Abstract
In this review we focus on sublingual immunotherapy (SLIT), toll like receptor-9 (TLR-9) vaccines, and anti-IL-5 as novel immunomodulating therapies in allergy. SLIT provides a novel oral route of administering an allergen to induce tolerance to inhaled allergens. Studies of SLIT in allergic rhinitis demonstrate that it reduces symptoms and medication use and is associated with a low incidence of systemic allergic reactions. Initial phase II studies with TLR-9 vaccines conjugated to a ragweed allergen demonstrate that they reduce symptoms of allergic rhinitis during the ragweed season. Anti-IL-5 is effective as a corticosteroid sparing agent in the hypereosinophilic syndrome. In contrast, anti-IL-5 has not shown benefit in moderate asthmatics with persistent symptoms, but may reduce features of airway remodeling in asthma. At present all these immunomodulating approaches (SLIT, TLR-9 vaccines, and anti-IL-5) are investigational in the USA and require further study to determine their safety and effectiveness.
Keywords: sublingual immunotherapy, subcutaneous immunotherapy, anti-IL-5, TLR-9, tolerance
INTRODUCTION
Allergic diseases are very common and affect approximately 20% of the population of the United States (1,2). IgE mediated allergic diseases range from potentially life threatening allergic reactions (i.e. anaphylaxis, severe asthma) to chronic allergic diseases associated with significantly reduced quality of life (i.e. eczema and allergic rhinitis). Allergic diseases are the sixth leading cause of chronic disease in the United States (2). The development of allergy frequently starts in early childhood with initial eczema, followed by the subsequent sequential development of food allergy, allergic rhinitis, and asthma, the so-called “atopic march” (1). Allergic diseases are an example of a gene environment interaction where individuals with a particular genotype make IgE responses to environmental allergens (i.e. pollens, dust mite, cat, foods, etc). As allergic disease frequently run in families, the search for genes that contribute to atopy (i.e. the ability to make an IgE response), as well as to specific allergic diseases (i.e. asthma, allergic rhinitis, eczema) has received a great deal of attention. In general, a large number of genes have been identified that are linked to atopic disease, but each identified gene only contributes a small amount to the observed phenotype (3). For example, in asthma over 100 genes have been linked to asthma with no single gene contributing more than 5% to the observed phenotype (3).
Current therapy for allergic diseases include allergen avoidance where possible, medications (i.e. antihistamines, leukotriene inhibitors, topical and/or oral corticosteroids), and immunotherapy (subcutaneous allergen immunotherapy, anti-IgE) in selected patients. In this review we focus on the development of novel immunomodulating therapeutic approaches (i.e. SLIT, CpG-allergen conjugates, and anti-IL-5), which are all currently investigational in the USA, and discuss results of human studies in terms of effectiveness and safety profiles.
ACHIEVING ALLERGEN SPECIFIC TOLERANCE
The concept of vaccinating allergic individuals to prevent allergy was initially described in 1911 in the Lancet by Noon (4), an immunologist at St Mary's Hospital in London. Noon demonstrated that he could immunize patients who had allergic rhinitis with subcutaneous injections of a grass pollen allergen to prevent symptoms during the grass pollen season (4). Since that time, the goal of allergen immunization in clinical practice has been to induce allergen specific tolerance to individual allergens so that exposure to a particular allergen is not associated with symptoms (5, 6). The main limitation of this allergen immunization approach has been the potential to induce systemic allergic reactions, necessitating that allergen immunization always be conducted in a clinic where a physician is immediately available to treat any reaction to the administered allergen immunotherapy. In order to understand whether novel approaches to inducing allergen specific tolerance (i.e. SLIT, CpG DNA) are an advance over currently available therapies to induce allergen specific tolerance (i.e. subcutaneous immunotherapy, abbreviated SCIT) we will briefly review the evidence for the efficacy and safety of SCIT in allergic diseases.
SUBCUTANEOUS IMMUNOTHERAPY TO INDUCE ALLERGEN SPECIFIC TOLERANCE
The goal of SCIT is to induce allergen specific clinical tolerance to the offending environmental allergen (5, 6). For example, patients with fall seasonal allergic rhinitis due to ragweed exposure are administered SCIT containing the major ragweed allergen Amb a I with the goal of inducing clinical tolerance to ragweed such that during the ragweed season patients would not have characteristic symptoms of allergic rhinitis (i.e. sneezing, rhinorrhoea, nasal congestion, post-nasal drip) during the ragweed season. For SCIT to be effective patients must have an appropriate history of allergic symptoms on exposure to the offending allergen, as well as evidence of IgE antibodies to the specific allergen (as demonstrated by immediate hypersensitivity skin test or blood test such as IgE RAST). Patients with allergic rhinitis are frequently sensitized to more than one environmental allergen (i.e. grass pollen, ragweed, cat, etc). Thus, SCIT frequently utilizes multiple allergens administered in one or two injections, provided the history is consistent with symptoms upon exposure to each allergen, and IgE responses to each allergen are documented.
SCIT Clinical Efficacy
SCIT has been demonstrated to be clinically effective in significantly reducing symptoms in patients with allergic rhinitis, bee venom allergy, and asthma (5,6). A Cochrane meta-analysis of SCIT in allergic rhinitis (51 double blind placebo controlled studies of 2,871 subjects) demonstrated a mean reduction in symptoms of 73%, and a mean reduction in medication use of 57% (7) (Table 1). Studies have demonstrated that after receiving SCIT for 3–5 years, there is long term remission of allergic rhinitis symptoms for at least 3–5 years following discontinuation of SCIT (8). In addition, SCIT decreases the onset of new allergic sensitizations in children (9), and in subjects with allergic rhinitis alone, reduces the likelihood of the progression of their disease from allergic rhinitis to asthma (10,11).
TABLE 1.
| SCIT1 | SLIT2 | |
|---|---|---|
| Double blind studies (number) | 51 | 22 |
| Study subjects (number) | 2,871 | 979 |
| Symptom reduction (%) | 73% | 42% |
| Medication reduction (%) | 57% | 43% |
Calderon MA, etal. Cochrane Review (2007) 1 :CD001936
Wilson DR, et al. Allergy (2005) 60 : 4–12
Meta analysis studies of SCIT in asthma (75 randomized controlled studies of 3,188 subjects) have also demonstrated that SCIT induces a significant reduction in asthma symptoms, asthma medication use, and a significant improvement in bronchial hyperreactivity (12). However, there are also individual studies in children that have shown no discernible benefit from SCIT in allergic children with perennial asthma who were receiving appropriate medical treatment (13). One of the limitations of using SCIT in asthma is that it's administration is limited for safety reasons to asthmatics whose FEV1 is > 70% of predicted. Thus, many of the moderate to severe asthmatics who might most benefit from an adding an immunotherapeutic approach to the treatment of their disease, have a contra-indication to receive SCIT.
