Abstract
The construction of CNS active imidazo[1,5-a][1,4]benzodiazepines has been improved in a one-pot annulation process.
Keywords: GABAA/BzR; imidazo[1,5-a][1,4]benzodiazepines; ethyl isocyanoacetate; annulation
Introduction
Imidazo[1,5-a][1,4]benzodiazepines are well docmented to exhibit potent activity at GABAA/Bz receptors. This series belongs to one of the very few chemical families which have been extensively investigated for GABAA/Bz receptor mediated activity.1,2 Flumazenil (Ro 15-1788), an imidazo[1,5-a][1,4]benzodiazepine, was earlier shown to bind to central GABAA receptors with little or no intrinsic agonist activity but with the ability to block the activity of an agonist or inverse agonist at GABAA/Bz receptors.2 It is employed principally as an antidote to reverse the effect of exogenous benzodiazepines.3 More recently ligands such as flumazenil have been shown to behave as weak inverse agonists or weak agonists depending on the biological paradigm employed. Many procedures have been reported to date to synthesize this imidazo-type of structure.4,5 Most of them have been achieved in low yield through an iminophosphate/chloride intermediate.
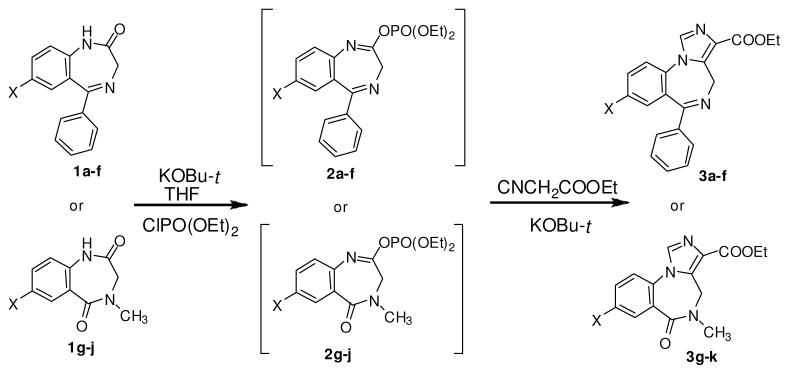
As part of a program directed toward the development of clinically relevant imidazo[1,5-a][1,4]-benzodiazepines,1,6 the construction of the imidazo-ring was considered a crucial step for the synthesis of gram quantities of imidazobenzodiazepine analogs. The previous process of using ethyl isocyanoacetate and iminophosphates/chlorides7,8 was employed coupled with different solvents and bases, but the yields of these reactions were very low (15–30%). Since this step was highly convergent and at the very end of the synthetic route, it affected the overall economy of the route in a dilatorious manner. It was, therefore, of interest to improve the annulation sequence for this series of CNS-active ligands.
Certainly a one-pot annulation process for the construction of imidazobenzodiazepines had been developed.(7,8) This procedure permited the condensation of the less stable iminophosphates with ethyl isocyanoacetate under basic conditions without isolation of the less stable iminophosphates and the pre-formation of the carbanion of ethyl isocyanoacetate. For instance, Watjen et al.7a had reported the formation of iminophosphates by using NaH or LDA in DMF, and then directly reacting this mixture with ethyl isocyanoacetate and potassium tert-butoxide to offer the imidazo molecules in 47% yield. Fryer and coworkers7b,c had reported the use of potassium tert-butoxide as the base in both steps, and DMF as well as THF were chosen as solvents in these cases; however, this process gave the ligands in only 44% yield. The latest report from Fryer’s procedure gave the target imidazo framework in 30% yield.8
Scope and Limitations
To improve the one-pot annulation reaction, the procedure was modified and after many attempts it was found the ratio of the reagent combination and reaction temperature were critical for good yields on a consistent basis. As shown in scheme 1, the initial amount of potassium tert-butoxide used to form the iminophosphates should be kept at 1.1 equivalents as compared to the amide (1a–k). The yield of the reaction was much lower if more potassium tert-butoxide (>1.1 equ-ivalents) was employed. The amount of diethylchlorophosphate (used as received) employed was only slightly higher (1.3 equivalents) than the potassium tert-butoxide to convert all the starting amide into the desired iminophosphates (1a–k). Importantly, the addition of ethyl isocyanoacetate, followed by the second addition of potassium tert-butoxide should be carried out at low temperature (−35 °C is recommended). However, a temperature lower than −35 °C (such as −78 °C) was also acceptable in this procedure and the second addition of potassium tert-butoxide was kept at 1.1 equivalents. This procedure made the separation of the product much easier which was critical for gram scale reactions. Most of the desired imidazobenzodiazepines were precipitated from ether after work-up for no chromatography was needed.
