Abstract
Bone marrow (BM) suppression is the important dose-limiting side effect of chemotherapy and radiotherapy for cancer. Although acute myelosuppression is an immediate concern for patients undergoing cancer therapy, its management has been improved significantly in recent years by the use of various hematopoietic growth factors. However, many patients receiving chemotherapy and/or ionizing radiation (IR) also develop residual (or long-term) BM injury (a sustained decrease in HSC reserves due to an impairment in HSC self-renewal) after the recovery from acute myelosuppression. Unlike acute myelosuppression, residual BM injury is latent and long lasting and shows little tendency for recovery. Following additional hematopoietic stress such as subsequent cycles of consolidation cancer treatment or autologous BM transplantation, residual BM injury can deteriorate to become a hypoplastic or myelodysplastic syndrome. This article review some of the new developments in elucidating the cellular and molecular mechanisms whereby chemotherapy and radiotherapy cause residual BM injury. Particularly, we discuss the role of induction of hematopoietic stem cell (HSC) senescence via the p53-p21Cip1/Waf1 and/or p16Ink4a-RB pathways in the induction of the injury and the therapeutic potential of molecularly targeting these pathways for amelioration of chemotherapy- and radiotherapy-induced long-term BM toxicity.
Keywords: ionizing radiation, chemotherapy, myelosuppression, hematopoietic stem cells, senescence
Myelosuppression is the most common dose-limiting side effect of conventional cancer therapy, particularly with certain alkylating agents and/or ionizing radiation (IR). It can lead to dose reductions and delays and adversely affect the quality of life of patients undergoing cancer treatment. For example, a recent study by Lyman et al. revealed that about a third of patients with potentially curable breast cancer received less than 85% of the minimum dose considered optimal for treatment and a quarter of the patients had treatment delayed for more than a week. Overall, this results in about 56% of the patients receiving less than 85% of the targeted dose intensity [1]. In a large portion of these cases, the dose reductions and delays may be attributable to the increased risks in developing neutropenia resulting from chemotherapy-induced bone marrow (BM) suppression. A similar finding was observed in aggressive non-Hodgkin’s lymphoma patients undergoing chemotherapy [2]. Dose reductions and delays can compromise treatment outcomes and decrease overall survival and disease-free survival. Therefore, alleviation of myelosuppression not only can improve the quality of cancer patients’ life but also has the potential to significantly increase the therapeutic efficacy of chemotherapy and radiotherapy.
The Structure of the Hematopoietic System
The hematopoietic system is organized in a hierarchical manner, in which the rare hematopoietic stem cells (HSCs) initiate the hierarchy and have the ability to self-renew, proliferate and differentiate into different lineages of peripheral blood cells though hematopoietic progenitor cells (HPCs) [3-5]. HSCs are quiescent under steady-state conditions and serve as reserves to protect the hematopoietic system from exhaustion under various stress conditions. In addition, they are more resistant to the cytotoxic effects of various chemotherapeutic agents and IR than are HPCs. In contrast, HPCs are rapidly proliferating cells with limited self-renewal ability. The proliferation and differentiation of HPCs satisfies the need of normal hematopoiesis and also allows the hematopoietic system to react swiftly and effectively to meet the demand for increasing the output of mature cells during hematopoietic crisis, such as loss of blood, hemolysis or infection. If HPCs are depleted by chemotherapy and/or IR during cancer therapy, acute myelosuppression occurs. Under this circumstance, HSCs can undergo self-renewing proliferation and differentiation to repopulate HPCs and to restore homeostasis. However, if the self-renewal ability of HSCs is impaired, a long-term or permanent damage to the hematopoietic system occurs and BM failure and death of the organism may ensue. This can occur when BM is exposed to chemotherapeutic agents that are selectively toxic to HSCs and/or to a high dose of IR [6].
Cancer therapy-induced myelosuppression
The majority of chemotherapeutic agents can cause myelosuppression in a dose-dependent manner. Among these compounds, alkylating agents, pyramidine analogs, anthracyclines, anthraquinones, nitrosoureas, methotrexate, hydroxyurea and mitomycin C are highly cytotoxic to BM [6,7]. In addition, exposure to a moderate or high dose of IR also suppresses BM hematopoietic function [6]. Acute myelosuppression occurs shortly after chemotherapy and/or IR and constitutes an immediate concern during cancer treatment. However, its management has been improved significantly in recent years by the use of hematopoietic growth factors (HGFs) such as granulocyte-colony stimulating factor (G-CSF), granulocyte/macrophage-colony stimulating factor (GM-CSF) or erythropoietin (EPO) [8]. These HGFs have the ability to promote the recovery of BM hematopoietic function primarily by stimulating HPC proliferation and differentiation.
Although many patients recover rapidly from an acute myelosuppression after chemotherapy and/or IR with or without HGF treatment, some will develop residual (or long-term) BM injury manifested by a decrease in HSC reserves and an impairment in HSC self-renewal [6,9,10]. The occurrence of long-term BM injury is more prevalent in patients and experimental animals receiving treatment with carboplatinum, busulfan (BU), bis-chloronitrosourea (BCNU) and/or total body irradiation (TBI). It is less common after receiving a single injection of agents that are relatively less cytotoxic to primitive HSCs, such as cytosine arabinoside (Ara-C), 5-fluorouracil (5-FU) or cyclophosphamide (CTX) [9,11-13]. However, repeated administration of some of these agents (such as 5-FU) can still cause significant long-term residual damage to BM [9,11-13].
Unlike acute myelosuppression, residual BM damage is latent. Patients and animals with residual BM injury usually have normal blood cell counts under normal homeostatic conditions in spite of a decrease in HSC reserves [9,10,14]. Because of this latency, the clinical implications of the residual BM injury have been largely overlooked. Moreover, the importance of long-term BM damage is further obscured by the seemingly complete recovery of peripheral blood cell counts, BM cellularity and the number of colony-forming units (CFUs) especially after the use of HGFs. In fact, the use of HGFs may worsen chemotherapy- and IR-induced residual BM damage by promoting HSC and HPC proliferation and differentiation at the expense of HSC self-renewal [12,15,16]. This could lead to an accelerated exhaustion of HSCs and further compromise the long-term recovery of BM hematopoietic function. Although residual BM damage is latent, it is long lasting and shows little tendency for recovery. It can lead to the development of hypoplastic marrow or a myelodysplastic syndrome at later times or following additional hematopoietic stress such as subsequent cycles of consolidation cancer treatment or autologus BM transplantation [9,10,14].