SCIT is not indicated for the treatment of several allergic conditions associated with IgE responses to allergens (i.e. food allergy, atopic dermatitis) as at present there is insufficient evidence of efficacy and/or safety data for administering SCIT for these allergic conditions.
How does SCIT induce immune tolerance ?
In inducing tolerance to inhaled allergens, SCIT has effects on T cell as well as B cell immune responses (5, 6). SCIT induces immune deviation from Th2 immune responses (characteristic of allergic inflammation)(sidebar 1) to Th1 immune responses, and also induces Treg cells (sidebar 2) which have the potential to downregulate Th2 immune responses (14). SCIT effects on B cells include a blunting of the seasonal increase in IgE levels, as well as induction of IgG4 antibodies (5, 6). Associated with these effects of SCIT on T cell and B cell immune responses, SCIT suppresses the number and activation of effector cells including mast cells, basophils, and eosinophils in target organs such as the nasal mucosa in allergic rhinitis (5,6).
SCIT Side Effects
To minimize the risk of allergic responses to the allergen(s) administered in SCIT, initial dosages are diluted 10,000 to 100,000 fold from the target effective dose which for most allergens ranges from 6–20 μg to induce tolerance (5, 6). To reach the target SCIT maintenance dose necessitates a slow up-dosing phase of weekly SCIT injections for 4–6 months in a clinic setting where systemic allergic reactions can be immediately treated with epinephrine. Approximately 0.1% of subjects receiving SCIT develop significant systemic reactions that require epinephrine administration (7). A variety of strategies to reduce the allergenicity of allergen immunotherapy have failed in the past because, because reductions in allergenicity of SCIT extracts reduced immunogenicity and clinical effectiveness (i.e. allergoids which are denatured allergens with reduced allergenicity have reduced immunogenicity)(5, 6). A variety of novel strategies (SLIT, CpG-Allergen, peptides) are currently being investigated to improve on the safety and effectiveness of immunotherapy.
SUBLINGUAL IMMUNOTHERAPY
SLIT Clinical Efficacy
SLIT (sublingual immunotherapy) is currently the focus of considerable investigation (16–19) because of the ease of oral administration of SLIT compared to the subcutaneous injection route of SCIT. In addition to the ease of administration, SLIT appears to have a good safety profile with the main side effects being local (oral itching) as opposed to systemic allergic reactions (20–22). In most studies, the reported oral itching is mild, self-resolving, and does not frequently lead to patients discontinuing using SLIT. A meta-analysis of SLIT in allergic rhinitis (22 double blind placebo controlled studies of 979 subjects) demonstrated a mean reduction in symptoms of 42%, and a mean reduction in medication use of 43% (23). Based on comparison of results of meta-analysis studies of SLIT and SCIT in allergic rhinitis it appears that SCIT is clinically more effective, and that SLIT is associated with fewer systemic adverse reactions (Table 1). However, at present there are no adequately powered double blind studies that have directly compared the safety and efficacy of SLIT vs SCIT. Although a double blind , placebo controlled study of SLIT vs SCIT in birch pollen allergic rhinitis demonstrated no significant difference in therapeutic efficacy, the study was not adequately powered to detect a difference if such a difference was present (24).
Sublingual immunotherapy is usually administered as soluble tablets or drops to be kept under the tongue for 1–2 minutes and then swallowed (25). SLIT has been administered either prior to the spring or fall allergy pollen season, or continuously throughout the year to prevent symptoms from perennial allergens such as dust mite. Optimal dosing regimens and duration of therapy with SLIT still require further investigation.
At present there are no double blind studies with SLIT demonstrating that like SCIT it can prevent the development of sensitization to new allergens, or that it has long lasting immunomodulating effects that result in sustained clinical remission after SLIT is discontinued. Open label studies with SLIT evaluating these end-points (26), need to be validated in placebo controlled double blind studies.
SLIT Side Effects
As safety is a key rationale for the use of SLIT (instead of SCIT) the safety record of SLIT in clinical trials has been examined in several reviews (21, 25). Overall, the review of the literature on SLIT suggests that SLIT is generally safe with no fatal adverse events being reported in the 20 years that SLIT has been administered predominantly in Europe (21, 25). Based on a review of published studies of SLIT in 3,984 patients, 14 SLIT related reported serious adverse events (mainly asthma exacerbations) were reported (21, 25). There are 3 reported cases of anaphylaxis associated with SLIT administration (25, 27, 28) necessitating awareness of the potential of SLIT to be associated with these significant adverse events in a minority of subjects. None of these reported adverse events with SLIT have been associated with hypotension or death (25). SLIT is not currently approved for use in the USA.
Immune response induced by SLIT
Studies have investigated whether SLIT like SCIT induces clinical tolerance by influencing B cell and T cell immune responses (29, 30). In general, the B cell immune response to SLIT in terms of IgG4 response is more limited than that induced by SCIT (29, 30) and is dependent upon the dose and duration of SLIT administration (31). Recent studies of T cell responses to SLIT in a small number of birch pollen allergic subjects who received SLIT for 1 year, demonstrated that SLIT induced Tregs within a month of receiving SLIT, and that after one year of SLIT there was evidence that SLIT had induced immune deviation (i.e. inhibition of Th2 and induction of Th1 immune response)(32).
