Abstract
Background:
Worldwide, the Brugada syndrome has been recognized as an important cause of sudden cardiac death at a relatively young age. Importantly, many drugs have been reported to induce the characteristic Brugada syndrome-linked ECG abnormalities and/or (fatal) ventricular tachyarrhythmias.
Objective:
To review the literature on the use of drugs in Brugada syndrome patients, to make recommendations based on the literature and expert opinion regarding drug safety, and to ensure worldwide online and up-to-date availability of this information to all physicians who treat Brugada syndrome patients.
Methods:
We have performed an extensive review of the literature, formed an international expert panel to produce a consensus recommendation to each drug, and initiated a website (www.brugadadrugs.org).
Results:
The literature search yielded 506 reports to be considered. Drugs were categorized to one of four categories: 1) drugs to be avoided (n=18), 2) drugs preferably avoided (n=23), 3) antiarrhythmic drugs (n=4) and 4) diagnostic drugs (n=4). Level of evidence for most associations was C (only consensus opinion of experts, case studies, or standard-of-care) as there are no randomized studies and few non-randomized studies in Brugada syndrome patients.
Conclusions:
Many drugs have been associated with adverse events in Brugada syndrome patients. We have initiated a website (www.brugadadrugs.org) to ensure worldwide availability on safe drug use in Brugada syndrome patients.
Introduction
Worldwide, the Brugada syndrome (BrS) has been recognized as an important cause of sudden cardiac death at a relatively young age. BrS is diagnosed in the presence of specific electrocardiographic abnormalities (known as the type-1 BrS-ECG, figure 1) combined with an absence of gross structural abnormalities and several other criteria.1,2 In addition, BrS often shows familial aggregation.
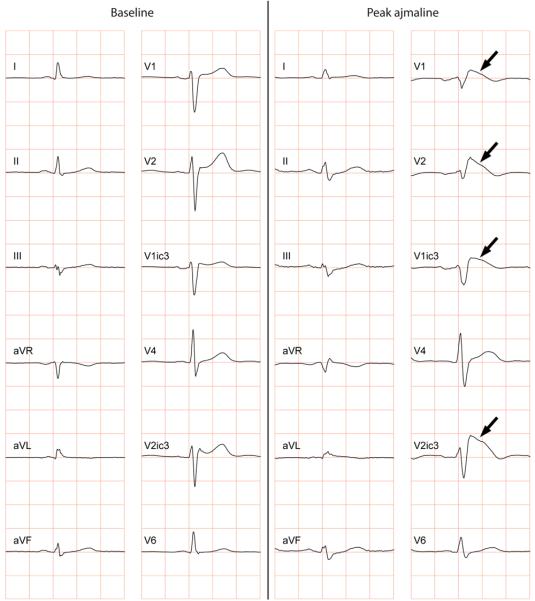
Figure 1.
Conversion of a normal ECG into a type-1 BrS-ECG during ajmaline challenge. Note the coved-type ST-segments (arrows) in the right precordial ECG leads at peak ajmaline (note V3 is placed in the 3rd intercostal space above V1 [V1ic3], and V5 is placed in the 3rd intercostal space above V2 [V2ic3]).
The presence of this type-1 BrS-ECG in particular has been linked to an increased risk for ventricular tachyarrhythmias, cardiac arrest and sudden death in BrS-patients.3 Importantly, many drugs have been reported to induce the type-1 BrS-ECG and/or (fatal) arrhythmias in BrS-patients (figure 2). Therefore, patients with BrS should be advised not to use these drugs or to use them only under controlled conditions.
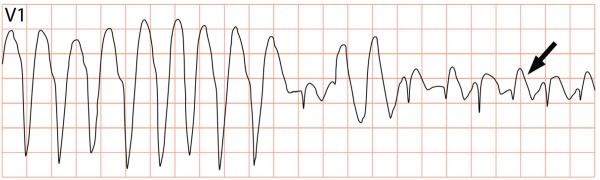
Figure 2.
Non-sustained ventricular tachycardia in a patient who was given flecainide for paroxysmal atrial fibrillation, note the coved-type ST-segments (arrow). The patient was diagnosed with BrS during an ajmaline provocation test.
Although the most appropriate treatment in BrS is under discussion,4,5 avoidance of potential proarrhythmic drugs and fever (a well-known trigger of cardiac events in Brugada syndrome)6,7 are generally accepted to be an important part of (prophylactic) treatment. However, some patients may (only) be appropriately treated with an implantable cardioverter-defibrillator. In contrast, there are also drugs that may have antiarrhythmic effects and may thus be used favorably in the acute or chronic setting.8-10 As BrS has a rather low prevalence (estimated at 1 in 2000, varying in different regions in the world),1 these and other critical characteristics of BrS are certainly not common knowledge for many physicians.11
With the aim of aiding all physicians who treat patients with BrS, we discussed the interaction between drugs and BrS, performed an extensive review of the literature, formed an international expert panel to produce a consensus recommendation for each drug, and initiated a website (www.brugadadrugs.org, figure 3) to ensure world-wide online and up-to-date availability of this knowledge base.
Figure 3.
Screenshot of the website at www.brugadadrugs.org
Methods
Literature review
PubMed (Text: Brugada; MeSH Terms: Chemicals and Drugs Category; only reports in English were considered) and expert knowledge was employed to investigate drugs that have been associated with the type-1 BrS-ECG, with arrhythmias or with antiarrhythmic properties in BrS-patients. Although there is large variation in the extent to which different drugs have been associated with BrS, we aimed to investigate the first reported drug-BrS association for each drug, but favored larger, combined clinical-experimental or otherwise important studies (e.g., those that report arrhythmias). Thus, we refer to many, but not to all reports that describe a certain drug-BrS association. Furthermore, we sought drugs with cardiac ion channel blocking effects that, hypothetically, have the potential to have deleterious effects in BrS-patients, but that have not yet been reported deleterious effects. Finally, for most drugs with clinical association with BrS, we were able to retrieve confirmatory experimental studies showing the effects of the drug on the cardiac electrophysiology.
Recommendations
As there are no randomized clinical trials in BrS, the level of evidence (ACC/AHA/ESC format) for most associations is C (only consensus opinion of experts, case studies, or standard-of-care) and for some associations B (non-randomized studies). To ascertain validity of recommendations given, the authors formed an international expert panel (the ‘BrugadaDrugs.org Advisory Board’) to summarize the clinical and experimental evidence and expert opinion. The classification of recommendation is expressed in a modified ACC/AHA/ESC format:
Class I: There is evidence and/or general agreement that a given treatment is potentially proarrhythmic (or potentially antiarrhythmic) in BrS-patients.
