Abstract
AIM: The aim of the study was to identify the prevalence of diabetic retinopathy (DR) and diabetic cataract (DC) in type 1 and type 2 diabetic patients within the Russian Federation. Also, the stage of DR at the time of its identification and the proportion of new cases diagnosed with DR or DC were to be determined. METHODS: A random sample of 7,186 adult patients with diabetes was screened for DR and DC using fundoscopy and fundus photography. Levels of HbA1c, total cholesterol, triglycerides, creatinine and urinary albumin excretion rate were assessed. RESULTS: In diabetic patients, the prevalence of DR and DC was 45.9% and 30.6%, respectively. These complications appeared significantly more frequently in patients with type 1 diabetes than in type 2 diabetes. The prevalence of background, preproliferative and proliferative DR among diabetic patients was 28.1%, 8.1%, and 6.7%, respectively. Patients with DR were older, had a longer duration of diabetes, higher HbA1c, elevated plasma total cholesterol, increased triglicerides, and higher systolic BP, compared with patients without DR. Microalbuminuria and proteinuria were more prevalent among patients with DR compared with non-DR patients. CONCLUSIONS: The results showed that diabetic retinopathy and cataract are wide-spread complications among diabetic patients in Russia. However, the disease course is more aggressive and accelerated in patients with type 1 diabetes than in those having type 2 diabetes. Therefore, it is important to prevent DR by identifying diabetes and signs of retinopathy at the earliest possible stage of progression for timely and adequate retina laser coagulation or surgical treatment, compensation of carbohydrate and lipid metabolism, and normalization of blood glucose and pressure.
Keywords: type 1 diabetes, type 2 diabetes, diabetic retinopathy, diabetic cataract, proliferative retinopathy, coagulation, glaucoma, eye fundus
Introduction
Diabetic retinopathy (DR) is a specific vascular complication of diabetes resulting from retinal vessel damage caused by poor blood glucose control, with subsequent changes to the fundus of the eye. These changes can be in the form of micro-aneurysms, hemorrhages, hard and soft exudates, proliferation of newly-formed vessels, retinal detachment, and the development of secondary rubeotic glaucoma [1]. DR is one of the most common and serious vascular complications in diabetes, and one of the leading causes of total loss of sight [2]. The clinical signs of DR are classified into background, preproliferative, and proliferative stages [3]. Modern classifications subdivide DR into five stages of diabetic retinopathy: non-proliferative (DR I), preproliferative (DR II), proliferative (DR III), partial or total retinal detachment (DR IV), diabetic retinopathy and secondary neovascular glaucoma (DR V). DR I is a reversible stage characterized by blood vessel changes in the ocular fundus such as microaneurysms, hemorrhages, hard exudates, and maculopathy. Poor glycemic control with frequent fluctuations of hypo- and hyperglycemia, together with arterial hypertension, results in the progression of DR I to more advanced stages.
International data show that 30-60% of diabetic patients have DR, with 3-10% of them in the proliferative stage. In type 1 diabetic patients, clinical symptoms of DR are found 5-7 years after disease onset in 15-20% of cases. The symptoms are present in 50-60% of cases after 10 years, and in virtually all patients after 30 years. In type 2 diabetes, DR symptoms are registered in 15-30% of cases at the same time as diabetes is diagnosed. This high occurrence of DR symptoms directly on diagnosis is because type 2 diabetes is generally diagnosed later than type 1 diabetes, i.e. when the damaging effect on blood vessels and nerves has proceeded unnoticed for a long time. 50-70% of type 2 diabetes cases show DR symptoms after 10 years, and 90% of these patients after 30 years [4, 5].
Another complication leading to loss of sight is cataract. It is characterized by an opacification of the translucent media of the lens that progresses rapidly in diabetic patients. Also, it leads to a sharp diminution in sight, constituting 50% of all blindness in the world. Approximately 20% of patients who have had operative treatment for cataract have diabetes [6].
The aims of this study were firstly to determine the actual prevalence of diabetic retinopathy, and its stage at the time of identification; secondly, to determine the incidence of cataract in diabetic patients, and thirdly to determine the proportion of new DR and DC cases.