TOLL LIKE RECEPTOR-9 VACCINES
CpG DNA, Toll Like Receptor-9, and Innate Immune response
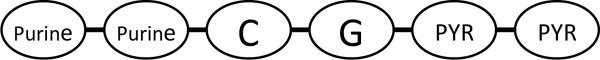
The use of bacterial derived products for immunotherapy to prevent allergy gained momentum with the development of the “hygiene hypothesis” which proposes that microbial exposure in early childhood protects against the development of allergy (1,33). Subsequently, the identification that several molecularly defined bacterial products are recognized by different Toll like receptors (TLRs) expressed by cells of the innate immune system provided an impetus to study whether these well defined bacterial products could be used as immunomodulators in the therapy of allergy (33, 34). For example, CpG (cytosine phosphorothioate guanosine) DNA is a non-coding six base pair sequence of DNA (Figure 1A) that is highly enriched in bacteria and binds with great specificity to its receptor TLR-9 expressed by cells of the innate immune system such as dendritic cells (33, 34). Activation of TLR-9 in dendritic cells leads to activation of intracellular signaling pathways including MAPK, NF-κB, cytokine gene transcription (IFN-α, IFN-β, IL-10, IL-12), and expression of co-stimulatory molecules (e.g. CD40, B7) which can all influence the adaptive immune response to allergens away from a pro-allergic Th2 immune response characterized by high levels of Th2 cytokines including IL-4, IL-5 (33, 34). Studies in mouse models of allergy and asthma have demonstrated that administration of the TLR-9 ligand CpG DNA inhibits Th2 cytokine responses, eosinophilic airway inflammation, mucus expression, airway remodeling, and airway hyperreactivity (35, 36). Additional studies, have demonstrated that conjugating the allergen to CpG DNA (as compared to administering the allergen and the CpG DNA separately) enhances the immune response to the allergen by approximately 100 fold to the same dose of allergen (37). The reason for the enhanced immunogenicity is presumed to be due to the allergen and the CpG DNA localizing to the same antigen presenting cell when administered as a conjugate, and localizing to different antigen presenting cells when administered separately (38). The localization of the allergen and CpG DNA to the same antigen presenting cell results in enhanced immunogenicity as both stimuli activate the same cell as opposed to different cells.
Figure 1A. CpG DNA.
CpG DNA is a six base pair sequence of non-coding DNA that comprise a central cytosine (C) linked to a guanosine (G) through a phosphorothioate linkage (abbreviated p in CpG). To have immunodulating properties the CpG sequence must be flanked on the 51 end by two purines, and on the 31 end by two pyrimidine base pairs.
TLR-9 Vaccines and Allergy
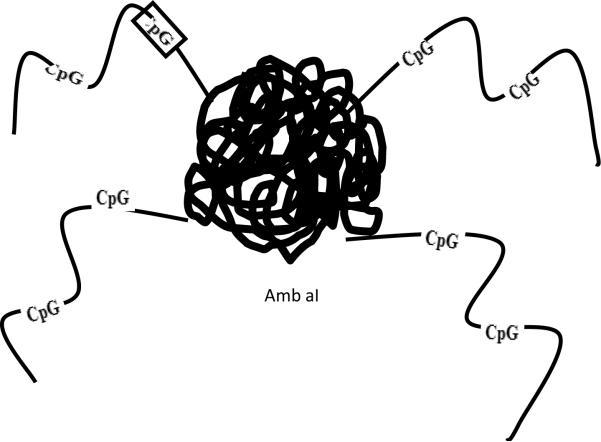
Studies in human subjects with ragweed induced allergic rhinitis have investigated whether a conjugate of the major ragweed protein allergen Amb a I conjugated to CpG DNA (conjugate referred to as Amb a I immunostimulatory DNA conjugate, abbreviated AIC)(Fig 1B) would inhibit Th2 cytokine responses as well as reduce allergic rhinitis symptoms during the ragweed season. Initial studies demonstrated that the AIC vaccine inhibited Th2 responses in peripheral blood (39, 40, 41) as well as Th2 cytokine responses and eosinophilic inflammation in the nasal mucosa of ragweed allergic subjects challenged with ragweed intranasally out of the ragweed season (42). In a subsequent double blind placebo controlled study, subjects with allergic rhinitis who received the AIC vaccine prior to the ragweed season had significantly reduced allergic rhinitis symptoms and used less allergy relief medication during the ragweed season compared to placebo treated subjects (43). Interestingly, although subjects only received the AIC vaccine before the first ragweed season, the protective effect lasted through the second ragweed season (43). In contrast to the slow six month buildup phase required with administration of SCIT to safely reach the target dose of 6–12 μg of Amb a I, the AIC vaccine was administered in only 6 weekly injections to reach a target dose of 12 μg of Amb a I without evidence of inducing any systemic allergic responses (43). A potential reason for the reduced allergenicity of the TLR-9 vaccine is suggested from the structure of the AIC vaccine which contains a central allergen protein Amb a I conjugated to four CpG containing DNA consequences radiating peripherally (Fig 1B). When an allergen such as Amb a I is injected subcutaneously it can cross link IgE bound to mast cells and induce an allergic response. In contrast, when the AIC vaccine is injected subcutaneously the four radiating sequences of CpG DNA reduce the ability of the central Amb a I allergen protein to bind and cross-link IgE bound to mast cells. In vitro studies in which basophils were incubated with either Amb a I allergen, or the same concentration of Amb a I allergen conjugated to CpG DNA, have demonstrated that whereas the Amb a I allergen alone readily binds to IgE affixed to basophils and induces basophil degranulation, the conjugate induces significantly less basophil histamine release (44). Although these initial early phase II studies of CpG DNA conjugated to Amb a I are encouraging in terms of demonstrating the potential for TLR-9 vaccines in allergic rhinitis, the number of subjects included in these studies is small and therefore further studies are needed with larger numbers of subjects to confirm these observations. In addition, as TLR-9 vaccines induce Th1 immune responses the development of autoimmune disease also needs to be carefully monitored in such studies. As yet, there are no reports of induction of auto-antibodies or autoimmune disease in subjects who have received the TLR-9 vaccine.
Figure 1B. B. Amb a1 conjugated to CpG DNA.
A ragweed based TLR-9 vaccine was constructed by chemically linking the major ragweed protein allergen Amb a I to four strands of DNA. Each of the four strands of DNA contain two CpG sequences. The CpG sequences bind to TLR-9 receptors expressed intracellulary by cells of the innate immune system such as dendritic cells which take up injected allergens.
Studies have also examined whether administration of CpG DNA alone, not conjugated to allergen, can reduce allergen induced responses in human asthmatics as CpG DNA has been shown previously to be effective in reducing airway inflammation, remodeling, and airway responsiveness in mouse (35), and primate (45) models of allergen induced asthma. In studies of mild asymptomatic asthmatics with normal pulmonary function, administration of nebulized CpG DNA did not reduce the number of eosinophils in sputum during the late phase response to allergen challenge, nor reduce airway hyperreactivity to methacholine and the late phase reduction in FEV1(46)(Sidebar 3). Further studies are needed to determine whether CpG DNA would be effective in reducing asthma symptoms in patients with persistent symptoms as opposed to studies using allergen challenge in asymptomatic asthmatics.
