Abstract
Background
Adiponectin, a circulating adipocyte protein, is associated with lower obesity. We have previously shown that adiponectin is present in human milk. This study determined whether higher milk adiponectin is associated with infant growth and investigated milk adiponectin's oligomeric form.
Design and Methods
This is a study of two parallel longitudinal cohorts of breastfed infants born between 1998 and 2005. Forty-five mother–infant pairs from Cincinnati, OH and 277 mother–infant pairs from Mexico City, Mexico were analyzed. All participants were healthy, term infants breastfed at least 1 month who completed 6 months of follow-up. Monthly milk samples (n = 1,379) up to 6 months were assayed for adiponectin by radioimmunoassay. Infant weight-for-age, length-for-age, and weight-for-length Z-scores up to 6 months of age were calculated using World Health Organization standards. Repeated-measures analysis was conducted. The structural form of human milk adiponectin was assessed by western blot.
Results
In the population studies, initial milk adiponectin was 24.0 ± 8.6 μg/L and did not differ by cohort. Over the first 6 months, higher milk adiponectin was associated with lower infant weight-for-age Z-score (−0.20 ± 0.04, p < 0.0001) and weight-for-length Z-score (−0.29 ± 0.08, p = 0.0002) but not length-for-age Z-score, adjusted for covariates, with no difference by cohort. By western blot, human milk adiponectin was predominantly in the biologically active high-molecular-weight form.
Conclusions
Our data suggest milk adiponectin may play a role in the early growth and development of breastfed infants.
Introduction
Obesity is a serious public health concern worldwide, with the rate of childhood obesity doubling in the United States in the past 20 years.1 Given the epidemic increases in childhood obesity, early preventive measures are needed. Breastfeeding is one such measure. Breastfeeding appears to have long-term effects on obesity, as it is associated with small but clinically meaningful decreases in childhood, adolescent, and adult obesity.2–4 Some controversy about these findings remains, however. A large randomized clinical trial to increase breastfeeding duration found no effect of breastfeeding on adiposity among 6-year olds,5 but the difference in breastfeeding behaviors by treatment group was only modest.6
Specific components of milk, which vary in concentration from mother to mother, may play a role in regulating growth and obesity risk. Human milk contains adiponectin, an adipocyte-derived hormone, in quantities consistent with a potential physiologic effect (10–20 μg/L).7–9 High levels of circulating adiponectin are associated with less obesity and diabetes, and adiponectin plays significant roles in insulin sensitivity and fat metabolism.10,11 Furthermore, adiponectin occurs in several oligomeric forms in serum,12 of which the high-molecular-weight (HMW) form appears to be the most biologically active.13,14
The objective of the present study was to determine the association between maternal milk adiponectin and infant weight and body proportionality in the first 6 months of life, the period of greatest human milk exposure. In addition, the first 6 months represents a period of rapid weight gain associated with later obesity.15 The biologic plausibility of a physiologic role of human milk adiponectin in infant growth is inferred from its oligomeric structure.
Materials and Methods
Human populations
Cincinnati. The Cincinnati Breastfeeding Cohort is a longitudinal study of breastfeeding mother–infant dyads.9,16 Subject inclusion criteria were full-term infants born ≥2.5 kg without congenital defects, at least predominant breastfeeding, and residence within 25 miles of the academic medical center. To be included in this analysis, mothers had to have a milk sample collected monthly up to 6 months postpartum. A total of 305 milk samples from 45 of 46 (98%) mothers were included in this study. The Institutional Review Board at the Cincinnati Children's Hospital Medical Center (Cincinnati, OH) approved this study, and all participant mothers provided written informed consent.
Mexico. From March 1998 to April 2003, 306 infants in San Pedro Martir, Mexico City, were enrolled and monitored prospectively from birth to 2 years of age.17 All enrolled infants were healthy, full-term infants born ≥2.2 kg without congenital defects, whose mothers intended to breastfeed. The current study included mother–infant pairs who breastfed for at least 1 month and had at least two milk samples collected between 1 week (baseline) and 6 months postpartum. Samples collected at baseline and months 1, 3, 5, and 6 were assayed. A total of 1,074 milk samples from 277 of 306 (90%) mothers were included in this study. This study was approved by the Institutional Review Boards of the National Institute of Medical Sciences and Nutrition (Mexico City, Mexico) and the Cincinnati Children's Hospital Medical Center, and all participant mothers provided written informed consent.
