Abstract
OBJECTIVES: The aim of this study was to compare the ability of fasting plasma glucose (FPG), post-load plasma glucose values and glycated hemoglobin (HbA1c) to predict progression to diabetes in non-diabetic first-degree relatives (FDR) of patients with type 2 diabetes. METHODS: A total of 701 non-diabetic FDR of diabetic patients aged 20-70 years surveyed in 2003 to 2005 were followed until 2008 for the onset of type 2 diabetes mellitus. At baseline and at follow-ups, participants underwent a standard 75 g 2-hour oral glucose tolerance test (OGTT). Prediction of progression to type 2 diabetes was assessed by using area under the receiver-operating characteristic (ROC) curves based upon measurement of FPG, post-load glucose values and HbA1c. RESULTS: The incidence of type 2 diabetes was 33.9 per 1000 person-years in men and 48.6 in women. The incidence rates were 4.6, 50.7, and 99.7 per 1000 person-years in FDR with normal glucose tolerance, impaired fasting glucose and impaired glucose tolerance respectively. FPG value was a better predictor of progression to diabetes than any post-load glucose values or HbA1c. The areas under the ROC curves were 0.811 for fasting, 0.752 for 1/2-hour, 0.782 for 1-hour and 0.756 for 2-hour glucose vs. 0.634 for HbA1c (p < 0.001). CONCLUSIONS: FPG had more discriminatory power to distinguish between individuals at risk for diabetes and those who were not at risk than post-load glucose values during OGTT or HbA1c. Our findings support the American Diabetes Association recommendation of using FPG concentration to diagnose diabetes.
Keywords: type 2 diabetes, first-degree relatives, OGTT, HbA1c, fasting plasma glucose, post-load plasma glucose, impaired glucose tolerance, IGT, IFG, MetS
Introduction
Diabetes prevention has become a major public health priority in both developed and developing nations. There is great interest in identifying individuals at high risk of developing diabetes. Subjects with glucose intolerance are at increased risk for future type 2 diabetes. Glucose intolerance is based on either a 2-hour 75 g oral glucose tolerance test (OGTT) for impaired glucose tolerance (IGT), or fasting plasma glucose (FPG) levels indicative of impaired fasting glucose (IFG) [1]. Many studies have shown that both states of glucose intolerance are good predictors of diabetes [2-8]. However, there is also considerable inconsistency between the subjects classified by these criteria [2-10]. Limited overlap between the IFG and IGT groups suggests the possibility that these are distinct conditions with different etiologies [2, 11]. A recent study has concluded that 1-hour plasma glucose concentration is superior to FPG in predicting type 2 diabetes [12], and the use of FPG as a focus of screening for metabolic risk modification has become questionable.
Glycated hemoglobin (HbA1c) is useful for monitoring glycemic control in diabetic patients [13]. However, the significance of HbA1c in identifying persons at future risk of diabetes remains unknown [14, 15]. The question then arises whether FPG is a better predictor of diabetes risk than post-load glucose values or HbA1c. Measuring FPG is less expensive, more convenient and more reproducible than performing an OGTT [16]. For these reasons, the guidelines published by the American Diabetes Association (ADA) recommend FPG as the preferred test for diagnosing diabetes [13]. In contrast, OGTT has been the preferred test for diagnosing diabetes in epidemiological studies for over 40 years, despite the general recognition that this test is expensive and inconvenient. This lack of agreement emphasizes that the question of optimally predicting diabetes risk is still unanswered to some degree and needs further clarification. Our study contributes to this question by comparing the ability of FPG, post-load glucose values and HbA1c to predict the incidence of type 2 diabetes in non-diabetic first-degree relatives (FDR) of patients with type 2 diabetes.
It is important to note in this regard that the relative contributions of insulin resistance and impaired beta-cell function may vary among various ethnic groups [16]. No comprehensive data are available at present for developing countries. This study makes an important contribution at the ethnological level, therefore, by characterizing the occurrence of diabetes in a specific population from central Iran.