ANTI-IL-5
The presence of tissue eosinophils is a prominent feature of several allergic diseases including allergic rhinitis, asthma, eosinophilic esophagitis, as well as the idiopathic hypereosinophilic syndrome. Although the eosinophil has the capacity to generate a variety of pro-inflammatory mediators which could theoretically contribute to the pathogenesis of diseases associated with tissue eosinophilia, without therapeutic interventions that narrowly target eosinophils it has not previously been possible to determine the role of the eosinophil in the pathogenesis of individual diseases. The identification that IL-5 is a lineage specific growth factor for eosinophils (47) has provided a novel therapeutic target to specifically reduce eosinophilic inflammation in particular diseases. Evidence to support a role for IL-5 in eosinophilic inflammation is derived from studies of mutant mice deficient in IL-5 which have significantly reduced levels of eosinophils (48). Increased levels of IL-5 and eosinophils are noted in the airway of asthmatics and exogenous administration of IL-5 by the inhalation route to asthmatics induces sputum eosinophila. Studies administering anti-IL-5 to human subjects have demonstrated that a single dose can reduce blood and sputum levels of eosinophils by >90% for approximately three months (48, 49).
Anti-IL-5 and asthma
Based on these encouraging results of anti-IL-5 depleting eosinophils, further studies with anti-IL-5 were performed in asymptomatic asthma patients undergoing allergen challenge (49), as well as in subjects with moderate asthma who had persistent symptoms (50). The studies in mild asymptomatic asthmatics demonstrated that anti-IL-5 did significantly reduce levels of blood and sputum eosinophils by > 90%, but anti-IL-5 did not reduce the late phase response to inhalation allergen challenge, or airway responsiveness (49). Subsequent studies of anti-IL-5 in patients with moderate persistent asthma also did not demonstrate improvements in asthma symptoms, or pulmonary function, when anti-IL-5 was added to inhaled corticosteroid therapy (50).
Anti-IL-5 and airway remodeling in asthma
In contrast to these studies which did not demonstrate a benefit for anti-IL-5 in asthma (49, 50), studies have demonstrated that anti-IL-5 can reduce selected features of airway remodeling in asthma (51). Airway remodeling in asthma is characterized by the development of subepithelial fibrosis, deposition of extracellular matrix proteins beneath the epithelium, smooth muscle hypertrophy/hyperplasia, mucus metaplasia, and angiogenesis. These structural changes, which occur in a subset of asthmatic subjects, may be due to persistent airway inflammation and/or impaired tissue repair mechanisms. The importance of IL-5 and eosinophils to airway remodeling is suggested from studies In mouse models in which chronic allergen challenge induces features of airway remodeling characteristic of asthma that are significantly reduced in IL-5 deficient mice which are deficient in eosinophils (48). As eosinophils are a significant source of the pro-fibrotic growth factor TGF-β1, reduction of eosinophilic inflammation and the number of TGF-β1+ eosinophils may explain the reduced airway remodeling in IL-5 deficient mice (48). Studies in human asthmatics have also suggested an important role for IL-5 in airway remodeling. In a double blind placebo controlled study of human asthmatics treated with anti-IL-5 airway biopsies and bronchoalveolar lavage fluid was obtained at baseline pre-anti-IL-5 therapy and repeated three months later after the anti-IL-5 therapeutic intervention (51). Anti-IL-5 significantly reduced airway biopsy eosinophils by approximately 60%, as well as levels of the extracellular matrix protein tenascin and lumican which are deposited in increased amounts beneath the airway epithelium in remodeled airways. As in mice deficient in IL-5, anti-IL-5 therapy decreased the number of eosinophils expressing TGF-β and the total levels of TGF-β1 (51). The contribution of TGF-β1 to allergen induced airway remodeling is suggested from the significant reductions in airway remodeling noted in wild type mice treated with an anti-TGF-β1 Ab (52), as well as in SMAD 2/3 deficient mice (which do not respond to TGF-β1)(53). Thus, eosinophil expression of TGF-β may be an important contributor to airway remodeling and further studies investigating whether anti-IL-5 reduces additional features of airway remodeling in addition to deposition of extracellular matrix components beneath the airway epithelium are needed. It should also be noted that anti-IL-5 is much more effective in reducing blood and sputum eosinophils (reduction > 90%) compared to its effect on reducing airway eosinophils (reductions of 50 to 60%)(54). The reduced effectiveness of anti-IL-5 in reducing airway eosinophils may be due to the presence of alternate eosinophil growth factors such as GM-CSF being present in the airway and maintaining the viability of eosinophils when IL-5 is neutralized. Thus, current studies do not support a role for anti-IL-5 in the therapy of asthma as anti-IL-5 does not reduce the late phase response to allergen challenge, nor reduce symptoms in symptomatic asthmatics (49, 50). Preliminary studies do suggest that anti-IL-5 has an effect on reducing selected features of airway remodeling (51). Further studies with anti-IL-5 and/or other novel therapies which more completely deplete airway eosinophils (i.e. chemokine antagonists which block eosinophil migration) are needed to finally determine the role of eosinophils in allergic asthma
Anti-IL-5 and the Idiopathic Hypereosinophilic Syndrome
In addition to studies investigating the use of anti-IL-5 in asthma, studies have also examined the role of anti-IL-5 in the idiopathic hypereosinophilic syndrome (HES) (55–57). The HES are a group of diseases characterized by persistent blood eosinophilia (> 1,500 eosinophils/μl) and evidence of end organ damage with no identifiable cause such as parasitic infection or other known causes of hypereosinophilia (58, 59). Imatinib mesylate, a tyrosine kinase inhibitor, is considered to be first line therapy for the myeloproliferative variant of HES which is associated with the fusion gene Fip1-like 1 platelet derived growth factor receptor α [FIP1L1-PDGFRA] (60). Systemic corticosteroids, hydroxyurea, and interferon alfa have been the mainstay of therapy in HES but are associated with considerable adverse effects and are not always effective (57). As eosinophil proliferation is induced by IL-5, studies have investigated whether patients with the HES who do not have the FIP1L1-PDGFRA gene benefit from anti-IL-5 therapy. In a double blind placebo controlled study, subjects with HES were stabilized in terms of symptoms and eosinophil count (< 1,000/μl) on monotherapy with prednisone and then randomized to receive either anti-IL-5 or placebo on a monthly basis for eight months (57). The primary end point was the ability of HES subjects to reduce the dose of prednisone to ≤10 mg/day for at least two months. Starting at week one of anti-IL-5 therapy, the prednisone dose was tapered using a predetermined alogorithm based on eosinophil counts and symptoms. Significantly more patients with HES receiving anti-IL-5 (84%) compared to placebo (43%) were able to taper their dose of prednisone to ≤10 mg/day for at least two months (57)(Table 2). Prior to receiving anti-IL-5 or placebo therapy the mean prednisone dose of HES subjects was approximately 30 mg/day whereas following anti-IL-5 therapy the mean dose of prednisone was 6 mg in the anti-IL-5 therapy group and 22 mg in the placebo group (57). Forty seven % of HES subjects treated with anti-IL-5 were able to completely taper off of prednisone, whereas this was the case in only 5% of HES subjects receiving placebo (57). Over the nine month treatment period, the rates of adverse events other than those due to the hypereosinophilic syndrome were similar in the anti-IL-5 and placebo groups. These studies suggest that anti-IL-5 therapy may be of benefit as a corticosteroid sparing agent in patients with HES negative for the FIP1L1-PDGFRA fusion gene. At present no studies have determined whether anti-IL-5 is effective in patients with acute presentations of HES, HES that is unresponsive to corticosteroids, or in HES patients with the FIP1L1-PDGFRA fusion gene (61).