Scheme 1.
A variety of different amidobenzodiazepines (1a–h) were chosen as substrates for this study (See Table-1). Both the 5-phenyl-benzodiazepines (1a–f) and N-methyl-6-oxo-benzodiazepines (1g–h) readily condensed with ethyl isocyanoacetate to give the desired imidazobenzodiazepines (3a–k) in 70–89% yield in this improved process. The substituents in ring-A such as F, Cl, Br of 1a–h did not effect the yield of the process. The α-substituted amidobenzodiazepines (1c–e, and 1i), which are more hindered than their unsubstituted parents (1a–b and 1f–1j), also gave the imidazo analogs in 70–81% yield. In the case of optically active substrates (1c–e, and 1i), this procedure provided the desired imidazo products without loss in optical activity. This is key for the R and S isomers have much different BzR/GABAergic receptor binding profiles. All of these reactions can be scaled up with no difficulty. The lesser amounts of 3d–3g simply reflect the lesser need for this material. When this process was employed for reactions of tert-butyl isocyanoacetate, the corresponding imidazobenzodiazepines were obtained in 70% yield.
Table 1.
Examples of Imidazo[1,5-a][1,4]benzodiazepines Obtained Using the General Procedure
| Starting material | Product | Yield (%) Scale |
|---|---|---|
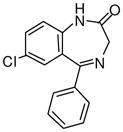 1a
1a
|
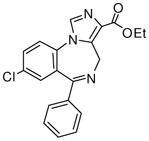 3a
3a
|
87 (11 g scale) |
 1b
1b
|
 3b
3b
|
77 (15 g scale) |
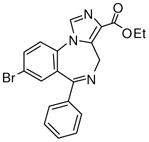
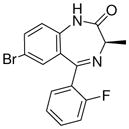 1c
1c
|
 3c
3c
|
74 (10 g scale) |
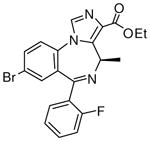
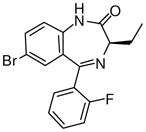 1d
1d
|
 3d
3d
|
73 (3.6 g scale) |
 1e
1e
|
 3e
3e
|
72 (1 g scale) |
 1f
1f
|
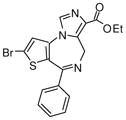 3f
3f
|
75 (5 g scale) |
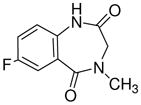 1g
1g
|
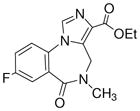 3g
3g
|
75 (2 g scale) |
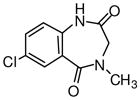 1h
1h
|
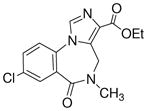 3h
3h
|
89 (15 g scale) |
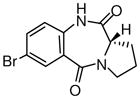 1i
1i
|
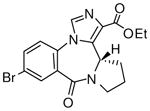 3i
3i
|
81 (15 g scale) |
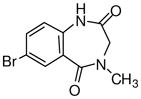 1j
1j
|
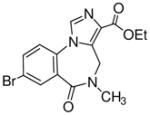 3j
3j
|
72 (15 g scale) |
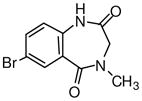 1k
1k
|
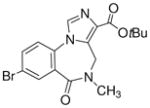 3k
3k
|
70 (20 g scale) |
An efficient, practical, improved, one-pot annulation reaction for the construction of potent BzR active imidazobenzodiazepines is described. A variety of substrates were successfully employed in this procedure. This one-pot process required neither the isolation of the unstable intermediates nor does it require the pre-formation of the carbanion of the isocyanocetate. Moreover, potassium tert-butoxide is a safer and easier-to-handle base for scale-up, as compared to other bases such as NaH, LHMDS and LDA. In addition, no chromatography was required for this process.