Residual BM injury-Mechanisms of induction
Residual BM injury is best defined by a significant reduction in HSC reserves resulting from a defect in HSC self-renewal. Early studies showed that the frequency of BM CFU-spleen (CFU-S) were reduced to 15-30% of control values in mice that had apparently recovered from acute myelosuppression long (weeks or months) after receiving TBI, BU or BCNU treatment [13,17,18]. BM cells from these mice showed severe impairment in long-term engraftment after BM transplantation as compared to the cells from control mice. In addition, BM cells from BU-treated mice supported fewer rounds of serial BM transplantation than control cells. Following mechanisms have been proposed to explain how chemotherapy and IR impair HSC self-renewal function and reduce HSC reserves: (1) quantitative reduction in HSCs due to induction of cell death or apoptosis; (2) qualitative changes in HSC replicative function resulting from induction of HSC senescence; and/or (3) damage to BM stromal cells or HSC niches that support HSC self-renewal [9,10,14,19].
(1) Induction of HSC Apoptosis
Apoptosis is an orderly and regulated form of cell death via a genetically controlled process [20,21]. In coordination with cell proliferation and differentiation, apoptosis has been assumed to contribute to the maintenance of hematopoietic homeostasis by regulating the size of hematopoietic lineages [22]. Dysregulation of apoptosis in hematopoietic cells can result in many pathological conditions [22]. Particularly, inappropriate and excessive spontaneous and activation-induced apoptosis can lead to myelosuppression and result in myelodysplasia, thrombocytopenia, leukopenia and lymphopenia. The induction of BM hematopoietic cell apoptosis, particularly in the HPC compartment, is largely responsible for chemotherapy-and IR-induced acute BM suppression [22-24]. However, the role of HSC apoptosis in chemotherapy- and IR-induced residual BM injury remains controversial. First, HSCs are quiescent cells that are relatively more resistant to the induction of apoptosis by chemotherapy and/or IR than HPCs [17,25]. Secondly, we have found that incubation of HSCs with z-VAD, a broad-spectrum caspase inhibitor, abrogates IR-induced apoptosis in HSCs, but only slightly attenuates IR-induced inhibition of HSC hematopoietic function, indicating that induction of HSC apoptosis per se is unlikely to be solely responsible for IR-induced residual BM injury [26,27]. Finally, BU, a potent inducer of residual BM injury, does not induce significant HSC apoptosis while severely diminishing HSC function, indicating that BU induces myelosuppression in an apoptosis-independent manner [26,27].
(2) Induction of HSC senescence
Cells undergo senescence after extensive replication or exposure to a genotoxic or oncogenic stress [28-30]. Although senescent cells remain metabolically active, they are no longer capable of dividing and thus are considered as non-functioning cells from a reproductive view [28-30]. It has been hypothesized that chemotherapy and IR cause residual BM injury primarily by induction of HSC senescence which impairs HSC replication and self-renewal leading to the reduction in HSC reserves [13,18,19]. Impairment in HSC self-renewal has been well documented in patients and animals after exposure to TBI or treatment with various chemotherapeutic agents that can cause residual BM injury [9,10,14,18,19,31]. For example, BM HSCs from carboplatinum-, BU-, BCNU- or IR-treated mice generated fewer CFU-S and repopulating units in lethally irradiated recipients after BM transplantation [11,31-33]. Similar impairments of HSC self-renewal capacity and long-term repopulating ability were observed in patients undergoing dose-intensified chemotherapy and autologous transplantation [34-36]. However, direct evidence to demonstrate that HSCs undergo senescence after exposure to IR or a chemotherapeutic agent was lacking until our recent studies. In these studies, we have found that BU, a potent myelosuppressive chemotherapeutic agent that causes severe long-term BM suppression, induces senescence but not apoptosis in BM hematopoietic cells in vitro [26,27]. Induction of HSC senescence also occurs after exposure to IR, another effector of residual BM injury [26,27]. The senescent HSCs induced by BU and IR have diminished clonogenic activity and express increased levels of SA-β-gal, p16Ink4a and Arf. SA-β-gal is the best-known biomaker of senescent cells and increased expression of p16Ink4a and Arf has been implicated in the establishment and maintenance of cellular senescence [30,37,38].
(3) Damage to BM stroma
A complex of cellular and molecular components constitutes the niches of BM stroma and is crucial for the maintenance of HSC self-renewal capacity and reserves [39-41]. Damage to BM stroma has been observed after exposure to IR or treatment with BU or CTX [42]. However, compared to HSCs and HPCs, BM stroma cells are relatively more resistant to chemotherapy and IR [19,19,43,44]. In particular, it has been shown that BM cells from normal mice transplanted into BU-treated mice were capable of restoring hematopoietic function to near normal levels, while transplantation of BM cells from BU-treated mice failed to do so [19,44]. Similar results were also found in BM cells from irradiated animals, which were unable to engraft as efficiently as un-irradiated cells after transplantation [19,44]. Therefore, this finding suggests that an intrinsic damage to HSCs is primarily responsible for the induction of residual BM injury by chemotherapy and IR and that the damage to BM stroma plays only a minor role in this event.
In summary, the studies discussed above suggest that induction of HSC senescence may play a key role in chemotherapy and IR-induced long-term BM injury. In contrast, damage to BM stroma appears less important to the residual BM injury as compared to HSC damage by chemotherapy and IR. However, the role of HSC apoptosis in chemotherapy- and/or IR-induced residual BM injury has yet to be clarified.
HSC Senescence
(1) Cellular Senescence
In the early 1960s, Hayflick and Moorhead demonstrated that normal human diploid fibroblasts (HDFs) have a finite growth potential [45]. After cells have undergone an intrinsically defined number of cell doublings (about 50-70), they enter into an irreversible or permanent growth-arrest state that is termed replicative senescence [28,29]. Senescent cells are incapable of dividing yet remain metabolically active [28-30]. They exhibit some unique changes in cellular morphology and biochemistry, including an enlarged and flattened appearance, increased SA-β-gal activity and elevated expression of the proteins encoded by the Ink4a-Arf locus [28-30]. The intrinsic replicative lifespan of a cell appears to be determined by telomere length [28]. Telomeres are the repetitive DNA sequences that protect the ends of chromosomes and keep them from being treated like a piece of broken DNA [28,46,47]. Without the expression of telemorase, telomeric sequences shorten each time DNA replicates in most somatic cells [28]. When the telomeres reach a critically short length (about 4kb) after a certain number of cell doublings, cells stop dividing and are irreversibly arrested at the G1 phase of the cell cycle to enter replicative senescence [28,29]. Replicative senescence has been considered a tumor suppressive mechanism. Alternatively, it has been regarded as an underlying cellular mechanism of organismal aging. The occurrence of replicative senescence has been demonstrated for most cell types, with a few exceptions including embryonic stem cells and the majority of tumor-derived cell lines [28,29]. This is because these cells express high levels of telomerase. A moderate level of telomerase activity is detectable in HSCs [48-51]. This activity is needed to maintain the normal function of HSCs, since a deficiency in telomerase activity can lead to telomere shortening and reduction in HSC transplantation ability as seen in late-generations of telomerase RNA component (TERC) null mice [51]. In addition, the development of aplastic anemia or marrow failure has been found in patients with telomerase deficiency due to mutation in telomerase reverse transcriptase (TERT) or TERC [52]. However, whether HSCs have a finite cell replicative ability and undergo replicative senescence after extensive proliferation remains intensely debated [53,54].
