Synopsis
AMP-activated protein kinase (AMPK) is a phylogenetically conserved fuel-sensing enzyme that is present in all mammalian cells. During exercise, it is activated in skeletal muscle in humans, and at least in rodents, also in adipose tissue, liver and perhaps other organs by events that increase the AMP/ATP ratio. When activated AMPK stimulates energy generating processes such as glucose uptake and fatty acid oxidation and decreases energy consuming processes such as protein and lipid synthesis. Exercise is perhaps the most powerful physiological activator of AMPK and a unique model for studying its many physiological roles. In addition, it improves the metabolic status of rodents with a metabolic syndrome phenotype, as does treatment with AMPK activating agents; therefore, it is tempting to attribute the therapeutic benefits of regular physical activity to activation of AMPK. Here we review the acute and chronic effects of exercise on AMPK activity in skeletal muscle and other tissues. We also discuss the potential role of AMPK activation in mediating the prevention and treatment by exercise of specific disorders associated with the metabolic syndrome including type 2 diabetes and Alzheimer’s disease.
Keywords: AMP activated protein kinase, exercise, muscle, metabolic syndrome, AMPK
(1) Introduction
AMP-activated protein kinase (AMPK) is a phylogenetically conserved fuel-sensing enzyme that is present in both primitive unicellular organisms and mammals [59]. It is activated by stresses that increase the cellular concentration of AMP relative to ATP due to either limited ATP production (e.g. glucose deprivation or hypoxia) or increased energy expenditure (e.g. muscle contraction). When this occurs, AMPK sets in motion processes that potentially both increase ATP generation such as fatty-acid oxidation and glucose transport, and decreases others that consume ATP, but are not acutely required for survival, such as lipid and protein synthesis and cell growth and proliferation [59;87]. In addition, it may specifically stimulate glycolysis in cardiac muscle [115]. Recent evidence suggests that AMPK may have a much wider range of actions. For instance, it is involved in the regulation of such diverse events as mitochondrial biogenesis [83;211], angiogenesis[138], cell polarity [209] and the control of food intake and whole-body energy expenditure at the level of the hypothalamus [123]. In addition, AMPK activation in peripheral tissues seems to counteract many of the cellular abnormalities observed in animal models of the metabolic syndrome including insulin resistance, inflammation and ectopic lipid deposition [16;60;112;170;180]. Conversely, its dysregulation (defined as decreased activity or impaired activation) may contribute to these abnormalities [167]
Exercise, which is perhaps the most extreme metabolic stress experienced by normal humans, leads to activation of AMPK in skeletal muscle [21;46;218] and, at least in rodents in intraabdominal adipose tissue, liver [24;141] and probably other organs (J.Cacicedo, M-S. Gauthier, N.Ruderman, unpublished observations). It also has effects on insulin sensitivity [121;156;157] and gene and protein expression in various tissues [67;141;144;206], and in humans it reduces overall morbidity and mortality in an otherwise sedentary population [11]. In contrast, physical inactivity is known to be a powerful risk factor for many diseases and is beginning to be considered as a disease by itself [110]. It can be hypothesized that many of the beneficial effects of exercise and the adverse effects of physical inactivity are related, respectively, to the activation or lack of activation of AMPK. From a biochemical perspective, exercise can be used both as a model to study the mechanisms by which AMPK is activated in skeletal muscle and other tissues and as a tool to unravel its physiological roles in vivo. In this review, we will examine these possibilities, with special emphasis on the apparent ability of exercise to prevent and treat various diseases.
(2) Regulation of AMPK in muscle by exercise/contraction (Fig 1)
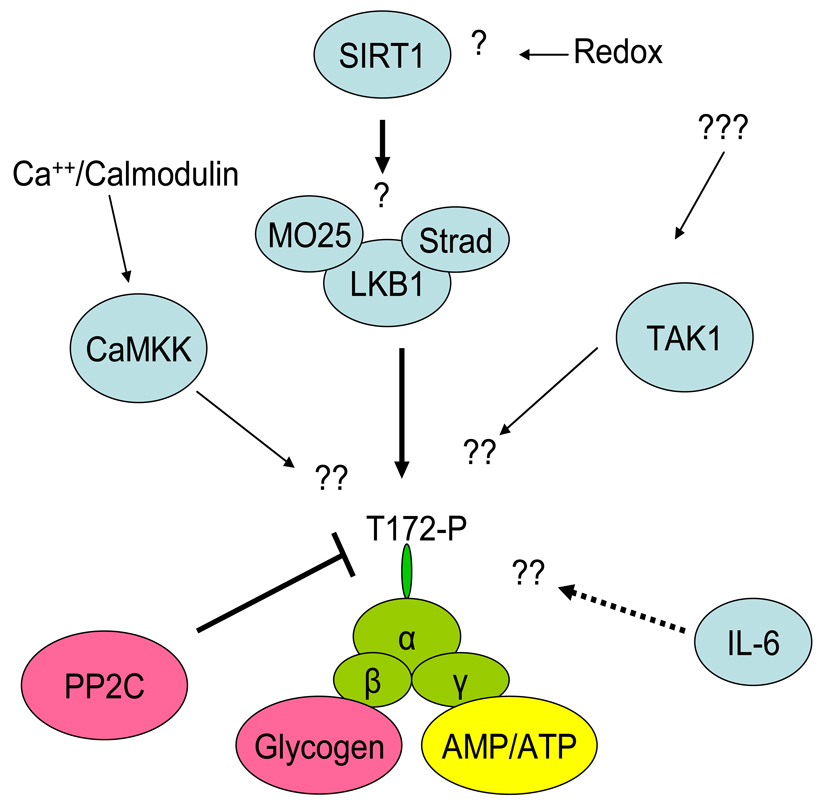
Figure 1.
Activation of AMPK in skeletal muscle during contraction/exercise. Although LKB1, the main kinase acting on α2 AMPK, is thought to be constitutively active, the possibility exists that SIRT1 may regulate LKB1 activity perhaps during prolonged exercise. In muscle, CaMKK may act as an AMPK kinase especially towards α1 AMPK during conditions in which AMP accumulation is limited. The role of TAK1 in muscle is uncertain. Binding of AMP to the γ subunit of AMPK makes AMPK a poor substrate for PP2C resulting in increased net phosphorylation of AMPK. IL-6 produced by contracting muscle may increase AMPK activity in this or adjacent muscle by an as yet unknown mechanism (broken line). The β subunit of AMPK has a glycogen binding domain and high glycogen levels in muscle inhibit AMPK activation during exercise.
AMPK is an heterotrimeric enzyme composed of a catalytic α-subunit and regulatory β and γ subunits. The α and β-subunits each exist in two isoforms (α1; α2 and β1; β2), and the γ subunit in 3 isoforms (γ 1; γ 2 and γ3). The γ subunit contains two pairs of Bateman (CBS) domains that bind AMP and ATP. According to recent findings, based on the crystal structure of the γ subunit it has been suggested that two sites bind either AMP or ATP, whereas a third site contains a tightly bound AMP that does not exchange [222]. Furthermore, under conditions in which the cell’s energy state is not depressed, it is believed that ATP is predominantly bound to the two Bateman domains and that most AMPK molecules are inactive [222].
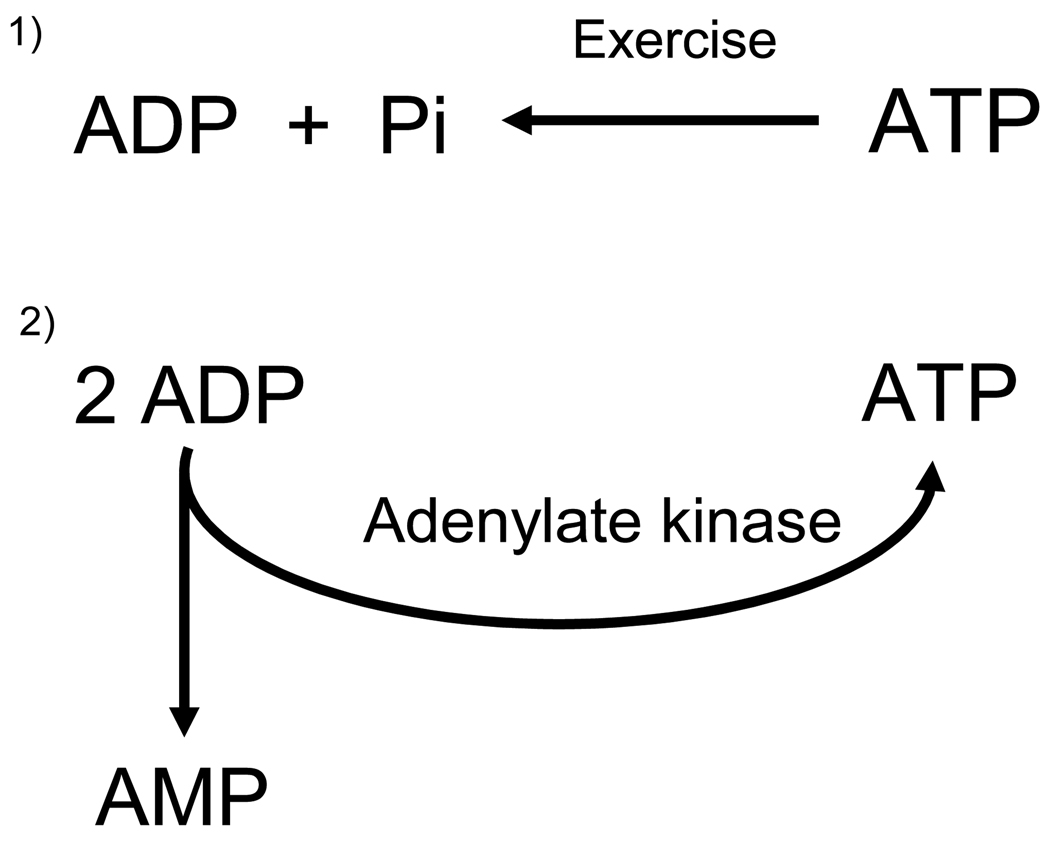
Physiological activation of AMPK occurs in skeletal muscle during exercise in response to increased binding of AMP and decreased binding of ATP to the γ subunit (Figure 1). Exercise can be characterized by a large (>100 fold) increase in muscle energy turnover and by alterations in nucleotide status. Although ADP is the direct product of the hydrolysis of ATP during muscle contraction, it is rapidly converted to AMP via the adenylate kinase reaction (Figure 2). This in turn leads to large increases in AMP concentration dependent on the intensity and duration of the exercise (181), even though free cytosolic AMP is buffered by protein binding and deamination to IMP [197]. In contrast, ATP concentrations change little during exercise, unless the exercise is very intense. AMP activates AMPK and ATP antagonizes this effect. Therefore, as already noted, it is the ratio between AMP and ATP that is of importance for AMPK activation [61]. AMP binding to the Bateman domains can stimulate AMPK allosterically, but apparently this only has a moderate activating effect (<10 -fold)[18]. More importantly, AMP binding leads to increased AMPK phosphorylation at Thr 172 of the α-subunit which can enhance AMPK activity more than 100-fold [62]. The importance of an increase in the AMP/ATP ratio in mediating AMPK phosphorylation during exercise is underscored by the fact that it is impaired in adenylate kinase deficient mice in which AMP generation is decreased during muscle contractions [58]. The mechanisms by which AMP activates AMPK have been challenged recently. Previous observations suggested that AMP binding to the Bateman domains of the γ-subunit of AMPK, in addition to allosterically activating AMPK, make it a much better substrate for upstream AMPK kinases and a poorer substrate for phosphatases [61]. More recent studies, however, suggest that the major effect of AMP binding to AMPK is to inhibit the action of phosphatases, [176;186]. According to this scheme, the major upstream kinase of AMPK, LKB-1 is constitutively active in muscle and in the basal state AMPK is continuously phosphorylated and dephosphorylated in a futile cycle. Although this may seem energetically wasteful, the energy cost of this cycling is quantitatively negligible and it allows for greater and more rapid changes in the phosphorylation status and activity of AMPK in response to various stimuli.
Figure 2.
During exercise ATP is degraded to ADP. ADP in turn is in part reconverted to ATP and AMP via the adenylate kinase reaction. The AMP/ATP ratio is very sensitive to changes in high energy phosphates because the adenylate kinase reaction is in equilibrium. Thus, [ATP][AMP]/[ADP]2 = K and [ATP][AMP] = K × [ADP]2. If both sides of the latter equation are divided by [ATP]2 we get [AMP]/[ATP] ≈ ([ADP]/[ATP])2 indicating that the AMP/ATP ratio varies approximately as the square of the ADP/ATP ratio.
LKB1
has been identified as an important upstream AMPK kinase in muscle and most other cells [63;221]. Its importance during electrically-induced muscle contractions has been demonstrated by the severely blunted activation of AMPKα2 and ACCβ phosphorylation in muscle-specific LKB-1 KO mice [96;174]. Interestingly, AMPKα1 activation was less affected [96;174]. In addition, exercise capacity in LKB-1 KO mice is markedly impaired compared to WT mice [193]. Although there is ample evidence for the importance of LKB-1 for AMPK activation in muscle, the activity of LKB-1 does not appear to be increased in muscle during exercise [74;174], supporting the notion that it is constitutively active.
CAMKK, TAK-1 and SIRT1
Although LKB1 is the predominant AMPK kinase in most cells, a Ca2+-dependent CAMKKβ has been found to phosphorylate AMPK at T172 in brain, endothelium and lymphocytes [64;220]. Recently, it has been reported that CaMKK may also act as an upstream AMPK kinase early during contractions in skeletal muscle [78] and that CaMKKα may be the important isoform in muscle [212],in contrast to other tissues in which CaMKKβ seems to be the dominant isoform [64;220]. Also of note, when the intracellular Ca++-concentration was increased in skeletal muscle by incubation with caffeine, it resulted in increased AMPK activity, primarily due to activation and phosphorylation of α1-AMPK [77]. Although definitive genetic studies are lacking, collectively these observation suggest that CaMKK in fact acts as an upstream AMPK kinase in skeletal muscle, perhaps mainly for α1-AMPK. They also suggest that an intensity- and/or time-dependent switch may occur in the relative importance of AMPKKs during contraction.