General procedure for synthesis of imidazo[1,5-a][1,4]benzodiazepines
Potassium t-butoxide (1.1 mmol) was added to a solution of the amidobenzodiazepine (1.0 mmol) in THF (20 mL) at 0 °C under argon. After stirring the mixture at 0 °C for 20 min, the reaction mixture was cooled to −35 °C and diethylchlorophosphate (1.3 mmol) was added slowly. After stirring this mixture at 0 °C for 30 min, the reaction flask was cooled to −35 °C and ethyl isocyanoacetate (1.1 mmol) was added, followed by addition of potassium t-butoxide (1.1 mmol). After stirring this mixture at ambient temperature for 4 hours, the reaction solution was quenched with a saturated aqueous solution of NaHCO3 (30 mL) and extracted with EtOAc (50 mL × 3). The combined organic layers were dried (Na2SO4), concentrated and precipitated from ether to give most of the imidazobenzodiazepine. The mother liquor was purified by flash chromatography on silica gel (40–60 % EtOAc in hexanes) to afford additional imidazobenzodiazepine (yields 70–89%).
Scale up procedure for synthesis of imidazo[1,5-a][1,4]benzodiazepines
Potassium t-butoxide (7.5g, 66.91 mmol) was added to a solution of 1j (15g, 55.76 mmol) in 1500mL anhydrous THF at 0°C and stirred for 20 min. The reaction mixture was cooled to −35°C and diethylchlorophosphate (12.5-mL, 72.49 mmol) was added slowly. After stirring at 0°C for 30 min, the reaction mixture was cooled to −78°C and ethyl isocyanoacetate (8.19mL, 72.49 mmol) was added followed by the addition of potassium t-butoxide (6.88g, 61.33 mmol). After stirring at room temperature for 4h, the reaction was quenched with a saturated aqueous solution of NaHCO3 and extracted with EtOAc. The combined organic layers were dried (Na2SO4) and concentrated to get a solid residue. This solid residue was treated with ether and the product 3j was precipitated as an off-white solid. The mother liquor was further purified by flash chromatography on silica gel (gradient elution 40–60% EtOAc in hexane) to afford additional product 3j with overall yield of 72% (14.61g).
Ethyl-8-chloro-6-phenyl-4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylate (3a)
Mp: 180–182 °C. 1H NMR (300 MHz, CDCl3) δ 7.93 (s, 1H), 7.68 (dd, 1H, J = 2.3, 2.3 Hz), 7.58 (s, 1H), 7.55-7.37 (m, 6H), 6.06 (d, 1H, J = 12.3 Hz), 4.70 (m, 2H), 4.15 (d, 1H, J = 14Hz), 1.46 (t, 3H). HRMS for C20H16ClN3O2: (M+1) 366.1007. Found: 366.1000.
