mol. Gen. Genet. 200 252–257. [DOI] [PubMed] [Google Scholar]
- Szilagyi, Z., G. Batta, K. Enczi and M. Sipiczki, 2005. Characterisation of two novel fork-head gene homologues of Schizosaccharomyces pombe: their involvement in cell cycle and sexual differentiation. Gene 348 101–109. [DOI] [PubMed] [Google Scholar]
- Woods, A., T. Sherwin, R. Sasse, T. H. MacRae, A. J. Baines et al., 1989. Definition of individual components within the cytoskeleton of Trypanosoma brucei by a library of monoclonal antibodies. J. Cell Sci. 93 491–500. [DOI] [PubMed] [Google Scholar]
- Ye, Y., M. Fujii, A. Hirata, M. Kawamukai, C. Shimoda et al., 2007. Geranylgeranyl diphosphate synthase in fission yeast is a heteromer of farnesyl diphosphate synthase (FPS), Fps1, and an FPS-like protein, Spo9, essential for sporulation. Mol. Biol. Cell 18 3568–3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo, B. Y., G. B. Calleja and B. F. Johnson, 1973. Ultrastructural changes of the fission yeast (Schizosaccharomyces pombe) during ascospore formation. Arch. Microbiol. 91 1–10. [DOI] [PubMed] [Google Scholar]