Another potentially relevant enzyme that has been studied in cultured cardiomyocytes, is the mitogen activated, TAK-1 (TGFbeta-activated protein kinase). It has been suggested that TAK1 is either a LKB-1 regulating kinase or a functional AMPK kinase [223]. Its possible role in skeletal muscle is not known.
Recently it has been suggested that silent information regulator 1 (SIRT1), a NAD+ dependent histone/protein deacetylase that has been linked to increased longevity caused by calorie restriction [120] may be able to deacetylate LKB-1 leading to its activation and that of AMPK in HEK293T cells and rat liver, in vivo [108]. The relevance of such a mechanism to AMPK activation in skeletal muscle under various conditions including exercise has not been systematically examined. However, recent studies have indicated that SIRT1 expression is increased after both a single prolonged bout of exercise [187] as well as after physical training [40]. Likewise, increases in SIRT1 mRNA have been observed in human muscle after 6 months of caloric restriction either with or without concomitant regular exercise [23]. Thus, it is conceivable that under certain conditions exercise may also affect LKB-1 activity in skeletal muscle and perhaps other tissues via SIRT1-induced deacetylation.
The above described mechanisms for activation of AMPK in muscle during exercise are directly related to the increase in sarcoplasmic calcium concentration and the ensuing metabolic perturbations (e.g increased AMP/ATP ratio) caused by the contractile activity (Figure 1). However, there may be additional mechanisms that increase AMPK activity in contracting muscle. For instance, increases in AMPK activity in muscle and adipose tissue caused by swimming is decreased in IL-6 KO mice [89;168] suggesting that IL-6, which is synthesized and released by the muscle cell during sustained exercise, may be involved in AMPK activation in various tissues. Also, in keeping with this notion, it has been shown that AMPK activation in the vastus lateralis muscle of humans correlates closely with IL-6 release from the leg during ergometer cycling [113].
Exercise intensity and heterotrimeric complexes
AMPK is activated in an intensity-dependent manner, such that its activation is observed acutely at exercise intensities above ≈ 60% of maximal aerobic capacity [21;128;141;185;215;218]. On the other hand, AMPK activation can also be observed if exercise at a lower intensity is very prolonged [216].
Recent findings in humans indicate that the various trimeric AMPK complexes are activated very differently during exercise. Thus, in human muscle only 3 heterotrimeric AMPK complexes are expressed: α1β2γ1, α2β2γ1 and α2β2γ3 and during intense exercise of up to 20 min duration only the α2β2γ3 complex is activated. The other complexes, which comprise as much as 80% of the total AMPK pool are unchanged or even decreased in activity [9]. Only after moderate intensity exercise of 60 min or more does the activity of the α2β2γ1 complex increase [194]. Interestingly, increases in the activity of α2β2γ3 correlated well with increased ACCβ phosphorylation suggesting that this trimeric complex plays a major role in the regulation of fatty acid oxidation [194]. In contrast, increases in the activity of the α2β2γ1 complex were shown to correlate with phosphorylation of the rab-gap protein AS160, an AMPK target that has been linked to the regulation of glucose transport [103;196]. Collectively, these findings suggest both that the regulation of the various trimeric complexes in muscle during exercise is very different and that the complexes have different downstream substrates and probably distinct biological actions.
AMPK regulation and glycogen content
In addition to exercise intensity, the magnitude of AMPK activation during exercise also depends on the content of glycogen in muscle (Figure 1). When muscle glycogen is low, AMPK activity is elevated at rest and it increases significantly more during exercise than when glycogen is high [31;162;184;214]. This dependency on glycogen content is also apparent when AMPK is activated by AICAR [81;213]. Interestingly, AMPK has a glycogen binding domain on its β-subunit. Although glycogen binding to this domain has not yet been demonstrated to alter AMPK activity in vitro [73;146]; as mentioned above, studies in vivo strongly suggest that glycogen- directly or indirectly- inhibits AMPK activity.
Differences between men and women
Interestingly, AMPK activation during physical activity is less in women than in men when exercising at the same relative exercise intensity [161]. This is likely because women are less metabolically stressed as indicated by nucleotide status [161], possibly due to the fact that they have both a higher percentage of oxidative type I muscle fibers [182] and a greater capillary density [161] than men. Although not studied, it would seem likely that at maximal exercise intensity, women and men would be equally stressed and presumably would have the same AMPK activation.
Metabolic effects of AMPK in muscle during exercise
Glucose uptake
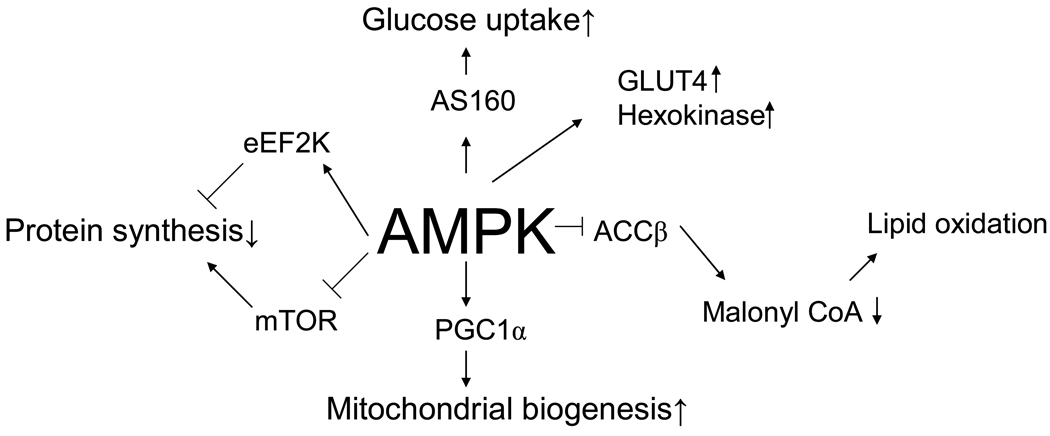
Activation of AMPK by AICAR in resting muscle results in increased glucose uptake [119], an effect that is lost when α2 or γ3-AMPK expression is deficient [5;85;126]. Thus, it is logical to assume that AMPK activation during exercise is responsible for the observed increase in muscle glucose uptake (Figure 3). Supportive evidence for this conclusion has been obtained in mice with various deficiencies in AMPK activity, including AMPK α1 and α2 and γ3 whole body KO mice, muscle specific LKB1 KO mice and transgenic mice overexpressing dominant negative AMPK constructs in muscle as discussed below. The picture is not clear, because a partial deficiency of AMPK, such as occurs in germline α2 AMPK KO [85] and γ3 KO mice [5] is associated with a normal rate of glucose uptake during contractions [85]. In contrast, in mice overexpressing a dominant negative α2AMPK construct in muscle [47;79;126;179] and in the muscle specific LKB1 KO mice in which α2 AMPK activation is completely blunted [96;175] glucose uptake in muscle during electrical stimulation is impaired. In α1 AMPK KO mice, glucose uptake during twitch contractions was recently shown to be decreased compared to wild type [80], in agreement with previous studies in which a modest decrease in glucose uptake during tetanic contractions was observed in the soleus muscle of α1 AMPK KO mice [85]. Taken together, the available data indicate that AMPK partially mediates the increase in glucose uptake during electrical stimulation of muscle. To date all of these experiments have been performed with muscles in which contraction was induced by electrical stimulation in vitro or in situ via the sciatic nerve. It does not automatically follow that the same results would be obtained during voluntary exercise in vivo during which the muscle recruitment pattern is very different and the systemic response to exercise has to be taken into account.
Figure 3.
Proposed roles of AMPK in regulation of metabolism and gene expression in skeletal muscle. Although there is clear evidence for these roles of AMPK in resting muscle, proof of this during exercise has so far been difficult to establish unequivocally using genetic mouse models with partial ablation of AMPK activity. See text for details.
AMPK belongs to a family of AMPK related kinases (ARKS), all of which are activated by LKB-1. Although several of these ARKS (QSK, QIK, MARK2/3, and MARK4) do not appear to be activated during electrically-induced muscle contractions [173], it was recently reported in a preliminary communication that expression of a phosphorylation impaired mutant AMPK- related kinase, SNARK (NUAK2), blunts electrically stimulated contraction-induced glucose uptake [95].
In recent years a direct target of Akt termed “Akt Substrate of 160 kDa” (AS160) has been implicated in insulin-mediated GLUT4 translocation and glucose uptake in both adipocytes and skeletal muscle [104;177]. It has been suggested that AS160 is also involved in the regulation of glucose transport during contraction/exercise, since it has been found to be an AMPK target in contracting muscle [104;196] (Figure 3). In addition. mutations in AS160 that prevent it from being phosphorylated decrease muscle glucose uptake during contractions [104]. Although these observations suggest an important role for AS160 in contraction induced glucose uptake, increased muscle AS160 phosphorylation has not been observed in human muscle until after 60 min of exercise, suggesting that it may not be an initiating event [32;195]. Furthermore, recent data in incubated rat muscle showed that AS160 phosphorylation is transient despite maintained glucose uptake during 60 min of electrical stimulation [50]. To date, 8 phosphorylation sites have been identified in AS160 [53]. Since the antibody (PAS) that has been used to detect phosphorylation of AS160 may bind to several but not all of these sites, the possibility exists that AS160 is phosphorylated on sites that are important for exercise induced glucose transport but are not detected by the PAS antibody. Site specific phosphospecific antibodies will be needed to address this issue. Recently, another Akt target Tbc1d1 has been shown to be expressed heavily in muscle and seems to be involved in insulin stimulated glucose uptake [158;190]. It is also phosphorylated during muscle contraction [50;190], but whether it is involved in regulating contraction-stimulated glucose uptake remains to be seen.
Fatty acid oxidation
In parallel to its effects on glucose uptake, AICAR increases fatty acid oxidation in resting skeletal muscle [119] and in part for this reason AMPK activation has been thought to participate in the regulation of fatty acids during exercise. Whole-body and muscle fatty acid oxidation increase during exercise (Figure 3) and in particular sustained exercise of moderate intensity [22]. However, in contrast to glucose oxidation, which increases with increasing exercise intensity [166], whole body fatty acid oxidation seems to plateau at around 60% of maximal aerobic capacity and at higher intensities it then decreases [202]. On the other hand, recent evidence suggests that when fatty acid uptake and oxidation are measured directly across a relatively small muscle group such as the vastus lateralis, fatty acid oxidation is not decreased at high exercise intensities [65] suggesting that that whole-body data do not accurately reflect local muscle metabolism.
In rodents, exercise and electrically-induced muscle contractions decrease the concentration of malonyl CoA in muscle [141;171;210] presumably due to the phosphorylation and inhibition by AMPK of ACC2, the ACC isoform which catalyzes the synthesis of the pool of malonylCoA that inhibits carnitine palmitoyltransferase 1 (CPT1) [15;118]. Also, the phosphorylation and activation of malonyl CoA decarboxylase by AMPK and perhaps other factors operative during exercise could enhance this effect [171]. Initial studies failed to find decreases in muscle malonyl CoA during exercise in humans [133;134]; however, more recent studies have described modest decreases in its concentration in human muscle both after acute exercise [29;160] and physical training [107]. Although AMPK activation and the ensuing decrease in malonyl CoA concentration in muscle may be an important mediator of the increase in muscle fatty acid oxidation during the transition from rest to exercise, it does not seem to play a key regulatory role during exercise. Thus, during exercise of increasing intensity muscle, AMPK activity increases [218], but as mentioned above, whole body fatty acid oxidation decreases [202] or remains stable when measured across an exercising muscle [65]. Furthermore, when muscle glycogen stores are decreased prior to exercise, muscle lipid oxidation is greater than when exercise is performed by muscle with full glycogen stores but malonyl CoA concentrations are similar [160]. Recent studies in mice overexpressing a kinase dead α2 AMPK construct in heart and skeletal muscle showed that both during in vitro electrical stimulation of muscle and during in vivo exercise impaired AMPK signalling was not accompanied by decreased fatty acid oxidation [36]. Data obtained in perfused rat skeletal muscle has suggested an important role for Ca++ signalling and ERK activation in regulating fatty acid uptake and oxidation during electrically-induced muscle contractions [151;152]. Thus, fine tuning of lipid oxidation may not be provided by AMPK. Other studies suggest that such fine tuning takes place at the level of carnitine availability, which like AMPK is regulated by exercise intensity and carbohydrate availability [160;172]. A low level of free carnitine in muscle limits the possibility for CPT1 catalyzed conversion of long chain fatty acids to fatty acylcarnitines in which form they can enter the mitochondria for oxidation.
AMPK activation during exercise may also decrease triglyceride synthesis in adipose tissue and liver by inhibiting the enzyme sn-glycerol-3-phosphate acyltransferase (GPAT) [127;141]. In addition, activation of AMPK in isolated cardiomyocytes results in translocation of the putative fatty acid transporter FAT/CD36 to the cell membrane which could increase fatty acid uptake as well as its oxidation [57]. In keeping with this possibility, AMPK stimulation in resting muscle has been shown to increase fatty acid oxidation in a CD36 dependent manner [12]. Thus, it appears that AMPK activation during exercise affects multiple enzymes and other molecules to increase the uptake as well as the oxidation of fatty acids.