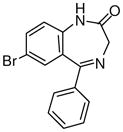
Ethyl-8-bromo-6-phenyl-4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylate (3b)
Mp: 180–182 °C. 1H NMR (300 MHz, CDCl3) δ 7.95 (s, 1H), 7.82 (dd, 1H, J = 2.2, 8.6 Hz), 7.60 (d, 1H, J = 2.2Hz), 7.53-7.40 (m, 6H), 6.08 (d, 1H, J = 12.3 Hz), 4.49-4.38 (m, 2H), 4.09 (d, 1H, J = 12.1 Hz), 1.44 (t, 3H, J = 7.1Hz). EIMS m/e (relative intensity, %) 411 (M+1, 34), 410 (M+, 8), 409 (34), 365 (61), 337 (100), 335 (100), 285 (21), 232 (17). Anal. Calcd. for C20H16BrN3O2: C, 58.55; H, 3.93; N, 10.24. Found: C, 58.30; H, 3.91; N, 9.94.
(R)-Ethyl-8-bromo-4-methyl-6-(2′fluorophenyl)-4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylate (3c)
White solid. Mp: 261–262 °C. [α]D25 −10.9 (c, 0.54, EtOAc); 1H NMR (300 MHz, CDCl3) δ 7.92 (s, 1H), 7.72 (dd, 1H, J = 1.5, 8.2 Hz), 7.60 (t, 1H, J = 6.9 Hz), 7.48 (d, 1H, J = 8.5 Hz), 7.49-7.42 (m, 2H), 7.29-7.23 (m, 1H), 7.05 (t, 1H, J = 9.3 Hz), 6.71 (q, 1H, J = 7.3 Hz), 4.41 (m, 2H), 1.42 (t, 3H, J = 7.1 Hz), 1.29 (d, 3H, J = 7.2 Hz). EIMS m/e (relative intensity, %) 442 (M+, 5), 428 (7), 381 (58), 355 (100). Anal. Calcd. for C21H17BrFN3O2: C, 57.03; H, 3.87; N, 9.50. Found: C, 57.13; H, 3.89; N, 9.51.
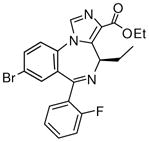
(R)-Ethyl-8-bromo-4-ethyl-6-(2′-fluorophenyl)-4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylate(3d)
White solid. Mp: 253–254 °C; 1H NMR (300 MHz, CDCl3): δ 7.93 (s, 1H), 7.72 (dd, 1H, J = 8.1 Hz), 7.59 (t, 1H, J = 7.5 Hz), 7.48-7.42 (m, 2H), 7.28-7.23 (m, 1H), 7.06 (t, 1H, J = 9.3 Hz), 6.51 (q, 1H, J = 7.8 Hz), 4.43 (m, 2 H), 1.76-1.52 (m, 3H), 1.43 (t, 3H, J = 7.2 Hz), 0.96 (t, 3H, J = 7.2 Hz). HRMS Calcd. for C22H19BrFN3O2: 456.0723; Found: 456.0709.
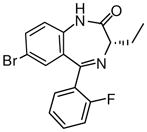
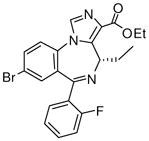
(S)-Ethyl-8-bromo-4-ethyl-6-(2′-fluorophenyl)-4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylate (3e)
White solid. Mp: 254–255 °C; 1H NMR (300 MHz, CDCl3): δ 7.92 (s, 1H), 7.72 (dd, 1H, J = 7.2 Hz), 7.59 (t, 1H, J = 6.9 Hz), 7.48-7.41 (m, 2H), 7.28-7.23 (m, 1H), 7.06 (t, 1H, J = 9.3 Hz), 6.51 (m, 1H), 4.45-4.37 (m,2H), 1.75-1.54 (m,3H), 1.42 (t, 3H, J = 6.9 Hz), 0.94 (t, 3H, J = 7.2 Hz). HRMS Calcd. for C22H19BrFN3O2: 456.0723; Found: 456.0703.