Patients and methods
Participants and data collection
The Isfahan Diabetes Prevention Study (IDPS) is an ongoing cohort study performed in central Iran to assess the efficacy of intensive diet and exercise to prevent or delay the onset of diabetes in FDR of patients with type 2 diabetes. The study was performed between 2003 and 2005. 2368 FDR (614 men and 1754 women) drawn from a consecutive sample of patients with type 2 diabetes attending our clinics at Isfahan Endocrine and Metabolism Research Center were included in the study. The participants completed laboratory tests including standard 75 g 2-hour OGTT and a questionnaire on their health status and on various potential risk factors for diabetes. The participants received follow-up tests according to a medical care standard in diabetes [13] to update information on demographic, anthropometric, and lifestyle factors and on newly diagnosed diabetes. Accordingly, if OGTT was normal at baseline, repeat testing was carried out at least at 3-year intervals. Otherwise, repeat testing was conducted annually. The IDPS baseline methods have been described in detail previously [8]. The participants included siblings and children of diabetic patients. The tenets of the Declaration of Helsinki were followed, institutional ethical committee approval was granted and an informed consent form was signed by each participant.
Diagnosis of diabetes
Cases of diabetes were identified from baseline and follow-up OGTT according to ADA criteria [1, 17]. Pregnant women were excluded. This study used data obtained from 701 FDR (150 men and 551 women), who were free of diabetes at registration and had at least one subsequent review in the mean follow-up period of 2.3 (range 1-4) years.
The procedures used to diagnose diabetes were as follows: data on age, gender, body size, HbA1c, cholesterol, low-density lipoprotein (LDL) cholesterol, high density lipoprotein (HDL) cholesterol, triglyceride, blood pressure (BP), family and personal medical history were collected at baseline and through follow-up. The same methodology was used for both the prevalence and incidence studies. Participants reported to clinics in the morning after an overnight fast. Subjects were asked to abstain from vigorous exercise in the evening before and in the morning of the investigations. Smokers were encouraged to abstain from smoking in the morning of the investigations. On arrival at the clinic, the information given by the participants in the questionnaire on family history was verified. Then, height, weight, waist, and hip circumference were measured using standard apparatus with the subjects in light clothes and without shoes. Weight was measured to the nearest 0.1 kg. Height, waist, and hip circumference were measured to the nearest 0.5 cm. Waist was measured midway between the lower rib margin and iliac-crest at the end of a gentle expiration. Hip circumference was measured over the greater trochanters directly over the underwear. Resting BP was measured after subjects had been seated for 10 minutes, using standard techniques. FPG was measured using the glucose oxidase method. All subjects underwent a standard OGTT (75 g glucose 2-hour) at baseline and follow-up assessments. Venous blood was sampled at fasting and at 30, 60, and 120 min after oral glucose administration. Plasma samples were analyzed the same day.
HbA1c (measured by ion-exchange chromatography), total cholesterol, triglyceride, HDL, and LDL (calculated by the Friedewald Equation [18]) were also assessed. All blood sample procedures were performed in the central laboratory of the Isfahan Endocrine and Metabolism Research Center using an enzyme-linked method.
Definitions
We calculated body mass index (BMI) as the ratio of weight (kg) to height squared (m2), the latter being assessed at baseline only. Normal BMI was defined as BMI < 25, overweight as BMI of 25-29.99, and obesity as BMI ≥ 30. Abdominal obesity was defined by waist circumference (≥102 cm in men and ≥88 cm in women) or by the waist-to-hip ratio (WHR) (≥0.95 in men and ≥0.80 in women).
Diabetes was defined by:
i) two FPG ≥126 mg/dl or
ii) one 2-hour plasma glucose ≥200 mg/dl or
iii) self-reporting of diabetic treatment.
IGT was defined as FPG < 126 mg/dl, but with a 2-hour plasma glucose concentration ≥140 and <200 mg/dl. If FPG was in the range from 100 to 126 mg/dl and 2-hours plasma glucose was <140 mg/dl, it was considered to be IFG. However, a FPG below 100 mg/dl and a 2-hours plasma glucose below 140 mg/dl was considered a sign of normal glucose tolerance (NGT) [1, 17].
An established definition of the metabolic syndrome (MetS) was used [19]. According to this definition, MetS was diagnosed if at least three of the following criteria were met:
i) BP ≥ 130/85 mmHg or a history of hypertension and current antihypertensive treatment,
ii) waist girth > 102 cm for men and >88 cm for women,
iii) serum triglyceride ≥150 mg/dl (≥1.7 mmol/l) and/or HDL cholesterol <40 mg/dl (<0.9 mmol/l) for men and <50 mg/dl (<1.0 mmol/l) for women,
iv) FPG levels ≥110 mg/dl (6.1 mmol/l).