Class IIa: There is conflicting evidence and/or divergence of opinion about the drug, but the weight of evidence/opinion is in favor of a potentially proarrhythmic (or potentially antiarrhythmic) effect in BrS-patients.
Class IIb: There is conflicting evidence and/or divergence of opinion about the drug and the potential proarrhythmic (or potentially antiarrhythmic) effect in BrS-patients is less well established by evidence/opinion.
Class III: There is very little evidence and/or agreement that a drug is potentially proarrhythmic (or potentially antiarrhythmic) in BrS-patients
Subsequently, we have listed the drugs into four groups:
Drugs to be avoided by BrS-patients
Drugs preferably avoided by BrS-patients
Potential antiarrhythmic drugs in BrS-patients
Diagnostic drugs in BrS
Within these groups, we differentiated between different drug classes (e.g., antiarrhythmic drugs and psychotropic drugs).
Results
The PubMed search yielded 563 reports, including 506 written in English. The BrugadaDrugs.org Advisory Board selected approximately 15% of these reports as adding considerably to our knowledge and understanding of drug effects in BrS. The drugs and accompanying recommendations are listed in Tables 1 through 4.
Table 1.
Drugs to be avoided by Brugada syndrome patients
| Drug category | Drug (generic) | Recommendation |
|---|---|---|
| Antiarrhythmic drugs: | Ajmaline26-29 | Class I |
| Flecainide30-34 | Class I | |
| Pilsicainide35-38 | Class I | |
| Procainamide17,26,39,40 | Class I | |
| Propafenone41-45 | Class IIa | |
|
| ||
| Psychotropic drugs | Amitriptyline46-49 | Class IIa |
| Clomipramine50,51 | Class IIa | |
| Desipramine52-55 | Class IIa | |
| Lithium52,56 | Class IIa | |
| Loxapine47,57 | Class IIa | |
| Nortriptyline55,58,59 | Class IIa | |
| Trifluoperazine47,60 | Class IIa | |
|
| ||
| Anesthetic drugs | Bupivacaine61-64 | Class IIa |
| Propofol62,65-67 | Class IIb | |
|
| ||
| Other substances | Acetylcholine17,68,69 | Class IIa |
| Alcohol (toxicity)47,70,71 | Class IIb | |
| Cocaine72-75 | Class IIa | |
| Ergonovine68,76 | Class IIb | |
Recommendation: Class I: convincing evidence/opinion; Class IIa: evidence/opinion less clear; Class IIb: conflicting evidence/opinion.
Table 4.
Diagnostic drugs in Brugada syndrome
Discussion
In this study we reviewed the literature on the use of drugs in BrS-patients and made recommendations about their safety that were based on the literature and expert opinion. We also initiated a website (www.brugadadrugs.org) where these drugs and the recommendations can be accessed by all physicians who treat patients with BrS and by others with possible interest (e.g., patients). On this website, we provide more detailed information on drugs in BrS than reviewed in this manuscript. In addition, the website is updated frequently (drugs added or removed, recommendations changed) according to the latest evidence.
Patients with BrS should be advised not to take the drugs from the ‘avoid’ and ‘preferably avoid’ lists or to use these drugs only after extensive consideration and/or in controlled conditions. We advise patients to give a list of these drugs to all of their health care providers (including their general practitioner, dentist and pharmacist). In many BrS-patients, avoidance of these drugs (and treatment of fever)6,7 is probably an appropriate and safe treatment. Furthermore, some BrS-patients seem to perform well on quinidine.8-10 Recently, a prospective registry has started investigating the use of empiric quinidine therapy for asymptomatic BrS-patients (ClinicalTrials.gov identifier NCT00789165).12 Further, the QUIDAM study (HydroQuinidine to Decrease Arrhythmic events in Brugada syndrome patients, ClinicalTrials.gov identifier NCT00927732), a French national double blinded randomized study, is currently performed on the role of quinidine therapy to improve the outcome in high risk BrS-patients. Reports have postulated an antiarrhythmic effect of other drugs (amrinone,13 bepridil,14,15 clarithromycin,13 denopamine,15 dimethyl lithospermate B,16 mexiletine,17,18 milrinone,13 phentolamine,17 prazosin,17 sotalol,19,20 tedisamil13,21 and 4-aminopyridine13). We consider the evidence on use of these drugs as antiarrhythmic treatment in Brugada syndrome patients currently to be too low.
An important issue regarding ventricular tachyarrhythmias in Brugada syndrome patients is that they can present as an epileptic seizure and that the cerebral hypoperfusion may create a clinical picture easily confused with a postictal phase. Therefore, in patients with seizures both epilepsy and arrhythmia syndromes such as Brugada syndrome7 (or, e.g., Long-QT syndrome)22 are part of the differential diagnosis. Many antiepileptic drugs, such as carbamazepine or phenytoin, act through cerebral ion channel blockade but will also result in cardiac ion channel blockade.23-25 The latter may have a deleterious (and possibly fatal) effect in patients with an arrhythmia syndrome such as Brugada syndrome. Therefore, it is important to exclude arrhythmia syndromes such as Brugada syndrome in patients suspected of epilepsy before a possible harmful treatment is started.
We hope that the website will be of help to physicians who are in need of this information and we welcome your suggestions and/or documentation on the safe or unsafe use of drugs in BrS-patients. Further, we hope that the information on our website will prevent BrS-patients from suffering a cardiac arrest or sudden cardiac death initiated by drugs that should be avoided.
Limitations
The principal limitation of the association between certain drugs, BrS and arrhythmias, is the limited number of case reports and experimental studies suggesting an effect in BrS. Further, there may be conflicting results and large variability may exist between BrS-patients in their response to certain drugs. This response may also vary in different conditions (e.g., with or without fever, drug in therapeutic range, overdosed or in combination with other drugs etc.). Therefore, clinical decision making should be based on more than the presence or absence of a (single) association in another patient. Additionally, it remains important for health care providers to recognize the active substances in medicines containing a combination of drugs, and to be aware of the drug category (e.g., many tricyclic antidepressants will be potentially proarrhythmic in BrS-patients).
Table 2.
