Abstract
Objective
To compare home-based pencil push-ups (HBPP), home-based computer vergence/accommodative therapy and pencil push-ups (HBCVAT+), office-based vergence/accommodative therapy with home reinforcement (OBVAT), and office-based placebo therapy with home reinforcement (OBPT) as treatments for symptomatic convergence insufficiency (CI).
Methods
In a randomized clinical trial, 221 children 9 to 17 years with symptomatic CI were assigned to one of four treatments.
Main Outcome Measures
Convergence Insufficiency Symptom Survey (CISS) score after 12 weeks of treatment. Secondary outcomes were near point of convergence (NPC) and positive fusional vergence at near (PFV).
Results
After 12 weeks of treatment the OBVAT group’s CISS score (15.1) was statistically significantly lower than the HBCVAT+, HBPP, and OBPT groups’ scores of 21.3, 24.7, and 21.9, respectively (P < 0.001). The OBVAT group also demonstrated a significantly improved NPC and PFV compared with the other groups (P <= 0.005). A successful or improved outcome for the OBVAT, HBPP, HBCVAT+, and OBPT groups was found in 73%, 43%, 33%, and 35%, respectively.
Conclusion
Twelve weeks of OBVAT results in a significantly greater improvement in symptoms and clinical measures of NPC and PFV and a greater percentage of patients reaching pre-determined criteria of success when compared with HBPP, HBCVAT+, or OBPT.
Keywords: convergence insufficiency, asthenopia, vision therapy, orthoptics, vergence/accommodative therapy, pencil push-ups, computer vergence/accommodative therapy, placebo therapy, exophoria, eyestrain, symptom survey
Introduction
Convergence insufficiency (CI) is a common binocular vision disorder (1-4) that is often associated with a variety of symptoms including eyestrain, headaches, blurred vision, diplopia, sleepiness, difficulty concentrating, movement of print, and loss of comprehension after short periods of reading or performing close activities. (5-13) Various treatments (10, 14-23) are commonly prescribed including passive treatment with base-in prism reading glasses and active treatments such as home-based therapy using pencil push-ups alone, home-based therapy using pencil push-ups plus other therapy techniques, office-based vision therapy, and orthoptics. Consensus regarding the most effective treatment is lacking and there are considerable differences amongst treatments in time and cost. Recent studies surveying the ophthalmic community suggest that home-based therapy using pencil push-ups alone is the most commonly prescribed treatment by both ophthalmologists and optometrists for young patients with symptomatic CI. (24-26)
Active therapies for the treatment of symptomatic CI typically involve the purposeful, controlled manipulation of target blur, vergence demand, and/or target proximity with the aim of normalizing the accommodative and vergence systems and their mutual interactions. (27) The various active treatment approaches for CI differ in: 1) ability to control and manipulate stimulus parameters (e.g., vergence and accommodative demand), 2) dosage, 3) mode of administration, and 4) use of motor learning theory and patient feedback. It is unknown, however, whether these differences affect the outcome of treatment.
Until recently, there has been a scarcity of rigorously performed scientific studies documenting the effectiveness of treatments for CI. In preparation for the randomized clinical trial reported herein, the Convergence Insufficiency Treatment Trial (CITT) investigator group completed two pilot studies which were placebo-controlled, randomized trials investigating the effectiveness of passive and active treatments for symptomatic CI in children. (28, 29) In the trial evaluating the effectiveness of base-in prism reading glasses prescribed according to Sheard’s criteria (convergence amplitudes less than twice the near phoria), (30) prism glasses were found to be no more effective than placebo reading glasses. (28) The other randomized trial comparing the effectiveness of home-based pencil push-ups, office-based vision therapy/orthoptics, and office-based placebo vision therapy/orthoptics, found office-based vision therapy/orthoptics to be more effective than pencil push-up or placebo therapy in improving both the signs and symptoms associated with CI. (29) A limitation of the latter study was a 19% (9 of 47) loss to follow up before treatment completion. In addition, it was suggested that a more intensive home-based vision therapy/orthoptics regimen should have been included as a treatment arm. (31)
The purpose of this randomized clinical trial was to further evaluate the commonly used active treatments for CI. We compared the effectiveness of 12 weeks of treatment using home-based pencil push-ups, home-based computer vergence/accommodative therapy and pencil push-ups, office-based vergence/accommodative therapy with home reinforcement, and office-based placebo therapy in improving symptoms and signs associated with symptomatic CI in children.
Patients and Methods
The tenets of the Declaration of Helsinki were followed throughout the study. The institutional review boards of all participating centers approved the protocol and informed consent forms. The parent or guardian (subsequently referred to as “parent”) of each study patient gave written informed consent and each patient gave assent to participation. There was an initial consent process for performing an eligibility examination followed by a second consent for the enrollment and randomization of eligible patients into the trial. Health Insurance Portability and Accountability Act (HIPAA) authorization was obtained from the parent. Study oversight was provided by an independent data and safety monitoring committee (see Appendix). This study is registered at ClinicalTrials.gov as the Convergence Insufficiency Treatment Trial (CITT). (32)
Patient Selection
Major eligibility criteria for the trial included children ages 9 to 17 years, exodeviation at near at least 4Δ greater than at far, a receded near point of convergence (NPC) break (6 cm or greater), and insufficient positive fusional vergence at near (PFV) (convergence amplitudes) (i.e., failing Sheard’s criterion [PFV less than twice the near phoria] (30) or minimum PFV of ≤15Δ base-out blur or break), and a CI Symptom Survey (described in Outcome Measures section below) score of ≥16. Because patients with symptomatic CI often have an associated accommodative insufficiency (12), patients with symptomatic CI associated with accommodative insufficiency were included in the study. However, children with monocular accommodative amplitudes <5D were excluded because the severity of their accommodative insufficiency may indicate an organic etiology. Table 1 provides a complete listing of eligibility and exclusion criteria.
Table 1. Eligibility and Exclusion Criteria.
| Eligibility Criteria: |
|
| Exclusion Criteria |
|
A refractive correction was prescribed for patients if a significant refractive error was present or a significant change in refractive correction was found. A significant refractive error/change was defined as ≥1.50 D hyperopia, ≥0.50 D myopia, ≥0.75 D astigmatism, ≥0.75 D anisometropia in spherical equivalent or ≥1.50 D anisometropia in any meridian (based on cycloplegic refraction). For hyperopes the investigator had the discretion to reduce the prescription up to 1.25 D. For myopia full correction was required. After wearing the glasses for at least two weeks, eligibility testing was repeated to determine if the patient still met the eligibility criteria. Thus, the CI Symptom Survey and eligibility testing were always performed with appropriate refractive correction in place.
Examination Procedures
Eligibility testing included administration of the CI Symptom Survey to identify whether the child was symptomatic. (12, 13, 33, 34) Other eligibility tests included: best-corrected visual acuity at distance and near, a sensorimotor examination (cover testing at distance and near, NPC, positive and negative fusional vergence at near (fusional convergence and divergence amplitudes), near stereoacuity, monocular accommodative amplitude, and monocular accommodative facility (the ability to quickly achieve clear vision while alternately viewing 20/30 print through +2 D and -2 D lenses), a cycloplegic refraction, and an ocular health evaluation. CITT-trained and certified ophthalmologists or optometrists using a previously described standardized protocol performed all testing. (35) Eligible patients who consented to participate were enrolled into the study and the measures taken at their eligibility examination were used as the study baseline measures.
Randomization
Eligible patients who consented to participate were randomly assigned with equal probability using a permuted block design to either home-based pencil push-ups, home-based computer-based vergence/accommodative therapy and pencil push-ups, office-based vergence/accommodative therapy with home reinforcement, or office-based placebo therapy. Randomization was achieved using a secure website created and managed by the Data Coordinating Center (DCC). To ensure approximately equal numbers of patients in each treatment arm by site, randomization was stratified by clinical site.
Treatment Protocols
The therapy regimens were each 12 weeks in duration. Patients were taught their assigned therapy procedures by CITT-trained and -certified therapists. Therapists were either optometrists, vision therapists, or orthoptists with at least one year of experience and most optometrists were residency trained. For home therapy procedures, patients were required to demonstrate their understanding and ability to perform the techniques in the office before they were prescribed for home. Instructional handouts also were provided for the home treatment procedures. Patients in all groups maintained a home therapy log and recorded their performance for each home therapy session. Monthly office visits were scheduled for children assigned to the two home-based therapy groups; at these visits the therapists answered questions, reviewed home therapy procedures, and estimated adherence (compliance). In addition, the therapist contacted the patients by phone on a weekly basis during which time the home therapy procedures and home logs were reviewed, and attempts were made to motivate the patients to adhere to treatment. Those assigned to office-based therapy groups were scheduled for weekly office therapy visits.
All treatments included time for instruction, feedback, review of the home log, and discussion about adherence. For the office-based groups this all occurred during the weekly office visits. For the home-based groups, these interactions occurred every 4 weeks in the office and weekly via a phone call with the therapist. The total treatment time for each group included the time spent in therapy at home or in the office plus the contact with the therapist via the weekly phone calls for the home-based therapy groups.