Data collection
Data collection was standardized between the Cincinnati and Mexico cohorts. Maternal and household characteristics, as well as infant birth and delivery characteristics, were ascertained by baseline questionnaire. Data on the infant's diet (e.g., total number of feedings of breast milk, water, formula, and other beverages or solid food) was ascertained by 24-hour recall conducted weekly in Mexico and monthly in Cincinnati. Monthly home visits were made by a trained research nurse for milk collection and anthropometric measurements.9
Anthropometric assessments
Infant birth weight was obtained by maternal report. Measurements of infant weight (±0.1 kg) and length (±0.1 cm) were collected monthly. In Cincinnati, infants were weighed in a clean diaper using a Baby Checker Scale (Medela, McHenry, IL). Supine length was measured using the Folding Lightweight Measuring Board (Hopkins Medical Products, Baltimore, MD). In Mexico, hanging scales were used to weigh infants, clothed in a clean T-shirt and clean diaper. Supine length was measured on a recumbent board, with one person stabilizing the infant's head and trunk and a second person straightening the child's legs and taking the measurement. Maternal anthropometry was collected in Cincinnati only. Maternal height was measured at the initial visit (±0.1 cm), and maternal weight was measured monthly (±0.1 kg; E-Z Carry Portable Digital Scale, Hopkins Medical Products).9
Milk collection
Milk samples were collected at home visits by a single study nurse weekly for the first month and then monthly for the duration of lactation.9 Milk collection involved draining an entire breast using an electric pump between 1000 h and 1300 h. Milk samples were stored on ice for transportation, aliquoted, and frozen at −70°C. Samples included in the current analysis experienced only a single freeze-thaw cycle.
Assay of milk adiponectin
Milk adiponectin was measured in skimmed milk by radioimmunoassay (Linco Research, St. Charles, MO) as described previously.9 Adiponectin assay kit controls had inter- and intra-assay coefficients of variation of 8.5% and 3.9%, respectively. Milk samples were assayed batched into two kit lots. Variability between kit lots was controlled by standardizing one kit lot to another using the percentage difference between 20 non-study milk samples assayed with each batch.
Calculated variables
“Percentage breastmilk” was defined as the proportion of feedings consisting of human milk during the past 24 hours. Exclusive breastfeeding for the first 4 months was calculated as a dichotomous variable, based on the World Health Organization (WHO) definition of achieving 100% of all feeds consisting of breastmilk.
Body mass index (BMI) was calculated as weight divided by the square of height (kg/m2). Infant anthropometrics were standardized to the WHO Child Growth Standards,18 and the resulting Z-scores for weight-for-age (WA), length-for-age (LA), weight-for-length (WFL), and BMI were analyzed. Prevalence of overweight (BMI Z-score ≥2), underweight (WA Z-score ≤ −2), stunting (LA Z-score ≤ −2), and wasting (WFL Z-score ≤ −2) were calculated according to WHO standards.19
Statistical analysis
All analyses were conducted using SAS version 9.1. (SAS Institute, Cary, NC). For statistical analysis, milk adiponectin values were natural log (ln) transformed for normality, but Figure 2 shows back-transformed values for ease of interpretation. Baseline comparisons between the study cohorts were achieved using unpaired Student's t tests (means), χ2 or Fisher's exact tests (categorical), or Mann-Whitney U tests (medians), as appropriate.
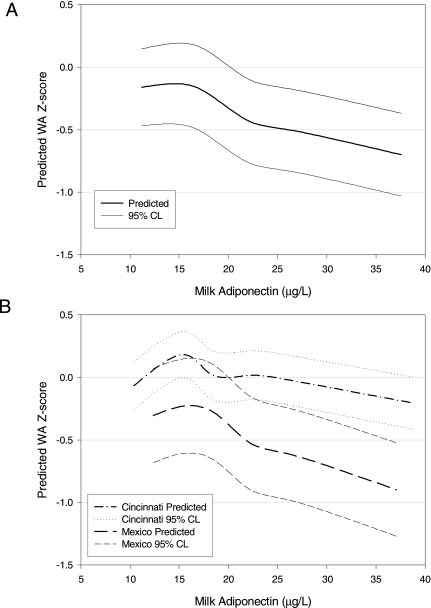
FIG. 2.
(A) Predicted WA Z-score (±95% confidence limits [CL]) from combined model, presented in Table 3. Milk adiponectin was modeled using natural log transformation and then back-transformed to original units. (B) Predicted WA Z-scores from cohort-specific models including the same covariates as the combined model.
Cross-sectional analysis was conducted for months 0 (baseline), 1, 3, 5 and 6, as these months include full data from both cohorts. Regression models were adjusted for sex, age in days, birthweight, cohort, and length (for WA Z-score) or weight (for LA Z-score).
Longitudinal analysis was conducted using repeated-measures models (SAS PROC MIXED), which account for both the intra- and inter-individual variability present in these data, resulting in a larger, and more valid, estimates of standard error.20 Each cohort was modeled separately, and then the cohorts were combined to test for common effects and interactions. Combined model results are reported throughout. The intercept and infant's growth trajectory across time (slopes) were allowed to vary by individual, while milk adiponectin and other covariates were modeled as fixed effects. A quadratic month term was used to test for a nonlinear component of growth during the first 6 months. Other covariates considered for model inclusion were: infant sex, age in days, cohort, birth weight, percentage breastmilk, exclusive breastfeeding for ≥4 months, maternal age at delivery, parity, type of delivery, maternal education, and maternal marital status. Maternal BMI was also tested in the Cincinnati cohort. Covariates were tested individually and retained in multivariate models if bivariate p ≤ 0.10. All final models, unless otherwise noted, were adjusted for a standard set of significant covariates: month, month2, sex, age in days, cohort, and birth weight.