Drugs preferably avoided by Brugada syndrome patients
| Drug category | Drug (generic) | Recommendation |
|---|---|---|
| Antiarrhythmic drugs: | Amiodarone77-79 | Class IIb |
| Cibenzoline80-82 | Class IIb | |
| Disopyramide14,17,83-85 | Class IIb | |
| Lidocaine17,86* | Class IIb | |
| Propranolol17,18,70,87,88 | Class IIb | |
| Verapamil17,89,90 | Class IIb | |
|
| ||
| Psychotropic drugs | Carbamazepine91,92 | Class IIb |
| Cyamemazine47,93 | Class IIb | |
| Doxepin48,94 | Class IIb | |
| Fluoxetine47,51 | Class IIb | |
| Imipramine95 | Class IIb | |
| Maprotiline46,96 | Class IIb | |
| Perphenazine46,97 | Class IIb | |
| Phenytoin98,99 | Class IIb | |
| Thioridazine100 | Class IIb | |
|
| ||
| Antianginal drugs | Diltiazem1,101-103 | Class III |
| Nicorandil1,104 | Class III | |
| Nifedipine1,105 | Class III | |
| Nitroglycerine1,106,107 | Class III | |
| Sorbidnitrate1,89,108 | Class III | |
|
| ||
| Other substances | Dimenhydrinate109-111 | Class IIb |
| Edrophonium17,18 | Class IIb | |
| Indapamide112 | Class IIb | |
Recommendation: Class I: convincing evidence/opinion; Class IIa: evidence/opinion less clear; Class IIb: conflicting evidence/opinion; Class III: very little evidence.
Note that lidocaine use for local anesthesia seems to be safe if the amount administered is low and if combined with adrenaline which results in a local effect only.
Table 3.
Potential antiarrhythmic drugs in Brugada syndrome patients
| Drug category | Drug (generic) | Recommendation |
|---|---|---|
| Antiarrhythmic drugs | Isoproterenol / Isoprenaline15,17,113,114* |
Class I |
| Orciprenaline115 | Class IIa | |
| Quinidine8-10,15,116,117† | Class I | |
|
| ||
| Other substances | Cilostazol118-120 | Class IIb |
Recommendation: Class I: convincing evidence/opinion; Class IIa: evidence/opinion less clear; Class IIb: conflicting evidence/opinion.
In adults an isoproterenol regimen of 0.003±0.003 μg/kg/min has been used by Ohgo et al.15 and 0.01 to 0.02 μg/kg/min has been used by Kasanuki et al.18
Aim at quinidine plasma levels of 1-3 μg/mL or 3.5-11 μmol/L.
Acknowledgements
The authors gratefully acknowledge Cardionetworks (non-profit organization based in The Netherlands, founded in 2007 with the aim to provide unbiased and up-to-date medical knowledge to the global community), and in particular its chair Jonas S.S.G. de Jong, MD, for hosting the website.
The inspiration for the website comes from www.qtdrugs.org, which contains lists of drugs associated with the Long-QT syndrome.
Funding: Netherlands Heart Foundation (grant 2005T024 to P.G.P.), Fondation Leducq Trans-Atlantic Network of Excellence, Preventing Sudden Death (grant 05-CVD-01 to A.A.M.W.); CHU de Nantes, France and Société Française de Cardiologie (grant P.H.R.C. 2004 R20/07 to V.P.); US National Institutes of Health (grant HL65962 to D.M.R.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Antzelevitch C, Brugada P, Borggrefe M, et al. Brugada syndrome: report of the second consensus conference. Heart Rhythm. 2005;2:429–440. doi: 10.1016/j.hrthm.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Wilde AA, Antzelevitch C, Borggrefe M, et al. Proposed diagnostic criteria for the Brugada syndrome: consensus report. Circulation. 2002;106:2514–2519. doi: 10.1161/01.cir.0000034169.45752.4a. [DOI] [PubMed] [Google Scholar]
- 3.Gehi AK, Duong TD, Metz LD, Gomes JA, Mehta D. Risk stratification of individuals with the Brugada electrocardiogram: a meta-analysis. J Cardiovasc Electrophysiol. 2006;17:577–583. doi: 10.1111/j.1540-8167.2006.00455.x. [DOI] [PubMed] [Google Scholar]
- 4.Paul M, Gerss J, Schulze-Bahr E, et al. Role of programmed ventricular stimulation in patients with Brugada syndrome: a meta-analysis of worldwide published data. Eur Heart J. 2007;28:2126–2133. doi: 10.1093/eurheartj/ehm116. [DOI] [PubMed] [Google Scholar]
- 5.Eckardt L, Probst V, Smits JP, et al. Long-term prognosis of individuals with right precordial ST-segment-elevation Brugada syndrome. Circulation. 2005;111:257–263. doi: 10.1161/01.CIR.0000153267.21278.8D. [DOI] [PubMed] [Google Scholar]
- 6.Amin AS, Meregalli PG, Bardai A, Wilde AA, Tan HL. Fever increases the risk for cardiac arrest in the Brugada syndrome. Ann Intern Med. 2008;149:216–218. doi: 10.7326/0003-4819-149-3-200808050-00020. [DOI] [PubMed] [Google Scholar]
- 7.Skinner JR, Chung SK, Nel CA, et al. Brugada syndrome masquerading as febrile seizures. Pediatrics. 2007;119:e1206–e1211. doi: 10.1542/peds.2006-2628. [DOI] [PubMed] [Google Scholar]
- 8.Belhassen B, Glick A, Viskin S. Efficacy of quinidine in high-risk patients with Brugada syndrome. Circulation. 2004;110:1731–1737. doi: 10.1161/01.CIR.0000143159.30585.90. [DOI] [PubMed] [Google Scholar]
- 9.Probst V, Denjoy I, Meregalli PG, et al. Clinical Aspects and Prognosis of Brugada Syndrome in Children. Circulation. 2007;115:2042–2048. doi: 10.1161/CIRCULATIONAHA.106.664219. [DOI] [PubMed] [Google Scholar]
- 10.Mizusawa Y, Sakurada H, Nishizaki M, Hiraoka M. Effects of low-dose quinidine on ventricular tachyarrhythmias in patients with Brugada syndrome: low-dose quinidine therapy as an adjunctive treatment. J Cardiovasc Pharmacol. 2006;47:359–364. doi: 10.1097/01.fjc.0000206437.27854.65. [DOI] [PubMed] [Google Scholar]