Protein synthesis
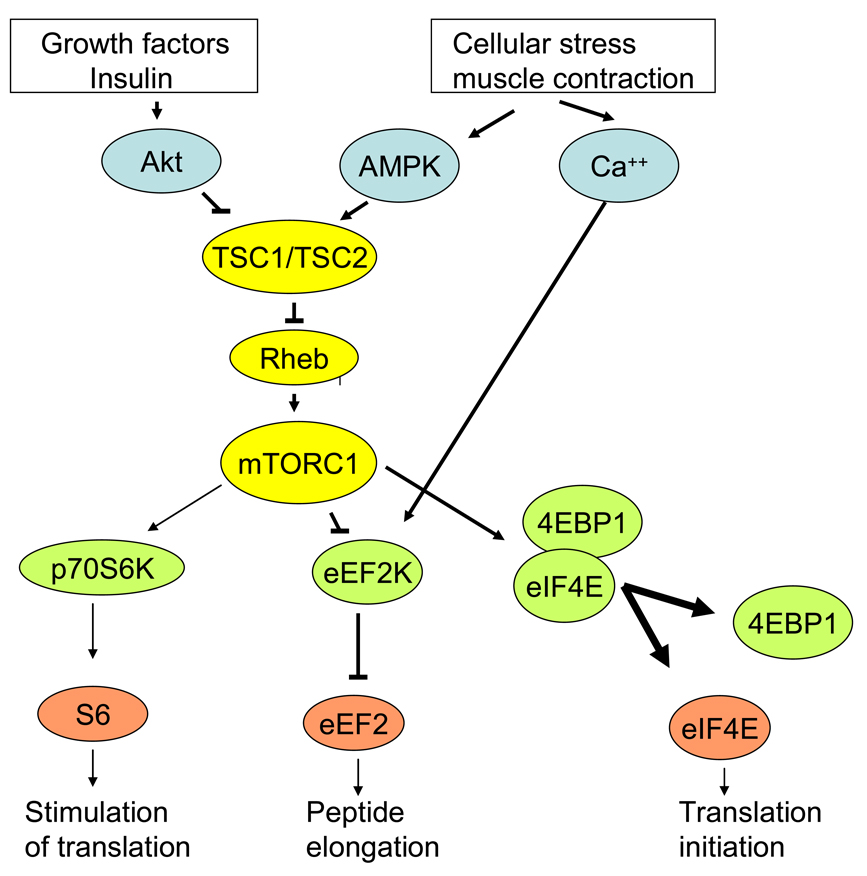
Protein synthesis is markedly diminished in skeletal muscle during contraction [17;35;154] (Figure 3). It has been proposed that AMPK activation inhibits protein synthesis in liver and ischemic heart muscle by activating of eEF2kinase leading to increased eEF2 phosphorylation and inhibition of peptide elongation [69;70]. Inhibition of peptide elongation in muscle during exercise has also been suggested by the finding of a rapid increase in eEF2 phosphorylation at the onset of bicycle ergometer exercise [165]. This appears to precede an increase in AMPK activity and is probably rather due to Ca++- dependent CaMKIII (eEF2 kinase) activation [165]. Interestingly, immediately following strength training an increased eEF2 phosphorylation has not been observed [30;35]. The reason for the different responses after endurance exercise and strength training is unclear. In addition to inhibition of peptide elongation, translation initiation may also be inhibited during exercise since decreased phosphorylation of 4E BP1 in muscle has been described both during resistance exercise training [30;35] and endurance exercise [164], perhaps due to increased α2 AMPK activity and TSC2 phosphorylation (Figure 4).
Figure 4.
Model describing the regulation of protein synthesis by AMPK, Ca++ and Akt -signaling pathways. Phosphorylation of TSC2 by Akt induces activation of the Rheb GTPase and the mTORC1 pathway promoting protein synthesis. In contrast, phosphorylation of TSC2 by AMPK results in Rheb inactivation and mTORC1 inhibition. Activated mTORC1 phosphorylates and activates p70S6kinase and phosphorylates and inactivates eEF2kinase (an inhibitor of eEF2), resulting respectively in the stimulation of peptide translation and elongation. In addition, activated mTORC1 phosphorylates 4EBP1 which binds to and inhibits the initiation factor eIF4E. This in turn inhibits 4EBP1 binding to eIF4E and enhances its ability to initiate translation. Thus mTORC1 coordinates the regulation of protein synthesis at the levels of initiation, translation and elongation. Same colours indicate same level in signal transduction.
In contrast to the inhibition of protein synthesis during exercise, after a bout of exercise, protein synthesis is typically increased [35;155] (as is glycogen synthesis) despite persistent increases in AMPK activity [35], casting doubt about the primacy of AMPK in regulating protein synthesis in human skeletal muscle in this situation. In rodents and humans the sensitivity to insulin of several metabolic pathways, including protein [8] and glycogen synthesis [156] amino acid transport [227] and glucose uptake [156;157] are increased after exercise, and perhaps this compensates for the increase in AMPK activity. In agreement with this interpretation, it has recently been shown that elements of the molecular pathway controlling peptide elongation are increased during insulin stimulation 4 hours after one-legged endurance exercise, compared to the non-exercised control leg [45].
Decreased running performance in AMPK deficient mouse muscle
Although it has been difficult to pinpoint defects in substrate metabolism, there is no doubt that mice with defects in AMPK activation, such as muscle specific kinase dead mice [49;125], α2 AMPK germ line KO mice [82] and LKB-1 muscle specific KO mice [192], have poor running capacity in vivo and disturbed nucleotide levels in muscle during exercise/contraction [49;82;86;93;175]. Thus, a partial absence of AMPK in muscle somehow results in poor exercise performance. The reason for this is not immediately obvious, but could be related to decreased mitochondrial capacity in the muscles of these mice rather than to an acute effect on metabolic regulation [49;84;93;192]. Studies of substrate metabolism during running in these genetic mouse models have not been reported. The possibility that decreased running performance in these mice is the result of decreased cardiac performance during exercise also cannot be excluded, since the genetic abnormalities in these mice also target the heart. On the other hand, this possibility seems less likely since heart specific AMPK DN mice appear to have a normal exercise capacity [129].
(3) Acute effects of exercise in tissues other than muscle
Liver and adipose tissue
It has long been appreciated that an acute bout of exercise is associated with changes in the metabolism of liver and adipose tissue that result in an increased provision of fuel for the contracting muscle. Thus, the liver increases the release into the circulation of glucose (initially derived from glycogenolysis and later from gluconeogenesis) and the adipocyte increases the hydrolysis of its triglyceride stores and the release of long chain nonesterified fatty acids (LCFA) into the circulation. A large body of evidence from both humans and experimental animals has linked these changes temporally to activation of the sympathetic nervous system (norepinephrine), which occurs very early during moderate and intense exercise and later to both this and increases in plasma levels of glucagon and epinephrine (for review see [51]). In addition, IL-6 released from muscle during exercise appears to stimulate the hydrolysis of fuel reservoirs in muscle, liver and adipose tissue and causes AMPK activation in these tissues (See below).
AMPK activation decreases the expression of phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase in the liver, two rate-limiting enzymes of the gluconeogenic pathway (for review see [205]). In addition, AMPK appears to inhibit hormone sensitive lipase (Reviewed by [27]), a key enzyme that controls lipolysis in the fat cell and other tissues. Thus, it was surprising when studies in rodents revealed that exercise activates rather than inhibits AMPK in liver [19;141] and intraabdominal adipose tissue [88;141] since AMPK activation theoretically should have inhibited gluconeogenesis and lipolysis. In both tissues exercise also caused a decrease in the energy state as reflected by an increase in the AMP/ATP ratio [19;97]. The most plausible explanation for the findings in liver is that the increase in the AMP/ATP ratio caused by exercise reflects the high energy cost of gluconeogenesis (6 ATPs per mole of glucose generated from lactate) and that AMPK acts acutely (i.e. before its effects on gluconeogenic gene expression becomes dominant) to maintain gluconeogenesis by enhancing ATP generation from fatty-acid oxidation. A similar line of reasoning could explain the findings in adipose tissue. Thus, recent studies with cultured adipocytes have demonstrated that the stimulation of lipolysis by agents that increase cAMP generation produce the same changes in AMPK activity and energy state in these cells [52], as does exercise in vivo [97]. It was shown that inhibition of lipolysis with an siRNA for adipose tissue triglyceride lipase (ATGL) or a chemical inhibitor (orlistat) prevented both of these changes from occurring, as did incubation with triacsin C, an inhibitor of acyl-CoA synthetase [52]. Based on these findings, it was postulated that the decrease in energy state in these cells was due to the high ATP requirement for fatty acid reesterification (7–9 ATP molecule of TG resynthesized), since 30–40% of the FFA generated by lipolysis in a fat cell is reesterified even when the lipolytic rate is increased. Interestingly, chemical inhibition of AMPK with compound C [52] or treatment of the adipocytes with siRNA for AMPK (M.S. Gauthier, N. Ruderman, unpublished data) caused a large increase in oxidant stress which increased markedly when lipolysis was stimulated. Presumably in this setting AMPK acts to restore cellular energy state and prevent lipotoxicity. Whether it does this by increasing fatty acid oxidation, inhibiting reesterification or modestly restraining lipolysis remains to be determined [52]. Also requiring study is whether a failure of AMPK to restore the energy state of the adipocyte predisposes it to apoptosis and inflammation [55].
A number of aspects of the acute exercise-induced changes in AMPK and energy state observed in rodent liver and epididymal adipose tissue require further study. One of them is whether similar phenomenon occur in humans As noted earlier, in one study, exercise of moderate to high intensity did not activate AMPK in human subcutaneous adipose tissue [105], whereas a small increase in phosphorylation of AMPK was reported in another [208]. Studies in rats suggest that long term exercise stimulates AMPK activity (and subunit expression) in liver and in intraabdominal, but not subcutaneous adipose tissue [189]. Whether a similar difference in adipose tissue occurs in humans is not known. Also requiring investigation is whether factors other than the sympathetic nervous system contribute to these exercise-induced changes in AMPK activity. In this context, it has been demonstrated [39] that the cytokine interleukin 6 is synthesized and released by skeletal muscle of both humans and rodents during prolonged exercise and that in humans an IL-6 infusion to simulate this effect increased adipose-tissue lipolysis [201]. Of specific relevance to this discussion, IL-6 administration both in vivo and in vitro has been shown to activate AMPK activity in both skeletal muscle and adipose tissue of rodents [168]. In addition, in IL-6 knockout mice, the baseline activity of AMPK in muscle and epididymal adipose tissue is substantially diminished, and the increase in AMPK activity caused by exercise is attenuated, although not completely prevented [88].
Hypothalamus
It has been demonstrated that leptin diminishes AMPK activity in the hypothalamus and that this is responsible for its ability to decrease food intake and increase sympathetic nervous system activity in the periphery [116;123]. Conversely, endocannabinoids [100] and ghrelin [1] increase hypothalamic AMPK activity and food intake. In an early study [2], an effect of exercise on AMPK activity in the hypothalamus was not observed. On the other hand, Flores et al [42] later reported that exercise increases the ability of leptin and insulin, acting at the level of the hypothalamus, to diminish food intake and Park et al [142] have shown that exercise prevents dexamethasone induced impairment of insulin and leptin signalling in the hypothalamus and in doing so it reverses the phosphorylation of AMPK induced by dexamethasone. How exercise achieves these effects is not known.
(4) Role of AMPK in mediating the chronic effects of exercise
Skeletal Muscle
In seminal studies, Winder’s group demonstrated that repeated activation of AMPK by daily injections of AICAR in rats increased GLUT4 and hexokinase protein content, and the expression of several mitochondrial enzymes in skeletal muscle [68;211].Subsequently, it was shown that the feeding of mice with beta-guanidinopropionic acid (GPA), a creatine analog that leads to increases in the intramuscular AMP/ATP ratio and AMPK activity, increased mitochondrial biogenesis in control mice, but not in mice overexpressing a dominant-negative AMPK in muscle [226]. These adaptations resemble those observed in skeletal muscle during endurance training and suggest that AMPK may play a key role in their causation. Other evidence speaks against this hypothesis, however. Thus, exercise training-induced increases in GLUT4 protein and mitochondrial enzymes were normal in both mice overexpressing a dominant negative AMPK construct in muscle [159] and mice with a germline deletion of α2 AMPK [84]. In addition, in the latter, exercise-induced increases in the mRNA for a number of mitochondrial enzymes were similar to those of WT mice [86]. On the other hand, both in these mice [84;93] and mice overexpressing the dominant negative AMPK construct [159], mitochondrial protein expression was decreased by 15–20% in the untrained basal state, suggesting that AMPK activity is important for basal mitochondrial enzyme expression. In contrast to these findings, muscle fiber type expression does not seem to be significantly altered under baseline conditions in mice overexpressing a dominant negative AMPK construct in muscle [149;159]. On the other hand, the switch to a more oxidative muscle fiber type elicited by endurance training was inhibited in these mice [159] and in mice overexpressing a constitutively active γ1 mutated AMPK in muscle, it has been shown that the fiber type composition of the gastrocnemius became more oxidative [159]. In summary, the available information suggests that in the basal state when its activity is low, AMPK appears to mediate changes in mitochondrial protein expression, but not fiber type. In contrast, when AMPK activity is high, such as after exercise training or overexpression of a constitutively active AMPK, it appears to regulate muscle fiber type. This moderate effect on muscle fiber type expression is not too surprising considering that regulation of muscle fiber type expression is very complex involving several other signalling molecules (e.g. calcineurin, CaMK and p38), as well as transcription factors and co-activators (e.g. PGC1- α, NFAT and PPARδ) [178].
AMPK abundance itself may be influenced in muscle by endurance training. In humans, trained subjects have a higher expression of α1 AMPK than untrained individuals [132]. Furthermore, intense endurance training of young healthy males for 3 weeks results in increases in α1 and α2 AMPK protein expression and ACC-β phosphorylation, the latter strongly suggesting that the basal activity of AMPK was increased [44]. Interestingly, in contrast to these changes, γ3 subunit expression was decreased by training [44]. Increased AMPK activity, which can persist in trained individuals for some time between exercise bouts may be important in eliciting increased insulin sensitivity, since AMPK activation by AICAR has been shown to increase insulin action in rat muscle [41;75]. In middle-aged subjects less intense training did not reveal changes in AMPK protein expression and activity. When studied 24–36 h after the last bout of exercise, however, PGC1α and malonyl CoA decarboxylase mRNA expression, both of which can be AMPK mediated, were increased for at least 24–30 h [107].