TABLE 2.
Hypereosinophilic Syndrome: Anti-IL-5 Facilitates Corticosteroid Tapering1
| Anti-IL-5 | Placebo | |
|---|---|---|
| Study subjects (number) | 43 | 42 |
| Prednisone dose (baseline) | 30 mg | 30 mg |
| Prednisone dose (9 months) | 6 mg | 22 mg |
| Tapered prednisone < 10 mg (% of subjects) | 84% | 43% |
| Tapered off of prednisone (% of subjects) | 47% | 5% |
In a double blind placebo controlled study1, subjects with the idiopathic hypereosinophilic syndrome were stabilized in terms of symptoms and eosinophil count (< 1,000/ul) on monotherapy with prednisone and then randomized to receive either anti-IL-5 or placebo on a monthly basis for eight months. Starting at week one of anti-IL-5 therapy, the prednisone dose was tapered using a predetermined alogorithm based on eosinophil counts and symptoms.
Rothenberg ME, et al. New Eng J Med (2008) 358:1215
Anti-IL-5 and Eosinophilic Esophagitis
The potential utility of anti-IL-5 as a therapy for eosinophilic esophagitis (EE), a disorder associated with esophageal remodeling and strictures (62), has also been explored In a small open-label phase I/II study in 4 adult patients with EE and longstanding dysphagia and esophageal strictures (63). Esophageal eosinophilia decreased significantly with anti-IL-5 and EE patients reported a better clinical outcome and improved quality of life (63). Further double blind placebo controlled studies are needed to validate these observations and to determine whether anti-IL-5 is a promising therapeutic intervention for EE.
SUMMARY POINTS
1. SCIT
Administration of SCIT to induce clinical tolerance to allergens in patients with allergic rhinitis, asthma, and bee venom allergy is the current “gold standard” against which novel immunomodulator therapies need to be compared in terms of efficacy and safety.
2. SLIT
Meta-analysis studies of the administration of SLIT to subjects with allergic rhinitis demonstrate that SLIT reduces symptoms of rhinitis and medication usage (23). SLIT appears to be associated with fewer systemic allergic reactions than SCIT, but does not appear to be as effective in reducing symptoms as SCIT.
3. CpG DNA
Preliminary phase II studies with CpG DNA, a TLR-9 adjuvant, conjugated to the major ragweed allergen demonstrated that a reduced number of injections could be used to induce tolerance to ragweed allergen in subjects with allergic rhinitis.
4. Anti-IL-5
Anti-IL-5 demonstrated effectiveness as a corticosteroid sparing agent in patients with HES and no FIP1L1-PDGFRA fusion gene (57). In contrast anti-IL-5 has not shown benefit in mild asthmatics subjected to allergen challenge, or in moderate asthmatics in reducing asthma symptoms or improving lung function. Pilot studies demonstrate that anti-IL-5 reduces features of airway remodeling in asthma.
FUTURE ISSUES
1. SLIT
Studies directly comparing SLIT versus SCIT are needed to determine the relative effectiveness and safety of SLIT versus SCIT. In addition double blind placebo controlled studies are needed to determine whether SLIT like SCIT has long lasting immunomodulating properties once SLIT is discontinued. The optimal dose and duration of SLIT therapy, as well as the use of multiple allergens in SLIT also needs to be investigated.
2. CpG DNA
Large scale studies are needed to validate the results of small scale phase II studies of CpG DNA conjugated to Amb a I in allergic rhinitis. Long term observation in large numbers of treated subjects is also needed to demonstrate that inducing a Th1 immune response is not associated with the subsequent development of autoimmunity.
3. Anti-IL-5
Studies with anti-IL-5 are needed to determine its role in the acute presentation of HES and also in other eosinophil associated diseases such as eosinophilic esophagitis, airway remodeling in asthma, Churg Strauss syndrome, and eosinophilic pneumonia.
4. Early intervention
The successful development of a safe and easily administered immunomodulator agent holds significant potential for early intervention in early childhood in high risk populations to prevent the development of allergy.
SIDEBAR
1. Allergic inflammation
Sites of allergic inflammation are characterized by the uptake of allergens by antigen presenting cells which digest allergens and present fragments of the allergen to allergen specific CD4+ T cells which secrete Th2 cytokines such as IL-4 (a switch factor for IgE synthesis), and IL-5 (an eosinophil growth factor). Upon re-exposure to allergen, allergens activate Th2 cells to express Th2 cytokines, as well as cross link IgE affixed to mast cell high affinity IgE receptors and induce mast cell degranulation.