- 11.Perez Riera AR, Filho CF, Uchida AH, et al. Study of the extent of the information of cardiologists from Sao Paulo city, Brazil, regarding a low-prevalence entity: Brugada syndrome. Ann Noninvasive Electrocardiol. 2008;13:352–363. doi: 10.1111/j.1542-474X.2008.00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viskin S, Wilde AA, Tan HL, Antzelevitch C, Shimizu W, Belhassen B. Empiric quinidine therapy for asymptomatic Brugada syndrome: Time for a prospective registry. Heart Rhythm. 2009;6:401–404. doi: 10.1016/j.hrthm.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marquez MF, Salica G, Hermosillo AG, et al. Ionic basis of pharmacological therapy in Brugada syndrome. J Cardiovasc Electrophysiol. 2007;18:234–240. doi: 10.1111/j.1540-8167.2006.00681.x. [DOI] [PubMed] [Google Scholar]
- 14.Sugao M, Fujiki A, Nishida K, et al. Repolarization dynamics in patients with idiopathic ventricular fibrillation: pharmacological therapy with bepridil and disopyramide. J Cardiovasc Pharmacol. 2005;45:545–549. doi: 10.1097/01.fjc.0000159660.16793.84. [DOI] [PubMed] [Google Scholar]
- 15.Ohgo T, Okamura H, Noda T, et al. Acute and chronic management in patients with Brugada syndrome associated with electrical storm of ventricular fibrillation. Heart Rhythm. 2007;4:695–700. doi: 10.1016/j.hrthm.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Fish JM, Welchons DR, Kim YS, Lee SH, Ho WK, Antzelevitch C. Dimethyl lithospermate B, an extract of Danshen, suppresses arrhythmogenesis associated with the Brugada syndrome. Circulation. 2006;113:1393–1400. doi: 10.1161/CIRCULATIONAHA.105.601690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyazaki T, Mitamura H, Miyoshi S, Soejima K, Aizawa Y, Ogawa S. Autonomic and antiarrhythmic drug modulation of ST segment elevation in patients with Brugada syndrome. J Am Coll Cardiol. 1996;27:1061–1070. doi: 10.1016/0735-1097(95)00613-3. [DOI] [PubMed] [Google Scholar]
- 18.Kasanuki H, Ohnishi S, Ohtuka M, et al. Idiopathic ventricular fibrillation induced with vagal activity in patients without obvious heart disease. Circulation. 1997;95:2277–2285. doi: 10.1161/01.cir.95.9.2277. [DOI] [PubMed] [Google Scholar]
- 19.Bertaglia E, Michieletto M, Spedicato L, Pascotto P. Right bundle branch block, intermittent ST segment elevation and inducible ventricular tachycardia in an asymptomatic patient: an unusual presentation of the Brugada syndrome? G Ital Cardiol. 1998;28:893–898. [PubMed] [Google Scholar]
- 20.Glatter KA, Wang Q, Keating M, Chen S, Chiamvimonvat N, Scheinman MM. Effectiveness of sotalol treatment in symptomatic Brugada syndrome. Am J Cardiol. 2004;93:1320–1322. doi: 10.1016/j.amjcard.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 21.Perez Riera AR, Zhang L, Uchida AH, Schapachnik E, Dubner S, Ferreira C. The management of Brugada syndrome patients. Cardiol J. 2007;14:97–106. [PubMed] [Google Scholar]
- 22.Johnson JN, Hofman N, Haglund CM, Cascino GD, Wilde AA, Ackerman MJ. Identification of a possible pathogenic link between congenital long QT syndrome and epilepsy. Neurology. 2009;72:224–231. doi: 10.1212/01.wnl.0000335760.02995.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bahls FH, Ozuna J, Ritchie DE. Interactions between calcium channel blockers and the anticonvulsants carbamazepine and phenytoin. Neurology. 1991;41:740–742. doi: 10.1212/wnl.41.5.740. [DOI] [PubMed] [Google Scholar]
- 24.Cave G, Sleigh JW. ECG features of sodium channel blockade in rodent phenytoin toxicity and effect of hypertonic saline. Vet Hum Toxicol. 2003;45:254–255. [PubMed] [Google Scholar]
- 25.Segal MM, Douglas AF. Late sodium channel openings underlying epileptiform activity are preferentially diminished by the anticonvulsant phenytoin. J Neurophysiol. 1997;77:3021–3034. doi: 10.1152/jn.1997.77.6.3021. [DOI] [PubMed] [Google Scholar]
- 26.Brugada J, Brugada P. Further characterization of the syndrome of right bundle branch block, ST segment elevation, and sudden cardiac death. J Cardiovasc Electrophysiol. 1997;8:325–331. doi: 10.1111/j.1540-8167.1997.tb00796.x. [DOI] [PubMed] [Google Scholar]
- 27.Rolf S, Bruns HJ, Wichter T, et al. The ajmaline challenge in Brugada syndrome: diagnostic impact, safety, and recommended protocol. Eur Heart J. 2003;24:1104–1112. doi: 10.1016/s0195-668x(03)00195-7. [DOI] [PubMed] [Google Scholar]
- 28.Wolpert C, Echternach C, Veltmann C, et al. Intravenous drug challenge using flecainide and ajmaline in patients with Brugada syndrome. Heart Rhythm. 2005;2:254–260. doi: 10.1016/j.hrthm.2004.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bebarova M, O'Hara T, Geelen JL, et al. Subepicardial phase-0 block and discontinuous transmural conduction underlie right-precordial ST-segment elevation by a SCN5A loss-of-function mutation. Am J Physiol Heart Circ Physiol. 2008;295:H48–H58. doi: 10.1152/ajpheart.91495.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krishnan SC, Josephson ME. ST segment elevation induced by class IC antiarrhythmic agents: underlying electrophysiologic mechanisms and insights into drug-induced proarrhythmia. J Cardiovasc Electrophysiol. 1998;9:1167–1172. doi: 10.1111/j.1540-8167.1998.tb00088.x. [DOI] [PubMed] [Google Scholar]
- 31.Brugada R, Brugada J, Antzelevitch C, et al. Sodium channel blockers identify risk for sudden death in patients with ST-segment elevation and right bundle branch block but structurally normal hearts. Circulation. 2000;101:510–515. doi: 10.1161/01.cir.101.5.510. [DOI] [PubMed] [Google Scholar]
- 32.Gasparini M, Priori SG, Mantica M, et al. Flecainide test in Brugada syndrome: a reproducible but risky tool. Pacing Clin Electrophysiol. 2003;26:338–341. doi: 10.1046/j.1460-9592.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 33.Meregalli PG, Ruijter JM, Hofman N, Bezzina CR, Wilde AA, Tan HL. Diagnostic Value of Flecainide Testing in Unmasking SCN5A-Related Brugada Syndrome. J Cardiovasc Electrophysiol. 2006;17:857–864. doi: 10.1111/j.1540-8167.2006.00531.x. [DOI] [PubMed] [Google Scholar]