Home-Based Pencil Push-ups (HBPP)
The pencil push-ups procedure used a pencil with 20/60 size letters and a white index card placed in the background to provide a suppression check by using physiological diplopia awareness. The goal of the procedure was to move the pencil to within 2 to 3 cm of the brow, just above the nose on each push up while trying to keep the target single and clear. Patients were instructed to perform the pencil push-ups procedure 15 minutes per day, 5 days per week. They maintained home therapy log forms, recording the closest distance that they could maintain fusion after each 5 minutes of therapy
Home-based Computer Vergence/Accommodative Therapy and Pencil Push-ups (HBCVAT+)
Patients in this group were taught to perform the aforementioned pencil push-up procedure as well as procedures on the Home Therapy System (HTS/CVS) (www.visiontherapysolutions.com) computer software. Using this program, they performed fusional vergence and accommodative therapy procedures including vergence base in, vergence base out, auto-slide vergence, and jump ductions vergence programs using random dot stereopsis targets. The accommodative rock program was used for accommodative therapy. Much like a clinician would do at each follow-up visit, this computer program automatically modified the therapy program after each session based on the patient’s performance. Patients were instructed to do pencil push-ups 5 minutes per day and the HTS software program for 15 minutes per day, 5 days per week and to save their data on a disk provided by the study and to bring the disk to each follow-up visit.
Office-Based Vergence/Accommodative Therapy with Home Reinforcement (OBVAT)
The office-based vergence/accommodative therapy group received a weekly 60-minute in-office therapy visit with additional prescribed procedures to be performed at home for 15 minutes a day, 5 days per week. The therapy procedures are described in detail elsewhere (21) and those performed during the weekly, office-based vergence/accommodative therapy sessions are listed in Table 2. At each office-based therapy session, the patient performed 4-5 procedures with constant supervision and guidance from the therapist. There were no diagnostic tests performed during these sessions. The therapist followed a detailed and specific protocol from the CITT Manual of Procedures (accessed at http://optometry.osu.edu/research/CITT/4363.cfm); this document describes each procedure, amount of time used, expected performance, and criteria for ending the procedure and advancing to a more difficult level.
Table 2. Office-based Vergence/Accommodative Therapy Protocol.
 |
Office-Based Placebo Therapy (OBPT)
Patients in the office-based placebo therapy group received therapy during a weekly 60-minute office visit and were prescribed procedures to be performed at home for 15 minutes per day, 5 days per week. The placebo therapy program consisted of 16 in-office therapy procedures and 4 home therapy procedures, which were designed to look like real vergence/accommodative therapy procedures yet not stimulate vergence, accommodation or fine saccadic eye movement skills beyond normal daily visual activities. The therapist followed a detailed protocol from the CITT Manual of Procedures (accessed at http://optometry.osu.edu/research/CITT/4363.cfm). Five procedures were performed during each office therapy visit and two procedures were assigned for home therapy each week. Placebo procedures included traditional vergence/accommodative therapy procedures modified to be monocular rather than binocular, binocular procedures performed at zero vergence disparity, and testing procedures that did not require significant demand on the vergence, accommodative or fine saccadic eye movement systems. For example, in one placebo procedure, the patient wore the appropriate filter glasses and performed vergence therapy at zero vergence demand on the Computer Orthopter (http://www.computerorthoptics.com). Some procedures were designed to have increasing levels of “difficulty.” As in real therapy, patients frequently wore filter glasses and were told that the glasses ensured that both eyes were being used together. Objectives and goals were established for each placebo procedure to simulate real therapy. The therapist told the patient the objective of each procedure before beginning the technique for motivational purposes.
Masking of Therapists and Patients
Because experienced therapists provided the treatments, it was not feasible to mask them to patients’ assigned treatment. However, each therapist followed a well-defined protocol for all treatments and was instructed to interact in an identical fashion with all patients. Although patients were obviously aware of whether they were assigned to office- or home-based therapy, those receiving office-based treatment were masked regarding whether they were assigned to vergence/accommodative therapy or placebo therapy.
To determine the effectiveness of masking, patients assigned to either of the two office-based treatments, were queried at the completion of treatment whether they thought they were randomized into the “active” or the “placebo” treatment. To assess examiner masking, examiners were asked if they thought that they could identify the patient’s treatment assignment at the completion of each masked examination. In addition, at the completion of the 12-week outcome examination, examiners were asked to guess the patient’s group assignment and to report a level of confidence in the response.
Follow-up Examinations: 12-week Treatment Period
Protocol-specified follow-up visits were conducted after 4 and 8 weeks of treatment. The primary outcome assessment was made at the visit following the twelfth week of treatment. At these follow-up visits, an examiner who was masked to the patient’s treatment group administered the CI Symptom Survey and a sensorimotor examination that included cover testing at distance and near, NPC, PFV, accommodative amplitude, and accommodative facility testing. After the clinical testing was completed, the CI Symptom Survey was re-administered.
Treatment Adherence Data
To assess adherence with therapy performed at home, at each masked examination the therapist was asked, “What percent (0%, 1-24%, 25-49%, 50-74%, 75-99%, or 100%) of the time do you feel the patient adhered to the home protocol?” The therapists’ estimate was based on a review of the home log, electronic data from the computer therapy program, and a discussion with the patient about home therapy. Thus, this estimate was primarily based upon patient reports. The response options of 0%, 1-24%, 25-49%, and 50-74% were combined into one category (0-74%) for data analysis because only 16% of patients were categorized into the response options below 75%.
Maintenance Therapy
Patients who demonstrated sufficient improvement on the CI Symptom Survey to be considered “asymptomatic” (i.e., CI Symptom Survey score <16) at the 12-week outcome visit were prescribed maintenance therapy of 15 minutes per week using home therapy procedures specific to the patient’s assigned treatment group. Patients not demonstrating sufficient improvement on the CI Symptom Survey and considered “symptomatic” (i.e., CI Symptom Survey score ≥16) were referred to a non-CITT eye care provider to receive alternative treatment for CI.
Outcome Measures and Criteria for Success
CI patients who seek treatment usually do so because they are symptomatic (or perceived to be by their parents) and successful treatment should result in a lessening of or abatement of symptoms. Thus, we used symptom level (as measured by the CI Symptom Survey (CISS)) as the primary outcome measure (Figure 1). The questionnaire consisted of 15 items that were read aloud by the examiner to the child. The examiner read the questions while the child viewed a card with the answers and was instructed to choose one of five possible answers (never, infrequently, sometimes, fairly often, always). Each response was scored as 0 to 4 points, with 4 representing the highest frequency of symptom occurrence (i.e., always). The 15 items were summed to obtain the total CISS score. The lowest possible score (least symptoms) was 0 and the highest was 60 (most symptomatic). Based on our previous work (13, 36), a CI Symptom Survey score of less than 16 is considered “asymptomatic” and a decrease of at least 10 or more points is considered “improved.”
Figure 1. CI Symptom Survey.
Clinician instructions: Read the following subject instructions and then each item exactly as written. If subject responds with “yes” - please qualify with frequency choices. Do not give examples.
Subject instructions: Please answer the following questions about how your eyes feel when reading or doing close work.
The goal of treatment for CI is not only to eliminate patient symptoms, but also to improve the patient’s convergence ability. Thus, we used NPC and PFV as secondary outcome measures. A “normal” NPC was defined as less than 6 cm and an “improved” NPC was defined as an improvement (decrease) in NPC of more than 4 cm from baseline to the 12-week outcome examination. To be classified as having “normal” PFV a patient had to pass Sheard’s criteria (i.e., PFV blur or if no blur, then break value at least twice the near phoria magnitude) and have a PFV blur/break of more than 15Δ. Improvement in PFV was defined as an increase of 10Δ or more from baseline to the 12-week outcome examination.
To evaluate each treatment’s ability to improve both signs and symptoms, we also developed a composite outcome classification that considered the change in all three outcome measures from baseline to the 12-week outcome examination. A “successful” outcome was a score of <16 on the CI Symptom Survey, a normal NPC (i.e., less than 6 cm), and normal PFV (i.e., greater than 15Δ and passing Sheard’s criterion). “Improved” was defined as a score of <16 or a 10 point decrease in the CI Symptom Survey score, and at least one of the following: normal NPC, an improvement in NPC of more than 4 cm, normal PFV or an increase in PFV of more than 10Δ. Patients who did not meet the criteria for “successful” or “improved” were considered “non-responders.”
Statistical Methods
Sample size
All sample size calculations were performed using PASS 2000 software (37) and assuming a two-sided test with 90% power. For a given outcome measure, the common standard deviation obtained from the CITT pilot study (29) was used as an estimate of variability. To control for multiple comparisons (4 groups compared two-at-a-time = 6 pair-wise comparisons), the alpha level used for determining sample size was set at 0.05/6 = 0.0083.