In addition, many types of cells undergo senescence after exposure to a genotoxic stress that causes DNA damage (such as IR, UV, free radicals, or a chemotherapeutic agent) [29,30]. This also occurs when cells are subjected to an oncogenic stress that results in aberrant regulation of cell proliferation [30]. Cells undergoing stress-induced senescence have a shortened intrinsic replicative lifespan without significant erosion in telomeres. However, cells undergoing premature senescence are morphologically indistinguishable from replicatively senescent cells and exhibit many of the characteristics ascribed to replicatively senescent cells [28-30]. Moreover, premature and replicative senescence share common pathways for their induction (discussed below) [29,30]. A variety of normal cells and various tumor cells exhibit a senescent phenotype after exposure to IR or treatment with a chemotherapeutic agent in vitro and in vivo, indicating that induction of premature senescence is a general cellular response to these treatments [55-60].
(2) HSC senescence
HSCs are distinguished from other somatic cells by their ability to self-renew, proliferate and differentiate into various lineages of blood cells. The potential of HSC self-renewal appears enormous since HSCs have the capacity to sustain the lifelong production of mature blood cells under steady-state conditions and repeatedly repopulate lethally irradiated recipients for up to four rounds of serial BM transplantation. However, the self-renewing ability of HSCs may not be unlimited, since HSCs eventually fail to engraft and repopulate lethally irradiated recipients after the forth or fifth serial BM transplantation. Therefore, it has been hypothesized that HSCs also have a finite cell replicative ability and can undergo replicative senescence or exhaustion after forced extensive proliferation, such as that caused by serial transplantation [53,54]. However, whether HSCs have a finite replicative capacity and undergo replicative senescence after serial BM transplantation remains intensely debated because several findings undermine the notion. First, it has been shown that a single retrovirally marked stem cell clone is capable of multiple lineage reconstitution of BM for over a year and that transplantation of a single HSC is capable of long-term repopulation of the host’s ablated hematopoietic system. These observations demonstrate a seemingly unlimited potential of HSC self-renewal [61,62]. Second, calculations of HSC self-renewal based on the competitive repopulating unit have shown that serial transplantation does not reduce the ability of HSC self-renewal as previously suggested, because the number of long-term repopulating HSCs increases constantly during serial BM transplantation without any sign of lessening [63]. In addition, over expression of TERT in HSCs maintains the length of HSC telomeres but fails to extend HSC transplantation capacity [64]. Taken together, these findings imply that, at least in mice, the decline in HSC reproductive capacity following serial BM transplantation is likely not a result of telomere erosion and the induction of HSC replicative senescence, but rather attributable to HSC premature senescence associated with excessive stress generated by multiple rounds of BM transplantation procedures. However, extrapolation of this finding from mice into humans requires caution, because the telomeres of human cells are significantly shorter than those of mouse cells [50].
Similarly, it has been hypothesized that long-term BM injury induced by IR and/or chemotherapy is attributable to the induction of HSC replicative senescence resulting from severe depletion of HSCs [32,33,65]. This hypothesis is inconsistent with the observations discussed above which fail to demonstrate that HSCs have a finite ability to self-renew and can undergo replicative senescence or exhaustion after extensive replication. Alternatively, the residual effects of chemotherapy and IR on HSCs may be attributed to the induction of HSC premature senescence. This hypothesis is supported by recent findings in our and other laboratories, showing that HSCs undergo senescence after exposure to IR [27], but without significant changes in telomere length [49]. In addition, a growing body of evidence demonstrates that HSCs can lose their ability to self-renew by undergoing premature senescence. For example, mice lacking the Bmi1 gene develop progressive BM hypoplasia and die early (< 2 months) after birth [66]. Although Bmi1-/- mice have a normal pool of fetal liver HSCs, transplantation of their fetal liver HSCs to a lethally irradiated recipient results only in a transient reconstitution of the hematopoietic system [66]. This suggests that the mutated fetal liver HSCs have the ability to proliferate and differentiate into HPCs for transient BM reconstitution but cannot self-renew and generate HSCs to ensure long-term hematopoietic engraftment. Deficiency in self-renewal was also found in neural and leukemia stem cells that lack Bmi1, indicating that Bmi1 is a general regulator of stem cell self-renewal [67,68]. Bmi1 is a member of the Polycomb group of transcriptional repressors. Its downstream targets include the gene products of the Ink4a/Arf locus, e.g. p16Ink4a and Arf. HSCs from Bmi1-/- mice express increased levels of p16Ink4a and Arf [66]. Enforced expression of p16Ink4a and Arf in HSCs induces cell cycle arrest and apoptosis, respectively, whereas p16Ink4a knockout partially restores the ability of Bmi1-/- stem cells to self-renew [66,68]. These findings demonstrate that Bmi1 promotes HSC self-renewal in part by preventing the cells from undergoing premature senescence via inhibition of p16Ink4a expression. Similarly, it has been reported recently that ATM-/- mice have a defect in HSC self-renewal and exhibit a progressive decline in HSCs with age [69]. This defect is corrected by inhibition of reactive oxygen species (ROS) production using an antioxidant, down-regulation of p16Ink4a expression with Bmi1, or suppression of the p16Ink4a-Rb pathways by human papillomavirus (HPV) protein E7. However, overexpression of TERT in ATM-/- HSCs could not correct the deficiency [69]. These findings indicate that ATM regulates HSC self-renewal via a telomere-independent mechanism, probably by inhibiting ROS production and p16Ink4a expression to prevent HSCs from undergoing premature senescence.
Interestingly, a shortening of the intrinsic replicative capacity of HSCs or loss of HSC self-renewal in all the conditions discussed above (including IR) does not affect HSC differentiation to generate various HPCs and more mature progeny prior to their final exhaustion [66,69]. Moreover, HPCs from irradiated mice and Bmi1-/- or ATM-/- mice showed no abnormalities nor did they exhibit any senescent changes [66,69]. These findings indicate that hematopoietic cell senescence mainly occurs at the level of HSCs.