- 34.Stokoe KS, Balasubramaniam R, Goddard CA, Colledge WH, Grace AA, Huang CL. Effects of flecainide and quinidine on arrhythmogenic properties of Scn5a+/− murine hearts. J Physiol. 2007;581:255–275. doi: 10.1113/jphysiol.2007.128785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takenaka S, Emori T, Koyama S, Morita H, Fukushima K, Ohe T. Asymptomatic form of Brugada syndrome. Pacing Clin Electrophysiol. 1999;22:1261–1263. doi: 10.1111/j.1540-8159.1999.tb00612.x. [DOI] [PubMed] [Google Scholar]
- 36.Fujiki A, Usui M, Nagasawa H, Mizumaki K, Hayashi H, Inoue H. ST segment elevation in the right precordial leads induced with class IC antiarrhythmic drugs: insight into the mechanism of Brugada syndrome. J Cardiovasc Electrophysiol. 1999;10:214–218. doi: 10.1111/j.1540-8167.1999.tb00662.x. [DOI] [PubMed] [Google Scholar]
- 37.Takagi M, Doi A, Takeuchi K, Yoshikawa J. Pilsicanide-induced marked T wave alternans and ventricular fibrillation in a patient with Brugada syndrome. J Cardiovasc Electrophysiol. 2002;13:837. doi: 10.1046/j.1540-8167.2002.00837.x. [DOI] [PubMed] [Google Scholar]
- 38.Kimura M, Kobayashi T, Owada S, et al. Mechanism of ST elevation and ventricular arrhythmias in an experimental Brugada syndrome model. Circulation. 2004;109:125–131. doi: 10.1161/01.CIR.0000105762.94855.46. [DOI] [PubMed] [Google Scholar]
- 39.Joshi S, Raiszadeh F, Pierce W, Steinberg JS. Antiarrhythmic induced electrical storm in Brugada syndrome: a case report. Ann Noninvasive Electrocardiol. 2007;12:274–278. doi: 10.1111/j.1542-474X.2007.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villemaire C, Savard P, Talajic M, Nattel S. A quantitative analysis of use-dependent ventricular conduction slowing by procainamide in anesthetized dogs. Circulation. 1992;85:2255–2266. doi: 10.1161/01.cir.85.6.2255. [DOI] [PubMed] [Google Scholar]
- 41.Matana A, Goldner V, Stanic K, Mavric Z, Zaputovic L, Matana Z. Unmasking effect of propafenone on the concealed form of the Brugada phenomenon. Pacing Clin Electrophysiol. 2000;23:416–418. doi: 10.1111/j.1540-8159.2000.tb06774.x. [DOI] [PubMed] [Google Scholar]
- 42.Akdemir I, Davutoglu V, Aksoy M. Intermittent Brugada syndrome misdiagnosed as acute myocardial infarction and unmasked with propafenone. Heart. 2002;87:543. doi: 10.1136/heart.87.6.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hasdemir C, Olukman M, Ulucan C, Roden DM. Brugada-Type ECG Pattern and Extreme QRS Complex Widening with Propafenone Overdose. J Cardiovasc Electrophysiol. 2006;17:565–566. doi: 10.1111/j.1540-8167.2006.00453.x. [DOI] [PubMed] [Google Scholar]
- 44.Shan Q, Yang B, Chen M, et al. Short-term normalization of ventricular repolarization by transcatheter ablation in a patient with suspected Brugada Syndrome. J Interv Card Electrophysiol. 2008;21:53–57. doi: 10.1007/s10840-007-9193-y. [DOI] [PubMed] [Google Scholar]
- 45.Stark U, Stark G, Poppe H, et al. Rate-dependent effects of detajmium and propafenone on ventricular conduction and refractoriness in isolated guinea pig hearts. J Cardiovasc Pharmacol. 1996;27:125–131. doi: 10.1097/00005344-199601000-00020. [DOI] [PubMed] [Google Scholar]
- 46.Bolognesi R, Tsialtas D, Vasini P, Conti M, Manca C. Abnormal ventricular repolarization mimicking myocardial infarction after heterocyclic antidepressant overdose. Am J Cardiol. 1997;79:242–245. doi: 10.1016/s0002-9149(96)00727-8. [DOI] [PubMed] [Google Scholar]
- 47.Rouleau F, Asfar P, Boulet S, et al. Transient ST segment elevation in right precordial leads induced by psychotropic drugs: relationship to the Brugada syndrome. J Cardiovasc Electrophysiol. 2001;12:61–65. doi: 10.1046/j.1540-8167.2001.00061.x. [DOI] [PubMed] [Google Scholar]
- 48.Bebarta VS, Phillips S, Eberhardt A, Calihan KJ, Waksman JC, Heard K. Incidence of Brugada electrocardiographic pattern and outcomes of these patients after intentional tricyclic antidepressant ingestion. Am J Cardiol. 2007;100:656–660. doi: 10.1016/j.amjcard.2007.03.077. [DOI] [PubMed] [Google Scholar]
- 49.Nau C, Seaver M, Wang SY, Wang GK. Block of human heart hH1 sodium channels by amitriptyline. J Pharmacol Exp Ther. 2000;292:1015–1023. [PubMed] [Google Scholar]
- 50.Goldgran-Toledano D, Sideris G, Kevorkian JP. Overdose of cyclic antidepressants and the Brugada syndrome. N Engl J Med. 2002;346:1591–1592. doi: 10.1056/NEJM200205163462020. [DOI] [PubMed] [Google Scholar]
- 51.Pacher P, Bagi Z, Lako-Futo Z, Ungvari Z, Nanasi PP, Kecskemeti V. Cardiac electrophysiological effects of citalopram in guinea pig papillary muscle comparison with clomipramine. Gen Pharmacol. 2000;34:17–23. doi: 10.1016/s0306-3623(99)00048-8. [DOI] [PubMed] [Google Scholar]
- 52.Babaliaros VC, Hurst JW. Tricyclic antidepressants and the Brugada syndrome: an example of Brugada waves appearing after the administration of desipramine. Clin Cardiol. 2002;25:395–398. doi: 10.1002/clc.4950250809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chow BJ, Gollob M, Birnie D. Brugada syndrome precipitated by a tricyclic antidepressant. Heart. 2005;91:651. doi: 10.1136/hrt.2004.049593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Akhtar M, Goldschlager NF. Brugada electrocardiographic pattern due to tricyclic antidepressant overdose. J Electrocardiol. 2006;39:336–339. doi: 10.1016/j.jelectrocard.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 55.Sudoh Y, Cahoon EE, Gerner P, Wang GK. Tricyclic antidepressants as long-acting local anesthetics. Pain. 2003;103:49–55. doi: 10.1016/s0304-3959(02)00375-5. [DOI] [PubMed] [Google Scholar]