The CITT was powered to reject the null hypothesis of no difference between groups assuming that the true population differences between groups are 10 points on the CI Symptom Survey, 4 cm in NPC, and 10Δ in PFV. These differences were based on clinician expert opinion and the repeatability of each measure (13, 38) The sample size of 52 children per group was determined as the maximum required sample size for the three outcome variables and adjusting for a 10% loss to follow-up.
Data Analysis
All data analyses were performed using SAS (version 9.1, SAS Institute, Cary, NC). All analyses followed the intention-to-treat principle. The mean of the two measures of the CI Symptom Survey score and the three measures of both the NPC and PFV obtained at each study visit were used for analyses. PFV was obtained from the base-out to blur measure if present; otherwise, base-out to break was used.
As planned a priori, a 4 group by 3 time period repeated measures analysis of covariance (ANCOVA) was used to compare the treatment groups at week 12. Using data from both the 4-week and 8-week visits maximizes the degrees of freedom thus ensuring the most appropriate estimate of the mean square error used in group mean comparisons. The baseline value of the outcome measure was used as a covariate because our initial pilot data showed a strong correlation between baseline and all subsequent values. In addition, all clinical and demographic variables collected at baseline were examined as potential confounders of the true relationship between a particular outcome measure and treatment group. For these analyses, the alpha level for inclusion in the final ANCOVA model was set at 0.10. If the final ANCOVA model indicated a significant group effect or group by time interaction, Tukey’s method of adjustment for multiple pair-wise group comparisons was used to hold the overall error rate at α=0.05. The mean square error from the ANCOVA model was also used to construct 95% confidence intervals for the mean difference between groups.
A chi-square test was used to compare the percentage of patients in each group classified as “successful,” “improved,” or “non-responder.” Post-hoc pair-wise group comparisons of the percentage in each classification were achieved using Logistic regression models. The baseline value of each outcome measure was included in the regression model.
An unweighted kappa statistic and the 95% confidence interval were used to assess the agreement between the examiner’s guess and the patient’s actual group assignment.
Results
Enrollment
Between July 2005 and October 2006, 221 patients were enrolled in the study. The number of patients enrolled at the 9 sites ranged from 14 to 35 (median = 25). The mean (SD) age of the patients was 11.8 (2.3) years; 59%were female, 55% were white, 30% were African American, 34% were Hispanic. At baseline, the mean (SD) clinical findings were 2 Δ (2.84) exodeviation at distance; 9.3Δ (4.4) exodeviation at near; NPC break/recovery of 14.2 (7.5) cm/17.9 (8.2) cm; and PFV break/recovery at near of 12.7 (4.69)Δ/8.8 (4.5)Δ. Table 3 provides the study population demographics and pertinent clinical measures at baseline by treatment group. Although children with constant strabismus were excluded, patients with intermittent exotropia were eligible for the study and a small number (4 to 7) were randomized to each treatment group. Although there was an imbalance at baseline in medication used among the four groups (highest in the OBPT group), only the psychotropic medications had potential affects on accommodation and the groups were balanced for these medications. Based on initial bivariate analyses, no confounders were identified for inclusion in the ANCOVA model for any of the 3 outcome measures.
Table 3. CITT Study Population Demographics and Clinical Measures at Baseline.
| Characteristic | HBPP n=54 |
HBCVAT+ n=53 |
OBVAT n=60 |
OBPT n=54 |
|---|---|---|---|---|
| Mean (std) Age (years) | 11.9 (2.2) | 11.6 (2.3) | 12.0 (2.6) | 11.8 (2.2) |
| Mean (std) Convergence Insufficiency Symptom Survey score | 27.8 (7.6) | 31.7 (9.1) | 30.2 (9.8) | 29.8 (8.9) |
| Mean (std) Near Point of Convergence (cm) | 14.7 (8.4) | 14.4 (7.5) | 13.4 (6.6) | 14.4 (7.8) |
| Mean (std) Positive Fusional Vergence Blur/Break (Δ) | 11.3 (4.0) | 10.5 (4.2) | 11.0 (4.2) | 11.0 (3.1) |
| Mean (std) Negative Fusional Vergence Blur/Break(Δ) | 13.0 (5.5) | 11.3 (4.3) | 10.4 (4.9) | 10.2 (3.3) |
| Mean (std) Monocular Accommodative Amplitude (D) | 10.1 (3.8) | 10.0 (4.5) | 10.0 (4.0) | 9.4 (2.9) |
| Accommodative Insufficiency*, No. (%) | 27 (50) | 30 (57) | 36 (60) | 28 (52) |
| Mean (std) Monocular Accommodative Facility (cpm) | 6.9 (4.2) | 5.7 (4.3) | 6.5 (4.4) | 6.8 (4.8) |
| Mean (std) Near Phoria (Δ) | 9.9 exo (5.0) | 9.4 exo (4.5) | 8.8 exo (3.7) | 9.0 exo (4.5) |
| Mean (std) Distance Phoria (Δ) | 2.4 exo (3.4) | 2.0 exo (3.0) | 1.7 exo (2.2) | 1.8 exo (2.5) |
| Mean (std) Spherical Equivalent Refractive Error - Right Eye (D) | -0.34 (1.5) | 0.08 (1.5) | -0.20 (1.3) | 0.15 (1.5) |
| Female, No. (%) | 27 (50) | 31 (58) | 41 (68) | 32 (59) |
| Race, No. (%) | ||||
| American Indian / Alaskan Native | 0 (0) | 3 (6) | 2 (3) | 5 (9) |
| Asian / Pacific Islander | 2 (4) | 0 (0) | 2 (3) | 0 (0) |
| Black or African American | 18 (34) | 12 (23) | 15 (25) | 20 (37) |
| White | 30 (57) | 30 (57) | 35 (59) | 25 (46) |
| Other | 3 (6) | 8 (15) | 5 (8) | 4 (7) |
| Hispanic or Latino, No. (%) | 12 (22) | 23 (45) | 24 (41) | 16 (30) |
| Attention Deficit Hyperactivity Disorder (Parent Report), No. (%) | ||||
| Yes | 6 (11) | 9 (17) | 7 (12) | 12 (22) |
| No | 45 (83) | 42 (79) | 51 (85) | 40 (74) |
| Missing | 3 (6) | 2 (4) | 2 (3) | 2 (4) |
| Glasses wearers, No. (%) | 24 (44) | 16 (30) | 16 (27) | 20 (37) |
| Medication use | ||||
| Number (%) reporting use | 5 (9) | 15 (28) | 14 (23) | 21 (39) |
| Using psychotropic medications†, No. (%) | 2 (40) | 4 (27) | 3 (21) | 6 (29) |
| Using pulmonary medications†, No. (%) | 2 (40) | 5 (33) | 2 (14) | 10 (48) |
| Using allergy medications†, No. (%) | 1 (20) | 6 (40) | 4 (29) | 11 (52) |
Defined as monocular accommodative amplitude less than Hoffstetter s minimum accommodative amplitude criteria minus 2.0D
Percent among those who reported medication use
HBPP: Home-based pencil push-up therapy
HBCVAT+: Home-based computer vergence/accommodative therapy and pencil push-ups
OBVAT: Office-based vergence/accommodative therapy with home reinforcement
OBPT: Office-based placebo therapy with home reinforcement
Δ = prism diopters
cpm = cycles per minute
Patient Follow Up
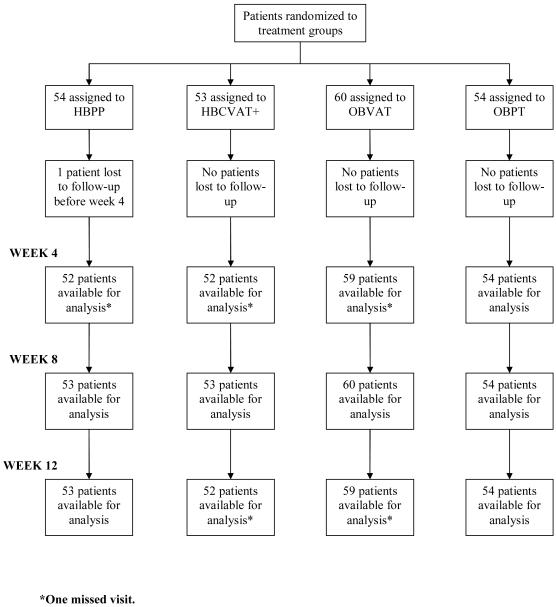
Of the 221 patients who entered the trial, 218 (99%) completed the 12-week outcome examination. Patient follow-up is shown in Figure 2. Less than 2% of all study visits through week 12 were missed. The highest percentage of missed visits occurred in the OBPT group (18 of 648 visits or 2.8%). Of the 720 study visits scheduled in the OBVAT group, only 17 (2.4%) were missed. In both the home-based treatment groups, the percentage of visits missed was less than 1.5% (1.3% of 639 visits in the HBPP group and 1.4% of 636 visits in the HBCVAT+ group).