Other tissues
Regular exercise leads to decreased inflammation, increased fibrinolytic activity, lowering of plasma triglycerides and increased HDL cholesterol [170]. To what extent these are chronic effects of repeated AMPK activation in tissues other than muscle is essentially an unanswered question. As already noted, long term exercise training (6 or12 weeks) induces increases in AMPK and ACC phosphorylation and α1 and α2 AMPK, mRNA, and protein in both rat visceral adipose tissue and liver [97;189]. Acutely, exercise-induced AMPK activation in these tissues is associated with decreases in the activities of a number of enzymes of lipid synthesis, including glycerophosphate acyl transferase, and acetyl CoA carboxylase and with an increase in malonyl CoA decarboxylase activity [141]. The cumulative effect of these changes would be an increase in fatty acid oxidation and a decrease in glycerolipid synthesis. Presumably, this occurs in liver and adipose tissue during physical training, however, to our knowledge this has not been directly studied.
(5) Inactivity
As already noted, inactivity is associated with an increased prevalence of various disorders including type 2 diabetes, coronary heart disease, Alzheimer’s disease and cancers of the colon and liver [10;191]. In addition, it is well established that bed rest for 3–7 days leads to glucose intolerance and insulin resistance [34;111;122]. Whether inactivity also leads to decreased activation of AMPK-mediated genes such as PGC1α [76;187] is not known. A less dramatic decrease in physical activity than with bed rest occurs when healthy free living subjects decrease their daily number of steps from 6,000–10.000 steps to 1500/day [135]. It was shown in this study that within 2–3 weeks of reduced stepping, oral glucose tolerance and insulin response to an OGTT were both decreased and plasma triglycerides were increased [135]. The relationship of such abnormalities to AMPK dysregulation is unclear. The predicted defect in AMPK in response to inactivity would be the absence of intermittent periods of AMPK activation caused by physical activity and possibly decreased AMPK activity at rest.
(6) Exercise and disease
Exercise, AMPK and the Metabolic Syndrome
The association between exercise, AMPK activation and disease prevention will be discussed primarily in the context of the Metabolic Syndrome. The Metabolic Syndrome is presently defined clinically as a disorder characterized by at least three of the following: central obesity, hyperglycaemia, hypertension, hypertriglyceridemia and a decrease in circulating levels of HDL cholesterol [56]. In addition, patients with this entity are typically insulin resistant and many present with a proinflammatory, procoagulant state, (increased IL-6, TNFα, PAI), all of which may antedate the clinical diagnosis by many years and contribute to its pathogenesis (Figure 5). Patients with the Metabolic Syndrome are predisposed to such disorders as type 2 diabetes, premature atherosclerotic heart disease, non-alcoholic fatty liver disease, various cancers [170], and neurodegenerative disorders, including Alzheimers and Parkinsons disease, all of which are less prevalent in individuals who are physically active [33;72;114;191]. As reviewed elsewhere [169;217;219] regular exercise has also been shown to increase insulin sensitivity and decrease inflammatory markers in people with the Metabolic Syndrome. In addition, in conjunction with diet therapy, exercise markedly diminishes progression from impaired glucose tolerance to overt diabetes in humans studied over 4–5 years [94;139;198] and it has shown efficacy in the prevention or therapy of many other metabolic-syndrome associated disorders (Fig 6). The linkage of these effects of exercise to AMPK has not been systematically examined. However, it is noteworthy that treatment with metformin and thiazolidinediones, antidiabetic agents that activate AMPK in human muscle [4;130] and diminish systemic insulin resistance [as does exercise] have also been demonstrated to decrease both progression from impaired glucose tolerance to overt diabetes [54;94;198] and risk factors for atherosclerotic heart disease [136]. In addition, metformin has been demonstrated to diminish the incidence of macrovascular disease by 39% when used as the sole initial therapy in individuals with new-onset type 2 diabetes in the UKPDS [199], although, in the same study a significantly higher incidence of macrovascular was observed in individuals treated with sulfonylureas and metformin [199]. More recently, a 35% decrease in cancer prevalence has been reported in patients with diabetes treated with metformin for many years [14;37] suggesting that AMPK may act as a tumor suppressor [112]. Regular exercise is known to be protective against some forms of cancer, including cancer of the breast, colon and likely the prostate [163] and the possibility exists that activation of AMPK by exercise may be involved in this association.
Figure 5.
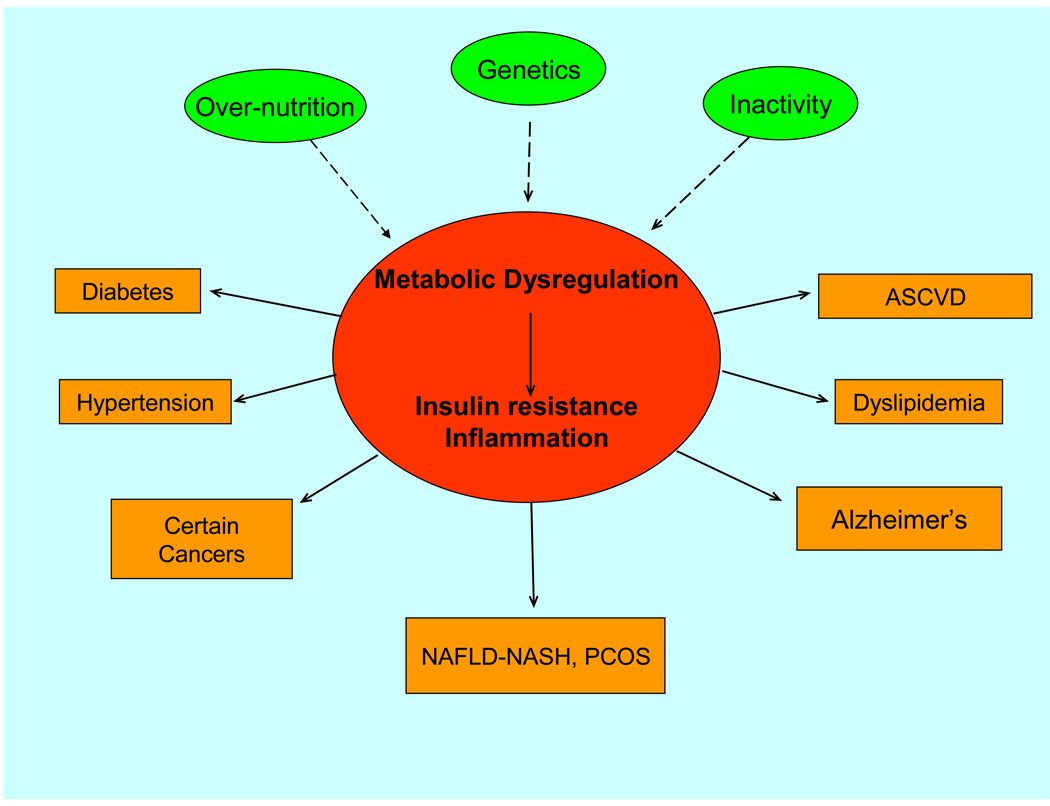
The Metabolic Syndrome can be defined as a disorder in which combinations of overnutrition, inactivity and as yet poorly defined genetic factors results in a state of metabolic dysregulation characterized by lipid abnormalities, insulin resistance and inflammation. This in turn can predispose to one or more of the shown disorders. Apart from inactivity being a causal factor, exercise has demonstrated efficacy in treating and preventing both the state of metabolic dysregulation and many of the diseases to which it predisposes. See text for details. NAFLD-NASH: non-alcoholic fatty liver disease. PCOS: Policystic ovary syndrome.
Figure 6.
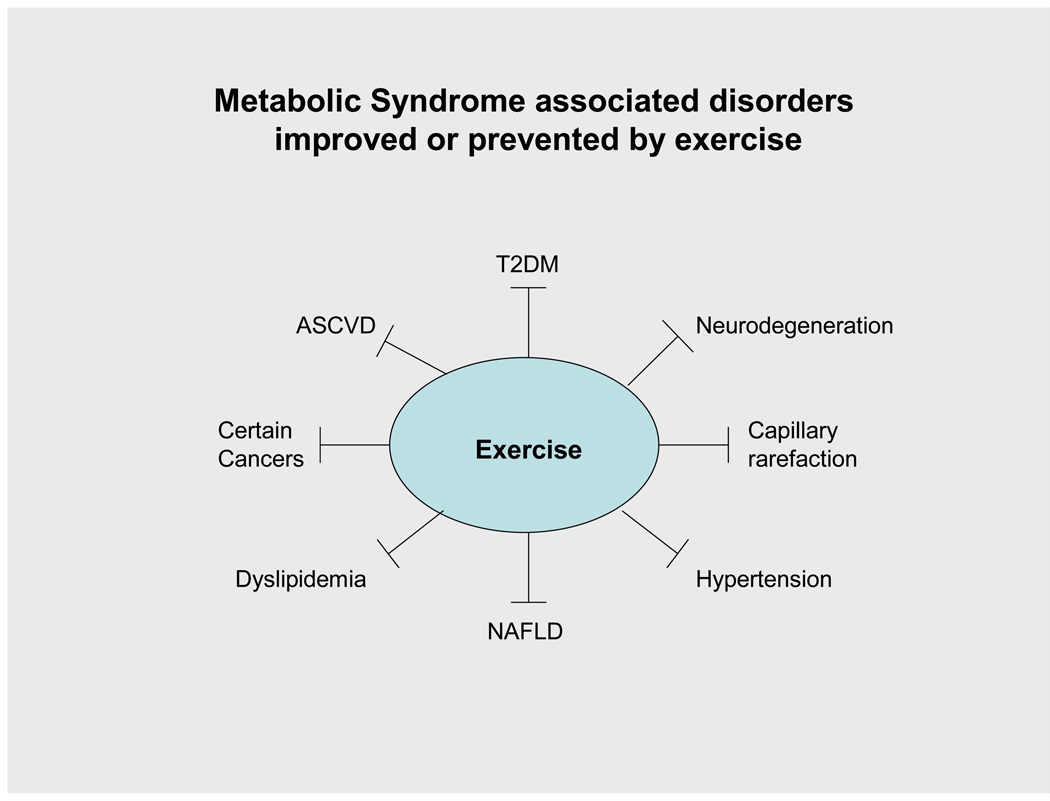
Some metabolic syndrome associated disorders that may be improved or prevented by exercise. Exercise, acting at least in part via AMPK, could exert these benefits by improving abnormalities in glucose and lipid metabolism, insulin secretion and action, inflammation, mitochondrial function, angiogenesis and other pathogenic factors.
Obesity, insulin resistance and type 2 diabetes
It must be emphasized that measurements of AMPK activity in humans with obesity and insulin resistance, with or without diabetes, have not painted a clear picture. Early studies from several laboratories failed to find abnormalities in AMPK activity in skeletal muscle of these patient groups [66;130]. Furthermore, no defect in the ability of AMP and AICAR to activate AMPK was observed in patients with obesity [183] and type 2 diabetes [98]. On the other hand it has recently been reported that the ability of exercise to activate AMPK is impaired in muscle of obese individuals with or without diabetes [28;181]. In one of these studies [28] increases in PGC1α mRNA and abundance induced by an acute bout of exercise in lean control subjects were markedly diminished in obese individuals; however, in the other [181] baseline PGC1α mRNA was diminished, but the percent increase induced by exercise was the same as in the control population. Likewise, decreased AMPK activity accompanied by increases in malonyl CoA and ACC activity (decreased PACC) have been reported in obese, insulin resistant humans with and without diabetes [4]. Interestingly, treatment with rosiglitazone (a thiazolidinedione) activated AMPK and diminished both the concentration of malonyl CoA and insulin resistance, as it had previously been reported to do in obese rodents [106;131]. Further studies are needed to confirm and extend these findings.
AMPK, exercise and the Metabolic Syndrome phenotype in rodents
Investigations in rodents are consistent with the notion that exercise may prevent disease by activating AMPK, although a causal role has not been established. Early studies by Berger and his co-workers [7] demonstrated that exercise diminishes insulin resistance and obesity in the Zucker fatty rat (fa/fa), a rodent later found to have a genetically defective leptin receptor [200] and a decrease in tissue AMPK activity [224]. As shown in [Table 1], decreased tissue AMPK activity has been found in many rodents with a metabolic syndrome phenotype. One of these is the ZDF rat, an obese rodent with the same genetic defect in the leptin receptor as the fa/fa rat and with a second as yet undefined genetic defect that causes it to develop diabetes between 10–13 weeks of age. Treatment of the ZDF rat with the AMPK activator AICAR [145;224] or with regular exercise [145], beginning at 5 wks of age, has been shown to diminish insulin resistance and ectopic lipid deposition in liver, muscle and pancreatic islets and prevent the development of diabetes in the fa/fa rat. Similar changes have been observed in mice subjected to caloric restriction, or treated with TZDs or metformin [200] suggesting diminished AMPK activity is a pathogenetic factor.
Table 1.
Some rodents in which decreased AMPK activity is associated with the metabolic syndrome
| Rodent | Comment | Refs |
|---|---|---|
| FaFa rat | Defective leptin receptor, obese, insulin resistant,non-diabetic | [224] |
| ZDF rat | Defective leptin receptor, obese, insulin resistant, diabetic | [224] |
| IL-6 KO mouse | Decreased AMPK activity and an impaired ability to exercise precedes obesity, dyslipidemia and impaired glucose tolerance |
[89;207] |
| Glucose-infused rat (5–24h) |
Decreased AMPK activity in muscle and liver appears concurrent with increased tissue triglyceride and insulin resistance |
[101] |
| ObOb mouse | Leptin deficient, obese, insulin resistant | [106;224] |
Another animal of note is the IL-6 KO mouse. The IL6 KO mouse appears normal at 3 months of age but has low AMPK activity in white muscle and adipose tissue [88], a low rate of fatty acid oxidation and an impaired ability to sustain exercise [207]. By 7–9 months of age, however, it is obese, glucose intolerant and dyslipidemic [207] and fatty acid oxidation has retuned to the same level as in control mice, although its ability to exercise is still impaired [38]. Whether treatment with IL-6, which activates AMPK in muscle and adipose tissue both in vivo and in vitro [168], can prevent these changes from occurring is not known. Studies by Wallenius et al. [207] suggested that intraperitoneally injected IL-6 has little effect on the phenotype of these mice, but that IL-6 administered into the 3rd ventricle increased energy expenditure and diminished their obesity. Of final note, IL-6 is synthesized and released from skeletal muscle during exercise and its concentration in plasma can increase transiently (hrs) by as much as 100-fold [39] when running a Marathon race, although much more modest increases are observed during more moderate exercise bouts [113]. In the IL-6 KO mouse, this increase in IL6 synthesis during exercise does not occur, and the activation of AMPK in both muscle and adipose tissue is markedly, although not totally impaired [88].