Materials and methods
We examined a random sample of patients with type 1 and type 2 diabetes from 20 regions of the Russian Federation. The patient sample set was selected with the use of random number drawings from the regional centers’ diabetes database. All patients had been diagnosed with diabetes (type 1 or 2). Diabetes classification was based on plasma glucose results, using the 1999 World Health Organization diabetes classification. Diabetes was diagnosed on the basis of fasting plasma glucose (≥ 7.0 mmol/l), 2-h plasma glucose (≥ 11 mmol/l), or current treatment with insulin or oral hypoglycemic medication. The total number of the patients examined was 7,186, all with diabetes. Among these patients were 2,683 men and 4,503 women; 3,455 had type 1 and 3,731 type 2 diabetes.
Ophthalmologic examination included lens biomicroscopy and vitreous body assessment by the slit lamp SL-930 (C.S.O., Italy). The Beta 200 (Heine, Germany) ophthalmoscope was used to perfrom direct and indirect ophthalmoscopy. Fundus photo examination was performed using the fundus-camera Genesis (KOWA, Japan). The international classification of DR was used to diagnose DR [7].
The urinary albumin excretion (UAE) rate was measured by the laboratory analyzer NycoCard Reader II (Axis-Shield, Norway) in the absence of any urinary tract infection symptoms. The test was considered to be positive if urinary albumin concentration exceeded 20 mg/l (for microalbuminuria) and above 200 mg/l (for proteinuria) in three urine samples. The examination of the cardiovascular system included the determination of blood pressure (BP) levels by the Korotkov method. Hypertension was defined when a systolic BP > 140 mmHg or a diastolic BP > 90 mmHg was measured 3 times in a 5 min interval, or when the patient was taking antihypertensive drugs.
Plasma total cholesterol (reference range 3.3-5.2 mmol/l), triglycerides (reference range 0.1-2.2 mmol/l), and serum creatinine (reference range 62.0-106.0 µmol/l) were measured using an analyzer (Reflotron Plus, Roche Company, Switzerland). HbA1c was measured by analyzer DS5 Glycomat (Drew Scientific, United Kingdom), and a reference range of 4-6% was applied.
Statistical analyses were carried out using the STATISTICA application program package (StatSoft Inc., USA). Differences between groups were tested by the chi-squared test and Mann-Whitney U test. Correlations were analyzed using Spearman's test. Results were considered significant if p < 0.05. Results are presented as median and quantiles [25%;75%].
Results
Prevalence of diabetic retinopathy
The overall prevalence of DR among diabetic patients was 45.9%. The prevalence of DR was significantly higher in type 1 than in type 2 diabetes (54.6% versus 34.2%, p < 0.0001). Female type 1 diabetes patients were significantly more affected by DR than male (56.2% vs. 50.5%, p < 0.001, Table 1).
Table 1. Clinical characteristics of type 1 and type 2 diabetic patients.
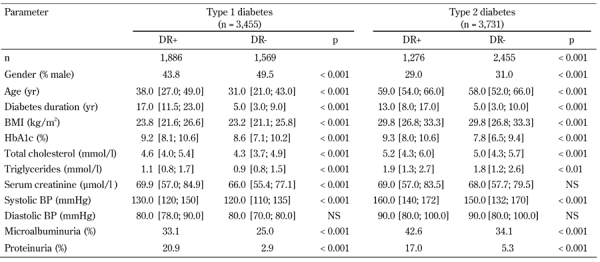
Data are median and quantiles, numbers, or percentages. DR: diabetic retinopathy. BMI: body mass index.
At a disease duration of 5 years or less, 5.8% of type 1 and 12.5% of type 2 diabetic patients had DR (p < 0.0001). At a duration of 10-14 years, the prevalence of DR was 72.2% among type 1 diabetes patients. The prevalence increased to 87.8% in cases where the disease continued for 15 years or more (Figure 1). A similar trend was seen for type 2 diabetes: 50.9% of patients with a disease duration of 10-14 years had DR, while the prevalence increased to 64.1% after a diabetes duration of 15 years or more. Blindness because of DR was significantly higher in type 1 than in type 2 diabetes (2.5% and 0.3%, respectively; p < 0.001, Figure 2).
Figure 1.