Pathways Leading to Senescence Induction
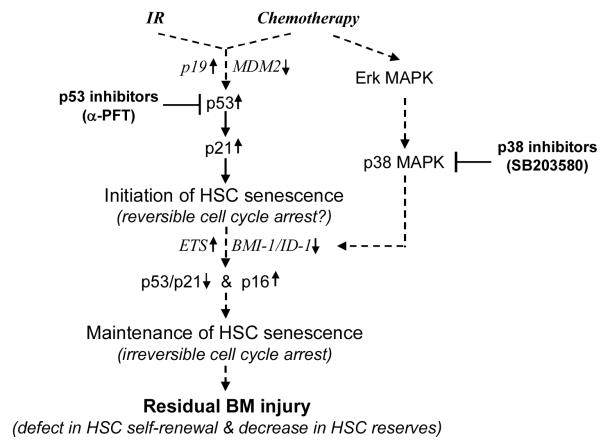
Two major pathways have been implicated in the induction of both replicative and premature senescence (Fig. 1). These are (1) the p53-p21Cip1/Waf1 pathway triggered by DNA damage or telomere shortening and (2) the p16Ink4a-Rb pathway activated by the Ras-Raf-MEK-Erk/p38 MAPK cascade [28-30,70]. Activation of either pathway is capable of inducing senescence. However, extensive cross talk exists at multiple levels between these two pathways. Frequently, the two pathways work in concert to induce replicative and premature senescence.
Fig. 1.
Hypothetic cellular and molecular mechanisms whereby IR and chemotherapy induce residual BM injury and potential novel therapeutic strategies.
(1) The p53-p21Cip1/Waf1 pathway
p53 is a tumor suppressor that functions as a universal sensor of genotoxic stress or DNA damage (including telomere shortening) [28,71,72]. Normally, p53 is associated with its negative regulator MDM2 which ubiquitinates p53 and then targets it for degradation by the proteasomes. When DNA is damaged by UV, ROS, IR or chemotherapeutic agents, ATM/ATR/DNA-PK/JNK are activated leading to p53 phosphorylation at its N-terminus by these kinases [71,72]. In addition, critically shortened telomeres in replicatively senescent cells can also be recognized as damaged DNA to cause p53 phosphorylation [28,29]. The phosphorylation of p53 leads to its stabilization via disruption of the MDM2–p53 interaction [71-74]. In addition, Arf can directly bind to MDM2 and cause the accumulation of p53 by segregating MDM2 from p53 and inhibiting MDM2’s E3 ubiquitin protein ligase activity for p53 [38,75,76]. Therefore, an increase in Arf expression also causes elevation of p53. Activation of the transactivation activity of p53 is further facilitated through the phosphorylation–acetylation cascade at the p53 C-terminus by CK2/TFII-H/p300/CBP [71,72]. Phosphorylated and acetylated p53 induces the transcription of various down-stream proteins, which leads to either cellular apoptosis or senescence depending on which down-stream proteins are induced [71,72]. The transcription of Bax and/or other proapoptotic proteins by p53 leads to apoptosis, while elevated p21Cip1/Waf1 expression induced by p53 causes cell cycle arrest and senescence [71,72]. The role for p53-p21Cip1/Waf1 in senescence is well established by numerous [28-30]. However, activation of p53 and p21Cip1/Waf1 in cells undergoing senescence is transient [77,78]. Increased expression of p53 and p21Cip1/Waf1 usually occurs during the onset of senescence and then subsides when the expression of p16Ink4a starts rising [77-79]. Before p16Ink4a up-regulation, inactivation of p53 can prevent senescence induction in some cells. However, once p16Ink4a is highly expressed, cell cycle arrest becomes irreversible simply by down regulation of p53 [79,80]. This suggests that p53 and p21Cip1/Waf1 play an important role in the initiation of senescence, but may not be required for the maintenance of senescence.
In agreement with this suggestion, we showed that IR-induced activation of p53 and up-regulation of p21Cip1/Waf1 occurred prior to the increased expression of p16Ink4a and Arf in murine BM HSCs [27]. While p53 activation and p21Cip1/Waf1 up-regulation gradually declined within a few weeks after IR, p16Ink4a and Arf expression remained elevated and the cells became positive for SA-β-gal staining, indicating that they had become senescent. Interestingly, the induction of premature senescence in HSCs by BU is not associated with activation of p53 and up-regulation of p21Cip1/Waf1, suggesting that IR induces HSC senescence in a p53-p21Cip1/Waf1-dependent manner while BU bypasses the p53-p21Cip1/Waf1 pathway [27]. It has yet to be determined if other chemotherapeutic agents also induce HSC senescence in an agent-specific manner via a p53-p21Cip1/Waf1-dependent or independent mechanism and whether inactivation of p53 by a pharmacological agent can prevent HSC senescence induced by IR and certain chemotherapeutic agents.
(2) The p16Ink4a-Rb pathway
The Ink4a-Arf locus encodes two tumor suppressors, p16Ink4a and Arf (p19Arf in mouse and p14Arf in human) [38,75]. The transcripts for these proteins have different first exons (α for p16Ink4a and β for Arf) but share exons 2 and 3. However, there is no amino acid sequence similarity between these two proteins due to the use of alternative reading frames for their translation [38,75]. The regulation of p16Ink4a and Arf transcription has not been well established. Their expression is up-regulated by transfection with oncogenic Ras. Activation of Erk/p38 MAPK appears to act downstream of Ras [38,75]. It was shown that high intensity of Ras activation stimulates sustained and high levels of Erk activity, which in turn leads to the activation of p38 MAPK and up-regulation of p16Ink4a [81]. Constitutive activation of p38 MAPK induces senescence via the p16Ink4a-Rb pathway, whereas inhibition of p38 MAPK activity attenuates Ras-induced cellular senescence [81,82]. Similarly, we have found that Erk or p38 MAPK inhibitor can affectively attenuate BU-induced senescence in HDFs, while inhibition of JNK has no significant effect (unpublished results). These results indicate that Erk/p38 MAPKs play a key role in the induction of cellular senescence by oncogenic stress and certain chemotherapeutic agents, such as BU. However, the signaling pathway through which p38 up-regulates p16Ink4a has yet to be elucidated. The positive and negative transcriptional factors that may be involved in p16Ink4a transcription include the Jun, Ets, Id, and BMI families [60,75]. Ras-induced Arf expression may be attributed to an up-regulation of the transcriptional factor DMP1 and/or down-regulation of JunD and TBX2 [60,75]. In addition, increased expression of p16Ink4a and/or Arf occurs following the initiation of senescence by activation of p53 in cells undergoing extensive replication or DNA damage [30,60,75]. However, the mechanisms of p16Ink4a and/or Arf induction in these situations are unknown.
p16Ink4a binds to cyclin-dependent kinases (CDK) 4/6 to prevent their interaction with cyclin D and thus, inhibits CDK4/6 activity [75]. This leads to hypophosphorylation of Rb and the formation of senescence-associated heterochromatic foci (SAHF), which in turn decreases the expression of E2F-dependent genes and restricts G1/S cell cycle progression [83]. Once SAHF are formed after the engagement of the p16Ink4a-Rb pathway, the growth arrest of senescent cells becomes permanent and cannot be reversed by subsequent inactivation of p53 [80,83]. This indicates that diverse stimuli can induce cellular senescence via various up-stream signal transduction cascades but eventually converge on the p16Ink4a-Rb pathway whose activation provides an inescapable obstacle to prevent senescent cells from re-entering the cell cycle. The observations supporting a key role for p16Ink4a in senescence are abundantly documented [30,38,75,84,85].