In the mice with a Metabolic Syndrome phenotype listed in Table 1, the decreases in AMPK activity were all secondary to another factor, such as a defective leptin receptor, or an oversupply of glucose or other nutrients. Studies of mice with a germline deletion of α1 or α2 AMPK to date have not yielded similar phenotypes, although the α2 AMPK KO mouse is insulin resistant in vivo, possibly the result of an increase in circulating catecholamines due to a decrease in hypothalamic AMPK [204]. Whether a second stress, such as age or prolonged overnutrition is needed for a metabolic syndrome phenotype to occur is not known. Overfeeding a high fat diet has been found to cause a greater increase in obesity in mice with a germline α2 AMPK deficiency; however, it failed to produce insulin resistance as it did in control mice [203] for reasons unknown. In contrast, in mice with a muscle-specific α2 AMPK ablation a similar high fat diet caused substantially more glucose intolerance and insulin resistance in muscle (Akt phosphorylation) than it did in control mice [48] indicating that ablation of α2 AMPK activity in muscle in fact exacerbates sensitivity to the deleterious effects of a high fat diet. Why muscle specific deletion of α2 AMPK has this effect when germ line deletion of α2 AMPK does not is at present unclear.
Some less studied disorders that are improved by physical activity (Figure 6)
As reviewed elsewhere [167;170], the metabolic syndrome has been linked to a wide array of diseases in addition to those already described, including non-alcoholic fatty liver disease/non-alcoholic steatohepatitis (NAFLD/NASH), polycystic ovary syndrome and cancers of the colon, breast and uterus. Furthermore, exercise has shown benefit in the prevention and therapy of many of these disorders (Fig 6). In the following sections we will discuss two of the disorders that have been more recently linked both to exercise and AMPK, capillary rarefaction and Alzheimer’s disease.
Capillary Rarefaction
Decreased capillary density in skeletal muscle (rarefaction) has been described in humans with obesity, diabetes and inactivity and it can be at least partially reversed by caloric restriction and exercise [90;148]. Similar findings have been observed in rodents. The most studied rodent has been the obese Zucker rat (fa/fa) in which a 75% decrease in muscle capillary density at age 6–7 weeks was substantially (50–60%) corrected after 10 weeks of regular exercise (treadmill running for 1 h/day, 6 days a week at a rate of ~22 m/min) [43]. Interestingly, AMPK activity is diminished in several tissues of the fa/fa rat [224]. AMPK has been implicated in the regulation of angiogenesis in response to ischemia by Walsh and his coworkers [137] and others have demonstrated an association between impaired angiogenesis and capillary rarefaction. Impaired angiogenesis in skeletal muscle of mice with experimental diabetes is associated with a decrease in the expression of VEGF [92] a vascular growth factor that can activate AMPK [153]. Exercise increases nitric oxide (NO) generation and endothelial nitric oxide synthase (eNOS) mRNA in skeletal and cardiac muscle [99] possibly via AMPK activation [20;124] and its ability to increase angiogenesis and muscle capillary density is lost following treatment with eNOS inhibitors [43]. Likewise, the histone/protein deacetylase SIRT1 has been shown to stimulate NO production by endothelial cells [117] and a lack of SIRT1 impairs angiogenesis [147]. In addition, exercise has been shown to increase SIRT1 activity and antioxidant enzymes in the heart of aging rats [40]. As reviewed earlier, SIRT1 may be an upstream regulator of AMPK. Thus, it will be of interest to determine whether the link between SIRT1 activity and capillarity is AMPK-mediated.
Alzheimer’s disease and dementia
Alzheimer’s disease and other neurodegenerative disorders that lead to dementia are more prevalent in people with the metabolic syndrome and type 2 diabetes than in the general population [33]. Although the evidence is not universally consistent, both observational and prospective studies strongly suggest that the risk for these disorders is diminished in humans who are more physically active [102]. In addition, improvement in cognitive function by physical activity has recently been observed in a randomized prospective trial in older adults at risk for Alzheimer’s disease [109].
Alzheimer’s disease has been linked to such factors as insulin resistance in the brain [102], and loss of neurons in the parietal and temporal lobes including the hippocampus [102]. In a recent study [143], 3 months of aerobic training in humans was associated with MRI measures of increased cerebral blood volume (and presumably blood flow) in the dentate gyrus of the hippocampus. Likewise, in mice exercise increased neurogenesis in the dentate gyrus [143]. Other studies in rodents have shown that regular exercise: (1) has cognitive effects similar to those it has in humans [102]; (2) increases mitochondrial biogenesis and decreases various manifestations of oxidative stress in the brain [13]; (3) diminishes cerebral inflammation in the Tg 2576 mouse, a rodent model of Alzheimer’s disease [140]; and (4) increases the synthesis and/or effects of BDNF (brain derived neurotrophic factor), IGF-1 (insulin-like growth factor-1) and VEGF (vascular endothelial cell growth factor), which collectively result in neuroprotection, angiogenesis, cell proliferation and changes in cortical morphology [102]. AMPK in brain was not measured in these studies: however, its activation has been shown to cause similar effects in other tissues. Finally, caloric restriction (60% of control), which has been shown to activate AMPK in the hippocampus of the rat has also been found to increase neurogenesis and improve cognition [25].
SIRT1, an histone/protein deacetylase that has been linked to the increase in longevity associated with caloric restriction has also been reported to provide neuronal protection in rodents with Alzheimer’s disease [3;91]. In some of these studies, resveratrol, a SIRT1 activator was demonstrated to diminish neurodegeneration in rodent models of both Alzheimer’s [91;150] and Huntington’s disease [3]. Recent reports suggest that SIRT1 may function, at least in part by activating AMPK [71;71;225]; indeed, resveratrol, which was used in some of the above mentioned rodent studies [3;6] has clearly been demonstrated to activate AMPK in various tissues and cells [6;225]. On the other hand, the role of SIRT1 in mediating the neuroprotective effect of resveratrol has recently been questioned. Thus, both resveratrol and the AMPK activator AICAR promoted robust neurite outgrowth in Neuro2A cells, and the effects of both agents were blocked by compound C, a compound commonly used to inhibit AMPK. However, the effects of resveratrol were still evident in cells lacking SIRT1 [26]. Whether this signifies that resveratrol does not require a sirtuin to activate AMPK (eg. at high concentrations it can inhibit mitochondrial ATP synthase), or another sirtuin assumed its function in these cells remains to be determined. With regard to the latter possibility, resveratrol has been shown to activate SIRT2 in neuronal cells [188]. In any event, studies of the effect of exercise on SIRT1 and AMPK activity in various regions of the brain will clearly be of interest.
Current Challenges and Concluding Remarks
Physical activity has unequivocal health benefits related to the prevention and treatment of lifestyle disorders associated with obesity and insulin resistance, including type 2 diabetes, cardiovascular and neurodegenerative diseases and some cancers. In addition, regular physical activity is associated with decreases in all cause mortality. That AMPK plays a key role in mediating these benefits of exercise has been suggested. The fact that exercise causes activation of AMPK in muscle and, at least in rodents adipose tissue and liver, coupled with the observation that pharmacological activation of AMPK leads to marked metabolic improvements in animal models of the metabolic syndrome has led to the assumption that its activation by exercise is a major reason for these health-promoting effects of physical activity. Also in support of this notion, decreased AMPK activity has been shown to accompany or precede the appearance of a metabolic syndrome phenotype in many of these rodents. The situation in humans is less clear. As already noted, both normal and decreased AMPK activity and normal and impaired activation of AMPK by exercise have been reported in obese individuals both with and without diabetes and the reason for these differing results is unclear. Likewise, the assumption that in sedentary people, the lack of even modest, AMPK activation could have long-term consequences for mitochondrial function and other events that lead to cellular dysfunction and disease requires further study. Studies of genetic mouse models with partial deficiencies in AMPK (total KO of AMPK leads to embryonic death) have so far not consistently demonstrated that these mice become insulin resistant or are more prone to obesity, diabetes and other disorders associated with the metabolic syndrome. However, germ line deficient mouse models may not adequately reflect the effect of the loss of AMPK protein since compensatory mechanisms may operate from conception. In addition, decreased AMPK activity in the hypothalamus could hypothetically mask some of the effects of peripheral AMPK deficiency by increasing sympathetic nervous system activity. Organ-specific knockdown of AMPK may prove useful in addressing this issue. Indeed, as already noted, in a recent study an increase in insulin resistance compared to control animals was observed in mice with a muscle-specific ablation of α2 AMPK when fed a high-fat diet for an extended time period. Finally, in humans, there is still a significant need to clarify the role of AMPK in regulating physiological responses in health and disease and in particular the response to exercise. We predict that future research will address questions such as: how significant are exercise-induced increases in AMPK activity in regulating the metabolism and function of muscle and tissues other than muscle; does dysfunction of AMPK play a role in the pathogenesis of insulin resistance and type 2 diabetes; is AMPK a target for the prevention and treatment of type 2 diabetes, Alzheimer’s disease and certain cancers; and what is the precise physiological role of the sirtuins and their relationship with AMPK?
Acknowledgements
The authors thank Dr. Laurie Goodyear for critically reviewing the manuscript and for her many helpful suggestions.
Funding: EAR is supported by grants from the Danish Medical Research Council, the Lundbeck Foundation, Novo-Nordisk Research Foundation, the European Commission Grant COST BM 0602 and by an Integrated Project EXGENESIS funded by the European Union. NBR is supported b U.S. Public Health Service grants RO1 DK19514 and 67509 and PO1 HL 68758 and a Mentor-based training grant from the American Diabetes Association 7-08-MN-50.