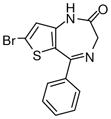
Ethyl-7-bromo-6-phenyl-4H-imidazo[1,5-a]-thieno-[2,3-f][1,4]diazepine-3-carboxylate(3f)
White solid. Mp: 180–182 °C. 1H NMR (300 MHz, CDCl3) δ 8.33 (s, 1 H), 8.02 (s, 1H), 7.63-7.35 (m, 5 H), 5.31 (s, 2H), 4.34-4.27 (q, 2H, J = 7.1 Hz), 1.32 (t, 3H, J = 7.1 Hz). HRMS for C18H14BrN3O2S: 416.0068. Found: 416.0049.
Ethyl-8-fluoro-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylate (3g)
Off-white solid. Mp: 200–202 °C. 1H NMR (300 MHz, CDCl3) δ 7.88 (s, 1H), 7.81-7.78 (m, 1H), 7.74-7.36 (m, 2H), 5.25 (br s, 1H), 4.44 (q, 2H, J = 7.3 Hz), 4.38 (br s, 1H), 3.27 (s, 3H), 1.47 (t, 3H, J = 7.3 Hz). HRMS for C15H14FN3O3: (M+1) 304.1097. Found: 304.1091. The spectral data for 3g were identical to the published values.2,4
Ethyl-8-chloro-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylate (3h)
Mp: 200–202 °C. 1H NMR (300 MHz, CDCl3) δ 8.10 (s, 1H), 7.90 (s, 1H), 7.62 (dd, 1H, J = 8.6, 2.5 Hz), 7.40 (d, 1H, J = 8.6 Hz), 5.23 (br s, 1H), 4.46 (q, 2H, J = 7.1 Hz), 4.13 (br s, 1H), 3.27 (s, 3H), 1.47 (t, 3H, J = 7.1 Hz).). EIMS for C15H14ClN3O2: m/e (relative intensity, %) 319 (M+, 100).
(S)-Ethyl 7-bromo-11,12,13,13a-tetrahydro-9-oxo-9H-imidazo[1,5-a]pyrrolo[2,1-d][1,4]benzodiazepine-1-carboxylate (3i)
White solid. Mp: 248.5–249 °C; [α]D25+45 (c, 1.0, CHCl3); 1H NMR (300 MHz, CDCl3) δ 8.23 (s, 1H), 7.82 (s, 1H), 7.75 (d, 1H, J = 7.5 Hz), 7.26 (d, 1H, J = 10.0 Hz), 4.72 (d, 1H, J = 6.0 Hz), 4.38 (q, 2H, J = 7.5 Hz), 3.75 (m, 1H), 3.56-3.48 (m, 2H), 2.27-2.14 (m, 3H), 1.41 (t, 3H, J = 7.5 Hz). EIMS m/e (relative intensity, %) 390 (M+, 10), 345 (60), 316 (100), 314 (98), 154 (24). Anal. Calcd. for C17H16BrN3O3: C, 52.32; H, 4.13; N, 10.77. Found: C, 52.70; H, 4.48; N, 10.64. HRMS for C17H16BrN3O3: 389.0375. Found: 389.0373.
Ethyl-8-bromo-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylate (3j)
Off-white solid. Mp: 192–193 °C. 1H NMR (300 MHz, CDCl3) δ 8.23 (s, 1H), 7.79 (s, 1H), 7.77 (d, 1H, J = 2.3 Hz), 7.35 (d, 1H, J = 6.4 Hz), 5.17 (br s, 1H), 4.43 (m, 3H), 3.28 (s, 3H), 1.45 (t, 3H, J = 7.1 Hz). EIMS for C15H14BrN3O3: m/e (relative intensity, %) 364 (M+, 100).
tert-Butyl-8-bromo-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylate (3k)
Mp: 180–183 °C. 1H NMR (300 MHz, DMSO-d6) δ 8.33 (s, 1H), 7.92 (s, 1H), 7.69 (d, 1H, J = 10.8 Hz), 7.03 (d, 1H, J = 8.7 Hz), 4.71 (br s, 1H), 4.12 (br s, 1H), 3.10 (s, 3H), 1.56 (s, 9H). EIMS for C17H18BrN3O3: m/e (relative intensity, %) 394 (M+, 100).