In contrast, Arf functions as a cell cycle negative regulator and tumor suppressor mainly by interacting with MDM2 and the p53-p21Cip1/Waf1 pathway [38,75,76]. Therefore, activation of p53 by Arf can induce not only cellular senescence but also apoptosis, depending on which gene down-stream of p53 is induced following p53 activation. In addition, Arf can also induce cell growth arrest in a p53-independent manner, which is less clearly defined [86].
Since both p16Ink4a and Arf are involved in cellular senescence, their relative importance to one another in the induction of senescence has generated some controversy [75,84,85,87,88]. For example, MEFs from Ink4a/Arf null mice which lack expression of both p16Ink4a and Arf due to the deletion of Ink4a/Arf exons 2 and 3, are immortal and unable to undergo senescence after exposure to oncogenic stress induced by Ras [84]. In contrast, MEFs from Ink4a null or mutated mice are relatively normal as they undergo replicative senescence and resist transformation by oncogonic Ras [85,88]. However, MEFs from Arf null mice exhibit the same phenotype as those from Ink4a/Arf null mice, indicating that Arf plays a more important role than p16Ink4a in induction of cellular senescence in MEFs [84,85,87]. In contrast, in HDFs, p16Ink4a seems to have a more preeminent role than Arf in both replicative senescence and Ras-induced premature senescence [30,75,85]. Moreover, even in the mouse, different cell types may utilize either p16Ink4a or Arf for the induction of senescence. For instance, only Arf is required to impose replicative arrest in mouse BM pre-B cells whereas both p16Ink4a and Arf provide an effective barrier to the unlimited growth of mouse BM-derived macrophages [89]. These results suggest that senescence is a tightly regulated process that may require different proteins and pathways for its induction in a cell type-dependent manner.
Increased expression of p16Ink4a and Arf has been found in Bmi1-/- HSCs [66]. The expression of p16Ink4a is important for the induction of Bmi1-/- HSC senescence, while the expression of Arf contributes to the induction of Bmi1-/- HSC apoptosis via the p53-mediated apoptotic machinery [66]. This suggests that the expression of both proteins can lead to the impairment of HSC self-renewal. Indeed, it was found that HSCs/HPCs from Ink4a/Arf null mice exhibited a significant increase in clonal expansion in vitro but a modest increase in HSC self-renewal in vivo [90,91]. However, lack of Arf alone did not provide any advantage for HSC/HPCs expansion and self-renewal, indicating that p16Ink4a may play a more significant role in regulation of HSC self-renewal than Arf [91]. This suggestion is in agreement with the findings in ATM mutated mice [69,91]. It was shown that the mutation of the ATM gene also resulted in up-regulation of p16Ink4a and Arf in HSCs. Inactivation of the p16Ink4a-Rb pathway by retroviral transfection of HPV E7 proteins restored the reproductive function of ATM-/- HSCs, while inhibition of the Arf-p53 pathway by E6 transfection had no such effect, indicating that p16Ink4a may play a more important role than Arf in the loss of ATM-/- HSC self-renewal. Increased expression of p16Ink4a and Arf has been found in senescent HSCs induced by IR and BU [27]. However, it has yet to be determined which of these proteins is more important for chemotherapy- and IR-induced HSC premature senescence.
Implication for cancer therapy
The cellular responses to chemotherapy and IR include apoptosis and senescence. In a multicellular organism, these responses act synergistically to eliminate damaged cells and those that are predisposed to becoming cancerous. However, these responses must be tightly controlled to ensure optimal survival for the organism, e.g. eliminating cells with a high risk of cancer while preserving a subset of stem cells necessary for adequate tissue repair. This is of particular importance in the maintenance of hematopoietic homeostasis since failure to eliminate damaged cells may lead to hematopoietic malignancy, while excessive cellular destruction of HPCs and HSCs will lead to acute myelosuppression and residual BM injury. In this regard, p53 and p16Ink4a may work as a “double-edged sword” at the center of this regulation. If p53 activation is dysregulated or the cells are incapable of terminating p53 activation after DNA repair, it could be detrimental. For example, it has been found that “super” p53 (p53+/m) mice which express a mutant but constitutively active form of p53, have a shortened life span [92]. It has been suggested that this constitutive activation gradually depletes adult tissue stem cells, leading to accelerated senescence or aging. Likewise, we hypothesize that the inability to appropriately regulate and terminate p53 activation and its down-stream events in HSCs may make these cells extremely sensitive to chemotherapy- and IR-induced senescence and lead to residual BM injury. Similarly, the p16Ink4a-Rb pathway functions as an alternative safeguard that inhibits tumorigenesis by induction of senescence in cells affected by genotoxic or oncogenic stress. However, if this pathway is overly activated or improperly regulated, it can also lead to stem cell exhaustion and premature aging [86]. As such, we believe that the inability to properly regulate the activity of the p16Ink4a-Rb pathway may be partially responsible for IR- and chemotherapy-induced HSC senescence and residual BM injury. Therefore, targeted inhibition of the p53-p21Cip1/Waf1 and/or p16Ink4a-Rb pathways may be exploitable as innovative strategies to reduce chemotherapy- and IR-induced BM toxicity if the inhibition does not increase the risk of hematopoietic malignancy (Fig. 1). This may be achievable by inhibiting p53 and p38 with α-PFT and SB203580, respectively.
Overall, this review summarizes some of the new developments in elucidating the cellular and molecular mechanisms whereby chemotherapy and IR cause residual BM injury. A better understanding of the mechanisms will allow us to develop new interventions to circumvent chemotherapy- and IR-induced long-term BM toxicity. This will result in a significant reduction in the potentially life threatening long-term effects of conventional cancer therapy on the hematopoietic system, increase the compliance of cancer patients to subsequent consolidation cancer treatments and facilitate long-term engraftment and recovery of hematopoietic function following autologous and allogeneic BM transplantation.
Acknowledgements
This study was supported in part by grants from the National Institutes of Health (R01CA102558 and R01CA86688) and a grant (GC-3319-05-4498CM) from the Department of Defense through the Hollings Cancer Center to Dr. Daohong Zhou. Dr. Yong Wang is a recipient of the Abney Foundation Scholarship.