Reference List
- 1.Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ. AMP-activated protein kinase plays a role in the control of food intake. J.Biol.Chem. 2004;279:12005–12008. doi: 10.1074/jbc.C300557200. [DOI] [PubMed] [Google Scholar]
- 2.Andersson U, Treebak JT, Nielsen JN, Smith KL, Abbott CR, Small CJ, Carling D, Richter EA. Exercise in rats does not alter hypothalamic AMP-activated protein kinase activity. Biochem.Biophys.Res.Commun. 2005;329:719–725. doi: 10.1016/j.bbrc.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 3.Anekonda TS, Reddy PH. Neuronal protection by sirtuins in Alzheimer's disease. J Neurochem. 2006;96:305–313. doi: 10.1111/j.1471-4159.2005.03492.x. [DOI] [PubMed] [Google Scholar]
- 4.Bandyopadhyay GK, Yu JG, Ofrecio J, Olefsky JM. Increased malonyl-CoA levels in muscle from obese and type 2 diabetic subjects lead to decreased fatty acid oxidation and increased lipogenesis; thiazolidinedione treatment reverses these defects. Diabetes. 2006;55:2277–2285. doi: 10.2337/db06-0062. [DOI] [PubMed] [Google Scholar]
- 5.Barnes BR, Marklund S, Steiler TL, Walter M, Hjalm G, Amarger V, Mahlapuu M, Leng Y, Johansson C, Galuska D, Lindgren K, Abrink M, Stapleton D, Zierath JR, Andersson L. The 5'-AMP-activated protein kinase gamma3 isoform has a key role in carbohydrate and lipid metabolism in glycolytic skeletal muscle. J.Biol.Chem. 2004;279:38441–38447. doi: 10.1074/jbc.M405533200. [DOI] [PubMed] [Google Scholar]
- 6.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le CD, Shaw RJ, Navas P, Puigserver P, Ingram DK, de CR, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker-Zimmermann K, Berger M, Berchtold P, Gries FA, Herberg L, Schwenen M. Treadmill training improves intravenous glucose tolerance and insulin sensitivity in fatty Zucker rats. Diabetologia. 1982;22:468–474. doi: 10.1007/BF00282592. [DOI] [PubMed] [Google Scholar]
- 8.Biolo G, Williams BD, Fleming RY, Wolfe RR. Insulin action on muscle protein kinetics and amino acid transport during recovery after resistance exercise. Diabetes. 1999;48:949–957. doi: 10.2337/diabetes.48.5.949. [DOI] [PubMed] [Google Scholar]
- 9.Birk JB, Wojtaszewski JF. Predominant {alpha}2/{beta}2/{gamma}3 AMPK activation during exercise in human skeletal muscle. J.Physiol. 2006;577:1021–1032. doi: 10.1113/jphysiol.2006.120972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blair SN, Kohl HW, III, Barlow CE, Paffenbarger RS, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality. Jama. 1995;273:1093–1098. [PubMed] [Google Scholar]
- 11.Blair SN, Kohl HW, III, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. Jama. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 12.Bonen A, Han XX, Habets DD, Febbraio M, Glatz JF, Luiken JJ. A null mutation in skeletal muscle FAT/CD36 reveals its essential role in insulin- and AICAR-stimulated fatty acid metabolism. Am.J.Physiol Endocrinol.Metab. 2007;292:E1740–E1749. doi: 10.1152/ajpendo.00579.2006. [DOI] [PubMed] [Google Scholar]
- 13.Boveris A, Navarro A. Systemic and mitochondrial adaptive responses to moderate exercise in rodents. Free Radic.Biol.Med. 2008;44:224–229. doi: 10.1016/j.freeradbiomed.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29:254–258. doi: 10.2337/diacare.29.02.06.dc05-1558. [DOI] [PubMed] [Google Scholar]
- 15.bu-Elheiga L, marza-Ortega DB, Baldini A, Wakil SJ. Human acetyl-CoA carboxylase 2. Molecular cloning, characterization, chromosomal mapping, and evidence for two isoforms. J Biol.Chem. 1997;272:10669–10677. doi: 10.1074/jbc.272.16.10669. [DOI] [PubMed] [Google Scholar]
- 16.Buhl ES, Jessen N, Pold R, Ledet T, Flyvbjerg A, Pedersen SB, Pedersen O, Schmitz O, Lund S. Long-term AICAR administration reduces metabolic disturbances and lowers blood pressure in rats displaying features of the insulin resistance syndrome. Diabetes. 2002;51:2199–2206. doi: 10.2337/diabetes.51.7.2199. [DOI] [PubMed] [Google Scholar]
- 17.Bylund-Fellenius AC, Ojamaa KM, Flaim KE, Li JB, Wassner SJ, Jefferson LS. Protein synthesis versus energy state in contracting muscles of perfused rat hindlimb. Am J Physiol. 1984;246:E297–E305. doi: 10.1152/ajpendo.1984.246.4.E297. [DOI] [PubMed] [Google Scholar]
- 18.Carling D, Zammit VA, Hardie DG. A common bicyclic protein kinase cascade inactivates the regulatory enzymes of fatty acid and cholesterol biosynthesis. FEBS Lett. 1987;223:217–222. doi: 10.1016/0014-5793(87)80292-2. [DOI] [PubMed] [Google Scholar]
- 19.Carlson CL, Winder WW. Liver AMP-activated protein kinase and acetyl-CoA carboxylase during and after exercise. J Appl.Physiol. 1999;86:669–674. doi: 10.1152/jappl.1999.86.2.669. [DOI] [PubMed] [Google Scholar]
- 20.Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999;443:285–289. doi: 10.1016/s0014-5793(98)01705-0. [DOI] [PubMed] [Google Scholar]
- 21.Chen ZP, Stephens TJ, Murthy S, Canny BJ, Hargreaves M, Witters LA, Kemp BE, McConell GK. Effect of exercise intensity on skeletal muscle AMPK signaling in humans. Diabetes. 2003;52:2205–2212. doi: 10.2337/diabetes.52.9.2205. [DOI] [PubMed] [Google Scholar]
- 22.Christensen EH, Hansen O. Respiratorisher Quotient und O2 -Aufname. Skandinavisches Archiv fur Physiologie. 1939;81:180–189. [Google Scholar]
- 23.Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, Ravussin E. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS.Med. 2007;4:e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coven DL, Hu X, Cong L, Bergeron R, Shulman GI, Hardie DG, Young LH. Physiological role of AMP-activated protein kinase in the heart: graded activation during exercise. Am J Physiol Endocrinol Metab. 2003;285:E629–E636. doi: 10.1152/ajpendo.00171.2003. [DOI] [PubMed] [Google Scholar]
- 25.Dagon Y, Avraham Y, Magen I, Gertler A, Ben-Hur T, Berry EM. Nutritional status, cognition, and survival: a new role for leptin and AMP kinase. J Biol.Chem. 2005;280:42142–42148. doi: 10.1074/jbc.M507607200. [DOI] [PubMed] [Google Scholar]
- 26.Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc.Natl.Acad.Sci.U.S.A. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daval M, Foufelle F, Ferre P. Functions of AMP-activated protein kinase in adipose tissue. J Physiol. 2006;574:55–62. doi: 10.1113/jphysiol.2006.111484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Filippis E, Alvarez G, Berria R, Cusi K, Everman S, Meyer C, Mandarino LJ. Insulin-resistant muscle is exercise resistant: evidence for reduced response of nuclear-encoded mitochondrial genes to exercise. Am J Physiol Endocrinol Metab. 2008;294:607–614. doi: 10.1152/ajpendo.00729.2007. [DOI] [PubMed] [Google Scholar]
- 29.Dean D, Daugaard JR, Young ME, Saha A, Vavvas D, Asp S, Kiens B, Kim KH, Witters L, Richter EA, Ruderman N. Exercise diminishes the activity of acetyl-CoA carboxylase in human muscle. Diabetes. 2000;49:1295–1300. doi: 10.2337/diabetes.49.8.1295. [DOI] [PubMed] [Google Scholar]
- 30.Deldicque L, Atherton P, Patel R, Theisen D, Nielens H, Rennie MJ, Francaux M. Decrease in Akt/PKB signalling in human skeletal muscle by resistance exercise. Eur.J Appl.Physiol. 2008;104:57–65. doi: 10.1007/s00421-008-0786-7. [DOI] [PubMed] [Google Scholar]
- 31.Derave W, Ai H, Ihlemann J, Witters LA, Kristiansen S, Richter EA, Ploug T. Dissociation of AMP-activated protein kinase activation and glucose transport in contracting slow-twitch muscle. Diabetes. 2000;49:1281–1287. doi: 10.2337/diabetes.49.8.1281. [DOI] [PubMed] [Google Scholar]
- 32.Deshmukh A, Coffey VG, Zhong Z, Chibalin AV, Hawley JA, Zierath JR. Exercise-induced phosphorylation of the novel Akt substrates AS160 and filamin A in human skeletal muscle. Diabetes. 2006;55:1776–1782. doi: 10.2337/db05-1419. [DOI] [PubMed] [Google Scholar]
- 33.Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, Gandevia SC, Gomez-Pinilla F, Greenwood BN, Hillman CH, Kramer AF, Levin BE, Moran TH, Russo-Neustadt AA, Salamone JD, Van Hoomissen JD, Wade CE, York DA, Zigmond MJ. Neurobiology of Exercise[ast] Obesity. 2006;14:345–356. doi: 10.1038/oby.2006.46. [DOI] [PubMed] [Google Scholar]
- 34.Dolkas CB, Greenleaf JE. Insulin and glucose responses during bed rest with isotonic and isometric exercise. J Appl.Physiol. 1977;43:1033–1038. doi: 10.1152/jappl.1977.43.6.1033. [DOI] [PubMed] [Google Scholar]
- 35.Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. The Journal of Physiology Online. 2006;576:613–624. doi: 10.1113/jphysiol.2006.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dzamko NL, Schertzer JD, Ryall J, Steel R, Macaulay SL, Wee S, Chen ZP, Michell BJ, Oakhill JS, Watt MJ, Jorgensen SB, Lynch GS, Kemp BE, Steinberg GR. AMPK independent pathways regulate skeletal muscle fatty acid oxidation. J Physiol. 2008 doi: 10.1113/jphysiol.2008.159814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faldt J, Wernstedt I, Fitzgerald SM, Wallenius K, Bergstrom G, Jansson JO. Reduced exercise endurance in interleukin-6-deficient mice. Endocrinology. 2004;145:2680–2686. doi: 10.1210/en.2003-1319. [DOI] [PubMed] [Google Scholar]
- 39.Febbraio MA, Pedersen BK. Muscle-derived interleukin-6: mechanisms for activation and possible biological roles. FASEB J. 2002;16:1335–1347. doi: 10.1096/fj.01-0876rev. [DOI] [PubMed] [Google Scholar]
- 40.Ferrara N, Rinaldi B, Corbi G, Conti V, Stiuso P, Boccuti S, Rengo G, Rossi F, Filippelli A. Exercise training promotes SIRT1 activity in aged rats. Rejuvenation.Res. 2008;11:139–150. doi: 10.1089/rej.2007.0576. [DOI] [PubMed] [Google Scholar]
- 41.Fisher JS, Gao J, Han DH, Holloszy JO, Nolte LA. Activation of AMP kinase enhances sensitivity of muscle glucose transport to insulin. Am J Physiol Endocrinol Metab. 2002;282:E18–E23. doi: 10.1152/ajpendo.2002.282.1.E18. [DOI] [PubMed] [Google Scholar]
- 42.Flores MB, Fernandes MF, Ropelle ER, Faria MC, Ueno M, Velloso LA, Saad MJ, Carvalheira JB. Exercise improves insulin and leptin sensitivity in hypothalamus of Wistar rats. Diabetes. 2006;55:2554–2561. doi: 10.2337/db05-1622. [DOI] [PubMed] [Google Scholar]
- 43.Frisbee JC, Samora JB, Peterson J, Bryner R. Exercise training blunts microvascular rarefaction in the metabolic syndrome. Am J Physiol Heart Circ.Physiol. 2006;291:H2483–H2492. doi: 10.1152/ajpheart.00566.2006. [DOI] [PubMed] [Google Scholar]
- 44.Frosig C, Jorgensen SB, Hardie DG, Richter EA, Wojtaszewski JF. 5'-AMP-activated protein kinase activity and protein expression are regulated by endurance training in human skeletal muscle. Am.J.Physiol Endocrinol.Metab. 2004;286:E411–E417. doi: 10.1152/ajpendo.00317.2003. [DOI] [PubMed] [Google Scholar]
- 45.Frosig C, Sajan MP, Maarbjerg SJ, Brandt N, Roepstorff C, Wojtaszewski JF, Kiens B, Farese RV, Richter EA. Exercise improves phosphatidylinositol-3,4,5-trisphosphate responsiveness of atypical protein kinase C and interacts with insulin signalling to peptide elongation in human skeletal muscle. J Physiol. 2007;582:1289–1301. doi: 10.1113/jphysiol.2007.136614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fujii N, Hayashi T, Hirshman MF, Smith JT, Habinowski SA, Kaijser L, Mu J, Ljungqvist O, Birnbaum MJ, Witters LA, Thorell A, Goodyear LJ. Exercise induces isoform-specific increase in 5'AMP-activated protein kinase activity in human skeletal muscle. Biochem.Biophys.Res.Commun. 2000;273:1150–1155. doi: 10.1006/bbrc.2000.3073. [DOI] [PubMed] [Google Scholar]
- 47.Fujii N, Hirshman MF, Kane EM, Ho RC, Peter LE, Seifert MM, Goodyear LJ. AMP-activated protein kinase alpha2 activity is not essential for contraction- and hyperosmolarity-induced glucose transport in skeletal muscle. J Biol.Chem. 2005;280:39033–39041. doi: 10.1074/jbc.M504208200. [DOI] [PubMed] [Google Scholar]
- 48.Fujii N, Ho RC, Manabe Y, Jessen N, Toyoda T, Holland WL, Summers SA, Hirshman MF, Goodyear LJ. Ablation of AMP-activated Protein Kinase {alpha}2 Activity Exacerbates Insulin Resistance Induced by High-fat Feeding of Mice. Diabetes. 2008 doi: 10.2337/db07-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fujii N, Seifert MM, Kane EM, Peter LE, Ho RC, Winstead S, Hirshman MF, Goodyear LJ. Role of AMP-activated protein kinase in exercise capacity, whole body glucose homeostasis, and glucose transport in skeletal muscle -insight from analysis of a transgenic mouse model- Diabetes Res.Clin.Pract. 2007;77 Suppl 1:S92–S98. doi: 10.1016/j.diabres.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 50.Funai K, Cartee GD. Contraction-stimulated Glucose Transport in Rat Skeletal Muscle is Sustained despite Reversal of Increased PAS-phosphorylation of AS160 and TBC1D1. J Appl.Physiol. 2008 doi: 10.1152/japplphysiol.90838.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galbo H. Integrated endocrine responses and exercise. In: Groot De., editor. Endocrinology. 3rd ed. Philadelphia: W.B.Saunders; 1995. pp. 2692–2701. [Google Scholar]
- 52.Gauthier MS, Miyoshi H, Souza SC, Cacicedo JM, Saha AK, Greenberg AS, Ruderman NB. AMP-activated protein kinase (AMPK) is activated as a consequence of lipolysis in the adipocyte: potential mechanism and physiological relevance. J Biol Chem. 2008 doi: 10.1074/jbc.M708177200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Geraghty KM, Chen S, Harthill JE, Ibrahim AF, Toth R, Morrice NA, Vandermoere F, Moorhead GB, Hardie DG, Mackintosh C. Regulation of multisite phosphorylation and 14-3-3 binding of AS160 in response to IGF-1, EGF, PMA and AICAR. Biochem.J. 2007;407:231–241. doi: 10.1042/BJ20070649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, Hanefeld M, Hoogwerf B, Laakso M, Mohan V, Shaw J, Zinman B, Holman RR. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006;368:1096–1105. doi: 10.1016/S0140-6736(06)69420-8. [DOI] [PubMed] [Google Scholar]