Determination of diabetes incidence
Incidence of diabetes was expressed as the number of type 2 diabetes cases per 1000 person-years of follow-up. The relevant period was considered to start on the date when the baseline examination was performed sometime between 2003 and 2005 until either i) the onset of diabetes, ii) the date of the last completed follow-up, iii) death, or iv) end of follow-up on December 31, 2007, whichever came first.
Statistical analysis
Statistical methods used included the Student’s t-test, chi squared test and Cox’s proportional hazards model. Univariate and multivariate Cox’s proportional hazards models were fitted to identify predictors of new-onset diabetes using SPSS for Windows (SPSS Inc., Chicago, IL, USA). The variables age, BMI, waist circumference (WC), triglyceride, LDL, HDL, total cholesterol and BP were included in the multivariate-adjusted analyses as continuous variables, while gender, IGT, IFG and MetS were categorical.
Adjustment for age was examined in separate models. Age-adjusted means were calculated and compared using general linear models. The ability of FPG, 1/2-hour, 1-hour and 2-hour glucose values and HbA1c to predict the incidence of diabetes was examined by receiver operating characteristic (ROC) curve and their respective areas under the curve (AUC), in which sensitivity was plotted as a function of 1-specificity. Areas under the ROC curves were compared by the algorithm developed by DeLong et al. [20]. All tests for statistical significance were two-tailed, and performed assuming a type I error probability of <0.05.
Results
The baseline characteristics of the 331 (47.2%) participants with NGT, 315 (44.9%) with IGT, and 55 (7.8%) with IFG are shown in Table 1. As expected, participants with IGT or IFG were older at baseline than those with NGT. They also had higher age-adjusted mean BMI, WC, hip circumference, waist-to-hip ratio, FPG, plasma glucose at 30, 60 and 120 min, cholesterol, LDL, HDL and triglyceride as well as a higher proportion of obesity. Mean (SD) age was 43.5 (6.7) years for those with IGT, 42.9 (6.6.) years for those with IFG and 41.8 (5.8) years for those with NGT. MetS was present in more than a quarter of the participants (25.2%; 95% CI: 22.0-28.4).
Table 1. Age, age-adjusted and proportional characteristics of first-degree relatives of type 2 diabetes patients grouped for glucose tolerance status in the Isfahan Diabetes Prevention Study.
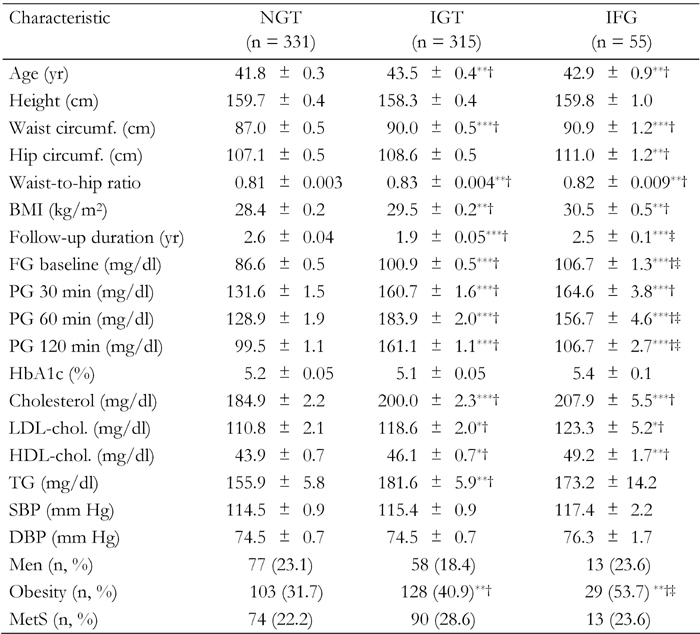
Age-adjusted means were calculated using general linear models. Data are expressed as mean ± SE or number (%). The difference in the mean or percentage of the variables is calculated for comparison with NGT (†) and IGT (‡). BMI: body mass index. FG: fasting glucose. PG: plasma glucose. TG: triglycerides. SBP: systolic blood pressure. DBP: diastolic blood pressure. MetS: metabolic syndrome. *p < 0.05, **p < 0.01, ***p < 0.001.