- 56.Darbar D, Yang T, Churchwell K, Wilde AA, Roden DM. Unmasking of Brugada syndrome by lithium. Circulation. 2005;112:1527–1531. doi: 10.1161/CIRCULATIONAHA.105.548487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kinugawa T, Kotake H, Mashiba H. Inhibitory actions of amoxapine, a tricyclic antidepressant agent, on electrophysiological properties of mammalian isolated cardiac preparations. Br J Pharmacol. 1988;94:1250–1256. doi: 10.1111/j.1476-5381.1988.tb11645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tada H, Sticherling C, Oral H, Morady F. Brugada syndrome mimicked by tricyclic antidepressant overdose. J Cardiovasc Electrophysiol. 2001;12:275. doi: 10.1046/j.1540-8167.2001.00275.x. [DOI] [PubMed] [Google Scholar]
- 59.Muir WW, Strauch SM, Schaal SF. Effects of tricyclic antidepressant drugs on the electrophysiological properties of dog Purkinje fibers. J Cardiovasc Pharmacol. 1982;4:82–90. doi: 10.1097/00005344-198201000-00014. [DOI] [PubMed] [Google Scholar]
- 60.Klockner U, Isenberg G. Calmodulin antagonists depress calcium and potassium currents in ventricular and vascular myocytes. Am J Physiol Heart Circ Physiol. 1987;253:H1601–H1611. doi: 10.1152/ajpheart.1987.253.6.H1601. [DOI] [PubMed] [Google Scholar]
- 61.Phillips N, Priestley M, Denniss AR, Uther JB. Brugada-type electrocardiographic pattern induced by epidural bupivacaine. Anesth Analg. 2003;97:264–7. doi: 10.1213/01.ane.0000067410.32384.3a. table. [DOI] [PubMed] [Google Scholar]
- 62.Vernooy K, Delhaas T, Cremer OL, et al. Electrocardiographic changes predicting sudden death in propofol-related infusion syndrome. Heart Rhythm. 2006;3:131–137. doi: 10.1016/j.hrthm.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de La Coussaye JE, Brugada J, Allessie MA. Electrophysiologic and arrhythmogenic effects of bupivacaine. A study with high-resolution ventricular epicardial mapping in rabbit hearts. Anesthesiology. 1992;77:132–141. [PubMed] [Google Scholar]
- 64.Berman MF, Lipka LJ. Relative sodium current block by bupivacaine and lidocaine in neonatal rat myocytes. Anesth Analg. 1994;79:350–356. doi: 10.1213/00000539-199408000-00027. [DOI] [PubMed] [Google Scholar]
- 65.Inamura M, Okamoto H, Kuroiwa M, Hoka S. General anesthesia for patients with Brugada syndrome. A report of six cases. Can J Anaesth. 2005;52:409–412. doi: 10.1007/BF03016285. [DOI] [PubMed] [Google Scholar]
- 66.Saint DA. The effects of propofol on macroscopic and single channel sodium currents in rat ventricular myocytes. Br J Pharmacol. 1998;124:655–662. doi: 10.1038/sj.bjp.0701876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robinson JD, Melman Y, Walsh EP. Cardiac conduction disturbances and ventricular tachycardia after prolonged propofol infusion in an infant. Pacing Clin Electrophysiol. 2008;31:1070–1073. doi: 10.1111/j.1540-8159.2008.01138.x. [DOI] [PubMed] [Google Scholar]
- 68.Noda T, Shimizu W, Taguchi A, et al. ST-segment elevation and ventricular fibrillation without coronary spasm by intracoronary injection of acetylcholine and/or ergonovine maleate in patients with Brugada syndrome. J Am Coll Cardiol. 2002;40:1841–1847. doi: 10.1016/s0735-1097(02)02494-4. [DOI] [PubMed] [Google Scholar]
- 69.Montgomery PR, Dresel PE. Conduction defects in experimental atrial arrhythmia. Am Heart J. 1974;88:191–197. doi: 10.1016/0002-8703(74)90009-x. [DOI] [PubMed] [Google Scholar]
- 70.Shimada M, Miyazaki T, Miyoshi S, et al. Sustained monomorphic ventricular tachycardia in a patient with Brugada syndrome. Jpn Circ J. 1996;60:364–370. doi: 10.1253/jcj.60.364. [DOI] [PubMed] [Google Scholar]
- 71.Habuchi Y, Furukawa T, Tanaka H, Lu LL, Morikawa J, Yoshimura M. Ethanol inhibition of Ca2+ and Na+ currents in the guinea-pig heart. Eur J Pharmacol. 1995;292:143–149. doi: 10.1016/0926-6917(95)90006-3. [DOI] [PubMed] [Google Scholar]
- 72.Littmann L, Monroe MH, Svenson RH. Brugada-type electrocardiographic pattern induced by cocaine. Mayo Clin Proc. 2000;75:845–849. doi: 10.4065/75.8.845. [DOI] [PubMed] [Google Scholar]
- 73.Ortega-Carnicer J, Bertos-Polo J, Gutierrez-Tirado C. Aborted sudden death, transient Brugada pattern, and wide QRS dysrrhythmias after massive cocaine ingestion. J Electrocardiol. 2001;34:345–349. doi: 10.1054/jelc.2001.26318. [DOI] [PubMed] [Google Scholar]
- 74.Bebarta VS, Summers S. Brugada electrocardiographic pattern induced by cocaine toxicity. Ann Emerg Med. 2007;49:827–829. doi: 10.1016/j.annemergmed.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 75.Xu YQ, Crumb WJ, Jr., Clarkson CW. Cocaethylene, a metabolite of cocaine and ethanol, is a potent blocker of cardiac sodium channels. J Pharmacol Exp Ther. 1994;271:319–325. [PubMed] [Google Scholar]
- 76.Muller-Schweinitzer E. The mechanism of ergometrine-induced coronary arterial spasm: in vitro studies on canine arteries. J Cardiovasc Pharmacol. 1980;2:645–655. doi: 10.1097/00005344-198009000-00013. [DOI] [PubMed] [Google Scholar]
- 77.Chalvidan T, Deharo JC, Dieuzaide P, Defaye P, Djiane P. Near fatal electrical storm in a patient equipped with an implantable cardioverter defibrillator for Brugada syndrome. Pacing Clin Electrophysiol. 2000;23:410–412. doi: 10.1111/j.1540-8159.2000.tb06772.x. [DOI] [PubMed] [Google Scholar]