Specific interaction terms were also tested and retained if significant, as follows. An interaction between month and cohort tested whether growth trajectory differed by cohort. An interaction of milk adiponectin by cohort tested whether the effect of milk adiponectin on anthropometry differed by cohort. Neither cohort interaction term was significant in any analysis. Determination of the final multivariate models was accomplished by comparing the Bayesian Information Criterion, with differences of at least 4 units favoring the model with the lower Bayesian Information Criterion.21 Predicted anthropometrics (e.g., WA Z-score) were calculated from the final models and plotted using the SigmaPlot (Systat Software, San Jose, CA) spline function. A value of p ≤ 0.05 was considered statistically significant.
Forms of adiponectin in milk
Human milk samples (1 mL) were centrifuged at 10,000 g for 20 minutes at 8°C, and the resulting aqueous layer was decanted from the fat layer and the pellet. For native polyacrylamide gele electrophoresis (PAGE), 13 μL of the aqueous milk preparation (e.g., 10.4 μL plus 2.6 μL of water) or 13 μL of a 1:60 dilution of serum was combined with 5 μL of 4× loading buffer and 2 μL of G250 and applied to each well of a native PAGE 3–12% Bis-Tris gel (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. The well-defined serum adiponectin bands served as standards for determining the oligomeric form of the unknown human milk adiponectin. For sodium dodecyl sulfate (SDS)-PAGE, 13 μL of aqueous milk preparation or 1:60 serum was mixed with 5 μL of 4× loading buffer and 2 μL of β-mercaptoethanol and applied to each well of a Nu-PAGE 4–12% BisTris gel. Molecular weight markers (Multimark, Invitrogen) were electrophoresed with each gel. The gels were transferred to polyvinylidene fluoride membrane and blotted against anti-human adiponectin antibody specific for the globular head region of human adiponectin (ALPCO Diagnostics, Salem, NH). The membrane was incubated in anti-rabbit tritiated secondary antibody (Cell Signaling, Beverly, MA) and overlaid with photographic film (BioMax XAR Scientific Imaging Film, Kodak, Rochester, NY), and the films were developed to visualize the bands that exhibited binding.
Results
Cohort studies
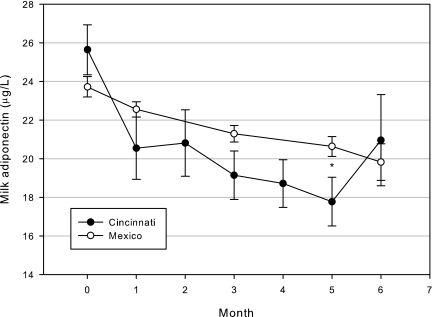
Table 1 shows cohort characteristics at baseline (1 week of age). Infants from Mexico had younger and less educated mothers (both p < 0.0001) and were shorter (p < 0.0001) and lighter (p = 0.0003) than their counterparts from Cincinnati. WFL Z-scores did not differ between the cohorts at baseline (p = 0.45). Overall, baseline milk adiponectin was 24.0 ± 8.6 μg/L and did not differ by cohort (p = 0.18). Consistent with previous analyses in subsets of these cohorts, milk adiponectin concentrations declined significantly during the first 6 months postpartum (β ± SE, −0.55 ± 0.12 μg/L milk adiponectin per month; Fig. 1), with no difference in slope by cohort (p = 0.3). Milk adiponectin was positively associated with maternal BMI in the Cincinnati cohort (β ± SE, 0.67 ± 0.30 μg/L milk adiponectin per BMI unit, p = 0.02) but not after adjusting for month and month2 (p = 0.11).
Table 1.