During 1630 (354 men and 1276 women) person-years of follow-up, 74 (10.6%) (12 men and 62 women) incident cases of type 2 diabetes occurred. The overall incidence of subsequent diabetes was 45.4 (95% CI: 35.8-56.7) per 1000 person-years. The incidence rate was higher in women (48.6, 95% CI: 37.4-61.8 per 1000 person-years) than men (33.9, 95% CI: 17.6-58.4), but the difference was not statistically significant. Of the 315 participants who had IGT at initial registration, 61 subsequently developed diabetes, giving an incidence of 99.7 (95% CI: 77.1-126.0) per 1000 person-years. This was much higher than the rate seen for NGT, which was 4.6 per 1000 person-years (95% CI: 1.28-11.7) (p < 0.001). Of the 55 participants who had IFG at initial registration, 7 subsequently developed diabetes, giving an incidence of 50.7 (95% CI: 20.7-102.0) per 1000 person-years. The incidence of type 2 diabetes in participants with MetS was 60.3 (95% CI 39.0-88.4) per 1000 person years. This was higher than the incidence rate seen for those without MetS, which was 40.4 per 1000 person-years (95% CI: 30.1-52.9), although this difference was not statistically significant.
The incidence of diabetes increased across the five subject groups, from 5.9 per 1000 person-years in the NGT and non-MetS group to 138.6 per 1000 person-years in the IGT and MetS group (Table 2). In addition, multivariate-adjusted relative risk (RR) of progression to diabetes in subjects with i) NGT and MetS, ii) IFG and MetS and iii) IGT with and without MetS increased in relation to those with NGT and without MetS (Table 2).
Table 2. Incidence rates and relative risks of type 2 diabetes by metabolic syndrome status in the Isfahan Diabetes Prevention Study, 2003-2008.
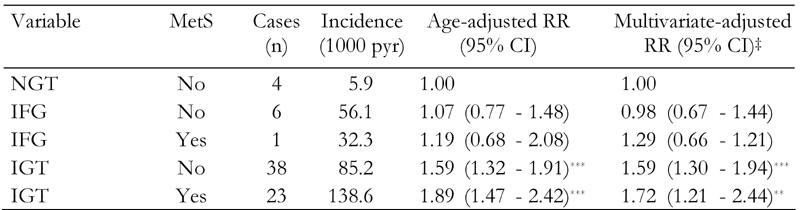
‡ Relative risk (95% CI) calculated by Cox’s proportional hazards model. Adjusted for age, gender, body mass index, waist circumference, triglyceride, LDL, HDL, total cholesterol, blood pressure, IGT and IFG. NGT: normal glucose tolerance. IFG: impaired fasting glucose. IGT: impaired glucose tolerance. CI = confidence interval. pyr: patient-years. * p < 0.05, ** p < 0.01, *** p < 0.001.
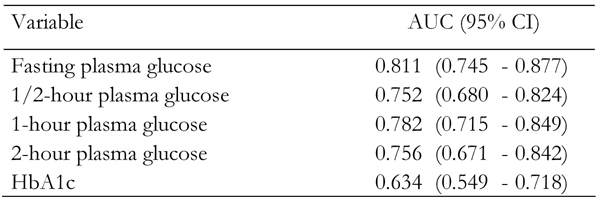
The areas under the ROC curves for incidence of type 2 diabetes were 0.811 (95% CI: 0.745-0.877), 0.752 (95% CI: 0.680-0.824), 0.782 (95% CI: 0.715-0.849), 0.756 (95% CI: 0.671-0.842) and 0.634 (95% CI: 0.549-0.718) for fasting, 1/2-hour, 1-hour, 2-hour glucose values and HbA1c respectively (Figure 1, Table 3). All plasma glucose concentrations during OGTT and HbA1c were significant predictors of future risk of type 2 diabetes (p < 0.001). The areas under the curves for FPG concentration were significantly greater than those for HbA1c (p < 0.001). FPG concentration had an area slightly but not significantly larger than that of 1/2-hour, 1-hour and 2-hour glucose concentrations. However, it is apparent that, in this population of FDR of patients with type 2 diabetes, FPG was the strongest predictor of future risk for type 2 diabetes.
Figure 1.