Figure 2. Flow Diagram of CITT Randomized Clinical Trial.
Treatment Adherence Data
At 12 weeks the percentage of CITT patients rated by therapists as compliant with the home therapy protocol at least 75% of the time was 67.3% in the HBCVAT group, 84.9% in the HBPP group, 87% in the OBPT group, and 91.4% in the OBVAT group (Table 6). Accounting for the observed differences in estimated adherence did not affect the results of the treatment group comparisons for symptom score, NPC, and PFV (data not shown).
Table 6. Number (%) of CITT patients rated by therapist as compliant with home therapy protocol at least 75% of the time by week.
| Week | HBPP | HBCVAT+ | OBVAT | OBPVT |
|---|---|---|---|---|
| 4 | 48 (92.3) | 37 (69.8) | 54 (94.7) | 52 (98.1) |
| 8 | 45 (84.9) | 35 (66.0) | 55 (91.7) | 50 (96.1) |
| 12 | 45 (84.9) | 35 (67.3) | 53 (91.4) | 47 (87.0) |
HBPP: Home-based pencil push-up therapy
HBCVAT+: Home-based computer vergence/accommodative therapy and pencil push-ups
OBVAT: Office-based vergence/accommodative therapy with home reinforcement
OBPT: Office-based placebo therapy
Placebo Treatment - Were Patients Masked?
Eighty-five percent of the patients assigned to placebo therapy and 93% of those assigned to vergence/accommodative therapy believed they had been assigned to the active therapy group.
Were Examiners Effectively Masked?
None of the examiners felt that they could identify the patients’ group assignment at the 4- and 8- week masked examinations, and only one examiner felt that he could identify the group assignment at outcome. One-third of the examiners responded that the patient was assigned to the OBVAT group, 24% responded HBCVAT+ and 21% to each of the other two groups. Examiners, when asked to guess, were correct in identifying the patient’s group assignment only 34% of the time which was less than what would have been expected by chance (i.e. 50% correct vs. incorrect, p<0.001). There was low agreement between the actual group assignment and the examiner’s guess of assigned treatment group (kappa = 0.11, 95% CI of 0.04 to 0.20).
Primary Outcome Measure: CI Symptom Score
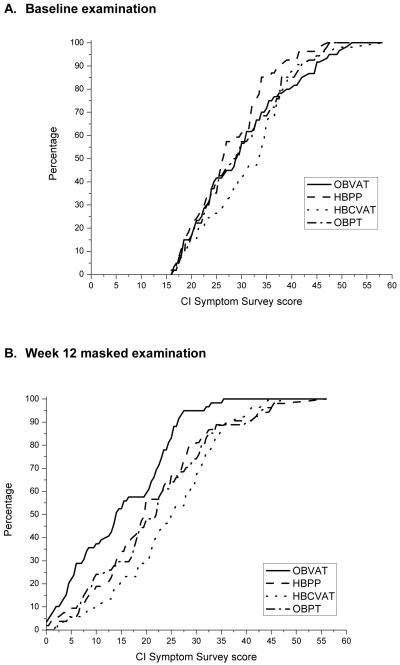
Figures 3a and 3b display the cumulative distribution plots of the mean symptom level for the four treatment groups at baseline and after 12 weeks of treatment, respectively. At the 12-week outcome exam, patients assigned to the OBVAT group reported a statistically significantly lower mean symptom level compared with patients in the other three treatment groups (Table 4). The mean CISS score for patients in the OBVAT group was 6.8 points lower than that observed among patients assigned to OBPT (95% confidence interval, 3.4 - 10.3; p < 0.0001). A mean difference of 7.9 points was found between the OBVAT and HBPP groups (95% confidence interval, 4.4 - 11.4; p < 0.0001). The largest difference in mean symptom level was 8.4 points (95% confidence interval, 4.9 - 11.9; p < 0.0001); this was observed between the OBVAT and HBCVAT+ groups. No significant differences were observed between the HBPP, HBCVAT+, and OBPT groups (pair-wise p-values all ≥ 0.38).
Figure 3. Cumulative distribution of CI Symptom Survey data collected during the eligibility examination and at the week 12 masked examination, by treatment group.
3A. Baseline examination
3B. Outcome Examination
Table 4. Mean and 95% confidence interval for each CITT outcome measure, by treatment group and time.
| Outcome / | HBPP | HBCVAT+ | OBVAT | OBPT | ||||
|---|---|---|---|---|---|---|---|---|
| Time | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI |
| CI Symptom Survey score | ||||||||
| At Baseline | 27.8 | 25.8, 29.8 | 31.7 | 29.3, 34.1 | 30.2 | 27.7, 32.7 | 29.8 | 27.4, 32.2 |
| At Week 12 | ||||||||
| Unadjusted | 21.3 | 18.0, 24.6 | 24.7 | 21.9, 27.5 | 15.1 | 12.6, 17.6 | 21.9 | 18.8, 25.0 |
| Adjusteda | 22.9 | 20.4, 25.5 | 23.5 | 20.9, 26.0 | 15.0 | 12.6, 17.4 | 21.9 | 19.3, 24.4 |
| Total Changea | -7.1 | -9.6, -4.5 | -6.0 | -8.6, -3.4 | -14.8 | -17.2, -12.4 | -7.8 | -10.4, -5.3 |
| Near point of convergence break (cm) | ||||||||
| At Baseline | 14.7 | 12.5, 16.9 | 14.4 | 12.4, 16.4 | 13.3 | 11.6, 15.0 | 14.4 | 12.3, 16.5 |
| At Week 12 | ||||||||
| Unadjusted | 8.0 | 6.1, 9.9 | 6.8 | 5.2, 8.4 | 3.5 | 3.0, 4.0 | 10.3 | 8.4, 12.2 |
| Adjusteda | 7.8 | 6.4, 9.2 | 6.8 | 5.4, 8.2 | 4.0 | 2.7, 5.3 | 10.3 | 8.9, 11.6 |
| Total Changea | -6.4 | -7.8, -5.0 | -7.5 | -8.9, -6.1 | -10.4 | -11.7, -9.0 | -3.9 | -5.3, -2.5 |
| Positive fusional vergence blur or break (Δ)b | ||||||||
| At Baseline | 11.3 | 10.2, 12.4 | 10.5 | 9.4, 11.6 | 11.0 | 9.9, 12.1 | 11.0 | 10.2, 11.8 |
| At Week 12 | ||||||||
| Unadjusted | 19.1 | 16.8, 21.4 | 22.8 | 19.8, 25.8 | 30.7 | 27.5, 33.9 | 17.8 | 15.5, 20.1 |
| Adjusteda | 18.9 | 16.2, 21.6 | 23.0 | 20.3, 25.7 | 30.5 | 28.0, 33.1 | 17.8 | 15.2, 20.5 |
| Total Changea | 7.9 | 5.2, 10.6 | 12.0 | 9.3, 14.8 | 19.7 | 17.1, 22.3 | 6.9 | 4.2, 9.5 |
HBPP: Home-based pencil push-up therapy
HBCVAT+: Home-based computer vergence/accommodative therapy and pencil push-ups
OBVAT: Office-based vergence/accommodative therapy with home reinforcement
OBPT: Office-based placebo therapy with home reinforcement
CI: Confidence Interval
Total Change: Week 12 minus baseline
Adjusted for measurement obtained at the baseline examination
The blur finding was used, but if the patient did not report a blur, the break finding was used
As seen in Table 5, the percentage of patients in each group considered asymptomatic (i.e., CI Symptom Survey score less than 16) or improved (i.e., change in score of 10 or more points at the outcome examination) was significantly higher in the OBVAT group compared with the other treatment groups (vs. HBPP: P = 0.013; vs. HBCVAT+: P < 0.001; vs. OBPT: P = 0.004). There was no significant difference in the percentage of patients considered asymptomatic or improved between the OBPT group and the two home-based groups (pair-wise p-values all greater than 0.60).