Baseline Characteristics by Cohort
| Characteristic | Cincinnati (n = 45) | Mexico (n = 277) | p |
|---|---|---|---|
| Maternal age (years, mean ± SD) | 32.2 ± 4.4 | 25.0 ± 5.7 | <0.0001 |
| Maternal education (n[%]) | |||
| ≤Elementary | 0 (0) | 102 (37) | |
| ≤High school | 2 (4) | 133 (48) | |
| ≤College | 24 (53) | 42 (15) | <0.0001 |
| >College | 19 (42) | 0 (0) | |
| Maternal marital status (n[%] married or common union) | 42 (93) | 161 (94) | 0.81 |
| Delivery (n[%]) | |||
| Vaginal | 34 (76) | 183 (66) | 0.21 |
| C-section | 11 (24) | 94 (34) | |
| Parity (n[%]) | |||
| 0 | 17 (38) | 90 (32) | |
| 1 | 15 (33) | 89 (32) | 0.67 |
| 2+ | 13 (29) | 98 (35) | |
| Maternal 1-month postpartum BMI (mean ± SD) | 25.4 ± 3.5 | N/A | N/A |
| Exclusive breastfeeding 4+ months (n[%]) | 27 (60) | 77 (28) | <0.0001 |
| Percentage breastmilk feeds during first 4 months (median [interquartile range]) | 100% (94.8–100%) | 98.9% (95.4–100%) | 0.03 |
| Milk adiponectin (μg/L, mean ± SD) | 25.6 ± 8.4 | 23.7 ± 8.6 | 0.18 |
| Infant sex (n[%] male) | 20 (44) | 125 (45) | 0.93 |
| Infant length (cm, mean ± SD) | 50.9 ± 2.0 | 49.6 ± 1.8 | <0.0001 |
| Birth weight (kg, mean ± SD) | 3.4 ± 0.5 | 3.2 ± 0.4 | 0.0003 |
| Infant Z-score (mean ± SD) | |||
| WA | −0.05 ± 0.9 | −0.57 ± 0.9 | 0.0003 |
| LA | 0.03 ± 1.0 | −0.71 ± 0.9 | <0.0001 |
| WFL | −0.44 ± 1.1 | −0.33 ± 0.9 | 0.45 |
| BMI | −0.11 ± 0.9 | −0.32 ± 0.9 | 0.16 |
| Growth indices (n[%]) | |||
| Overweight | 0 (0) | 0 (0) | N/A |
| Underweight | 1 (2) | 20 (7) | 0.33 |
| Stunting | 1 (2) | 19 (7) | 0.49 |
| Wasting | 3 (7) | 16 (6) | 0.73 |
N/A, not applicable.
FIG. 1.
Milk adiponectin concentrations by month and cohort. Data presented are unadjusted mean ± SE values. *p = 0.03.
The concentration of adiponectin in milk was significantly associated with lower infant WA Z-score at baseline and months 1 and 3, with a trend toward significance in month 5 (p = 0.08; Table 2). In longitudinal analysis, this association remained highly significant (β ± SE, −0.20 ± 0.04 Z-score units per μg/L milk adiponectin, p < 0.0001), adjusting for standard covariates plus length in the combined model (Table 3 and Fig. 2). This association was found in both the Cincinnati (−0.12 ± 0.05 WA Z-score units, p = 0.009) and Mexico (−0.25 ± 0.06 WA Z-score units, p < 0.0001) cohorts.
Table 2.
Cross-Sectional Associations Between Milk Adiponectin and Anthropometric Outcomes by Month
| Month 0 | Month 1 | Month 3 | Month 5 | Month 6 | |
|---|---|---|---|---|---|
| Number in model | 304 | 308 | 278 | 258 | 116 |
| WFL Z-score | −0.41 ± 0.16** | −0.78 ± 0.19** | −0.52 ± 0.20** | −0.31 ± 0.18 | −0.08 ± 0.22 |
| WA Z-score | −0.23 ± 0.08** | −0.50 ± 0.11** | −0.41 ± 0.14** | −0.24 ± 0.14 | −0.07 ± 0.17 |
| LA Z-score | 0.21 ± 0.13 | 0.31 ± 0.13* | 0.16 ± 0.14 | 0.22 ± 0.13 | 0.22 ± 0.18 |
Data are β ± SE values for the natural log of milk adiponectin from linear regression models, adjusted for sex, age in days, birth weight, cohort, and length (WA Z-score) or weight (LA Z-score).
*p ≤ 0.5, **p ≤ 0.01.
Table 3.
Final Combined Mixed Models for Standardized Outcomes
| |
Z-score |
||
|---|---|---|---|
| WA | LA | WFL | |
| Intercept | −10.7 ± 0.46** | −5.8 ± 0.33** | −1.69 ± 0.04** |
| Ln milk adiponectin (μg/L) | −0.20 ± 0.04** | 0.09 ± 0.06 | −0.29 ± 0.08** |
| Month | −0.04 ± 0.06 | −0.17 ± 0.08* | 0.50 ± 0.11** |
| Month2 | 0.03 ± 0.004** | 0.04 ± 0.005** | −0.03 ± 0.01** |
| Age (days) | −0.02 ± 0.002** | −0.02 ± 0.003* | −0.009 ± 0.004* |
| Sexa | 0.32 ± 0.04* | 0.31 ± 0.07** | 0.11 ± 0.08 |
| Birth weight (kg) | 1.26 ± 0.06** | 0.84 ± 0.08** | 0.71 ± 0.09** |
| Cohorta | −0.03 ± 0.06 | 0.37 ± 0.09** | −0.33 ± 0.11* |
| Length (cm) | 0.13 ± 0.01** | — | — |
| Weight (kg) | — | 0.55 ± 0.04** | — |
Data are β ± SE values.