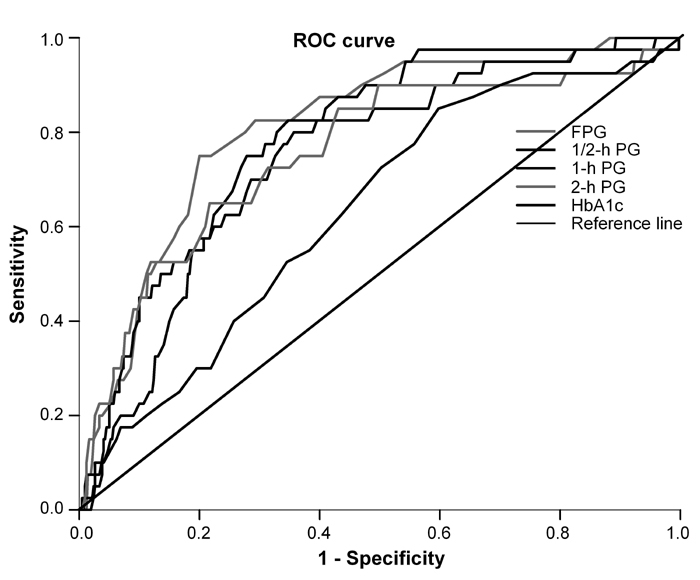
Receiver operating characteristic (ROC) curves for fasting, 1/2-hour, 1-hour and 2-hour glucose and HbA1c for prediction of type 2 diabetes in non-diabetic first-degree relatives of patients with type 2 diabetes. The estimated area under the ROC curves and their 95% confidence intervals are shown in Table 3.
Table 3. Area under the curve (95% confidence interval) of fasting, 30-min, 1-hour and 2-hour plasma glucose and HbA1c.
Discussion
Our study showed that the discriminatory power of FPG to distinguish between individuals at diabetes risk and those not at risk was superior to that of HbA1c and post-load plasma glucose values. HbA1c was an even weaker risk indicator than fasting and post-load plasma glucose values. The study thus confirms the reliability of FPG for diabetes prediction. Even though fasting and 1-hour glucose value had approximately the same predictive power in our study, FPG is superior in clinical practice because it is a simple test and no OGTT is needed. Choosing FPG as the test for diabetes avoids the cost and inconvenience associated with OGTT [1].
These results contradict the findings of others. Abdul-Ghani et al. showed that plasma glucose concentration at 1 hour during the OGTT is a stronger predictor of future risk for type 2 diabetes than FPG [12, 21]. Our findings are also inconsistent with those obtained by Tsuji et al. In screening tests for diabetes using the ROC curve analysis, they found that HbA1c has almost the same discriminatory power as FPG [22]. On the basis of our findings, fasting followed by 1-hour glucose, turned out to be the most reliable and simultaneously the most practical predictor of progression to diabetes.
Some studies suggest that the combined use of FPG and HbA1c predicts the incidence of diabetes more accurately than either test alone in individuals at risk of diabetes [23-25]. This is a question that has not been answered by our observations, but the higher discriminatory power of FPG indicates that further research is necessary to verify this statement.
The reason that HbA1c is inferior to FPG or post-load glucose values at predicting type 2 diabetes is because the existence of hemoglobin or red cell abnormalities can increase the variability of HbA1c values. This variability may contribute to its inferior prediction of diabetes compared with fasting or post-load glucose values. In addition, FPG and HbA1c may reflect different aspects of glucose metabolism. While HbA1c can reflect a variety of factors in glucose metabolism, FPG levels mainly depend on insulin resistance and hepatic glucose production [26].
Our results also demonstrate that FDR participants with IGT and with or without MetS (ATP III criteria) at baseline have a higher risk of progression to diabetes, emphasizing the role of glucose testing alone in predicting diabetes. Participants with IGT and MetS had a 90% higher risk of diabetes than those with NGT, even after controlling for other covariates (age, gender, BMI, waist circumference, triglyceride, LDL, HDL, total cholesterol, IFG and diastolic BP). Such individuals identified to be at high risk of developing diabetes need further monitoring and should be included in an early intervention program to rescue residual beta-cell function. Our findings are consistent with an ADA consensus statement [27] that the high-risk group could benefit from intervention programs employing diet and exercise and possibly pharmacotherapy to reduce future risk of diabetes. The high risk of developing type 2 diabetes in FDR with high fasting or 1-hour glucose underlines the importance of preventing type 2 diabetes in these individuals. Recent clinical trials demonstrate that lifestyle [28-31] and pharmaceutical [21, 28, 29] interventions in individuals with IGT can prevent the development of diabetes. This highlights the importance of identifying high-risk subjects so as to institute early lifestyle or pharmacological interventions.