References
- [1].Lyman GH, Dale DC, Crawford J. Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: a nationwide study of community practices. J Clin Oncol. 2003;21:4524–4531. doi: 10.1200/JCO.2003.05.002. [DOI] [PubMed] [Google Scholar]
- [2].Lyman GH, Dale DC, Friedberg J, Crawford J, Fisher RI. Incidence and predictors of low chemotherapy dose-intensity in aggressive non-Hodgkin’s lymphoma: a nationwide study. J Clin Oncol. 2004;22:4302–4311. doi: 10.1200/JCO.2004.03.213. [DOI] [PubMed] [Google Scholar]
- [3].Reya T. Regulation of hematopoietic stem cell self-renewal. Recent Prog Horm Res. 2003;58:283–295. doi: 10.1210/rp.58.1.283. [DOI] [PubMed] [Google Scholar]
- [4].Smith C. Hematopoietic stem cells and hematopoiesis. Cancer Control. 2003;10:9–16. doi: 10.1177/107327480301000103. [DOI] [PubMed] [Google Scholar]
- [5].Weissman IL, Anderson DJ, Gage F. Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu Rev Cell Dev Biol. 2001;17:387–403. doi: 10.1146/annurev.cellbio.17.1.387. [DOI] [PubMed] [Google Scholar]
- [6].Mauch P, Constine L, Greenberger J, et al. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys. 1995;31:1319–1339. doi: 10.1016/0360-3016(94)00430-S. [DOI] [PubMed] [Google Scholar]
- [7].Marsh JC. The effects of cancer chemotherapeutic agents on normal hematopoietic precursor cells: a review. Cancer Res. 1976;36:1853–1882. [PubMed] [Google Scholar]
- [8].Dempke W, Von Poblozki A, Grothey A, Schmoll HJ. Human hematopoietic growth factors: old lessons and new perspectives. Anticancer Res. 2000;20:5155–5164. [PubMed] [Google Scholar]
- [9].Gale RP. Myelosuppressive effects of antineoplastic chemotherapy. In: Testa NG, Gale RP, editors. Hematopoiesis: Long-term effects of chemotherapy and radiation. Marcel Dekker, Inc.; New York: 1988. pp. 63–73. [Google Scholar]
- [10].Testa NG, Hendry JH, Molineux G. Long-term bone marrow damage in experimental systems and in patients after radiation or chemotherapy. Anticancer Res. 1985;5:101–110. [PubMed] [Google Scholar]
- [11].Gardner RV, Lerner C, Astle CM, Harrison DE. Assessing permanent damage to primitive hematopoietic stem cells after chemotherapy using the competitive repopulation assay. Cancer Chemother Pharmacol. 1993;32:450–454. doi: 10.1007/BF00685889. [DOI] [PubMed] [Google Scholar]
- [12].van Os R, Robinson S, Sheridan T, Mislow JM, Dawes D, Mauch PM. Granulocyte colony-stimulating factor enhances bone marrow stem cell damage caused by repeated administration of cytotoxic agents. Blood. 1998;92:1950–1956. [PubMed] [Google Scholar]
- [13].Neben S, Hellman S, Montgomery M, Ferrara J, Mauch P, Hemman S. Hematopoietic stem cell deficit of transplanted bone marrow previously exposed to cytotoxic agents. Exp Hematol. 1993;21:156–162. [PubMed] [Google Scholar]
- [14].Lohrmann HPE, Schreml W. Long-term hematopoietic damage after cytotoxic drug therapy for solid tumors. In: Testa NG, Gale RP, editors. Hematopoiesis: Long-term effects of chemotherapy and radiation. Marcel Dekker, Inc.; New York: 1988. pp. 325–337. [Google Scholar]
- [15].Gardner RV, Begue R, McKinnon E. The effect of granulocyte-macrophage colony-stimulating factor (GM-CSF) on primitive hematopoietic stem cell (PHSC) function and numbers, after chemotherapy. Exp Hematol. 2001;29:1053–1059. doi: 10.1016/s0301-472x(01)00685-3. [DOI] [PubMed] [Google Scholar]
- [16].van Os R, Robinson S, Sheridan T, Mauch PM. Granulocyte-colony stimulating factor impedes recovery from damage caused by cytotoxic agents through increased differentiation at the expense of self-renewal. Stem Cells. 2000;18:120–127. doi: 10.1634/stemcells.18-2-120. [DOI] [PubMed] [Google Scholar]
- [17].Botnick LE, Hannon EC, Vigneulle R, Hellman S. Differential effects of cytotoxic agents on hematopoietic progenitors. Cancer Res. 1981;41:2338–2342. [PubMed] [Google Scholar]
- [18].Mauch P, Rosenblatt M, Hellman S. Permanent loss in stem cell self renewal capacity following stress to the marrow. Blood. 1988;72:1193–1196. [PubMed] [Google Scholar]
- [19].Hellman S, Botnick LE. Stem cell depletion: an explanation of the late effects of cytotoxins. Int J Radiat Oncol Biol Phys. 1977;2:181–184. doi: 10.1016/0360-3016(77)90028-1. [DOI] [PubMed] [Google Scholar]
- [20].Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995;146:3–15. [PMC free article] [PubMed] [Google Scholar]
- [22].Wickremasinghe RG, Hoffbrand AV. Biochemical and genetic control of apoptosis: relevance to normal hematopoiesis and hematological malignancies. Blood. 1999;93:3587–3600. [PubMed] [Google Scholar]
- [23].Dainiak N. Hematologic consequences of exposure to ionizing radiation. Exp Hematol. 2002;30:513–528. doi: 10.1016/s0301-472x(02)00802-0. [DOI] [PubMed] [Google Scholar]
- [24].Domen J. The role of apoptosis in regulating hematopoiesis and hematopoietic stem cells. Immunol Res. 2000;22:83–94. doi: 10.1385/IR:22:2-3:83. [DOI] [PubMed] [Google Scholar]
- [25].Ploemacher RE, van Os R, van Beurden CA, Down JD. Murine haemopoietic stem cells with long-term engraftment and marrow repopulating ability are more resistant to gamma-radiation than are spleen colony forming cells. Int J Radiat Biol. 1992;61:489–499. doi: 10.1080/09553009214551251. [DOI] [PubMed] [Google Scholar]
- [26].Meng A, Wang Y, Brown SA, Van Zant G, Zhou D. Ionizing radiation and busulfan inhibit murine bone marrow cell hematopoietic function via apoptosis-dependent and -independent mechanisms. Exp Hematol. 2003;31:1348–1356. doi: 10.1016/j.exphem.2003.08.014. [DOI] [PubMed] [Google Scholar]
- [27].Meng A, Wang Y, Van Zant G, Zhou D. Ionizing radiation and busulfan induce premature senescence in murine bone marrow hematopoietic cells. Cancer Res. 2003;63:5414–5419. [PubMed] [Google Scholar]
- [28].Campisi J, Kim SH, Lim CS, Rubio M. Cellular senescence, cancer and aging: the telomere connection. Exp Gerontol. 2001;36:1619–1637. doi: 10.1016/s0531-5565(01)00160-7. [DOI] [PubMed] [Google Scholar]
- [29].Marcotte R, Wang E. Replicative senescence revisited. J Gerontol A Biol Sci Med Sci. 2002;57:B257–B269. doi: 10.1093/gerona/57.7.b257. [DOI] [PubMed] [Google Scholar]