Referent categories: boys, Mexico cohort.
*p ≤ 0.05, **p ≤ 0.0002.
Further, milk adiponectin concentration was associated with lower WFL Z-score at baseline and months 1 and 3, with a trend toward significance in month 5 (p = 0.08; Table 2). These results were highly significant in longitudinal mixed models (β ± SE, −0.29 ± 0.08 units per μg/L milk adiponectin, p = 0.0002), adjusting for the standard covariates (Table 3). However, milk adiponectin was not significantly associated with infant length (β ± SE, 0.19 ± 0.11 cm, p = 0.08) or LA Z-score (0.09 ± 0.06 units, p = 0.09) in longitudinal models, adjusting for covariates.
Infant weight was added to the longitudinal models for WFL Z-score to test whether the relationship of milk adiponectin concentrations with WFL Z-score could be explained by its association with infant weight (data not shown). The effect of milk adiponectin on WFL Z-score was attenuated but remained significant (−0.13 ± 0.06 units per μg/L milk adiponectin, p = 0.04), suggesting an effect of milk adiponectin on body proportionality independent of infant weight.
Forms of adiponectin in milk
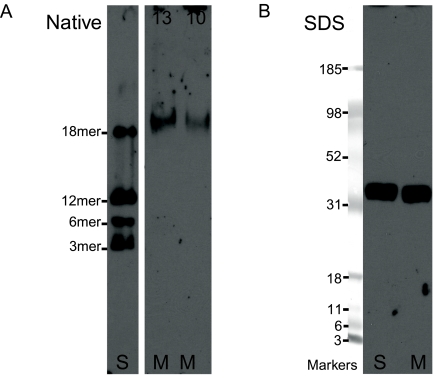
Analysis by PAGE/western blot demonstrated that human milk contains appreciable amounts of adiponectin. Western blots of native gels (Fig. 3A) indicated that the adiponectin found in human milk is primarily of the HMW octadecamer (18mer) form and provided no evidence for the intrinsic presence of monomeric, trimeric, hexameric, or dodecameric forms. In native gels of individual milk samples, milk adiponectin electrophoresed either slightly higher than or essentially similar to the HMW adiponectin oligomers of serum. Western blots of reduced adiponectin resolved on SDS-PAGE gels (Fig. 3B) indicated that both serum adiponectin and milk adiponectin reduce to subunits of a size consistent with the stable monomer.
FIG. 3.
Western blots of human milk adiponectin. (A) In native PAGE, 13 μL (13) or 10.4 μL (10) of milk (M) or serum (S) (13 μL of a 1:60 dilution) was applied to each well of a native PAGE 3–12% Bis-Tris gel. Bands were blotted and incubated with anti-adiponectin antibody and anti-rabbit secondary antibody, and overlaid photographic film was developed to visualize adiponectin bands. Human milk adiponectin is found as the 18mer, a form associated with highest activity for controlling many metabolic processes. (B) In SDS-PAGE, milk (M) or serum (S; 1:60) was applied to each well of a 4–12% gel; molecular weight markers are Multimark (Invitrogen). Both human serum adiponectin and human milk adiponectin reduce to a single band whose weight is consistent with a stable monomer.
Discussion
Consistent with adiponectin's known physiologic functions, we demonstrated that milk adiponectin is associated with lower infant adiposity by 6 months of age. In addition, we report that milk adiponectin occurs predominantly in the active HMW form. Milk adiponectin was significantly associated with lower infant WA Z-score and WFL Z-score in both the Cincinnati and Mexico City birth cohorts during the first 6 months of life. However, milk adiponectin was not associated with infant length. By the end of 6 months, the prevalence of overweight and underweight were low in our cohorts, so the association of milk adiponectin with infant growth appears to occur largely within the range of normal variation during this period.
In contrast to our findings, the only previous study of milk adiponectin and infant growth reported that higher levels of milk adiponectin measured at 6 weeks postpartum were associated with increased odds of overweight by 2 years of age, particularly in those breastfed for at least 6 months.22 Our data do not support that finding but are consistent with previous findings that circulating adiponectin is inversely associated with obesity in children.23,24 Given the concordance of evidence between our two independent cohorts, milk adiponectin levels appear to be associated with lower infant weight in the first 6 months of life. The apparently stronger cross-sectional association of milk adiponectin with infant weight in the first 3 months suggests that the effects may depend on current ingestion of milk. Follow-up of our cohorts will determine how this relationship may change between 6 months and 2 years of life.
Replication of findings between the two cohorts argues strongly against these findings being due to chance. The current study has the strength of two well-characterized international cohorts with standardized measurements and a large sample size. All of the study infants were breastfed for at least 1 month, and most were breastfed exclusively for 4–6 months, reducing heterogeneity within the study.