2. Treg
While Th2 cells may play a role in promoting allergic inflammation, regulatory T cells (Treg) have the ability to down-regulate Th2 cell function and thus potentially reduce levels of allergy. There are two broad categories of Tregs, a) natural Tregs and b) inducible or adaptive Tregs. Adaptive Tregs have many features in common with natural Tregs, but exhibit marked cytokine dependent suppressive mechanisms in vitro which are mediated through the secretion of IL-10 and TGF-β, which may suppress Th2 cells. Thus, immunotherapy strategies which induce Tregs may provide one mechanism of inducing tolerance to allergens.
3. Late phase response to allergen challenge
Under experimental research conditions a patient with mild asthma can be exposed to a defined dose of an inhaled allergen to which they are sensitized (i.e. cat) and their lung function monitored to determine whether they develop an immediate response (fall in FEV1 of ≥ 15% within 10-30 minutes of allergen challenge), as well as a late phase response to allergen challenge (fall in FEV1 of ≥ 15% 4–6 hours after initial allergen challenge). The early phase fall in FEV1 reflects mast cell activation, whereas the late phase fall in FEV1 is considered to reflect recruitment of inflammatory cells from the circulation. Study subjects pretreated with investigational therapies can be assessed as to whether the therapy blocks the early or late phase response to allergen challenge.
ACKNOWLEDGEMENT
Dr Broide is supported by NIH grants AI038425, AI072115, and AI070535.
ABBREVIATIONS
- SCIT
Subcutaneous immunotherapy
- SLIT
Sublingual immunotherapy
- Treg
Regulatory T cell
- TLR-9
Toll like receptor-9
- CpG
Cytosine phosphorothioate guanosine
- Amb a I
Ambrosia artemisiifolia (short ragweed) I pollen
- AIC
Amb a I immunostimulatory DNA conjugate
- HES
Hypereosinophilic syndrome
- IL-5
Interleukin-5
- EE
Eosinophilic esophagitis
Footnotes
DISCLOSURE STATEMENT The authors have no conflicts of interest to disclose in relation to the therapies discussed.
REFERENCES
- 1.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355:2226–35. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 2.Natahn RA, Meltzer EO, Selner JC, Storms W. Prevalence of allergic rhinitis in the United States. J Allergy Clin Immunol. 1997;99:S808–14. [PubMed] [Google Scholar]
- 3.Ober C, Hoffjan S. Asthma genetics 2006: the long and winding road to gene discovery. Genes Immunity. 2006;7:95–100. doi: 10.1038/sj.gene.6364284. [DOI] [PubMed] [Google Scholar]
- 4.Noon L. Prophylactic inoculation against hayfever. Lancet. 1911:1572–3. [Google Scholar]
- 5.Adkis M, Akdis CA. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2007;119:780–89. doi: 10.1016/j.jaci.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Larché M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006;6:761–71. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- 7.Calderon MA, Alves B, Jacobson M, Hurwitz B, Sheikh A, et al. Allergen injection immunotherapy for seasonal allergic rhinitis (Review) Cochrane Database of Systemic Reviews. 2007;1:CD001936. doi: 10.1002/14651858.CD001936.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durham SR, Walker SM, Varga EM, Jacobson MR, O'Brien F, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468–75. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- 9.Des Roches A, Paradis L, Menaro J-L, Bouges S, Duares J-P, et al. Immunotherapy with a standardized Dermatophagoides pteronyssinus extract, VI : specific immunotherapy prevents the onset of new sensitizations in children. J Allergy Clin Immunol. 1997;99:450–3. doi: 10.1016/s0091-6749(97)70069-1. [DOI] [PubMed] [Google Scholar]
- 10.Moller C, Dreborg S, Ferdousi HA, Halken S, Host A, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjuctivitis (the PAT Study) J Allergy Clin Immunol. 2002;9:251–6. doi: 10.1067/mai.2002.121317. [DOI] [PubMed] [Google Scholar]
- 11.Niggemann B, Jacobesn L, Dreborg S, Ferdousi HA, Halken S, et al. Five-year follow-up on the PAT study: specific immunotherapy and long-term prevention of asthma in children. Allergy. 2006;61:855–9. doi: 10.1111/j.1398-9995.2006.01068.x. [DOI] [PubMed] [Google Scholar]
- 12.Abramson MJ, Puy RM, Weiner JM. Allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2003;4:CD001186. doi: 10.1002/14651858.CD001186. [DOI] [PubMed] [Google Scholar]
- 13.Adkinson NF, Jr, Eggleston PA, Eney D, Goldstein EO, Schuberth KC, et al. A controlled trial of immunotherapy for asthma in allergic children. N Engl J Med. 1997;336:325–31. doi: 10.1056/NEJM199701303360502. [DOI] [PubMed] [Google Scholar]
- 14.Hawrylowicz CM, O'Gara A. Potential role of IL-10-secreting regulatory T cells in allergy and asthma. Nat Rev Immunol. 2005;5:271–83. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- 15.Durham SR, Yang WH, Pedersen MR, Johansen N, Rak S. Sublingual immunotherapy with once-daily grass tablets: a randomized controlled trial in seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;117:802–9. doi: 10.1016/j.jaci.2005.12.1358. [DOI] [PubMed] [Google Scholar]