- [30].Serrano M, Blasco MA. Putting the stress on senescence. Curr Opin Cell Biol. 2001;13:748–753. doi: 10.1016/s0955-0674(00)00278-7. [DOI] [PubMed] [Google Scholar]
- [31].van Os R, Robinson S, Sheridan T, Mislow JM, Dawes D, Mauch PM. Granulocyte colony-stimulating factor enhances bone marrow stem cell damage caused by repeated administration of cytotoxic agents. Blood. 1998;92:1950–1956. [PubMed] [Google Scholar]
- [32].Mauch P, Rosenblatt M, Hellman S. Permanent loss in stem cell self renewal capacity following stress to the marrow. Blood. 1988;72:1193–1196. [PubMed] [Google Scholar]
- [33].Neben S, Hellman S, Montgomery M, Ferrara J, Mauch P. Hematopoietic stem cell deficit of transplanted bone marrow previously exposed to cytotoxic agents. Exp Hematol. 1993;21:156–162. [PubMed] [Google Scholar]
- [34].Cartron G, Herault O, Benboubker L, et al. Quantitative and qualitative analysis of the human primitive progenitor cell compartment after autologous stem cell transplantation. J Hematother Stem Cell Res. 2002;11:359–368. doi: 10.1089/152581602753658547. [DOI] [PubMed] [Google Scholar]
- [35].Domenech J, Linassier C, Gihana E, et al. Prolonged impairment of hematopoiesis after high-dose therapy followed by autologous bone marrow transplantation. Blood. 1995;85:3320–3327. [PubMed] [Google Scholar]
- [36].Domenech J, Cartron G, Clement N, et al. Persistent decrease in proliferative potential of marrow CD34(+)cells exposed to early-acting growth factors after autologous bone marrow transplantation. Bone Marrow Transplant. 2002;29:557–562. doi: 10.1038/sj.bmt.1703512. [DOI] [PubMed] [Google Scholar]
- [37].Dimri GP, Lee X, Basile G, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sharpless NE, DePinho RA. The INK4A/ARF locus and its two gene products. Curr Opin Genet Dev. 1999;9:22–30. doi: 10.1016/s0959-437x(99)80004-5. [DOI] [PubMed] [Google Scholar]
- [39].Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- [40].Suda T, Arai F, Hirao A. Hematopoietic stem cells and their niche. Trends Immunol. 2005;26:426–433. doi: 10.1016/j.it.2005.06.006. [DOI] [PubMed] [Google Scholar]
- [41].Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- [42].Testa NG, Hendry J, Molineux G. Long-term bone marrow damage after cytotoxic treatment: stem cells and microenvironment. In: Testa NG, Gale RP, editors. Hematopoiesis: Long-term effects of chemotherapy and radiation. Marcel Dekker; New York: 1988. pp. 75–91. [Google Scholar]
- [43].Halka KG, Caro J, Erslev AJ. Long-term marrow cultures from mice with busulfan-induced chronic latent aplasia. J Lab Clin Med. 1987;109:698–705. [PubMed] [Google Scholar]
- [44].Morley A, Trainor K, Blake J. A primary stem cell lesion in experimental chronic hypoplastic marrow failure. Blood. 1975;45:681–688. [PubMed] [Google Scholar]
- [45].Hayflick L, Moorhead PS. The limited in vitro lifetime of human diploid cell strains. Experimental Cell Research. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- [46].Lo AW, Sprung CN, Fouladi B, et al. Chromosome instability as a result of double-strand breaks near telomeres in mouse embryonic stem cells. Mol Cell Biol. 2002;22:4836–4850. doi: 10.1128/MCB.22.13.4836-4850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Sprung CN, Reynolds GE, Jasin M, Murnane JP. Chromosome healing in mouse embryonic stem cells. Proc Natl Acad Sci U S A. 1999;96:6781–6786. doi: 10.1073/pnas.96.12.6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Allsopp RC, Morin GB, DePinho R, Harley CB, Weissman IL. Telomerase is required to slow telomere shortening and extend replicative lifespan of HSCs during serial transplantation. Blood. 2003;102:517–520. doi: 10.1182/blood-2002-07-2334. [DOI] [PubMed] [Google Scholar]
- [49].Goytisolo FA, Samper E, Martin-Caballero J, et al. Short telomeres result in organismal hypersensitivity to ionizing radiation in mammals. J Exp Med. 2000;192:1625–1636. doi: 10.1084/jem.192.11.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Greenwood MJ, Lansdorp PM. Telomeres, telomerase, and hematopoietic stem cell biology. Arch Med Res. 2003;34:489–495. doi: 10.1016/j.arcmed.2003.07.003. [DOI] [PubMed] [Google Scholar]
- [51].Samper E, Fernandez P, Eguia R, et al. Long-term repopulating ability of telomerase-deficient murine hematopoietic stem cells. Blood. 2002;99:2767–2775. doi: 10.1182/blood.v99.8.2767. [DOI] [PubMed] [Google Scholar]
- [52].Yamaguchi H, Calado RT, Ly H, et al. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med. 2005;352:1413–1424. doi: 10.1056/NEJMoa042980. [DOI] [PubMed] [Google Scholar]
- [53].Allsopp RC, Weissman IL. Replicative senescence of hematopoietic stem cells during serial transplantation: does telomere shortening play a role? Oncogene. 2002;21:3270–3273. doi: 10.1038/sj.onc.1205314. [DOI] [PubMed] [Google Scholar]
- [54].Effros RB, Globerson A. Hematopoietic cells and replicative senescence. Exp Gerontol. 2002;37:191–196. doi: 10.1016/s0531-5565(01)00183-8. [DOI] [PubMed] [Google Scholar]
- [55].Di Leonardo A, Linke SP, Clarkin K, Wahl GM. DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev. 1994;8:2540–2551. doi: 10.1101/gad.8.21.2540. [DOI] [PubMed] [Google Scholar]
- [56].Rave-Frank M, Virsik-Kopp P, Pradier O, Nitsche M, Grunefeld S, Schmidberger H. In vitro response of human dermal fibroblasts to X-irradiation: relationship between radiation-induced clonogenic cell death, chromosome aberrations and markers of proliferative senescence or differentiation. Int J Radiat Biol. 2001;77:1163–1174. doi: 10.1080/09553000110086372. [DOI] [PubMed] [Google Scholar]
- [57].Suzuki K, Mori I, Nakayama Y, Miyakoda M, Kodama S, Watanabe M. Radiation-induced senescence-like growth arrest requires TP53 function but not telomere shortening. Radiat Res. 2001;155:248–253. doi: 10.1667/0033-7587(2001)155[0248:rislga]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- [58].Gajdusek C, Onoda K, London S, Johnson M, Morrison R, Mayberg M. Early molecular changes in irradiated aortic endothelium. J Cell Physiol. 2001;188:8–23. doi: 10.1002/jcp.1091. [DOI] [PubMed] [Google Scholar]