- 78.Paul G, Yusuf S, Sharma S. Unmasking of the Brugada syndrome phenotype during the acute phase of amiodarone infusion. Circulation. 2006;114:e489–e491. doi: 10.1161/CIRCULATIONAHA.106.620799. [DOI] [PubMed] [Google Scholar]
- 79.Wu L, Rajamani S, Shryock JC, et al. Augmentation of late sodium current unmasks the proarrhythmic effects of amiodarone. Cardiovasc Res. 2008;77:481–488. doi: 10.1093/cvr/cvm069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tada H, Nogami A, Shimizu W, et al. ST segment and T wave alternans in a patient with Brugada syndrome. Pacing Clin Electrophysiol. 2000;23:413–415. doi: 10.1111/j.1540-8159.2000.tb06773.x. [DOI] [PubMed] [Google Scholar]
- 81.Sarkozy A, Caenepeel A, Geelen P, Peytchev P, de ZM, Brugada P. Cibenzoline induced Brugada ECG pattern. Europace. 2005;7:537–539. doi: 10.1016/j.eupc.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 82.Niwa R, Honjo H, Kodama I, Maruyama K, Toyama J. Na+ channel blocking effects of cibenzoline on guinea-pig ventricular cells. Eur J Pharmacol. 1998;352:317–327. doi: 10.1016/s0014-2999(98)00354-9. [DOI] [PubMed] [Google Scholar]
- 83.Chinushi M, Aizawa Y, Ogawa Y, Shiba M, Takahashi K. Discrepant drug action of disopyramide on ECG abnormalities and induction of ventricular arrhythmias in a patient with Brugada syndrome. J Electrocardiol. 1997;30:133–136. doi: 10.1016/s0022-0736(97)80021-0. [DOI] [PubMed] [Google Scholar]
- 84.Shimizu W, Antzelevitch C, Suyama K, et al. Effect of sodium channel blockers on ST segment, QRS duration, and corrected QT interval in patients with Brugada syndrome. J Cardiovasc Electrophysiol. 2000;11:1320–1329. doi: 10.1046/j.1540-8167.2000.01320.x. [DOI] [PubMed] [Google Scholar]
- 85.Grant AO, Chandra R, Keller C, Carboni M, Starmer CF. Block of wild-type and inactivation-deficient cardiac sodium channels IFM/QQQ stably expressed in mammalian cells. Biophys J. 2000;79:3019–3035. doi: 10.1016/S0006-3495(00)76538-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Barajas-Martinez HM, Hu D, Cordeiro JM, et al. Lidocaine-Induced Brugada Syndrome Phenotype Linked to a Novel Double Mutation in the Cardiac Sodium Channel. Circ Res. 2008;103:396–404. doi: 10.1161/CIRCRESAHA.108.172619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aouate P, Clerc J, Viard P, Seoud J. Propranolol intoxication revealing a Brugada syndrome. J Cardiovasc Electrophysiol. 2005;16:348–351. doi: 10.1046/j.1540-8167.2005.40564.x. [DOI] [PubMed] [Google Scholar]
- 88.Shimada M, Nakamura Y, Iwanaga S, et al. Nonischemic ST-segment elevation induced by negative inotropic agents. Jpn Circ J. 1999;63:610–616. doi: 10.1253/jcj.63.610. [DOI] [PubMed] [Google Scholar]
- 89.Chinushi M, Tagawa M, Nakamura Y, Aizawa Y. Shortening of the ventricular fibrillatory intervals after administration of verapamil in a patient with Brugada syndrome and vasospastic angina. J Electrocardiol. 2006;39:331–335. doi: 10.1016/j.jelectrocard.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 90.Fish JM, Antzelevitch C. Cellular Mechanism and Arrhythmogenic Potential of T-Wave Alternans in the Brugada Syndrome. J Cardiovasc Electrophysiol. 2008;19:301–308. doi: 10.1111/j.1540-8167.2007.01025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Megarbane B, Leprince P, Deye N, et al. Extracorporeal life support in a case of acute carbamazepine poisoning with life-threatening refractory myocardial failure. Intensive Care Med. 2006;32:1409–1413. doi: 10.1007/s00134-006-0257-8. [DOI] [PubMed] [Google Scholar]
- 92.Brau ME, Dreimann M, Olschewski A, Vogel W, Hempelmann G. Effect of drugs used for neuropathic pain management on tetrodotoxin-resistant Na(+) currents in rat sensory neurons. Anesthesiology. 2001;94:137–144. doi: 10.1097/00000542-200101000-00024. [DOI] [PubMed] [Google Scholar]
- 93.Crumb W, Llorca PM, Lancon C, Thomas GP, Garay RP, Hameg A. Effects of cyamemazine on hERG, INa, ICa, Ito, Isus and IK1 channel currents, and on the QTc interval in guinea pigs. Eur J Pharmacol. 2006;532:270–278. doi: 10.1016/j.ejphar.2005.12.079. [DOI] [PubMed] [Google Scholar]
- 94.Muir WW, Strauch SM, Schaal SF. Effects of tricyclic antidepressant drugs on the electrophysiological properties of drug Purkinje fibers. J Cardiovasc Pharmacol. 1982;4:82–90. doi: 10.1097/00005344-198201000-00014. [DOI] [PubMed] [Google Scholar]
- 95.Robert E, Bruelle P, de La Coussaye JE, et al. Electrophysiologic and proarrhythmogenic effects of therapeutic and toxic doses of imipramine: a study with high resolution ventricular epicardial mapping in rabbit hearts. J Pharmacol Exp Ther. 1996;278:170–178. [PubMed] [Google Scholar]
- 96.Igawa O, Kotake H, Kurata Y, et al. Electrophysiological effects of maprotiline, a tetracyclic antidepressant agent, on isolated cardiac preparations. J Cardiovasc Pharmacol. 1988;11:167–173. [PubMed] [Google Scholar]
- 97.Bebarova M, Matejovic P, Pasek M, et al. Effect of antipsychotic drug perphenazine on fast sodium current and transient outward potassium current in rat ventricular myocytes. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:125–133. doi: 10.1007/s00210-009-0420-1. [DOI] [PubMed] [Google Scholar]
- 98.Al Aloul B, Adabag AS, Houghland MA, Tholakanahalli V. Brugada pattern electrocardiogram associated with supratherapeutic phenytoin levels and the risk of sudden death. Pacing Clin Electrophysiol. 2007;30:713–715. doi: 10.1111/j.1540-8159.2007.00734.x. [DOI] [PubMed] [Google Scholar]
- 99.Xu YQ, Pickoff AS, Clarkson CW. Evidence for developmental changes in sodium channel inactivation gating and sodium channel block by phenytoin in rat cardiac myocytes. Circ Res. 1991;69:644–656. doi: 10.1161/01.res.69.3.644. [DOI] [PubMed] [Google Scholar]