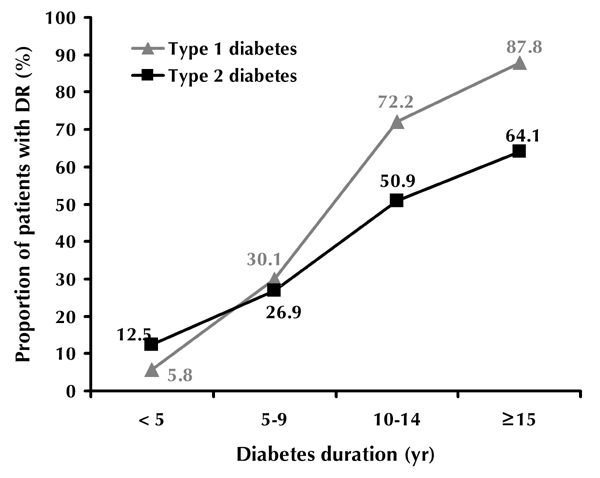
Prevalence of diabetic retinopathy according to diabetes type and duration.
Figure 2.

Prevalence of stages of diabetic retinopathy in diabetes patients by diabetes type. * p < 0.001.
The mean HbA1c level in type 1 diabetes patients with DR was 9.2 ± 0.1% compared with 8.2 ± 0.1% in patients without DR. Patients with DR were older, had longer disease duration, higher HbA1c, elevated total cholesterol, increased tri-glycerides, and higher systolic BP, compared with patients without DR. The prevalence of microalbuminuria and proteinuria among patients with DR was significantly higher than among patients without DR (Table 1). 88.6% of patients with DR had poor glycemic control (HbA1c ≥ 7.0%).
We performed a regression analysis and found that, in type 1 diabetes patients, diabetic retinopathy showed significant correlations with duration of diabetes (r = 0.66, p < 0.0001), HbA1c level (r = 0.15, p < 0.0001), systolic BP (r = 0.27, p < 0.0001), and UAE (r = 0.37, p < 0.0001). In type 2 diabetes patients, the correlation of clinical parameters with DR was also significant: DR vs. duration of diabetes: r = 0.43, p < 0.0001; DR vs. HbA1c; r = 0.30, p < 0.0001; DR vs. systolic BP: r = 0.11, p < 0.0001; DR vs. UAE: r = 0.23, p < 0.0001. The difference between the two correlation coefficients (r) in the type 1 and type 2 diabetes patient groups was statistically significant for all parameters (p < 0.0001).
The proportion of new DR cases identified by screening for diabetes was 65%. This was higher for type 2 than for type 1 diabetes (74.4% and 55.4% respectively, p = 0.04).
Stages of diabetic retinopathy
Background DR occurred in 28.1% of all diabetes patients. There was a statistically significant difference between background DR prevalence in patients with type 1 and type 2 diabetes (31.6% versus 24.7%; p < 0.001, Figure 2). With a disease duration of 5 years or less, background DR occured in 4.2% of type 1 and 9.1% of type 2 diabetic patients. With a disease duration of 15 years or more, background DR occurred in 43.0% of type 1 and 44.2% of type 2 diabetes (Table 2). DR levels were significantly higher among women than men (30.1% vs. 27.7%, respectively; p < 0.001).
Table 2. Proportion of patients with diabetic retinopathy by diabetes duration and complication stage.
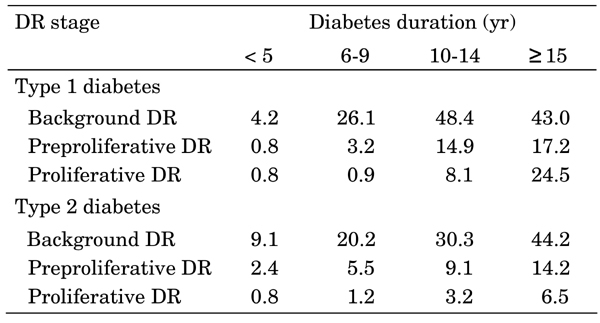
DR: diabetic retinopathy.
Preproliferative DR was seen in 8.1% of diabetic patients. It was significantly higher among type 1 diabetes than in type 2 diabetes patients (9.1% vs. 7.2%, respectively, p < 0.001, Figure 2). In patients with 5 years or less disease duration, the prevalence of preproliferative DR was 0.8% in type 1 and 2.4% in type 2 diabetes. The prevalence increased with duration of diabetes. In type 1 and type 2 diabetic patients with a disease duration of 15 years or more, preproliferative DR was seen in 17.2% and 14.2% of patients, respectively. Generally, the prevalence of preproliferative DR was higher in males than in women (type 1 diabetes: 10.9% vs. 7.5%, p < 0.0001; type 2 diabetes: 7.5% vs. 7.0%, p < 0.0001, Table 2).