Table 5. Percentage of CITT patients in each treatment group who are classified at the 12-week outcome examination as normal/asymptomatic or improved from baseline for each outcome measure.
| CI Symptom Survey * | |||||
|---|---|---|---|---|---|
| Treatment Group | N | CISS still ≥ 16 but improved ≥ 10 | CISS < 16 but improved < 10 | CISS < 16 and improved ≥ 10 | CISS < 16 and/or improved ≥ 10 |
| (A) | (B) | (C) | (A+B+C) | ||
| HBPP | 53 | 13.2 | 9.4 | 24.5 | 47.1 |
| HBCVAT+ | 52 | 15.4 | 5.8 | 17.3 | 38.5 |
| OBVAT | 59 | 17.0 | 6.8 | 49.2 | 72.9 |
| OBPT | 54 | 13.0 | 7.4 | 22.2 | 42.6 |
| Near point of convergence break†,* | |||||
|---|---|---|---|---|---|
| Treatment Group | N | Receded NPC but improved ≥ 4 cm | Normal NPC but improved < 4 cm | Normal NPC and improved ≥ 4 cm | Normal NPC and/or improved ≥ 4 cm |
| (A) | (B) | (C) | (A+B+C) | ||
| HBPP | 53 | 28.3 | 13.2 | 35.9 | 77.4 |
| HBCVAT+ | 52 | 23.1 | 5.8 | 48.1 | 77.0 |
| OBVAT | 59 | 8.5 | 8.5 | 78.0 | 95.0 |
| OBPT | 54 | 33.3 | 5.6 | 20.4 | 59.3 |
| Positive fusional vergence‡,* | |||||
|---|---|---|---|---|---|
| Treatment Group | N | Insufficient PFV but improved > 10Δ | Normal PFV but improved ≤ 10Δ | Normal PFV and improved > 10Δ | Normal PFV and/or improved > 10Δ |
| (A) | (B) | (C) | (A+B+C) | ||
| HBPP | 53 | 9.4 | 17.0 | 30.2 | 56.6 |
| HBCVAT+ | 52 | 7.7 | 7.7 | 44.2 | 59.6 |
| OBVAT | 59 | 3.4 | 10.2 | 69.5 | 83.1 |
| OBPT | 54 | 1.9 | 18.5 | 24.1 | 44.5 |
“Normal” is defined as NPC less than 6 cm; “Improved” is defined as a decrease in NPC of more than 4 cm; “Receded” is defined as NPC > 6cm
“Normal” is defined as PFV greater than 15Δ and passing Sheard’s criterion; “Improved” is defined as an increase in PFV of more than 10Δ;“Insufficient” is defined as PFV ≤ 15Δ or failing Sheard’s criterion.
Columns A, B and C are mutually exclusive
We also used an alternate definition of success in which patients who achieved a symptom score less than 16 were only considered a success if improvement was 10 or more points (column B of Table 5). This eliminated the chance that subjects with CI Symptom Survey scores just meeting the eligibility criteria (≥16) would be classified as a success when the change in the CI Symptom Survey score was within the normal variability of the survey. Sixty-six percent of patients in the OBVAT group met this criterion which was statistically significantly greater than that observed in any of the other treatment groups (vs. 38% in HBPP: p =0.003; vs. 33% in HBCVAT+: p= 0.0006; and vs. 35% in OBPT: p = 0.001); there were no statistical differences among the latter three treatment groups (pair-wise p-values all greater than 0.50).
Secondary Outcome Measures
Near Point of Convergence (NPC) Break
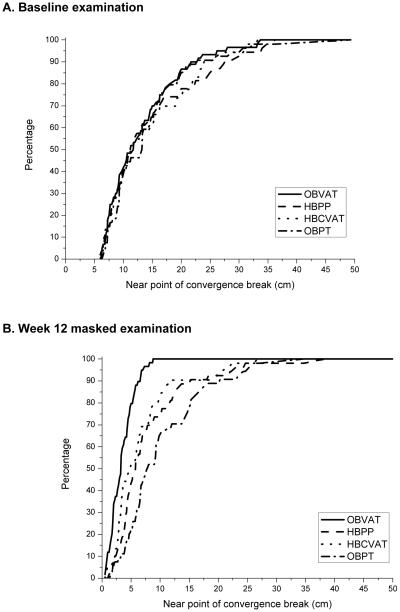
Figures 4a and b display the cumulative distribution plots of the mean NPC break for the four treatment groups at baseline and after 12 weeks of treatment, respectively. At the outcome visit, the mean NPC was statistically significantly improved in the OBVAT group compared with the other three groups (pair-wise p-values all ≤ 0.005) (Table 4). While the mean NPC of both home-based groups measured significantly closer than that of the OBPT group (pair-wise p-values all ≤ 0.013), there were no statistically significant differences (P = 0.33) between the two home-based therapy groups.
Figure 4. Cumulative distribution of near point of convergence (cm) data collected during the eligibility examination and at the week 12 masked examination, by treatment group.
4A. Baseline examination
4B. Outcome Examination
The percentage of patients who had normal (i.e., break less than 6cm) or improved (i.e., decrease of ≥ 4 cm) NPC at the 12-week outcome examination was significantly greater in the OBVAT group compared with the other treatment groups (vs. HBPP: P = 0.008; vs. HBCVAT+: P = 0.006; vs. OBPT: P < 0.001 (Table 5). There were slightly more patients with a normal or improved NPC in both the HBPP and HBCVAT+ groups compared with the OBPT group, however, the difference was not statistically significant (p = 0.056 and 0.070 respectively). There was no significant difference between the two home-based groups (p = 0.93).
Using an alternate definition of success in which patients who achieved a normal NPC were only considered a success if improvement was >4cm (Table 5, column B) resulted in 87% of patients in the OBVAT achieving this criterion, which was significantly higher than that found in any of the other treatment groups (vs. 71% in HBCVAT+: p = 0.023); vs. 64% in HBPP: p = 0.002; and vs. 54% in OBPT group: p < 0.001). There was also a significant difference between the HBCVAT+ group and the OBPT group (p = 0.032); no differences were found between the HBPP group and either the HBCVAT+ group (p = 0.37) or the OBPT (p = 0.20). This conservative estimate would not include some patients who would be considered clinically successful (e.g. 7 cm NPC at baseline which improves to 3.5 cm).
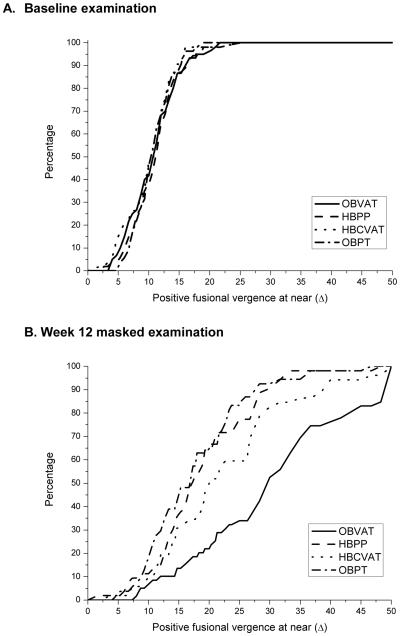
Positive Fusional Vergence (PFV) at Near
Figures 5a and b display the cumulative distribution plots of the mean PFV at near for the four treatment groups at baseline and after 12 weeks of treatment, respectively. At the outcome examination the mean PFV for patients in the OBVAT group was statistically significantly greater than all other groups (pair-wise p-values all < 0.001). The mean PFV in the HBCVAT+ group was significantly better (higher) than in the HBPP (p = 0.037) and OBPT groups (p = 0.008). There was no significant difference in response in the HBPP and OBPT groups (p = 0.57).
Figure 5. Cumulative distribution of positive fusional vergence (Δ) data collected during the eligibility examination and at the week 12 masked examination, by treatment group.
5A. Baseline examination
5B. Outcome Examination
As seen in Table 5, the percentage of patients with normal or improved PFV at the outcome examination was significantly higher in the OBVAT group compared with all other treatment groups (vs. HBPP: P =0.002; vs. HBCVAT+: P = 0.007; vs. OBPT: P < 0.001). There were no significant differences in the percentage normal or improved in the latter three treatment groups (pair-wise p-values all greater than 0.10).
As with CI Symptom Survey and NPC break, an alternate definition of success was used in which patients who achieved a normal PFV were only considered a success if improvement was >10Δ. (Table 5, column B). Seventy-three percent of patients in the OBVAT group achieved this criteria which was significantly higher than that in any of the other treatment groups (vs.52% in HBCVAT+: p = 0.022; vs. 40% in HBPP group: p =0.0005; and vs. 26% in OBPT group: p < 0.0001). There was also a significant difference between the HBCVAT+ and OBPT groups (p = 0.007), however, no other significant differences were detected (p > 0.10). Again, this conservative estimate would not include some patients who would be considered clinically successful (e.g. 10 exophoria at near with positive fusional vergence at near of 16Δ at baseline which improves to 25Δ).
Successful, Improved, and Non-Responder Criteria (Composite Outcome Classification)
Using the composite outcome classification that combines symptoms, NPC and PFV, the proportion of patients found to be “successful” or “improved” in the OBVAT group was statistically significantly greater than that in any of the other groups (p-values < 0.002). While nearly three-quarters of patients in the OBVAT group (73%) were either “successful” or “improved”, less than half the patients in the HBPP group (43%), one-third of the patients in the HBCVAT+ group (33%) and only slightly more than placebo group (35%) were similarly classified.
Secondary Measures Combined
Previous studies have assessed treatment effectiveness by evaluating improvements in NPC and PFV. The proportions of patients achieving both a normal NPC and PFV were 73%, 40%, 37%, and 22% in the OBVAT, HBPP, HBCVAT+, and the OBPT groups, respectively. The percentage achieving both a normal NPC and PFV was significantly higher for the OBVAT group compared with the other treatment groups (P < 0.001 for each pair-wise comparison). No other group differences were significant (P > 0.11 for each pair-wise comparison).