- 100.Copetti R, Proclemer A, Pillinini PP. Brugada-like ECG abnormalities during thioridazine overdose. Br J Clin Pharmacol. 2005;59:608. doi: 10.1111/j.1365-2125.2005.02362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Itoh E, Suzuki K, Tanabe Y. A case of vasospastic angina presenting Brugada-type ECG abnormalities. Jpn Circ J. 1999;63:493–495. doi: 10.1253/jcj.63.493. [DOI] [PubMed] [Google Scholar]
- 102.Sasaki T, Niwano S, Kitano Y, Izumi T. Two cases of Brugada syndrome associated with spontaneous clinical episodes of coronary vasospasm. Intern Med. 2006;45:77–80. doi: 10.2169/internalmedicine.45.1404. [DOI] [PubMed] [Google Scholar]
- 103.Miyazaki K, Adaniya H, Sawanobori T, Hiraoka M. Electrophysiological effects of clentiazem, a new Ca2+ antagonist, on rabbit hearts. J Cardiovasc Pharmacol. 1996;27:615–621. doi: 10.1097/00005344-199605000-00001. [DOI] [PubMed] [Google Scholar]
- 104.Robert E, Aya AG, de La Coussaye JE, et al. Dispersion-based reentry: mechanism of initiation of ventricular tachycardia in isolated rabbit hearts. Am J Physiol. 1999;276:H413–H423. doi: 10.1152/ajpheart.1999.276.2.H413. [DOI] [PubMed] [Google Scholar]
- 105.Hussain M, Orchard CH. Sarcoplasmic reticulum Ca2+ content, L-type Ca2+ current and the Ca2+ transient in rat myocytes during beta-adrenergic stimulation. J Physiol. 1997;505(Pt 2):385–402. doi: 10.1111/j.1469-7793.1997.385bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Matsuo K, Shimizu W, Kurita T, Inagaki M, Aihara N, Kamakura S. Dynamic changes of 12-lead electrocardiograms in a patient with Brugada syndrome. J Cardiovasc Electrophysiol. 1998;9:508–512. doi: 10.1111/j.1540-8167.1998.tb01843.x. [DOI] [PubMed] [Google Scholar]
- 107.Korth M. Influence of glyceryl trinitrate on force of contraction and action potential of guinea-pig myocardium. Naunyn Schmiedebergs Arch Pharmacol. 1975;287:329–347. doi: 10.1007/BF00500036. [DOI] [PubMed] [Google Scholar]
- 108.Atanassova R, Spassov G, Balansky R, Boev K. Effects of isosorbide-5-mononitrate and isosorbide-2-mononitrate on the contractile and electrical activity and on the content of cyclic nucleotides in isolated heart muscles of the guinea-pig and dog. J Pharm Pharmacol. 1992;44:663–666. doi: 10.1111/j.2042-7158.1992.tb05490.x. [DOI] [PubMed] [Google Scholar]
- 109.Pastor A, Nunez A, Cantale C, Cosio FG. Asymptomatic Brugada syndrome case unmasked during dimenhydrinate infusion. J Cardiovasc Electrophysiol. 2001;12:1192–1194. doi: 10.1046/j.1540-8167.2001.01192.x. [DOI] [PubMed] [Google Scholar]
- 110.Lopez-Barbeito B, Lluis M, Delgado V, et al. Diphenhydramine overdose and Brugada sign. Pacing Clin Electrophysiol. 2005;28:730–732. doi: 10.1111/j.1540-8159.2005.00154.x. [DOI] [PubMed] [Google Scholar]
- 111.Kuo CC, Huang RC, Lou BS. Inhibition of Na(+) current by diphenhydramine and other diphenyl compounds: molecular determinants of selective binding to the inactivated channels. Mol Pharmacol. 2000;57:135–143. [PubMed] [Google Scholar]
- 112.Mok NS, Tong CK, Yuen HC. Concomitant-acquired Long QT and Brugada syndromes associated with indapamide-induced hypokalemia and hyponatremia. Pacing Clin Electrophysiol. 2008;31:772–775. doi: 10.1111/j.1540-8159.2008.01085.x. [DOI] [PubMed] [Google Scholar]
- 113.Watanabe A, Kusano KF, Morita H, et al. Low-dose isoproterenol for repetitive ventricular arrhythmia in patients with Brugada syndrome. Eur Heart J. 2006;27:1579–1583. doi: 10.1093/eurheartj/ehl060. [DOI] [PubMed] [Google Scholar]
- 114.Ganesan AN, Maack C, Johns DC, Sidor A, O'Rourke B. Beta-adrenergic stimulation of L-type Ca2+ channels in cardiac myocytes requires the distal carboxyl terminus of alpha1C but not serine 1928. Circ Res. 2006;98:e11–e18. doi: 10.1161/01.RES.0000202692.23001.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kyriazis K, Bahlmann E, van der SH, Kuck KH. Electrical storm in Brugada syndrome successfully treated with orciprenaline; effect of low-dose quinidine on the electrocardiogram. Europace. 2009 doi: 10.1093/europace/eup070. [DOI] [PubMed] [Google Scholar]
- 116.Alings M, Dekker L, Sadee A, Wilde A. Quinidine induced electrocardiographic normalization in two patients with Brugada syndrome. Pacing Clin Electrophysiol. 2001;24:1420–1422. doi: 10.1046/j.1460-9592.2001.01420.x. [DOI] [PubMed] [Google Scholar]
- 117.Yan GX, Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST-segment elevation. Circulation. 1999;100:1660–1666. doi: 10.1161/01.cir.100.15.1660. [DOI] [PubMed] [Google Scholar]
- 118.Tsuchiya T, Ashikaga K, Honda T, Arita M. Prevention of ventricular fibrillation by cilostazol, an oral phosphodiesterase inhibitor, in a patient with Brugada syndrome. J Cardiovasc Electrophysiol. 2002;13:698–701. doi: 10.1046/j.1540-8167.2002.00698.x. [DOI] [PubMed] [Google Scholar]
- 119.Abud A, Bagattin D, Goyeneche R, Becker C. Failure of cilostazol in the prevention of ventricular fibrillation in a patient with Brugada syndrome. J Cardiovasc Electrophysiol. 2006;17:210–212. doi: 10.1111/j.1540-8167.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- 120.Matsui K, Kiyosue T, Wang JC, Dohi K, Arita M. Effects of pimobendan on the L-type Ca2+ current and developed tension in guinea-pig ventricular myocytes and papillary muscle: comparison with IBMX, milrinone, and cilostazol. Cardiovasc Drugs Ther. 1999;13:105–113. doi: 10.1023/a:1007779908346. [DOI] [PubMed] [Google Scholar]