The strengths of the present study include the prospective cohort design, the nature of the sample which included both men and women over a wide age range, the fact that diagnosis of diabetes was based on standard OGTT, and information on potential determinants of diabetes. Selection and information bias is considered unlikely by virtue of the prospective design. Our study focused on individuals at increased risk of developing type 2 diabetes, because they had FDR with the disease. Even though the study included more than 700 participants who were thoroughly examined and followed up, the three-year follow-up period may be controversial. Because the results obtained when assessing diabetes prediction continue to be conflicting, a long-term follow-up period of 3-6 years in a large cohort could serve to clarify the question further.
In conclusion, our study indicates that FPG was better than post-load glucose values and HbA1c for predicting new onset diabetes. One- and 2-hour glucose showed an almost equal discriminating ability.
Conflict of interest statement: The authors declare that they have no competing conflict of interests.
Acknowledgments
We are grateful to Mr. Majid Abyar for computer technical assistance. This study could not have been conducted without the contribution of the FDR of patients with type 2 diabetes who agreed to participate.
References
- 1.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 2.Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med. 2002;19(9):708–723. doi: 10.1046/j.1464-5491.2002.00835.x. [DOI] [PubMed] [Google Scholar]
- 3.Larsson H, Berglund G, Lindgärde F, Ahren B. Comparison of ADA and WHO criteria for diagnosis of diabetes and glucose intolerance. Diabetologia. 1998;41(9):1124–1125. doi: 10.1007/s001250051040. [DOI] [PubMed] [Google Scholar]
- 4.de Vegt F, Dekker JM, Jager A, Hienkens E, Kostense PJ, Stehouwer CD, Nijpels G, Bouter LM, Heine RJ. Relation of impaired fasting and postload glucose with incident type 2 diabetes in a Dutch population: The Hoorn Study. JAMA. 2001;285(16):2109–2113. doi: 10.1001/jama.285.16.2109. [DOI] [PubMed] [Google Scholar]
- 5.Shaw JE, Zimmet PZ, de Courten M, Dowse GK, Chitson P, Gareeboo H, Hemraj F, Fareed D, Tuomilehto J, Alberti KG. Impaired fasting glucose or impaired glucose tolerance. What best predicts future diabetes in Mauritius? Diabetes Care. 1999;22(3):399–402. doi: 10.2337/diacare.22.3.399. [DOI] [PubMed] [Google Scholar]
- 6.Vaccaro O, Ruffa G, Imperatore G, Iovino V, Rivellese AA, Riccardi G. Risk of diabetes in the new diagnostic category of impaired fasting glucose: a prospective analysis. Diabetes Care. 1999;22(9):1490–1493. doi: 10.2337/diacare.22.9.1490. [DOI] [PubMed] [Google Scholar]
- 7.Qiao Q, Lindström J, Valle TT, Tuomilehto J. Progression to clinically diagnosed and treated diabetes from impaired glucose tolerance and impaired fasting glycaemia. Diabet Med. 2003;20(12):1027–1033. doi: 10.1111/j.1464-5491.2003.01054.x. [DOI] [PubMed] [Google Scholar]
- 8.Amini M, Janghorbani M. Diabetes and impaired glucose regulation in first-degree relatives of patients with type 2 diabetes in Isfahan, Iran: prevalence and risk factors. Rev Diabet Stud. 2007;4(3):169–176. doi: 10.1900/RDS.2007.4.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakagami T DECODA Study Group. Hyperglycaemia and mortality from all causes and from cardiovascular disease in five populations of Asian origin. Diabetologia. 2004;47(3):385–394. doi: 10.1007/s00125-004-1334-6. [DOI] [PubMed] [Google Scholar]
- 10.Janus ED, Watt NM, Lam KS, Cockram CS, Siu ST, Liu LJ, Lam TH. The prevalence of diabetes, association with cardiovascular risk factors and implications of diagnostic criteria (ADA 1997 and WHO 1998) in a 1996 community-based population study in Hong Kong Chinese. Diabet Med. 2000;17(10):741–745. doi: 10.1046/j.1464-5491.2000.00376.x. [DOI] [PubMed] [Google Scholar]