The results are also unlikely to be explained by confounding, as several factors potentially affecting infant development were assessed and controlled for in multivariate models. We have particularly focused on the potential for our findings to have resulted from independent physiologic events, such as declining levels of milk adiponectin and increasing weight during this time frame. First, consistency between cross-sectional and longitudinal findings reduces the likelihood of time as a significant confounder. In addition, the use of standardized growth outcomes (e.g., Z-scores) minimizes individual change over time. Sensitivity analyses conducted to control for any residual change in standardized outcomes over time did not change our results (data not shown). Thus, our results appear robust to parallel temporal changes in the variables of interest.
Although spurious and confounded explanations for these findings are unlikely, the true nature of the relationship between milk adiponectin and infant growth is unclear and may be direct (causal) or indirect (associated). The potential for any human milk protein to affect infant development directly depends on its ability to pass through the infant digestive system. The presence of human milk adiponectin as HMW octadecamers is consistent with its potential functional relevance to the infant. Many milk proteins are highly glycosylated, aiding their protection against proteolytic degradation.25,26 The HMW form of adiponectin is the most highly glycosylated form, and this glycosylation is necessary for activation of downstream pathways.27 Circulating HMW adiponectin is recognized as the form most biologically active in many aspects of metabolic control. HMW adiponectin is more strongly correlated with weight loss and circulating high-density lipoprotein cholesterol than total adiponectin13 and better predicts response to the insulin-sensitizing drugs thiazolidinediones.28 Recently, adiponectin in cord blood has been described as predominantly in the HMW form.29 Thus, the predominance of human milk adiponectin in the HMW form is consistent with both resistance to digestion and biological activity in the infant.
Another adipose-related peptide hormone, leptin, when radioactively labeled and ingested by neonatal mice, is transferred to the stomach and then to the serum.30,31 Analogously, recombinantly expressed human adiponectin fed to 2-day-old mice subsequently appears in intestinal mucosa and serum in a dose-dependent manner (D.S.N., unpublished data, 2007), supporting the possibility that adiponectin in human milk is absorbed and transported intact into serum. Preliminary analysis of our cohorts also suggests a positive correlation between milk adiponectin and infant serum adiponectin (data not shown).
Despite several study strengths, limitations should also be acknowledged. The present data do not address, and should not imply, causality, and further studies are needed to clarify the biologic implications of milk adiponectin. As the concentration of adiponectin in milk is much lower than endogenous serum levels in the infant, we recognize that the role of milk adiponectin in infant growth may be indirect. Indeed, milk adiponectin may serve as a biomarker for other factors in human milk with a physiologic impact on infant growth, such as insulin-like growth factor-1 (IGF-1) or total protein. IGF-1 is present in human milk,32 and breastfed infants have lower circulating levels of IGF-1 than formula-fed infants.33,34 Furthermore, IGF-1 has been shown to negatively regulate the expression of adiponectin in fat tissue35 and is positively associated with growth velocity in infants36 and children.37 The cohorts also do not share all data elements, limiting our ability to estimate those effects in the combined analysis. In particular, maternal BMI was collected only in the Cincinnati cohort but was not a significant predictor of infant weight. However, we and others have reported a positive relationship between maternal BMI and milk adiponectin,8,9 suggesting that adiponectin could act to limit the growth of babies born to overweight mothers.
Conclusions
Higher adiponectin concentrations in human milk are associated with significantly lower weight and leaner body proportionality over the first 6 months of life in breastfed infants. These data, in conjunction with the occurrence of human milk adiponectin predominantly in the HMW biologically active form, suggest that human milk adiponectin may have a role in early regulation of neonatal weight gain. Alternatively, adiponectin may serve as a biomarker for other human milk bioactive factors that affect weight gain. Regardless, this study supports the concept that human milk components may influence the growth of breastfed infants. Additional analysis of adiponectin and its bioactive correlates is required to clarify their respective physiologic effects.
Acknowledgments
The authors wish to acknowledge the participants of both studies as well as Ms. Barbara Davidson, Ms. Hilda Ortega-Gallegos, Ms. Luz del Carmen Mendez, Ms. Rosa Maria Garcia-Loperena, Mr. Sahle Amsalu, and Mr. Walter Banach for valuable technical assistance. We would also like to acknowledge the work of field team members in both Cincinnati and Mexico. This work was supported by grants R21-054029 (to J.G.W.) and P01-13021 (to A.L.M.) from the NICHD, NIH, grant P30-DK40561 from the NIDDK, NIH, a Cincinnati Children's Hospital Medical Center Trustee Award (to J.G.W.), and a Cincinnati Children's Hospital Medical Center Lactation Grant.
Disclosure Statement
L.J.M. and A.L.M. are listed on a U.S. patent application claiming human milk adiponectin as an oral treatment for adiposity and inflammatory disorders, and L.J.M. received a portion of a licensing fee for this technology. D.S.N. is a principal shareholder of Glycosyn, Inc., and has resolved all identified conflicts of interest that emanate from its licensing the milk adiponectin intellectual property in the U.S. patent application. The remaining authors declare no existing conflicts of interest.