Attention Deficit Hyperactivity Disorder (ADHD)
Children with parent-reported ADHD scored higher on the CISS at baseline than children without parent-reported ADHD and there were slight differences in the distribution of these children among treatment groups at baseline. However, ADHD was not a confounder and did not affect the mean treatment differences among the groups. There was also no interaction between ADHD and treatment (p = 0.93). We examined the 3-way interaction between ADHD, treatment, and time and found no significant effect (p = 0.26).
Adverse Events
Six events were reported to include the eyes or vision. All were unexpected and further evaluations determined all six were not serious and unrelated to the study treatment.
Comment
We compared the effectiveness of three active vision therapy approaches in 221 children with symptomatic CI. Office-based vergence/accommodative therapy with home reinforcement was statistically significantly more effective than home-based pencil push-ups therapy, home-based computer vergence/accommodative therapy and pencil push-ups, and office-based placebo therapy in improving both the symptoms and clinical signs associated with symptomatic CI. Although symptoms did improve in the two home-based therapies, these treatments were no more effective in improving symptoms than office-based placebo therapy.
We established four criteria, a priori, to determine the clinical relevance of the data from this study: 1) the magnitude of the difference on the CI Symptom Survey between treatment groups at outcome, 2) the proportion of children who achieved a normal or improved symptom score on the CI Symptom Survey at outcome, 3) the magnitude of the change in secondary outcome measures, NPC and PFV (convergence amplitudes) at outcome, and 4) the proportion of patients classified as “successful” or “improved” when using the composite outcome classification (combining the treatment effects of all three outcome measures).
The first criterion, the treatment group difference in the CI Symptom Survey score at outcome, was difficult to establish a priori. Our survey instrument had not been incorporated into clinical practice, and consequently the magnitude of the difference between two treatment regimens that indicated clinical relevance had not been established. Based upon the group mean differences found for the CI Symptom Survey in our previous pilot study (29), the CITT was designed to have 90% power to reject the null hypothesis of no group mean differences if the true population difference between groups in the CI Symptom Survey score was 10 points. This difference of 10 points, along with data on the variability in CISS scores obtained from three separate randomized trials conducted by the CITT Group translates into an effect size of greater than 1SD.
In the present study, we did not find a difference in group means of 10 or more points on the CISS. Instead, we found statistically significant group differences ranging between 7 to 8.5 points between the office-based vergence/ accommodative therapy group and each of the other three treatment groups. This translates to an effect size ranging from 0.77 to 0.94 SD. Using Cohen’s (39) guidelines for interpretation of effect size (0.2 is small, 0.5 is medium, 0.8 is large), the group differences we found are considered large. Based on Sloan et al.’s (40) contention that an effect size of 0.5 is a conservative estimate of a clinically meaningful difference that is scientifically supportable and unlikely to be one that can be disregarded. The group differences observed in this study were considered clinically meaningful, although they were less than the a priori estimate of a 10 or more point change between groups. Looking retrospectively and reviewing the literature on effect size, the 10-point difference was a significant over-estimate of the potential treatment effect. Further study and refinement of the CI Symptom Study will help clarify the issue.
The second criterion used to assess clinical relevance was an assessment of whether there were differences among treatment groups in the ability to achieve a normal or improved symptom level on the CI Symptom Survey. After treatment, 73% of patients assigned to office-based vergence/accommodative therapy met this criterion, in contrast to 47% assigned to home-based pencil push-ups, 39% assigned to home-based computer vergence/accommodative therapy and pencil push-ups, and 43% assigned to office-based placebo therapy. Changing the criterion to require that patients achieve both a score of less than 16 and a change of 10 or more points on the CI Symptom Survey showed lower success rates for all groups, but the differences among treatment groups remained the same.
The third criterion used to evaluate clinical relevance was an evaluation of the secondary outcome measures, NPC and PFV (convergence amplitudes), as they are often used clinically to determine treatment success for CI. The proportion of patients who achieved a clinically normal level for both measures was 73% in the office-based vergence/accommodative therapy group versus no more than 40% in each of the other three treatment groups.
The fourth a priori criterion for determining clinical significance was the proportion of patients classified as “successful” or “improved” when using the composite outcome classification (combining the treatment effects of all three outcomes). A significantly higher proportion of children assigned to the office-based vergence/accommodative therapy (73%) as compared with the other three treatment groups were classified as “successful” or “improved.” No significant differences were observed between the two home-based and the placebo therapy groups. Thus, based on the analysis of all four a priori criteria we conclude that there are both statistically significant and clinically meaningful differences between the groups.
The results of this large, randomized clinical trial are similar to those from the only previous randomized trial of vision therapy/orthoptics for CI in children, (29) in which three treatment groups were studied: home-based pencil push-ups, office-based vision therapy/orthoptics, and office-based placebo therapy. In that pilot study, only the office-based vergence/accommodative therapy group experienced a significant improvement in symptoms, NPC, and PFV.
The current study was not designed to show the maximal improvement possible with treatment. A longer duration of treatment may have resulted in additional changes in signs and symptoms. Office-based vergence/accommodative therapy programs for CI are often 12 to 24 office visits. (19-21) Our 12-week treatment program was based on the assumption that this represented the maximum length of time that a symptomatic patient who was not improving would stay on assigned treatment. Because our 12-week treatment program is at the low end of the range of recommended office-based therapy time for CI, it is possible that office-based vergence/accommodative therapy might have been effective in more patients had the treatment program been of longer duration. Likewise, a longer treatment program may have resulted in additional improvements by those assigned to the home-based treatment groups. It is also possible that using more home-based therapy procedures, or prescribing longer periods of daily home-based therapy may have produced different results. Answers to these questions will have to await further study.
While a placebo effect could be associated with any of the four treatments due to the patient’s expectation that the treatment would be effective, it is possible that office-based therapy might be more susceptible to the placebo effect due to the enthusiasm, caring, and compassion of a therapist who spends 60 minutes per week with the patient. (41) However, this is the second randomized trial of office-based vergence/accommodative therapy that was designed to control for the effect of the “therapist as a placebo”, (42) by designing placebo therapy that simulated bonafide therapy procedures and training therapists to behave identically for patients in both the office-based therapy groups. The data reported herein confirm that we were successful in achieving this objective as 85% of the patients assigned to office-based placebo therapy believed they had been assigned to the actual office-based vergence/accommodative therapy group. This compares well with our previous pilot study in which 90% of the patients assigned to placebo therapy believed they had been assigned to actual therapy. (29) A “no treatment” group was not included; therefore, it is not known whether any improvements were due to regression to the mean or natural history of the disease. However, any such effects should have affected all treatment groups similarly because there were no statistically significant or clinically relevant differences in any primary or secondary outcome measure among the treatment groups at baseline. Therefore, the observed differences in effectiveness between the office-based vergence/accommodative therapy and placebo therapy groups are most likely attributable to treatment effect.
The office-based vergence/accommodative therapy treatment program used in this study represents a typical approach used in clinical practice. (21) We conclude that this specific therapy protocol was successful in this study and should be applicable to children with similar clinical findings. A better understanding of which procedures were most effective will require additional research.
While this study was not designed to determine which factors within a particular group contributed to the outcome, the procedures which comprise the office-based vergence/accommodative therapy provide the greatest ability to control and manipulate stimulus parameters (e.g., vergence amplitude and accommodative demand) and the greatest ability to incorporate motor learning theory (e.g., modeling and demonstration, transfer of training, patient feedback). The weekly visits with the therapist during office-based vergence/accommodative therapy also permit the inclusion of a variety of procedures, which stress convergence, and accommodative abilities not typically addressed in home therapy programs. There were also differences among the treatment groups in time spent performing therapy and interacting with the therapist. The two office-based groups had an average prescribed therapy time of 135 minutes per week, the home-based computer vergence/accommodative therapy and pencil push-ups group averaged 115 minutes, and the home-based pencil push-ups group averaged 90 minutes including the weekly phone calls with the therapist. However, this study was not designed to equalize time spent performing therapy and/or interacting with a therapist; rather, it was designed as an effectiveness study evaluating three clinical treatments as typically provided in clinical practice. It is possible that the difference in treatment effect found in this study could be related to the office-based vergence-accommodative therapy group having been prescribed more minutes of therapy per day than the home-based groups. However, having a patient perform a greater amount of daily home-based therapy, particularly pencil push-ups, is likely impractical.
There are limited data in the literature suggesting a relationship between CI and ADHD. (43, 44) Although we asked parents whether their child had ADHD (i.e., parental report), this study was not designed to assess the relationship between CI and ADHD, was not powered for such subgroup analyses, nor was the diagnosis of ADHD definitive. However, investigation of this possible association is of interest and merits additional research.