- 55.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin.Nutr. 2006;83:461S–465S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 56.Grundy SM. Does the metabolic syndrome exist? Diabetes Care. 2006;29:1689–1692. doi: 10.2337/dc05-2307. [DOI] [PubMed] [Google Scholar]
- 57.Habets DD, Coumans WA, Voshol PJ, den Boer MA, Febbraio M, Bonen A, Glatz JF, Luiken JJ. AMPK-mediated increase in myocardial long-chain fatty acid uptake critically depends on sarcolemmal CD36. Biochem.Biophys.Res.Commun. 2007;355:204–210. doi: 10.1016/j.bbrc.2007.01.141. [DOI] [PubMed] [Google Scholar]
- 58.Hancock CR, Janssen E, Terjung RL. Contraction-mediated phosphorylation of AMPK is lower in skeletal muscle of adenylate kinase-deficient mice. J Appl.Physiol. 2006;100:406–413. doi: 10.1152/japplphysiol.00885.2005. [DOI] [PubMed] [Google Scholar]
- 59.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat.Rev.Mol.Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 60.Hardie DG. Role of AMP-activated protein kinase in the metabolic syndrome and in heart disease. FEBS Lett. 2008;582:81–89. doi: 10.1016/j.febslet.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 61.Hardie DG, Salt IP, Davies SP. Analysis of the role of the AMP-activated protein kinase in the response to cellular stress. Methods Mol.Biol. 2000;99:63–74. doi: 10.1385/1-59259-054-3:63. [DOI] [PubMed] [Google Scholar]
- 62.Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003;546:113–120. doi: 10.1016/s0014-5793(03)00560-x. [DOI] [PubMed] [Google Scholar]
- 63.Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRADalpha/beta and MO25alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J.Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 65.Helge JW, Stallknecht B, Richter EA, Galbo H, Kiens B. Muscle metabolism during graded quadriceps exercise in man. J Physiol. 2007;581:1247–1258. doi: 10.1113/jphysiol.2007.128348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hojlund K, Mustard KJ, Staehr P, Hardie DG, Beck-Nielsen H, Richter EA, Wojtaszewski JF. AMPK activity and isoform protein expression are similar in muscle of obese subjects with and without type 2 diabetes. Am.J.Physiol Endocrinol.Metab. 2004;286:E239–E244. doi: 10.1152/ajpendo.00326.2003. [DOI] [PubMed] [Google Scholar]
- 67.Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl.Physiol. 1984;56:831–838. doi: 10.1152/jappl.1984.56.4.831. [DOI] [PubMed] [Google Scholar]
- 68.Holmes BF, Kurth-Kraczek EJ, Winder WW. Chronic activation of 5`-amp-activated protein kinase increases glut-4, hexokinase and glycogen in muscle. APStracts. 1999;6:379A. doi: 10.1152/jappl.1999.87.5.1990. (Abstract) [DOI] [PubMed] [Google Scholar]
- 69.Horman S, Beauloye C, Vertommen D, Vanoverschelde JL, Hue L, Rider MH. Myocardial ischemia and increased heart work modulate the phosphorylation state of eukaryotic elongation factor-2. J Biol.Chem. 2003;278:41970–41976. doi: 10.1074/jbc.M302403200. [DOI] [PubMed] [Google Scholar]
- 70.Horman S, Browne G, Krause U, Patel J, Vertommen D, Bertrand L, Lavoinne A, Hue L, Proud C, Rider M. Activation of AMP-activated protein kinase leads to the phosphorylation of elongation factor 2 and an inhibition of protein synthesis. Curr.Biol. 2002;12:1419–1423. doi: 10.1016/s0960-9822(02)01077-1. [DOI] [PubMed] [Google Scholar]
- 71.Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, Lan F, Walsh K, Wierzbicki M, Verbeuren TJ, Cohen RA, Zang M. SIRT1 Regulates Hepatocyte Lipid Metabolism through Activating AMP-activated Protein Kinase. J Biol.Chem. 2008;283:20015–20026. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hu FB, Sigal RJ, Rich-Edwards JW, Colditz GA, Solomon CG, Willett WC, Speizer FE, Manson JE. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: a prospective study. Jama. 1999;282:1433–1439. doi: 10.1001/jama.282.15.1433. [DOI] [PubMed] [Google Scholar]
- 73.Hudson ER, Pan DA, James J, Lucocq JM, Hawley SA, Green KA, Baba O, Terashima T, Hardie DG. A novel domain in AMP-activated protein kinase causes glycogen storage bodies similar to those seen in hereditary cardiac arrhythmias. Curr.Biol. 2003;13:861–866. doi: 10.1016/s0960-9822(03)00249-5. [DOI] [PubMed] [Google Scholar]
- 74.Hurst D, Taylor EB, Cline TD, Greenwood LJ, Compton CL, Lamb JD, Winder WW. AMP-activated protein kinase kinase activity and phosphorylation of AMP-activated protein kinase in contracting muscle of sedentary and endurance-trained rats. Am J Physiol Endocrinol Metab. 2005;289:E710–E715. doi: 10.1152/ajpendo.00155.2005. [DOI] [PubMed] [Google Scholar]
- 75.Iglesias MA, Ye JM, Frangioudakis G, Saha AK, Tomas E, Ruderman NB, Cooney GJ, Kraegen EW. AICAR administration causes an apparent enhancement of muscle and liver insulin action in insulin-resistant high-fat-fed rats. Diabetes. 2002;51:2886–2894. doi: 10.2337/diabetes.51.10.2886. [DOI] [PubMed] [Google Scholar]
- 76.Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc.Natl.Acad.Sci.U.S.A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jensen TE, Rose AJ, Hellsten Y, Wojtaszewski JF, Richter EA. Caffeine-induced Ca(2+) release increases AMPK-dependent glucose uptake in rodent soleus muscle. Am J Physiol Endocrinol Metab. 2007;293:E286–E292. doi: 10.1152/ajpendo.00693.2006. [DOI] [PubMed] [Google Scholar]
- 78.Jensen TE, Rose AJ, Jorgensen SB, Brandt N, Schjerling P, Wojtaszewski JF, Richter EA. Possible CaMKK-dependent regulation of AMPK phosphorylation and glucose uptake at the onset of mild tetanic skeletal muscle contraction. Am.J.Physiol Endocrinol.Metab. 2007 doi: 10.1152/ajpendo.00456.2006. [DOI] [PubMed] [Google Scholar]
- 79.Jensen TE, Rose AJ, Jorgensen SB, Brandt N, Schjerling P, Wojtaszewski JF, Richter EA. Possible CaMKK-dependent regulation of AMPK phosphorylation and glucose uptake at the onset of mild tetanic skeletal muscle contraction. Am J Physiol Endocrinol Metab. 2007;292:E1308–E1317. doi: 10.1152/ajpendo.00456.2006. [DOI] [PubMed] [Google Scholar]
- 80.Jensen TE, Schjerling P, Viollet B, Wojtaszewski JF, Richter EA. AMPK alpha1 activation is required for stimulation of glucose uptake by twitch contraction, but not by H2O2, in mouse skeletal muscle. PLoS.ONE. 2008;3:e2102. doi: 10.1371/journal.pone.0002102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jorgensen SB, Nielsen JN, Birk JB, Olsen GS, Viollet B, Andreelli F, Schjerling P, Vaulont S, Hardie DG, Hansen BF, Richter EA, Wojtaszewski JF. The alpha2–5'AMP-activated protein kinase is a site 2 glycogen synthase kinase in skeletal muscle and is responsive to glucose loading. Diabetes. 2004;53:3074–3081. doi: 10.2337/diabetes.53.12.3074. [DOI] [PubMed] [Google Scholar]
- 82.Jorgensen SB, Richter EA, Wojtaszewski JF. Role of AMPK in skeletal muscle metabolic regulation and adaptation in relation to exercise. J.Physiol. 2006;574:17–31. doi: 10.1113/jphysiol.2006.109942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jorgensen SB, Treebak JT, Viollet B, Schjerling P, Vaulont S, Wojtaszewski JF, Richter EA. Role of AMPKalpha2 in basal, training-, and AICAR-induced GLUT4, hexokinase II, and mitochondrial protein expression in mouse muscle. Am J Physiol Endocrinol Metab. 2007;292:E331–E339. doi: 10.1152/ajpendo.00243.2006. [DOI] [PubMed] [Google Scholar]
- 84.Jorgensen SB, Treebak JT, Viollet B, Schjerling P, Vaulont S, Wojtaszewski JF, Richter EA. Role of AMPK{alpha}2 in basal, training-, and AICAR-induced GLUT4, hexokinase II, and mitochondrial protein expression in mouse muscle. Am.J.Physiol Endocrinol.Metab. 2007;292:E331–E339. doi: 10.1152/ajpendo.00243.2006. [DOI] [PubMed] [Google Scholar]
- 85.Jorgensen SB, Viollet B, Andreelli F, Frosig C, Birk JB, Schjerling P, Vaulont S, Richter EA, Wojtaszewski JF. Knockout of the alpha2 but not alpha1 5'-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranosidebut not contraction-induced glucose uptake in skeletal muscle. J.Biol.Chem. 2004;279:1070–1079. doi: 10.1074/jbc.M306205200. [DOI] [PubMed] [Google Scholar]
- 86.Jorgensen SB, Wojtaszewski JF, Viollet B, Andreelli F, Birk JB, Hellsten Y, Schjerling P, Vaulont S, Neufer PD, Richter EA, Pilegaard H. Effects of alpha-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle. FASEB J. 2005;19:1146–1148. doi: 10.1096/fj.04-3144fje. [DOI] [PubMed] [Google Scholar]
- 87.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 88.Kelly M, Keller C, Avilucea PR, Keller P, Luo Z, Xiang X, Giralt M, Hidalgo J, Saha AK, Pedersen BK, Ruderman NB. AMPK activity is diminished in tissues of IL-6 knockout mice: the effect of exercise. Biochem.Biophys.Res.Commun. 2004;320:449–454. doi: 10.1016/j.bbrc.2004.05.188. [DOI] [PubMed] [Google Scholar]
- 89.Kelly M, Keller C, Avilucea PR, Keller P, Luo Z, Xiang X, Giralt M, Hidalgo J, Saha AK, Pedersen BK, Ruderman NB. AMPK activity is diminished in tissues of IL-6 knockout mice: the effect of exercise. Biochem Biophys Res Commun. 2004;320:449–454. doi: 10.1016/j.bbrc.2004.05.188. [DOI] [PubMed] [Google Scholar]
- 90.Kern PA, Simsolo RB, Fournier M. Effect of weight loss on muscle fiber type, fiber size, capillarity, and succinate dehydrogenase activity in humans. J Clin.Endocrinol Metab. 1999;84:4185–4190. doi: 10.1210/jcem.84.11.6090. [DOI] [PubMed] [Google Scholar]
- 91.Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, Puigserver P, Sinclair DA, Tsai LH. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kivela R, Silvennoinen M, Touvra AM, Lehti TM, Kainulainen H, Vihko V. Effects of experimental type 1 diabetes and exercise training on angiogenic gene expression and capillarization in skeletal muscle. FASEB J. 2006;20:1570–1572. doi: 10.1096/fj.05-4780fje. [DOI] [PubMed] [Google Scholar]
- 93.Klein D, Pilegaard H, Treebak JT, Jensen TE, Viollet B, Schjerling P, Wojtaszewski JF. Lack of {alpha}2 5'AMP Activated Protein Kinase enhances Pyruvate Dehydrogenase activity during exercise. Am J Physiol Endocrinol Metab. 2007 doi: 10.1152/ajpendo.00382.2007. [DOI] [PubMed] [Google Scholar]
- 94.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N.Engl.J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Koh H-J, Fujii N, Toyoda T, Jung MM, Hirshman MF, Goodyear LJ. The AMPK-related protein kinase SNARK mediates contraction-stimulated glucose transport in mouse skeletal muscle. Diabetes. 2008;57:A91, Ap2. doi: 10.1073/pnas.1008131107. (Abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Koh HJ, Arnolds DE, Fujii N, Tran TT, Rogers MJ, Jessen N, Li Y, Liew CW, Ho RC, Hirshman MF, Kulkarni RN, Kahn CR, Goodyear LJ. Skeletal muscle-selective knockout of LKB1 increases insulin sensitivity, improves glucose homeostasis, and decreases TRB3. Mol.Cell Biol. 2006;26:8217–8227. doi: 10.1128/MCB.00979-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koh HJ, Hirshman MF, He H, Li Y, Manabe Y, Balschi JA, Goodyear LJ. Adrenaline is a critical mediator of acute exercise-induced AMP-activated protein kinase activation in adipocytes. Biochem.J. 2007;403:473–481. doi: 10.1042/BJ20061479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Koistinen HA, Galuska D, Chibalin AV, Yang J, Zierath JR, Holman GD, Wallberg-Henriksson H. 5-amino-imidazole carboxamide riboside increases glucose transport and cell-surface GLUT4 content in skeletal muscle from subjects with type 2 diabetes. Diabetes. 2003;52:1066–1072. doi: 10.2337/diabetes.52.5.1066. [DOI] [PubMed] [Google Scholar]
- 99.Kojda G, Cheng YC, Burchfield J, Harrison DG. Dysfunctional regulation of endothelial nitric oxide synthase (eNOS) expression in response to exercise in mice lacking one eNOS gene. Circulation. 2001;103:2839–2844. doi: 10.1161/01.cir.103.23.2839. [DOI] [PubMed] [Google Scholar]
- 100.Kola B, Hubina E, Tucci SA, Kirkham TC, Garcia EA, Mitchell SE, Williams LM, Hawley SA, Hardie DG, Grossman AB, Korbonits M. Cannabinoids and ghrelin have both central and peripheral metabolic and cardiac effects via AMP-activated protein kinase. J Biol Chem. 2005;280:25196–25201. doi: 10.1074/jbc.C500175200. [DOI] [PubMed] [Google Scholar]
- 101.Kraegen EW, Saha AK, Preston E, Wilks D, Hoy AJ, Cooney GJ, Ruderman NB. Increased malonyl CoA and diacylglycerol content and reduced AMPK activity accompany insulin resistance induced by glucose infusion in muscle and liver of rats. Am J Physiol Endocrinol Metab. 2005 doi: 10.1152/ajpendo.00316.2005. [DOI] [PubMed] [Google Scholar]
- 102.Kramer AF, Erickson KI. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends Cogn Sci. 2007;11:342–348. doi: 10.1016/j.tics.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 103.Kramer HF, Witczak CA, Fujii N, Jessen N, Taylor EB, Arnolds DE, Sakamoto K, Hirshman MF, Goodyear LJ. Distinct signals regulate AS160 phosphorylation in response to insulin, AICAR, and contraction in mouse skeletal muscle. Diabetes. 2006;55:2067–2076. doi: 10.2337/db06-0150. [DOI] [PubMed] [Google Scholar]
- 104.Kramer HF, Witczak CA, Taylor EB, Fujii N, Hirshman MF, Goodyear LJ. AS160 regulates insulin- and contraction-stimulated glucose uptake in mouse skeletal muscle. J Biol.Chem. 2006;281:31478–31485. doi: 10.1074/jbc.M605461200. [DOI] [PubMed] [Google Scholar]