Proliferative DR was observed in 6.7% of the adult diabetic patients and was significantly higher in type 1 than type 2 diabetes (11.1% to 2.7%, p < 0.001, Figure 2). In the first 5 years after diabetes onset, the prevalence of proliferative DR was 0.8% for both type 1 and type 2 diabetes. However, as disease duration increased, the proportion of sufferers with irreversible DR stages rose above average. For patients with a disease duration of 15 years or more, proliferative DR was seen in 24.5% of type 1 and 6.5% of type 2 diabetes patients (Table 2). Proliferative DR was significantly higher in women than in males: 12.4% vs. 9.5% (p < 0.001) for type 1 diabetes and 2.8% vs. 2.7% (p < 0.001) for type 2 diabetes.
Prevalence of diabetic cataract
The overall prevalence of diabetic cataract (DC) among adult diabetes patients was 30.6%. It was significantly higher for type 1 than for type 2 diabetes (32.6% and 29.2%, respectively; p < 0.001, Figure 3), and directly correlated with the duration of diabetes. For type 1 diabetes with a duration of less than 5 years, 6-9 years, 10-14 years and more than 15 years, DC was found in 8.9%, 13.2%, 28.2% and 51.5% of patients, respectively. In type 2 diabetes patients with less than 5 years duration, 23.6% of patients had DC (Figure 3). In patients who suffered from type 2 diabetes for 15 years or more, the prevalence of DC was 39.8%. DC was observed in 30.9% of women and 27.5% of males with type 2 diabetes (p < 0.001). In type 1 diabetes, the prevalence of DC was 1.4 times greater in women than in men (34.8% and 24.7%, respectively; p < 0.01).
Figure 3.
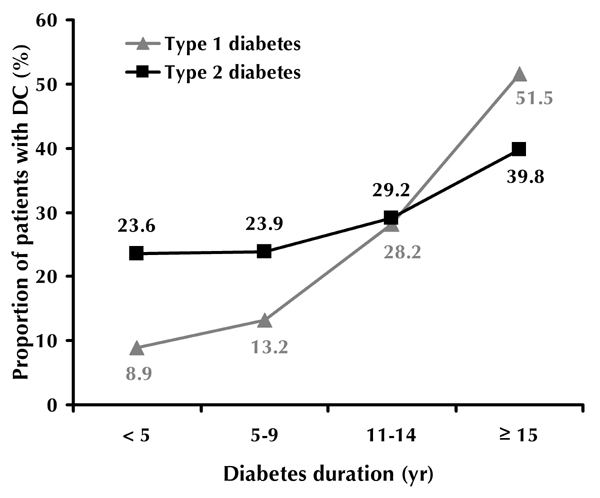
Prevalence of diabetic cataract according to diabetes type and duration.
Discussion
The prevalence of DR was significantly higher in type 1 than in type 2 diabetes (54.6% vs. 34.2%, p < 0.0001). Proliferative DR was higher in type 1 diabetes, while initial stage DR was higher in type 2 diabetes. The prevalence rate of DC among diabetic patients was 30.6%, with a significantly higher rate in type 1 than in type 2 diabetes.
The data emerging from our study are partly different to those from similar studies, conducted in other countries, examining epidemiological data. The overall prevalence of DR among diabetic patients was 33.2% in the USA, 34.5% in Japan, 16.1% in Germany, 33.6% in the UK, 17.4% in Slovakia, and 22.4% in the Ukraine [8-12]. The prevalence of DR in type 1 diabetes was lower in Russia than in the USA and Sweden, but higher than in Italy and New Zealand [13-16]. For type 2 diabetes, the prevalence of DR in our study was higher than that observed for the USA, France and Australia [17-19].
5.8% of type 1 and 12.5% of type 2 diabetes patients with a disease duration of 5 years or less had DR. These observations largely corresponded to data obtained in Sri-Lanka and Sweden [20, 21]. Also consistent with other reports, we found an increase in the prevalence of DR in patients with longer disease duration [22].
In the present study, we found significant differences in the duration of diabetes, HbA1c level, and systolic BP between the groups of patients with and without DR. In this regard, several studies have reported a statistically significant association between retinopathy and nephropathy in diabetes [23, 24]. Poor glycemic control and elevated blood pressure have been shown to be risk factors for the development of diabetic complications [25]. In our study, DR strongly correlated with higher UAE, increased HbA1c, and elevated systolic BP. In summary, this study showed that the prevalence of DR and risk factors associated with DR are similar to those found in other studies.