References
- 1.Stein CJ. Colditz GA. The epidemic of obesity. J Clin Endocrinol Metab. 2004;89:2522–2525. doi: 10.1210/jc.2004-0288. [DOI] [PubMed] [Google Scholar]
- 2.Owen CG. Martin RM. Whincup PH, et al. Effect of infant feeding on the risk of obesity across the life course: A quantitative review of published evidence. Pediatrics. 2005;115:1367–1377. doi: 10.1542/peds.2004-1176. [DOI] [PubMed] [Google Scholar]
- 3.Gillman MW. Rifas-Shiman SL. Camargo CA, Jr, et al. Risk of overweight among adolescents who were breastfed as infants. JAMA. 2001;285:2461–2467. doi: 10.1001/jama.285.19.2461. [DOI] [PubMed] [Google Scholar]
- 4.Ip S. Chung M. Raman G, et al. Evidence Report/Technology Assessment Number 153: Breastfeeding and Maternal and Infant Health Outcomes in Developed Countries. Agency for Healthcare Research and Quality; Rockville, MD: 2007. [Google Scholar]
- 5.Kramer MS. Matush L. Vanilovich I, et al. Effects of prolonged and exclusive breastfeeding on child height, weight, adiposity, and blood pressure at age 6.5 y: Evidence from a large randomized trial. Am J Clin Nutr. 2007;86:1717–1721. doi: 10.1093/ajcn/86.5.1717. [DOI] [PubMed] [Google Scholar]
- 6.Kramer MS. Chalmers B. Hodnett ED, et al. Promotion of Breastfeeding Intervention Trial (PROBIT): A randomized trial in the Republic of Belarus. JAMA. 2001;285:413–420. doi: 10.1001/jama.285.4.413. [DOI] [PubMed] [Google Scholar]
- 7.Weyermann M. Beermann C. Brenner H, et al. Adiponectin and leptin in maternal serum, cord blood, and breast milk. Clin Chem. 2006;52:2095–2102. doi: 10.1373/clinchem.2006.071019. [DOI] [PubMed] [Google Scholar]
- 8.Bronsky J. Karpisek M. Bronska E, et al. Adiponectin, adipocyte fatty acid binding protein, and epidermal fatty acid binding protein: Proteins newly identified in human breast milk. Clin Chem. 2006;52:1763–1770. doi: 10.1373/clinchem.2005.063032. [DOI] [PubMed] [Google Scholar]
- 9.Martin LJ. Woo JG. Geraghty SR, et al. Adiponectin is present in human milk and associated with maternal factors. Am J Clin Nutr. 2006;83:1106–1111. doi: 10.1093/ajcn/83.5.1106. [DOI] [PubMed] [Google Scholar]
- 10.Arita Y. Kihara S. Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 11.Berg AH. Combs TP. Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13:84–89. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- 12.Tsao TS. Tomas E. Murrey HE, et al. Role of disulfide bonds in Acrp30/adiponectin structure and signaling specificity. Different oligomers activate different signal transduction pathways. J Biol Chem. 2003;278:50810–50817. doi: 10.1074/jbc.M309469200. [DOI] [PubMed] [Google Scholar]
- 13.Bobbert T. Rochlitz H. Wegewitz U, et al. Changes of adiponectin oligomer composition by moderate weight reduction. Diabetes. 2005;54:2712–2719. doi: 10.2337/diabetes.54.9.2712. [DOI] [PubMed] [Google Scholar]
- 14.Pajvani UB. Du X. Combs TP, et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073–9085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 15.Dennison BA. Edmunds LS. Stratton HH, et al. Rapid infant weight gain predicts childhood overweight. Obesity (Silver Spring) 2006;14:491–499. doi: 10.1038/oby.2006.64. [DOI] [PubMed] [Google Scholar]
- 16.Geraghty SR. Davidson BS. Warner BB, et al. The development of a research human milk bank. J Hum Lact. 2005;21:59–66. doi: 10.1177/0890334404273162. [DOI] [PubMed] [Google Scholar]
- 17.Guerrero ML. Morrow RC. Calva JJ, et al. Rapid ethnographic assessment of breastfeeding practices in periurban Mexico City. Bull World Health Organ. 1999;77:323–330. [PMC free article] [PubMed] [Google Scholar]
- 18.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 19.de Onis M. Onyango AW. Borghi E, et al. Comparison of the World Health Organization (WHO) Child Growth Standards and the National Center for Health Statistics/WHO international growth reference: Implications for child health programmes. Public Health Nutr. 2006;9:942–947. doi: 10.1017/phn20062005. [DOI] [PubMed] [Google Scholar]
- 20.Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. J Educ Behav Stat. 1998;24:323–355. [Google Scholar]
- 21.Raftery A. Bayesian model selection in social research. Sociol Methodol. 1995:111–195. [Google Scholar]