- 16.Kinaciyan T, Jahn-Schmid B, Radakovics A, Zwolfer B, Schreliber C, et al. Successful sublingual immunotherapy with birch pollen has limited effects on concomitant food allergy to apple and the immune response to the Bet c1 homolog Mal d1. J Allergy Clin Immunol. 2007;119:937–44. doi: 10.1016/j.jaci.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Marogna M, Spadolini I, Massolo A, Canoica GW, Passalacqua G. Clinical, functional, and immunologic effects of sublingual immunotherapy in birch pollinosis: a 3-year randomized controlled study. J Allergy Clin Immunol. 2005;115:1184–8. doi: 10.1016/j.jaci.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 18.Novembre E, Gali E, Landi F, Caffarelli C, Pifferi M, et al. Coseasonal sublingual immunotherapy reduces the development of asthma in children with allergic rhinoconjuctivitis. J Allergy Clin Immunol. 2004;114:851–7. doi: 10.1016/j.jaci.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 19.Pajno GB. Sublingual immunotherapy: The optimism and the issues. J Allergy Clin Immunol. 2007;119:796–801. doi: 10.1016/j.jaci.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Dahl R, Kapp A, Colombo G, Monchy JG, Rak S, et al. Efficacy and safety of sublingual immunotherapy with once daily grass allergen tablets for seasonal allergic rhinoconjuctivitis. J Allergy Clin Immunol. 2006;118:434–40. doi: 10.1016/j.jaci.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Gidaro GB, Marcucci F, Sensi L, Incorvaia C, Frati F, et al. The safety of sublingual-swallow immunotherapy : an analysis of published studies. Clin Exp Allergy. 2005;35:565–71. doi: 10.1111/j.1365-2222.2005.02240.x. [DOI] [PubMed] [Google Scholar]
- 22.Passalacqua G, Durham SR, Global Allergy and Asthma European Network Allergic rhinitis and its impact on asthma update: allergen immunotherapy. J Allergy Clin Immunol. 2007;119:881–91. doi: 10.1016/j.jaci.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 23.Wilson DR, Lima MT, Durham SR. Sublingual immunotherapy for allergic rhinitis: systemic review and meta-analysis. Allergy. 2005;60:4–12. doi: 10.1111/j.1398-9995.2005.00699.x. [DOI] [PubMed] [Google Scholar]
- 24.Khinchi MS, Poulsen LK, Carat F, Andre C, Hansen AB, et al. Clinical efficacy of sublingual and subcutaneous birch pollen allergen-specific immunotherapy : a randomized, placebo-controlled, double-blind, double-dummy study. Allergy. 2004;59:45–53. doi: 10.1046/j.1398-9995.2003.00387.x. [DOI] [PubMed] [Google Scholar]
- 25.Cox LS, Linnemann DL, Nolte H, Weldon D, Finegold I, et al. Sublingual immunotherapy: a comprehensive review. J Allergy Clin Immunol. 2006;117:1021–35. doi: 10.1016/j.jaci.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 26.Di Rienzo V, Marcucci F, Puccinelli P, Parmiani S, Frati F, et al. Long-lasting effect of sublingual immunotherapy in children with asthma due to house dust mite : a 10-year prospective study. Clin Exp Allergy. 2003;33:206–10. doi: 10.1046/j.1365-2222.2003.01587.x. [DOI] [PubMed] [Google Scholar]
- 27.Antico A, Pagani M, Crema A. Anaphylaxis by latex sublingual immuotherapy. Allergy. 2006;61:1236–7. doi: 10.1111/j.1398-9995.2006.01155.x. [DOI] [PubMed] [Google Scholar]
- 28.Dunsky FH, Goldstein MP, Dvorin DJ, Belecanech GA. Anaphylaxis to sublingual immunotherapy. Allergy. 2006;61:1235. doi: 10.1111/j.1398-9995.2006.01137.x. [DOI] [PubMed] [Google Scholar]
- 29.Frew AJ. How does sublingual immunotherapy work? J Allergy Clin Immunol. 2007;120:533–6. doi: 10.1016/j.jaci.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Moingeon P, Batard T, Fadel R, Frati F, Sieber J, et al. Immune mechanisms of allergen-specific sublingual immunotherapy. Allergy. 2006;61:151–65. doi: 10.1111/j.1398-9995.2006.01002.x. [DOI] [PubMed] [Google Scholar]
- 31.Smith HE, White PJ, Annila I, Poole J, Andre C, et al. Randomised controlled trial of high dose sublingual immunotherapy to treat seasonal allergic rhinitis. J Allergy Clin Immunol. 2004;114:831–7. doi: 10.1016/j.jaci.2004.06.058. [DOI] [PubMed] [Google Scholar]
- 32.Bohle B, Kinaciyan T, Gestmayr M, Radakovics A, Jahn-Schmid B, et al. Sublingual immunotherapy indues IL-10-producing T regulatory cells. Allergen-specific T-cell tolerance and immune deviation. J Allergy Clin Immunol. 2007;120:707–13. doi: 10.1016/j.jaci.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 33.Horner AA, Redecke V, Raz E. Toll-like receptor ligands: hygiene, atopy and therapeutic implications. Curr. Opin. Allergy Clin. Immunol. 2004;4:555–61. doi: 10.1097/00130832-200412000-00014. [DOI] [PubMed] [Google Scholar]
- 34.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat. Rev. Drug Discov. 2006;5:471–84. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 35.Broide D, Schwarze J, Tighe H, Gifford T, Nguyen MD, et al. Immunostimulatory DNA sequences inhibit IL-5, eosinophilic inflammation, and airway hyperresponsiveness in mice. J. Immunol. 1998;161:7054–62. [PubMed] [Google Scholar]
- 36.Cho JY, Miller M, Baek KJ, Han JW, Nayar J, et al. Immunostimulatory DNA inhibits transforming growth factor-beta expression and airway remodeling. Am. J. Resp. Cell Mol. Biol. 2004;30:651–61. doi: 10.1165/rcmb.2003-0066OC. [DOI] [PubMed] [Google Scholar]
- 37.Santeliz JV, Van Nest G, Traquina P, Larsen E, Wills-Karp M. Amb a 1-linked CpG oligodeoxynucleotides reverse established airway hyperreponsiveness in a murine model of asthma. J. Allergy Clin. Immunol. 2002;109:455–62. doi: 10.1067/mai.2002.122156. [DOI] [PubMed] [Google Scholar]
- 38.Shirota H, Sano K, Hirasawa N, Terui T, Ohuchi K, et al. Novel roles of CpG oligodeoxynucleotides as a leader for the sampling and presentation of CpG-tagged antigen by dendritic cells. J. Immunol. 2001;167:66–74. doi: 10.4049/jimmunol.167.1.66. [DOI] [PubMed] [Google Scholar]
- 39.Tighe H, Takabayashi K, Schwartz D, Van Nest G, Tuck S, et al. Conjugation of Immunostimulatory DNA to the short ragweed allergen amb a 1 enhances its immunogenicity and reduces its allergenicity. J. Allergy Clin. Immunol. 2000;106:124–34. doi: 10.1067/mai.2000.107927. [DOI] [PubMed] [Google Scholar]