We could identify no other sources of bias or confounding factors to explain our findings. Accounting for slight differences in the distribution of baseline factors between groups in the analyses did not alter the interpretation of the results. The follow-up visit rate was excellent and almost identical in all four groups. The investigators performing the 4, 8 and 12-week examinations were masked to the treatment group and the patients in the two office-based treatment groups were effectively masked as well. We did have slight differences in adherence among the groups, however, accounting for these differences in estimated adherence did not affect the results of the treatment group comparisons for the CI Symptom Survey, NPC, or PFV. The placebo effect was accounted for by incorporating the office-based placebo treatment group.
In translating the results into clinical practice, it is important to recognize that the results of our study can only be applied to children 9 to 17 years old with symptomatic CI. Adults with symptomatic CI may respond differently as suggested by our pilot study. (45) The findings of this study indicate that the specific form of vision therapy/orthoptics described herein as office-based vergence/accommodative therapy with home reinforcement is the most effective of the treatments studied in this trial for symptomatic CI in children, with about 75% of patients achieving normalization of or improvement in symptoms and signs within a 12-week period.
In regard to home-based therapy it is important to note that the data reported in this study for the pencil push-ups group were derived from a therapy program designed with considerably closer follow-up than is typical in clinical practice. Patients were called on a weekly basis by a therapist, completed a home log, and returned for office visits every fourth week. It is possible that this treatment would be less effective if prescribed according to usual clinical practice, which does not include weekly telephone calls from a therapist and often has less frequent follow-up. The results of the CITT pilot study, in which the home-based pencil push-ups group did not receive weekly phone calls, provide some support for this hypothesis as none of the 11 patients (0/11) were classified as successful or improved. (29)
It is easy to understand the clinical popularity of home-based treatment because of its simplicity and cost effectiveness. Both home-based pencil push-ups and home-based computer vergence/accommodative therapy can be taught to the patient in a short time and require fewer follow-up visits than office-based therapy (4 visits for home-based treatments vs. 12 visits for office-based treatment). While our study was not designed to conduct a cost-utility analysis, this would be worthwhile to explore in future research.
There are a number of interesting clinical questions that cannot be answered at this time. It is possible that there may be psychological effects of the interaction between the therapist and the patient that could affect the office-based and home-based treatment group’s results differentially, if these effects were present, and if they were dependent upon patient-therapist contact time. In this study we did not have a placebo home-based therapy group and thus, do not know whether the changes found in the two home-based groups are due to a real or a placebo treatment effect. It is possible that different protocols that more closely monitor and encourage adherence would affect the outcomes. For the office-based vergence-accommodative therapy regimen, we do not know which procedures were most effective or why, and whether the treatment protocol can be modified to make it more effective. This includes understanding the nature of the synergistic role of the active home treatment component as well as the therapist interaction. It is also not known whether the treatment effect will be sustained over time. Therefore, a conclusion about the long-term benefit of treatment must await the results of the 12-month follow-up study we are conducting.
Conclusion
This large-scale multi-center, randomized clinical trial of treatments for symptomatic children with CI demonstrates that a 12 week regimen of office-based vergence/accommodative therapy with home reinforcement is more effective than a 12 week program of home-based pencil push-ups or home-based computer vergence/accommodative therapy and pencil push-ups in improving symptoms and signs associated with CI.
Acknowledgments
Supported by the National Eye Institute of the National Institutes of Health, Department of Health and Human Services
Appendix
The Convergence Insufficiency Treatment Trial Investigator Group
Writing Committee
Lead Authors: Mitchell Scheiman, OD; Susan Cotter, OD,MS, Gladys Lynn Mitchell, MAS, Marjean Kulp, OD, MS, Michael Rouse, OD, MS, Richard Hertle, MD, Maryann Redford, DDS, MPH
Additional Writing Committee Members (Alphabetical): Jeffrey Cooper, MS, OD, Rachel Coulter, OD, Michael Gallaway, OD, David Granet, MD, Kristine Hopkins, OD, Brian G. Mohney, MD, Susanna Tamkins, OD
Clinical Sites
Sites are listed in order of the number of patients enrolled in the study with the number of patients enrolled is listed in parentheses preceded by the site name and location. Personnel are listed as (PI) for principal investigator, (SC) for coordinator, (E) for examiner, and (VT) for therapist.
Study Center: Bascom Palmer Eye Institute (35)
Susanna Tamkins, OD (PI); Hilda Capo, MD (E); Mark Dunbar, OD (E); Craig McKeown, MD (CO-PI); Arlanna Moshfeghi, MD (E); Kathryn Nelson, OD (E); Vicky Fischer, OD (VT); Adam Perlman, OD (VT); Ronda Singh, OD (VT); Eva Olivares (SC); Ana Rosa (SC); Nidia Rosado (SC); Elias Silverman (SC)
Study Center: SUNY College of Optometry (28)
Jeffrey Cooper, MS, OD (PI); Audra Steiner, OD (E, Co-PI); Marta Brunelli (VT); Stacy Friedman, OD (VT); Steven Ritter, OD (E); Lily Zhu, OD (E); Lyndon Wong, OD (E); Ida Chung, OD (E); Kaity Colon (SC)
Study Center: UAB School of Optometry (28)
Kristine Hopkins, OD (PI); Marcela Frazier, OD (E); Janene Sims, OD (E); Marsha Swanson, OD (E); Katherine Weise, OD (E); Adrienne Broadfoot, MS, OTR/L (VT, SC); Michelle Anderson, OD (VT); Catherine Baldwin (SC)
Study Center: NOVA Southeastern University (27)
Rachel Coulter, OD (PI); Deborah Amster, OD (E); Gregory Fecho, OD (E); Tanya Mahaphon, OD (E); Jacqueline Rodena, OD (E); Mary Bartuccio, OD (VT); Yin Tea, OD (VT); Annette Bade, OD (SC)
Study Center: Pennsylvania College of Optometry (25)
Michael Gallaway, OD (PI); Brandy Scombordi, OD (E); Mark Boas, OD (VT); Tomohiko Yamada, OD (VT); Ryan Langan (SC), Ruth Shoge, OD (E); Lily Zhu, OD (E)
Study Center - The Ohio State University College of Optometry (24)
Marjean Kulp, OD, MS (PI); Michelle Buckland, OD (E); Michael Earley, OD, PhD (E); Gina Gabriel, OD, MS (E); Aaron Zimmerman, OD (E); Kathleen Reuter, OD (VT); Andrew Toole, OD, MS (VT); Molly Biddle, MEd (SC); Nancy Stevens, MS, RD, LD (SC)
Study Center: Southern California College of Optometry (23)
Susan Cotter, OD, MS (PI); Eric Borsting, OD, MS (E); Michael Rouse, OD, MS, (E); Carmen Barnhardt, OD, MS (VT); Raymond Chu, OD (VT); Susan Parker (SC); Rebecca Bridgeford (SC); Jamie Morris (SC); Javier Villalobos (SC)
Study Center: University of CA San Diego: Ratner Children’s Eye Center (17)
David Granet, MD (PI); Lara Hustana, OD (E); Shira Robbins, MD (E); Erica Castro (VT); Cintia Gomi, MD (SC)
Study Center: Mayo Clinic (14)
Brian G. Mohney, MD (PI); Jonathan Holmes, MD (E); Melissa Rice, OD (VT); Virginia Karlsson, BS, CO (VT); Becky Nielsen (SC); Jan Sease, COMT/BS (SC); Tracee Shevlin (SC)
CITT Study Chair
Mitchell Scheiman, OD (Study Chair); Karen Pollack (Study Coordinator); Susan Cotter, OD, MS (Vice Chair); Richard Hertle, MD (Vice Chair); Michael Rouse, OD, MS (Consultant)
CITT Data Coordinating Center
Gladys Lynn Mitchell, MAS, (PI); Tracy Kitts, (Project Coordinator); Melanie Bacher (Programmer); Linda Barrett (Data Entry); Loraine Sinnott, PhD (Biostatistician); Kelly Watson (Student worker); Pam Wessel (Office Associate)
National Eye Institute, Bethesda, MD
Maryann Redford, DDS., MPH
CITT Executive Committee
Mitchell Scheiman, OD; G. Lynn Mitchell, MAS; Susan Cotter, OD, MS; Richard Hertle, MD; Marjean Kulp, OD, MS; Maryann Redford, DDS., MPH; Michael Rouse, OD, MSEd
Data and Safety Monitoring Committee
Marie Diener-West, PhD, Chair, Rev. Andrew Costello, CSsR, William V. Good, MD, Ron D. Hays, PhD, Argye Hillis, PhD (Through March 2006), Ruth Manny, OD, PhD
Footnotes
Application To Clinical Practice: Office-based vergence accommodative therapy is an effective treatment for children with symptomatic CI.