- 11.Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care. 2006;29(5):1130–1139. doi: 10.2337/diacare.2951130. [DOI] [PubMed] [Google Scholar]
- 12.Abdul-Ghani MA, Lyssenko V, Tuomi T, DeFronzo RA, Groop L. Fasting versus postload plasma glucose concentration and the risk for future type 2 diabetes: results from the Botnia Study. Diabetes Care. 2009;32(2):281–286. doi: 10.2337/dc08-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Executive summary. Standard of Medical Care in Diabetes-2008. Diabetes Care. 2008;31(Suppl 1):S5–S11. [Google Scholar]
- 14.Davidson MB, Schriger DL, Lorber B. HbA(1c) measurements do not improve the detection of type 2 diabetes in a randomly selected population. Diabetes Care. 2001;24(11):2017–2018. doi: 10.2337/diacare.24.11.2017-a. [DOI] [PubMed] [Google Scholar]
- 15.Perry RC, Shankar RR, Fineberg N, McGill J, Baron AD. HbA1c measurement improves the detection of type 2 diabetes in high-risk individuals with nondiagnostic levels of fasting plasma glucose: the Early Diabetes Intervention Program (EDIP) Diabetes Care. 2001;24(3):465–471. doi: 10.2337/diacare.24.3.465. [DOI] [PubMed] [Google Scholar]
- 16.Abdul-Ghani MA, Matsuda M, Sabbah M, Sabbah M, Jenkinson C, Richardson D, Kaku K, DeFronzo TA. The relative contributions of insulin resistance and beta cell failure to the transition from normal to impaired glucose tolerance vary in different ethnic groups. Diabetes Metab Syndr Clin Res Rev. 2007;1:105–112. [Google Scholar]
- 17.Genuth S, Alberti KG, Bennett P, Buse J, DeFronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A. et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26(11):3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 18.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 19.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 20.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 21.Abdul-Ghani MA, Williams K, DeFronzo RA, Stern M. What is the best predictor of future type 2 diabetes? Diabetes Care. 2007;30:1544–1548. doi: 10.2337/dc06-1331. [DOI] [PubMed] [Google Scholar]
- 22.Tsuji I, Nakamoto K, Hasegawa T, Hisashige A, Inawashiro H, Fukao A, Hisamichi S. Receiver operating characteristic analysis on fasting plasma glucose, HbA1c, and fructosamine on diabetes screening. Diabetes Care. 1991;14(11):1075–1077. doi: 10.2337/diacare.14.11.1075. [DOI] [PubMed] [Google Scholar]
- 23.Ko GT, Chan JC, Tsang LW, Cockram CS. Combined use of fasting plasma glucose and HbA1c predicts the progression to diabetes in Chinese subjects. Diabetes Care. 2000;23(12):1770–1773. doi: 10.2337/diacare.23.12.1770. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi M, Kaji K, Togashi A, Onigo Y, Shibosawa T, Kawazu S. Usefulness of paired estimation of fasting plasma glucose and HbA1c; a long-term follow-up study of screened non-diabetic subjects. J Japan Diabet Soc. 2001;44:745–750. [Google Scholar]
- 25.Inoue K, Matsumoto M, Akimoto K. Fasting plasma glucose and HbA1c as risk factors for type 2 diabetes. Diabet Med. 2008;25(10):1157–1163. doi: 10.1111/j.1464-5491.2008.02572.x. [DOI] [PubMed] [Google Scholar]
- 26.Rothman DL, Magnusson I, Katz LD, Shulman RG, Shulman GI. Quantitation of hepatic glycogenolysis and gluconeogenesis in fasting humans with 13C NMR. Science. 1991;254(5031):573–576. doi: 10.1126/science.1948033. [DOI] [PubMed] [Google Scholar]
- 27.Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, Zinman B American Diabetes Association. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 2007;30(3):753–759. doi: 10.2337/dc07-9920. [DOI] [PubMed] [Google Scholar]
- 28.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 30.Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB. et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20(4):537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 31.Li G, Zhang P, Wang J, Gregg EW, Yang W, Gong Q, Li H, Li H, Jiang Y, An Y. et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet. 2008;371(9626):1783–1789. doi: 10.1016/S0140-6736(08)60766-7. [DOI] [PubMed] [Google Scholar]