- 40.Simons FE, Shikishima Y, Van Nest G, Eiden JJ, HayGlass KT. Selective immune redirection in humans with ragweed allergy by injecting Amb a 1 linked to immunostimulatory DNA. J. Allergy Clin. Immunol. 2004;113:1144–51. doi: 10.1016/j.jaci.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 41.Marshall JD, Abtahi S, Eiden JJ, Tuck S, Milley R, et al. Immunostimulatory sequence DNA linked to the Amb a 1 allergen promotes T(H)1 cytokine expression while downregulating T(H)2-cytokine expression in PBMCs from human patients with ragweed allergy. J. Allergy Clin. Immunol. 2001;108:191–97. doi: 10.1067/mai.2001.116984. [DOI] [PubMed] [Google Scholar]
- 42.Tulic MK, Fiset PO, Christodoulopoulos P, Vaillancourt P, Desrosiers M, et al. A m b a 1-immunostimulatory oligodeoxynucleotide conjugate immunotherapy decreases the nasal inflammatory response. J. Allergy Clin. Immunol. 2004;113:235–41. doi: 10.1016/j.jaci.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Creticos PS, Schroeder JT, Hamilton RG, Balcer-Whaley SL, Khattignavong AP, et al. Immunotherapy with a ragweed-toll-like receptor 9 agonist vaccine for allergic rhinitis. N. Engl. J. Med. 2006;355:1445–55. doi: 10.1056/NEJMoa052916. [DOI] [PubMed] [Google Scholar]
- 44.Creticos PS, Eiden JJ, Balcer SL, Van Nest G, Kagey-Sobotka A, et al. Immunostimulatory oligonucleotides conjugated to Amb a 1: Safety, skin test reactivity, and basophil histamine release. J. Allergy Clin. Immunol. 2000;105:S70. [Google Scholar]
- 45.Fanucchi MV, Scheledle ES, Baker GL, Evans MJ, McDonald RJ, et al. Immunostimulatory oligonucleotides attenuate airways remodeling in allergic monkeys. Am. J. Respir. Crit. Care Med. 2004;170:1153–57. doi: 10.1164/rccm.200404-533OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gauvreau GM, Hessel E, Boulet, Coffman, O'Byne P. Immunostimulatory sequences regulate interferon genes but not allergic airway responses. Am. J. Respir. Crit. Care Med. 2006;174:15–20. doi: 10.1164/rccm.200601-057OC. [DOI] [PubMed] [Google Scholar]
- 47.Lopez AF, Sanderson CJ, Gamble JR, Campbell HD, Young IG, et al. Recombinant human interleukin 5 is a selective activator of human eosinophil function. J Exp Med. 1988;167:219–24. doi: 10.1084/jem.167.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cho JY, Miller M, Baek KJ, Han JW, Nayar J, et al. Inhibition of airway remodeling in IL-5-deficient mice. J. Clin. Invest. 2004;113:551–60. doi: 10.1172/JCI19133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leckie M, Brinke A, Khan J, Diamant A, O'Connor BJ, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsìveness, and the late asthmatic response. Lancet. 2000;356:2144–48. doi: 10.1016/s0140-6736(00)03496-6. [DOI] [PubMed] [Google Scholar]
- 50.Flood-Page P, Swenson C, Faiferman I, Matthews J, Williams M, et al. A study to evaluate safety and efficacy of mepolizumab in patient with moderate persistent asthma. Am J Respir Crit Care Med. 2007;176:1062–71. doi: 10.1164/rccm.200701-085OC. [DOI] [PubMed] [Google Scholar]
- 51.Flood-Page P, Menzies-Gow A, Phipps S, Ying S, Wangoo A, et al. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112:1029–36. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McMillan SJ, Xanthou G, Lloyd CM. Manipulation of allergen-induced airway remodeling by treatment with anti-TGF-beta antibody: effect on the Smad signaling pathway. J. Immunol. 2005;174:5774–80. doi: 10.4049/jimmunol.174.9.5774. [DOI] [PubMed] [Google Scholar]
- 53.Le AV, Cho JY, Miller M, McElwain S, Golgotiu K, et al. Inhibition of allergen-induced airway remodeling in smad 3-deficient mice. J Immunol. 2007;178:7310–16. doi: 10.4049/jimmunol.178.11.7310. [DOI] [PubMed] [Google Scholar]
- 54.Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS. Eosinophil's role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med. 2003;167:199–204. doi: 10.1164/rccm.200208-789OC. [DOI] [PubMed] [Google Scholar]
- 55.Garrett JK, Jameson SC, Thomson B, Collins M, Wagoner L, et al. Anti-Interleukin-5 (mepolizumab) therapy for hypereosinophilic syndromes. J Allergy Clin Immunol. 2004;113:115–9. doi: 10.1016/j.jaci.2003.10.049. [DOI] [PubMed] [Google Scholar]
- 56.Plötz SG, Simon HU, Darsow U, Simon D, Vassina E, et al. Use of an anti-interleukin-5 antibody in the hypereosinophilic syndrome with eosinophilic dermatitis. N Engl J Med. 2003;349:2334–9. doi: 10.1056/NEJMoa031261. [DOI] [PubMed] [Google Scholar]
- 57.Rothenberg ME, Klion AD, Roufosse FE, Kahn JE, Weller PF, et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N. Engl. J. Med. 2008;358:1215–27. doi: 10.1056/NEJMoa070812. [DOI] [PubMed] [Google Scholar]
- 58.Wilkins HJ, Crane MM, Copeland K, William WV. Hypereosinophilic syndrome: an update. Am J Hematol. 2005;80:148–57. doi: 10.1002/ajh.20423. [DOI] [PubMed] [Google Scholar]
- 59.Gleich GJ, LEiferman KM. The hypereosinophilic syndromes: still more heterogeneity. Curr Opin Immunol. 2005;17:679–84. doi: 10.1016/j.coi.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 60.Cools J, DeAngelo DJ, Gotlib J. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003;348:12011–14. doi: 10.1056/NEJMoa025217. [DOI] [PubMed] [Google Scholar]
- 61.Stein ML, Collins MH, Villanueva JM, Kushner JP, Putnam PE, et al. Anti-IL-5 (mepolizumab) therapy for eosinophilic esophagitis. J Allergy Clin Immunol. 2006;118:1312–9. doi: 10.1016/j.jaci.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 62.Wechsler M. Combating the Eosinophil with Anti-Interleukin-5 Therapy. N Engl J Med. 2008;358:1293–4. doi: 10.1056/NEJMe0800524. [DOI] [PubMed] [Google Scholar]
- 63.Aceves SS, Newbury RO, Dohil R, Bastian JF, Broide DH. Esophageal remodeling in pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2007;119:206–12. doi: 10.1016/j.jaci.2006.10.016. [DOI] [PubMed] [Google Scholar]