Trial Registration: clinicaltrials.gov identifier: NCT00338611
References
- 1.Letourneau JE, Lapierre N, Lamont A. The relationship between convergence insufficiency and school achievement. Am J Optom Physiol Opt. 1979;56:18–22. doi: 10.1097/00006324-197901000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Letourneau JE, Ducic S. Prevalence of convergence insufficiency among elementary school children. Can J Optom. 1988;50:194–197. [Google Scholar]
- 3.Porcar E, Martinez-Palomera A. Prevalence of general binocular dysfunctions in a population of university students. Optom Vis Sci. 1997;74:111–113. doi: 10.1097/00006324-199702000-00023. [DOI] [PubMed] [Google Scholar]
- 4.Rouse MW, Borsting E, Hyman L, et al. Frequency of convergence insufficiency among fifth and sixth graders. Optom Vis Sci. 1999;76:643–649. doi: 10.1097/00006324-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 5.Daum KM. Convergence insufficiency. Am J Optom Physiol Opt. 1984;61:16–22. doi: 10.1097/00006324-198401000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Cooper J, Duckman R. Convergence insufficiency: incidence, diagnosis, and treatment. J Am Optom Assoc. 1978;49:673–680. [PubMed] [Google Scholar]
- 7.Kent PR, Steeve JH. Convergence insufficiency: incidence among military personnel and relief by orthoptic methods. Military Surgeon. 1953;112:202–205. [PubMed] [Google Scholar]
- 8.Poynter HL, Schor C, Haynes HM, Hirsch J. Oculomotor functions in reading disability. Am J Optom Physiol Optics. 1982;59:126–127. doi: 10.1097/00006324-198202000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Mazow ML. The convergence insufficiency syndrome. J Ped Ophthalmol. 1971;8:243–244. [Google Scholar]
- 10.Duke-Elder S, Wybar K. Ocular Motility and Strabismus. In: Duke-Elder S, editor. System of Ophthalmology. Mosby; St Louis: 1973. [Google Scholar]
- 11.Pickwell LD, Hampshire R. The significance of inadequate convergence. Ophthal Physiol Opt. 1981;1:13–18. [PubMed] [Google Scholar]
- 12.Borsting E, Rouse MW, Deland PN, et al. Association of symptoms and convergence and accommodative insufficiency in school-age children. Optometry. 2003;74(1):25–34. [PubMed] [Google Scholar]
- 13.Borsting EJ, Rouse MW, Mitchell GL, et al. Validity and reliability of the revised convergence insufficiency symptom survey in children aged 9-18 years. Optom Vis Sci. 2003;80:832–838. doi: 10.1097/00006324-200312000-00014. [DOI] [PubMed] [Google Scholar]
- 14.von Noorden GK. Binocular Vision and Ocular Motility: Theory and Management of Strabismus. Mosby; St Louis: 1996. [Google Scholar]
- 15.Abrams D. Duke-Elder’s practice of refraction. Churchill-Livingstone; Edinburgh: 1993. [Google Scholar]
- 16.Cibis G, Tongue A, Stass-Isern M. Decision making in pediatric ophthalmology. Mosby-Year Book; Philadelphia: 1993. [Google Scholar]
- 17.Pratt-Johnson JA, Tillson G. Management of Strabismus and Amblyopia. Thieme Medical Publishers; New York: 2001. [Google Scholar]
- 18.von Noorden G, Helveston E. Strabismus: A decision making approach. Mosby-Year Book; St. Louis: 1994. [Google Scholar]
- 19.Griffin JR, Grisham JD. Binocular Anomalies: Diagnosis and Vision Therapy. Butterworth-Heinemann; Boston: 2002. [Google Scholar]
- 20.Press LJ. Applied Concepts in Vision Therapy. Mosby-Year Book; St. Louis: 1997. [Google Scholar]
- 21.Scheiman M, Wick B. Clinical Management of Binocular Vision: Heterophoric, Accommodative and Eye Movement Disorders. Lippincott, Williams and Wilkins; Philadelphia: 2002. [Google Scholar]
- 22.Hugonnier R, Clayette-Hugonnier C. Strabismus, Heterophoria and Ocular Motor Paralysis. CV Mosby; St. Louis: 1969. [Google Scholar]
- 23.Gallaway M, Scheiman M, Malhotra K. The effectiveness of pencil pushups treatment for convergence insufficiency: a pilot study. Optom Vis Sci. 2002;79:265–267. doi: 10.1097/00006324-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Chin FH, Faibish B, Hisaka C, Thal L, Tsuda K. A survey of the treatment of convergence insufficiency. J Beh Optom. 1995;6:91–92. 109. [Google Scholar]
- 25.Scheiman M, Cooper J, Mitchell GL, et al. A survey of treatment modalities for convergence insufficiency. Opt Vis Sci. 2002;79:151–157. doi: 10.1097/00006324-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Scheiman M, Mitchell GL, Cotter S, et al. In Reply: Convergence Insufficiency Randomized Clinical Trial. Arch Ophthalmol. 2005;123:1760–1761. doi: 10.1001/archopht.123.1.14. [DOI] [PubMed] [Google Scholar]
- 27.Ciuffreda K. The scientific basis for and efficacy of optometric vision therapy in nonstrabismic accommodative and binocular vision disorders. Optometry. 2002;73:735–762. [PubMed] [Google Scholar]
- 28.Scheiman M, Cotter S, Rouse M, et al. Randomised clinical trial of the effectiveness of base-in prism reading glasses versus placebo reading glasses for symptomatic convergence insufficiency in children. Br J Ophthalmol. 2005;89:1318–1323. doi: 10.1136/bjo.2005.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheiman M, Mitchell GL, Cotter S, et al. A randomized trial of the effectiveness of treatments for convergence insufficiency in children. Arch Ophthalmol. 2005;123:14–24. doi: 10.1001/archopht.123.1.14. [DOI] [PubMed] [Google Scholar]
- 30.Sheard C. Zones of ocular comfort. Am J Optom. 1930;7:9–25. [Google Scholar]
- 31.Kushner B. Editorial: The treatment of convergence insufficiency. Arch Ophthalmol. 2005;123:100–101. doi: 10.1001/archopht.123.1.100. [DOI] [PubMed] [Google Scholar]
- 32.CITT Investigator Group . Convergence Insufficiency Treatment Trial. 2004. [Google Scholar]
- 33.Borsting E, Rouse MW, De Land PN, Convergence Insufficiency and Reading Study (CIRS) group Prospective comparison of convergence insufficiency and normal binocular children on CIRS symptom surveys. Optom Vis Sci. 1999;76(4):221–228. doi: 10.1097/00006324-199904000-00025. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell G, Scheiman M, Borsting E, et al. Evaluation of a symptom survey for convergence insufficiency patients. Optom Vis Sci. 2001;12:37 s. [Google Scholar]
- 35.The Convergence Insufficiency Treatment Trial Investigator Group The Convergence Insufficiency Treatment Trial: design, methods, and baseline data. Ophthal Epidemiology. 2008 doi: 10.1080/09286580701772037. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borsting E, Rouse MW, De Land PN, CIRS Group Prospective comparison of convergence insufficiency and normal binocular children on CIRS symptom surveys. Optom Vis Sci. 1999;76:221–228. doi: 10.1097/00006324-199904000-00025. [DOI] [PubMed] [Google Scholar]
- 37.Hintze J. NCSS and PASS, Kaysville, Utah, Number Cruncher Statistical Systems. 2000. [Google Scholar]
- 38.Rouse MW, Hyman L, Hussein M. Reliability of binocular vision measurements used in the classification of convergence insufficiency. Optom Vis Sci. 2002;79:254–264. doi: 10.1097/00006324-200204000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Cohen J. Statistical Power Analyses for the Behavioral Sciences. Earlbaum Associates; Hillsdale, NH: 1988. [Google Scholar]
- 40.Sloan JA, Cella D, RD H. Clinical significance of patient-reported questionnaire data: another step toward consensus. Journal of Clinical Epidemiology. 2005;58:1217–1219. doi: 10.1016/j.jclinepi.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 41.Brody H. The doctor as a therapeutic agent: a placebo effect research agenda. In: Harrington A, editor. The Placebo Effect: An Interdisciplinary Exploration. Harvard University Press; Cambridge: 1997. [Google Scholar]
- 42.Margo C. The placebo effect. Surv Ophthalmol. 1999;44:31–44. doi: 10.1016/s0039-6257(99)00060-0. [DOI] [PubMed] [Google Scholar]
- 43.Borsting E, Rouse M, Chu R. Measuring ADHD behaviors in children with symptomatic accommodative dysfunction or convergence insufficiency: a preliminary study. Optometry. 2005;76:588–592. doi: 10.1016/j.optm.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 44.Granet DB, Gomi CF, Ventura R, Miller-Scholte A. The relationship between convergence insufficiency and ADHD. Strabismus. 2005;13:163–168. doi: 10.1080/09273970500455436. [DOI] [PubMed] [Google Scholar]
- 45.Scheiman M, Mitchell GL, Cotter S, et al. A randomized clinical trial of vision therapy/orthoptics versus pencil pushups for the treatment of convergence insufficiency in young adults. Optom Vis Sci. 2005;82:583–595. doi: 10.1097/01.opx.0000171331.36871.2f. [DOI] [PubMed] [Google Scholar]