A more aggressive management of glycemia and hypertension could reduce the development and progression of microvascular complications in diabetes. The data from many studies suggest that controlling risk factors can help to prevent DR. Firstly, DR should be identified at an early stage to allow timely and adequate laser coagulation of the retina or surgical treatment (e.g., cataract extraction, vitrectomy). Secondly, the carbohydrate and lipid metabolism should be controlled, and finally blood glucose and BP should be normalized [26, 27].
This is one of the first population-based studies of diabetic retinopathy in the Russian Federation. The patients were randomly selected using the regional centers' diabetes databases. The type of diabetes was determined based on medical records from diabetes databases. However, C-peptide levels, as marker of β-cell destruction, as well as data on socio-economic, ethnic or smoking status were not recorded. These data may be included in subsequent population-based studies to enrich the analysis of risk factors for diabetic complications.
References
- 1.Dedov I, Shestakova MV. In: Diabetes mellitus. Universum Publishing; Moscow: 2003. Diabetic retinopathy; pp. 231–243. [Google Scholar]
- 2.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 3.Negi A, Vernon SA. Diabetic retinopathy. J R Soc Med. 2003;96(6):266–272. doi: 10.1258/jrsm.96.6.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dowse GK, Humphrey AR, Collins VR, Plehwe W, Garreboo H, Fareed D, Hemraj F, Taylor HR, Tuomilehto J, Alberti KG, Zimmet PZ. Prevalence and risk factors for diabetic retinopathy in the multiethnic population of Mauritius. Am J Epidemiol. 1998;147:448–457. doi: 10.1093/oxfordjournals.aje.a009470. [DOI] [PubMed] [Google Scholar]
- 5.de Fine Olivarius N, Nielsen NV, Andreasen AH. Diabetic retinopathy in newly diagnosed middle-aged and elderly diabetic patients. Prevalence and interrelationship with microalbuminuria and triglycerides. Graefes Arch Clin Exp Ophthalmol. 2001;239(9):664–672. doi: 10.1007/s004170100324. [DOI] [PubMed] [Google Scholar]
- 6.Segato T, Midena E, Grigoletto F, Zucchetto M, Fedele D, Piermarocchi S, Crepaldi G. The epidemiology and prevalence of diabetic retinopathy in the Vento region of north east Italy. Veneto Group for Diabetic Retinopathy. Diabet Med. 1991;8(Spec No):S11–S16. doi: 10.1111/j.1464-5491.1991.tb02149.x. [DOI] [PubMed] [Google Scholar]
- 7.Ekoe JM, Zimmet P, Robert D, Williamg R. In: The epidemiology of diabetes mellitus: an international perspective. Wiley; England: 2001. Long-term complications: diabetic retinopathy; pp. 349–367. [Google Scholar]
- 8.Wong TY, Klein R, Islam FM, Cotch MF, Folsom AR, Klein BE, Sharrett AR, Shea S. Diabetic retinopathy in a multi-ethnic cohort in the United States. Am J Ophthalmol. 2006;141(3):446–455. doi: 10.1016/j.ajo.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawano M, Omori Y, Katayama S, Kawakami M, Suzuki Y, Takahashi K, Takemura Y, Nagata N, Hiratsuka A, Matsuzaki F, Kanazawa Y, Akanuma Y. A questionnaire for neurological symptoms in patients with diabetes - cross-sectional multicenter study in Saitama Prefecture, Japan. Diabetes Res Clin Pract. 2001;54(Suppl):41–47. doi: 10.1016/s0168-8227(01)00256-x. [DOI] [PubMed] [Google Scholar]
- 10.Hesse L, Grusser M, Hoffstadt K, Jorgens V, Hartmann P, Kroll P. Population-based study of diabetic retinopathy in Wolfsburg. Ophthalmologe. 2001;98:1065–1068. doi: 10.1007/s003470170026. [DOI] [PubMed] [Google Scholar]
- 11.Broadbent DM, Scott JA, Vora JP, Harding SP. Prevalence of diabetic eye disease in an inner city population: the Liverpool Diabetic Eye Study. Eye. 1999;13:160–165. doi: 10.1038/eye.1999.43. [DOI] [PubMed] [Google Scholar]
- 12.Internetional Diabeties Federation. Diabetes Atlas. Belgium: 2003. pp. 102–105. [Google Scholar]