- [59].Oh CW, Bump EA, Kim JS, Janigro D, Mayberg MR. Induction of a senescence-like phenotype in bovine aortic endothelial cells by ionizing radiation. Radiat Res. 2001;156:232–240. doi: 10.1667/0033-7587(2001)156[0232:ioaslp]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- [60].Roninson IB. Tumor cell senescence in cancer treatment. Cancer Res. 2003;63:2705–2715. [PubMed] [Google Scholar]
- [61].Keller G, Snodgrass R. Life span of multipotential hematopoietic stem cells in vivo. J Exp Med. 1990;171:1407–1418. doi: 10.1084/jem.171.5.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- [63].Iscove NN, Nawa K. Hematopoietic stem cells expand during serial transplantation in vivo without apparent exhaustion. Curr Biol. 1997;7:805–808. doi: 10.1016/s0960-9822(06)00341-1. [DOI] [PubMed] [Google Scholar]
- [64].Allsopp RC, Morin GB, Horner JW, DePinho R, Harley CB, Weissman IL. Effect of TERT over-expression on the long-term transplantation capacity of hematopoietic stem cells. Nat Med. 2003;9:369–371. doi: 10.1038/nm0403-369. [DOI] [PubMed] [Google Scholar]
- [65].Hellman S, Botnick LE. Stem cell depletion: an explanation of the late effects of cytotoxins. Int J Radiat Oncol Biol Phys. 1977;2:181–184. doi: 10.1016/0360-3016(77)90028-1. [DOI] [PubMed] [Google Scholar]
- [66].Park IK, Qian D, Kiel M, et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423:302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- [67].Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature. 2003;423:255–260. doi: 10.1038/nature01572. [DOI] [PubMed] [Google Scholar]
- [68].Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ito K, Hirao A, Arai F, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- [70].Toussaint O, Medrano EE, von Zglinicki T. Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp Gerontol. 2000;35:927–945. doi: 10.1016/s0531-5565(00)00180-7. [DOI] [PubMed] [Google Scholar]
- [71].Norbury CJ, Hickson ID. Cellular responses to DNA damage. Annu Rev Pharmacol Toxicol. 2001;41:367–401. doi: 10.1146/annurev.pharmtox.41.1.367. [DOI] [PubMed] [Google Scholar]
- [72].Shen Y, White E. p53-dependent apoptosis pathways. Adv Cancer Res. 2001;82:55–84. doi: 10.1016/s0065-230x(01)82002-9. [DOI] [PubMed] [Google Scholar]
- [73].Kachnic LA, Wu B, Wunsch H, et al. The ability of p53 to activate downstream genes p21(WAF1/cip1) and MDM2, and cell cycle arrest following DNA damage is delayed and attenuated in scid cells deficient in the DNA-dependent protein kinase. J Biol Chem. 1999;274:13111–13117. doi: 10.1074/jbc.274.19.13111. [DOI] [PubMed] [Google Scholar]
- [74].Tang W, Willers H, Powell SN. p53 directly enhances rejoining of DNA double-strand breaks with cohesive ends in gamma-irradiated mouse fibroblasts. Cancer Res. 1999;59:2562–2565. [PubMed] [Google Scholar]
- [75].Lowe SW, Sherr CJ. Tumor suppression by Ink4a-Arf: progress and puzzles. Curr Opin Genet Dev. 2003;13:77–83. doi: 10.1016/s0959-437x(02)00013-8. [DOI] [PubMed] [Google Scholar]
- [76].Sherr CJ, Weber JD. The ARF/p53 pathway. Curr Opin Genet Dev. 2000;10:94–99. doi: 10.1016/s0959-437x(99)00038-6. [DOI] [PubMed] [Google Scholar]
- [77].Robles SJ, Adami GR. Agents that cause DNA double strand breaks lead to p16INK4a enrichment and the premature senescence of normal fibroblasts. Oncogene. 1998;16:1113–1123. doi: 10.1038/sj.onc.1201862. [DOI] [PubMed] [Google Scholar]
- [78].te Poele RH, Okorokov AL, Jardine L, Cummings J, Joel SP. DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res. 2002;62:1876–1883. [PubMed] [Google Scholar]
- [79].Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- [80].Beausejour CM, Krtolica A, Galimi F, et al. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22:4212–4222. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Deng Q, Liao R, Wu BL, Sun P. High intensity ras signaling induces premature senescence by activating p38 pathway in primary human fibroblasts. J Biol Chem. 2004;279:1050–1059. doi: 10.1074/jbc.M308644200. [DOI] [PubMed] [Google Scholar]
- [82].Wang W, Chen JX, Liao R, et al. Sequential activation of the MEK-extracellular signal-regulated kinase and MKK3/6-p38 mitogen-activated protein kinase pathways mediates oncogenic ras-induced premature senescence. Mol Cell Biol. 2002;22:3389–3403. doi: 10.1128/MCB.22.10.3389-3403.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Narita M, Nunez S, Heard E, et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–716. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]
- [84].Serrano M, Lee H, Chin L, Cordon-Cardo C, Beach D, DePinho RA. Role of the INK4a locus in tumor suppression and cell mortality. Cell. 1996;85:27–37. doi: 10.1016/s0092-8674(00)81079-x. [DOI] [PubMed] [Google Scholar]
- [85].Sherr CJ. Parsing Ink4a/Arf: “pure” p16-null mice. Cell. 2001;106:531–534. doi: 10.1016/s0092-8674(01)00486-x. [DOI] [PubMed] [Google Scholar]
- [86].Sharpless NE. Ink4a/Arf links senescence and aging. Exp Gerontol. 2004;39:1751–1759. doi: 10.1016/j.exger.2004.06.025. [DOI] [PubMed] [Google Scholar]
- [87].Kamijo T, Zindy F, Roussel MF, et al. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell. 1997;91:649–659. doi: 10.1016/s0092-8674(00)80452-3. [DOI] [PubMed] [Google Scholar]
- [88].Sharpless NE, Bardeesy N, Lee KH, et al. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature. 2001;413:86–91. doi: 10.1038/35092592. [DOI] [PubMed] [Google Scholar]
- [89].Randle DH, Zindy F, Sherr CJ, Roussel MF. Differential effects of p19(Arf) and p16(Ink4a) loss on senescence of murine bone marrow-derived preB cells and macrophages. Proc Natl Acad Sci U S A. 2001;98:9654–9659. doi: 10.1073/pnas.171217498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Lewis JL, Chinswangwatanakul W, Zheng B, et al. The influence of INK4 proteins on growth and self-renewal kinetics of hematopoietic progenitor cells. Blood. 2001;97:2604–2610. doi: 10.1182/blood.v97.9.2604. [DOI] [PubMed] [Google Scholar]
- [91].Stepanova L, Sorrentino BP. A limited role for p16Ink4a and p19Arf in the loss of hematopoietic stem cells during proliferative stress. Blood. 2005;106:827–832. doi: 10.1182/blood-2004-06-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Tyner SD, Venkatachalam S, Choi J, et al. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]