Acknowledgments
We thank the National Institute of Mental Health (MH46851) and the Lynde and Harry Bradley Foundation for generous financial support.
References
- 1.Clayton T, Chen JL, Ernst M, Richter L, Cromer BA, Morton CJ, Ng H, Kaczorowski CC, Helmstetter FJ, Furtmüller R, Ecker G, Parker MW, Sieghart W, Cook JM. Curr Med Chem. 2007;14:2755. doi: 10.2174/092986707782360097. [DOI] [PubMed] [Google Scholar]
- 2.Polc P, Laurent JP, Scherchlicht R, Haefely W. Naunyn Schmiedebergs Arch Pharmacol. 1981;316:317. doi: 10.1007/BF00501364. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman EJ, Warren EW. Clinical Pharmacy. 1993;12:641. [PubMed] [Google Scholar]
- 4.Hoffmann-La Roche F Co. A.-G., Switz. Diazepine Derivatives. Neth. Appl. 7803585, 1978. Chem Abstr. 1978;90:152254g. [Google Scholar]
- 5.Rogers-Evans M, Spurr P, Hennig M. Tetrahedron Lett. 2003;44:2425. and references cited therein. [Google Scholar]
- 6.(a) Cook JM, Han D, Clayton T. U.S. Pat. Appl US 2006258643 CAN 145:505485 ; (b) Li X, Yu J, Atack JR, Cook JM. Med Chem Res. 2004;13:259. [Google Scholar]; (c) Li X, Cao H, Zhang C, Furtmueller R, Fuchs K, Huck S, Sieghart W, Deschamps J, Cook JM. J Med Chem. 2003;42:5567. doi: 10.1021/jm034164c. [DOI] [PubMed] [Google Scholar]; (d) Li X, Ma C, He X, Yu J, Han D, Zhang C, Atack JR, Cook JM. Med Chem Res. 2002;11:504. [Google Scholar]; (e) Liu R, Hu RJ, Zhang P, Skolnick P, Cook JM. J Med Chem. 1996;39:1928. doi: 10.1021/jm950887n. [DOI] [PubMed] [Google Scholar]; (f) Zhang P, Zhang W, Liu R, Harris B, Skolnick P, Cook JM. J Med Chem. 1995;38:1679. doi: 10.1021/jm00010a013. [DOI] [PubMed] [Google Scholar]; (g) Zhang P, Liu R, McKernan RM, Wafford K, Cook JM. Med Chem Res. 1995;5:487. [Google Scholar]; (h) Liu R, Zhang P, McKernan RM, Wafford K, Cook JM. Med Chem Res. 1995;5:700. [Google Scholar]
- 7.(a) Watjen F, Baker R, Engelstoff M, Herbert R, MacLeod A, Knight A, Merchant K, Moseley J, Saunders J. J Med Chem. 1989;32(10):2282. doi: 10.1021/jm00130a010. [DOI] [PubMed] [Google Scholar]; (b) Fryer IR, Walser A. Eur Pat Appl EP. 1985:135770. [Google Scholar]; Chem Abstr. 1985;103:160545. [Google Scholar]; (c) Fryer IR, Gu ZQ, Wang CG. J Heterocyclic Chem. 1991;28(7):1661. [Google Scholar]
- 8.Anzini M, Braile C, Valenti S, Cappelli A, Vomero S, Marinelli L, Limongelli V, Novellino E, Betti L, Giannaccini G, Lucacchini A, Ghelardini C, Norcini M, Makovec F, Giorgi G, Fryer IR. J Med Chem. 2008;51:4730–4743. doi: 10.1021/jm8002944. [DOI] [PubMed] [Google Scholar]