- 13.Roy MS, Klein R, O'Colmain BJ, Klein BE, Moss SE, Kempen JH. The prevalence of diabetic retinopathy among adult type 1 diabetic persons in the United States. Arch Ophthalmol. 2004;122(4):546–551. doi: 10.1001/archopht.122.4.546. [DOI] [PubMed] [Google Scholar]
- 14.Lovestam-Adrian M, Agardh CD, Torffvit O, Agardh E. Diabetic retinopathy, visual acuity, and medical risk indicators: a continuous 10-year follow-up study in Type 1 diabetic patients under routine care. J Diabetes Complications. 2001;15(6):287–294. doi: 10.1016/s1056-8727(01)00167-2. [DOI] [PubMed] [Google Scholar]
- 15.Lepore G, Bruttomesso D, Nosari I, Tiengo A, Trevisan R. Glycaemic control and microvascular complications in a large cohort of Italian Type 1 diabetic out-patients. Diabetes Nutr Metab. 2002;15(4):232–239. [PubMed] [Google Scholar]
- 16.Florkowski CM, Scott RS, Coope PA, Graham PJ, Moir CL. Age at diagnosis, glycaemic control and the development of retinopathy in a population-based cohort of Type 1 diabetic subjects in Canterbury, New Zealend. Diabetes Res Clin Pract. 2001;52:125–131. doi: 10.1016/s0168-8227(00)00248-5. [DOI] [PubMed] [Google Scholar]
- 17.Harris MI, Klein R, Cowie CC, Rowland M, Byrd-Holt DD. Is the risk of diabetic retinopathy greater in non-Hispanic blacks and Mexican Americans than in non-Hispanic whites with type 2 diabetes? A U.S. population study. Diabetes Care. 1998;21:1230–1235. doi: 10.2337/diacare.21.8.1230. [DOI] [PubMed] [Google Scholar]
- 18.Delcourt C, Vauzelle-Kervroedan F, Cathelineau G, Papoz L. Low prevalence of long-term complications in non-insulin-dependent diabetes mellitus in France: a multicenter study. CODIAB-INSERM-ZENECA Pharma Study Group. J Diabetes Complications. 1998;12(2):88–95. doi: 10.1016/s1056-8727(97)98005-3. [DOI] [PubMed] [Google Scholar]
- 19.Tapp RJ, Shaw JE, Harper CA, Balkau B, McCarty DJ, Taylor HR, Welborn TA, Zimmet PZ. The prevalence of and factors associated with diabetic retinopathy in the Australian Population. Diabetes Care. 2003;26:1731–1737. doi: 10.2337/diacare.26.6.1731. [DOI] [PubMed] [Google Scholar]
- 20.Weerasuriya N, Siribaddana S, Dissanayake A, Subasinghe Z, Wariyapola D, Fernando DJ. Long-term complications in newly diagnosed Sri Lankan patients with type 2 diabetes mellitus. QJM. 1998;91(6):439–443. doi: 10.1093/qjmed/91.6.439. [DOI] [PubMed] [Google Scholar]
- 21.Gudbjörnsdottir S, Cederholm J, The Swedish National Diabetes Register 2005. Report 23. [Google Scholar]
- 22.UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317(7160):703–713. [PMC free article] [PubMed] [Google Scholar]
- 23.Stephenson JM, Fuller JH, Viberti GC, Sjolie AK, Navalesi R. Blood pressure, retinopathy and urinary albumin excretion in IDDM: the EURODIAB IDDM Complications Study. Diabetologia. 1995;38:599–603. doi: 10.1007/BF00400730. [DOI] [PubMed] [Google Scholar]
- 24.Gall MA, Hougaard P, Borch Johnsen K, Parving HH. Risk factors for development of incipient and overt diabetic nephropathy in patients with non-insulin dependent diabetes mellitus: prospective, observational study. BMJ. 1997;314:783–788. doi: 10.1136/bmj.314.7083.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stratton IM, Kohner EM, Aldington SJ, Turner RC, Holman RR, Manley SE, Mathews DR. UKPDS 50: risk factors for incidence and progression of retinopathy in type II diabetes over 6 years from diagnosis. Diabetologia. 2001;44(2):156–163. doi: 10.1007/s001250051594. [DOI] [PubMed] [Google Scholar]
- 26.Stratton IM, Adler AI, Neil HA, Yudkin JS, Matthews DR, Cull CA, Wright AD, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;12:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adler AI, Stratton IM, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;12:412–419. doi: 10.1136/bmj.321.7258.412. [DOI] [PMC free article] [PubMed] [Google Scholar]