- 22.Weyermann M. Brenner H. Rothenbacher D. Adipokines in human milk and risk of overweight in early childhood: A prospective cohort study. Epidemiology. 2007;18:722–729. doi: 10.1097/ede.0b013e3181567ed4. [DOI] [PubMed] [Google Scholar]
- 23.Asayama K. Hayashibe H. Dobashi K, et al. Decrease in serum adiponectin level due to obesity and visceral fat accumulation in children. Obes Res. 2003;11:1072–1079. doi: 10.1038/oby.2003.147. [DOI] [PubMed] [Google Scholar]
- 24.Stefan N. Bunt JC. Salbe AD, et al. Plasma adiponectin concentrations in children: Relationships with obesity and insulinemia. J Clin Endocrinol Metab. 2002;87:4652–4656. doi: 10.1210/jc.2002-020694. [DOI] [PubMed] [Google Scholar]
- 25.van Berkel PH. Geerts ME. van Veen HA, et al. Glycosylated and unglycosylated human lactoferrins both bind iron and show identical affinities towards human lysozyme and bacterial lipopolysaccharide, but differ in their susceptibilities towards tryptic proteolysis. Biochem J. 1995;312:107–114. doi: 10.1042/bj3120107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marinaro JA. Neumann GM. Russo VC, et al. O-glycosylation of insulin-like growth factor (IGF) binding protein-6 maintains high IGF-II binding affinity by decreasing binding to glycosaminoglycans and susceptibility to proteolysis. Eur J Biochem. 2000;267:5378–5386. doi: 10.1046/j.1432-1327.2000.01575.x. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y. Xu A. Knight C, et al. Hydroxylation and glycosylation of the four conserved lysine residues in the collagenous domain of adiponectin. Potential role in the modulation of its insulin-sensitizing activity. J Biol Chem. 2002;277:19521–19529. doi: 10.1074/jbc.M200601200. [DOI] [PubMed] [Google Scholar]
- 28.Pajvani UB. Hawkins M. Combs TP, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279:12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 29.Odden N. Morkrid L. High molecular weight adiponectin dominates in cord blood of newborns but is unaffected by pre-eclamptic pregnancies. Clin Endocrinol (Oxf) 2007;67:891–896. doi: 10.1111/j.1365-2265.2007.02981.x. [DOI] [PubMed] [Google Scholar]
- 30.Casabiell X. Pineiro V. Tome MA, et al. Presence of leptin in colostrum and/or breast milk from lactating mothers: A potential role in the regulation of neonatal food intake. J Clin Endocrinol Metab. 1997;82:4270–4273. doi: 10.1210/jcem.82.12.4590. [DOI] [PubMed] [Google Scholar]
- 31.Sanchez J. Oliver P. Miralles O, et al. Leptin orally supplied to neonate rats is directly uptaken by the immature stomach and may regulate short-term feeding. Endocrinology. 2005;146:2575–2582. doi: 10.1210/en.2005-0112. [DOI] [PubMed] [Google Scholar]
- 32.Burrin DG. Is milk-borne insulin-like growth factor-I essential for neonatal development? J Nutr. 1997;127(5 Suppl):975S–979S. doi: 10.1093/jn/127.5.975S. [DOI] [PubMed] [Google Scholar]
- 33.Larnkjaer A. Ingstrup HK. Schack-Nielsen L, et al. Early programming of the IGF-I axis: Negative association between IGF-I in infancy and late adolescence in a 17-year longitudinal follow-up study of healthy subjects. Growth Horm IGF Res. 2009;19:82–86. doi: 10.1016/j.ghir.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Savino F. Fissore MF. Grassino EC, et al. Ghrelin, leptin and IGF-I levels in breast-fed and formula-fed infants in the first years of life. Acta Paediatr. 2005;94:531–537. doi: 10.1111/j.1651-2227.2005.tb01934.x. [DOI] [PubMed] [Google Scholar]
- 35.Halleux CM. Takahashi M. Delporte ML, et al. Secretion of adiponectin and regulation of apM1 gene expression in human visceral adipose tissue. Biochem Biophys Res Commun. 2001;288:1102–1107. doi: 10.1006/bbrc.2001.5904. [DOI] [PubMed] [Google Scholar]
- 36.Skalkidou A. Petridou E. Papathoma E, et al. Growth velocity during the first postnatal week of life is linked to a spurt of IGF-I effect. Paediatr Perinat Epidemiol. 2003;17:281–286. doi: 10.1046/j.1365-3016.2003.00494.x. [DOI] [PubMed] [Google Scholar]
- 37.Ong K. Kratzsch J. Kiess W, et al. Circulating IGF-I levels in childhood are related to both current body composition and early postnatal growth rate. J Clin Endocrinol Metab. 2002;87:1041–1044. doi: 10.1210/jcem.87.3.8342. [DOI] [PubMed] [Google Scholar]