- 105.Kristensen JM, Johnsen AB, Birk JB, Nielsen JN, Jensen BR, Hellsten Y, Richter EA, Wojtaszewski JF. Absence of humoral mediated 5'AMP-activated protein kinase activation in human skeletal muscle and adipose tissue during exercise. J Physiol. 2007 doi: 10.1113/jphysiol.2007.143123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kubota N, Terauchi Y, Kubota T, Kumagai H, Itoh S, Satoh H, Yano W, Ogata H, Tokuyama K, Takamoto I, Mineyama T, Ishikawa M, Moroi M, Sugi K, Yamauchi T, Ueki K, Tobe K, Noda T, Nagai R, Kadowaki T. Pioglitazone ameliorates insulin resistance and diabetes by both adiponectin-dependent and -independent pathways. J Biol.Chem. 2006;281:8748–8755. doi: 10.1074/jbc.M505649200. [DOI] [PubMed] [Google Scholar]
- 107.Kuhl JE, Ruderman NB, Musi N, Goodyear LJ, Patti ME, Crunkhorn S, Dronamraju D, Thorell A, Nygren J, Ljungkvist O, Degerblad M, Stahle A, Brismar TB, Andersen KL, Saha AK, Efendic S, Bavenholm PN. Exercise training decreases the concentration of malonyl-CoA and increases the expression and activity of malonyl-CoA decarboxylase in human muscle. Am J Physiol Endocrinol Metab. 2006;290:E1296–E1303. doi: 10.1152/ajpendo.00341.2005. [DOI] [PubMed] [Google Scholar]
- 108.Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization and activity of LKB1; possible role in AMP-activated protein kinase activation. J.Biol.Chem. 2008 doi: 10.1074/jbc.M805711200. M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lautenschlager NT, Cox KL, Flicker L, Foster JK, van Bockxmeer FM, Xiao J, Greenop KR, Almeida OP. Effect of Physical Activity on Cognitive Function in Older Adults at Risk for Alzheimer Disease: A Randomized Trial. JAMA: The Journal of the American Medical Association. 2008;300:1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 110.Lees SJ, Booth FW. Physical inactivity is a disease. World Rev.Nutr.Diet. 2005;95:73–79. doi: 10.1159/000088274. [DOI] [PubMed] [Google Scholar]
- 111.Lipman R, Schnure J, Bradley E, Lecocq F. Impairment of peripheral glucose utilization in normal subjects by prolonged bed rest. J.Lab.Clin.Med. 1970;76:221–230. [PubMed] [Google Scholar]
- 112.Luo Z, Saha AK, Xiang X, Ruderman NB. AMPK, the metabolic syndrome and cancer. Trends Pharmacol.Sci. 2005;26:69–76. doi: 10.1016/j.tips.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 113.MacDonald C, Wojtaszewski JF, Pedersen BK, Kiens B, Richter EA. Interleukin-6 release from human skeletal muscle during exercise: relation to AMPK activity. J Appl.Physiol. 2003;95:2273–2277. doi: 10.1152/japplphysiol.00242.2003. [DOI] [PubMed] [Google Scholar]
- 114.Manson JE, Nathan DM, Krolewski AS, Stampfer MJ, Willett WC, Hennekens CH. A prospective study of exercise and incidence of diabetes among US male physicians. Jama. 1992;268:63–67. [PubMed] [Google Scholar]
- 115.Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF, Van den BG, Carling D, Hue L. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr.Biol. 2000;10:1247–1255. doi: 10.1016/s0960-9822(00)00742-9. [DOI] [PubMed] [Google Scholar]
- 116.Martin TL, Alquier T, Asakura K, Furukawa N, Preitner F, Kahn BB. Diet-induced obesity alters AMP kinase activity in hypothalamus and skeletal muscle. J.Biol.Chem. 2006;281:18933–18941. doi: 10.1074/jbc.M512831200. [DOI] [PubMed] [Google Scholar]
- 117.Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc.Natl.Acad.Sci.U.S.A. 2007;104:14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McGarry JD. Banting lecture 2001: dysregulation of Fatty Acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51:7–18. doi: 10.2337/diabetes.51.1.7. [DOI] [PubMed] [Google Scholar]
- 119.Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol. 1997;273:E1107–E1112. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]
- 120.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem.J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mikines K, Sonne B, Farrell P, Tronier B, Galbo H. Effect of physical exercise on sensitivity and responsiveness to insulin in humans. Am.J.Physiol. 1988;254:E248–E259. doi: 10.1152/ajpendo.1988.254.3.E248. [DOI] [PubMed] [Google Scholar]
- 122.Mikines KJ, Richter EA, Dela F, Galbo H. Seven days of bed rest decrease insulin action on glucose uptake in leg and whole body. J.Appl.Physiol. 1991;70:1245–1254. doi: 10.1152/jappl.1991.70.3.1245. [DOI] [PubMed] [Google Scholar]
- 123.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 124.Morrow VA, Foufelle F, Connell JM, Petrie JR, Gould GW, Salt IP. Direct activation of AMP-activated protein kinase stimulates nitric-oxide synthesis in human aortic endothelial cells. J Biol.Chem. 2003;278:31629–31639. doi: 10.1074/jbc.M212831200. [DOI] [PubMed] [Google Scholar]
- 125.Mu J, Barton ER, Birnbaum MJ. Selective suppression of AMP-activated protein kinase in skeletal muscle: update on 'lazy mice'. Biochem.Soc.Trans. 2003;31:236–241. doi: 10.1042/bst0310236. [DOI] [PubMed] [Google Scholar]
- 126.Mu J, Brozinick JT, Jr., Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol.Cell. 2001;7:1085–1094. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- 127.Muoio DM, Seefeld K, Witters LA, Coleman RA. AMP-activated kinase reciprocally regulates triacylglycerol synthesis and fatty acid oxidation in liver and muscle: evidence that sn-glycerol-3-phosphate acyltransferase is a novel target. Biochem.J. 1999;338(Pt 3):783–791. [PMC free article] [PubMed] [Google Scholar]
- 128.Musi N, Hayashi T, Fujii N, Hirshman MF, Witters LA, Goodyear LJ. AMP-activated protein kinase activity and glucose uptake in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2001;280:E677–E684. doi: 10.1152/ajpendo.2001.280.5.E677. [DOI] [PubMed] [Google Scholar]
- 129.Musi N, Hirshman MF, Arad M, Xing Y, Fujii N, Pomerleau J, Ahmad F, Berul CI, Seidman JG, Tian R, Goodyear LJ. Functional role of AMP-activated protein kinase in the heart during exercise. FEBS Lett. 2005;579:2045–2050. doi: 10.1016/j.febslet.2005.02.052. [DOI] [PubMed] [Google Scholar]
- 130.Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, Zhou G, Williamson JM, Ljunqvist O, Efendic S, Moller DE, Thorell A, Goodyear LJ. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51:2074–2081. doi: 10.2337/diabetes.51.7.2074. [DOI] [PubMed] [Google Scholar]
- 131.Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, Pang Z, Chen AS, Ruderman NB, Chen H, Rossetti L, Scherer PE. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol.Chem. 2006;281:2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- 132.Nielsen JN, Mustard KJ, Graham DA, Yu H, MacDonald CS, Pilegaard H, Goodyear LJ, Hardie DG, Richter EA, Wojtaszewski JF. 5'AMP-activated protein kinase activity and subunit expression in exercise-trained human skeletal muscle. J.Appl.Physiol. 2002:00642. doi: 10.1152/japplphysiol.00642.2002. [DOI] [PubMed] [Google Scholar]
- 133.Odland LM, Heigenhauser GJF, Lopaschuk GD, Spriet LL. Human skeletal muscle malonyl-CoA at rest and during prolonged submaximal exercise. Am.J.Physiol. 1996;270:E541–E544. doi: 10.1152/ajpendo.1996.270.3.E541. [DOI] [PubMed] [Google Scholar]
- 134.Odland LM, Howlett RA, Heigenhauser GJ, Hultman E, Spriet LL. Skeletal muscle malonyl-CoA content at the onset of exercise at varying power outputs in humans. Am.J.Physiol. 1998;274:E1080–E1085. doi: 10.1152/ajpendo.1998.274.6.E1080. [DOI] [PubMed] [Google Scholar]
- 135.Olsen RH, Krogh-Madsen R, Thomsen C, Booth FW, Pedersen BK. Metabolic responses to reduced daily steps in healthy nonexercising men. Jama. 2008;299:1261–1263. doi: 10.1001/jama.299.11.1259. [DOI] [PubMed] [Google Scholar]
- 136.Orchard TJ, Temprosa M, Goldberg R, Haffner S, Ratner R, Marcovina S, Fowler S. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med. 2005;142:611–619. doi: 10.7326/0003-4819-142-8-200504190-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol.Chem. 2004;279:1304–1309. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ouchi N, Shibata R, Walsh K. AMP-activated protein kinase signaling stimulates VEGF expression and angiogenesis in skeletal muscle. Circ.Res. 2005;96:838–846. doi: 10.1161/01.RES.0000163633.10240.3b. [DOI] [PubMed] [Google Scholar]
- 139.Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB, Liu PA, Jiang XG, Jiang YY, Wang JP, Zheng H, Zhang H, Bennett PH, Howard BV. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 140.Parachikova A, Nichol KE, Cotman CW. Short-term exercise in aged Tg2576 mice alters neuroinflammation and improves cognition. Neurobiol.Dis. 2008;30:121–129. doi: 10.1016/j.nbd.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Park H, Kaushik VK, Constant S, Prentki M, Przybytkowski E, Ruderman NB, Saha AK. Coordinate regulation of malonyl-CoA decarboxylase, sn-glycerol-3-phosphate acyltransferase, and acetyl-CoA carboxylase by AMP-activated protein kinase in rat tissues in response to exercise. J Biol.Chem. 2002;277:32571–32577. doi: 10.1074/jbc.M201692200. [DOI] [PubMed] [Google Scholar]
- 142.Park S, Jang JS, Jun DW, Hong SM. Exercise enhances insulin and leptin signaling in the cerebral cortex and hypothalamus during dexamethasone-induced stress in diabetic rats. Neuroendocrinology. 2005;82:282–293. doi: 10.1159/000093127. [DOI] [PubMed] [Google Scholar]
- 143.Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proceedings of the National Academy of Sciences. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pilegaard H, Ordway GA, Saltin B, Neufer PD. Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. Am J Physiol Endocrinol Metab. 2000;279:E806–E814. doi: 10.1152/ajpendo.2000.279.4.E806. [DOI] [PubMed] [Google Scholar]
- 145.Pold R, Jensen LS, Jessen N, Buhl ES, Schmitz O, Flyvbjerg A, Fujii N, Goodyear LJ, Gotfredsen CF, Brand CL, Lund S. Long-Term AICAR Administration and Exercise Prevents Diabetes in ZDF Rats. Diabetes. 2005;54:928–934. doi: 10.2337/diabetes.54.4.928. [DOI] [PubMed] [Google Scholar]
- 146.Polekhina G, Gupta A, Van Denderen BJ, Feil SC, Kemp BE, Stapleton D, Parker MW. Structural basis for glycogen recognition by AMP-activated protein kinase. Structure. 2005;13:1453–1462. doi: 10.1016/j.str.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 147.Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, Dequiedt F, Haendeler J, Mione M, Dejana E, Alt FW, Zeiher AM, Dimmeler S. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007;21:2644–2658. doi: 10.1101/gad.435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Prior BM, Yang HT, Terjung RL. What makes vessels grow with exercise training? J Appl.Physiol. 2004;97:1119–1128. doi: 10.1152/japplphysiol.00035.2004. [DOI] [PubMed] [Google Scholar]
- 149.Putman CT, Kiricsi M, Pearcey J, MacLean IM, Bamford JA, Murdoch GK, Dixon WT, Pette D. AMPK activation increases uncoupling protein-3 expression and mitochondrial enzyme activities in rat muscle without fibre type transitions. J Physiol. 2003;551:169–178. doi: 10.1113/jphysiol.2003.040691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Qin W, Yang T, Ho L, Zhao Z, Wang J, Chen L, Zhao W, Thiyagarajan M, MacGrogan D, Rodgers JT, Puigserver P, Sadoshima J, Deng H, Pedrini S, Gandy S, Sauve AA, Pasinetti GM. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol.Chem. 2006;281:21745–21754. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]
- 151.Raney MA, Turcotte LP. Evidence for the regulation of contraction-induced fatty acid oxidation via extracellular signal-regulated kinase 1/2 activation independent of changes in fatty acid uptake. Metabolism. 2007;56:1192–1200. doi: 10.1016/j.metabol.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 152.Raney MA, Turcotte LP. Evidence for the involvement of CaMKII and AMPK in Ca2+-dependent signaling pathways regulating FA uptake and oxidation in contracting rodent muscle. J Appl.Physiol. 2008;104:1366–1373. doi: 10.1152/japplphysiol.01282.2007. [DOI] [PubMed] [Google Scholar]
- 153.Reihill JA, Ewart MA, Hardie DG, Salt IP. AMP-activated protein kinase mediates VEGF-stimulated endothelial NO production. Biochem.Biophys.Res.Commun. 2007;354:1084–1088. doi: 10.1016/j.bbrc.2007.01.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rennie MJ. Why muscle stops building when it's working. J Physiol. 2005;569:3. doi: 10.1113/jphysiol.2005.099424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rennie MJ, Tipton KD. Protein and amino acid metabolism during and after exercise and the effects of nutrition. Annu.Rev.Nutr. 2000;20:457–483. doi: 10.1146/annurev.nutr.20.1.457. [DOI] [PubMed] [Google Scholar]
- 156.Richter EA, Garetto LP, Goodman MN, Ruderman NB. Muscle glucose metabolism following exercise in the rat. J.Clin.Invest. 1982;69:785–793. doi: 10.1172/JCI110517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Richter EA, Mikines KJ, Galbo H, Kiens B. Effect of exercise on insulin action in human skeletal muscle. J.Appl.Physiol. 1989;66:876–885. doi: 10.1152/jappl.1989.66.2.876. [DOI] [PubMed] [Google Scholar]
- 158.Roach WG, Chavez JA, Miinea CP, Lienhard GE. Substrate specificity and effect on GLUT4 translocation of the Rab GTPase-activating protein Tbc1d1. Biochem.J. 2007;403:353–358. doi: 10.1042/BJ20061798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rockl KS, Hirshman MF, Brandauer J, Fujii N, Witters LA, Goodyear LJ. Skeletal muscle adaptation to exercise training: AMP-activated protein kinase mediates muscle fiber type shift. Diabetes. 2007;56:2062–2069. doi: 10.2337/db07-0255. [DOI] [PubMed] [Google Scholar]
- 160.Roepstorff C, Halberg N, Hillig T, Saha AK, Ruderman NB, Wojtaszewski JF, Richter EA, Kiens B. Malonyl-CoA and carnitine in regulation of fat oxidation in human skeletal muscle during exercise. Am.J.Physiol Endocrinol.Metab. 2005;288:E133–E142. doi: 10.1152/ajpendo.00379.2004. [DOI] [PubMed] [Google Scholar]
- 161.Roepstorff C, Thiele M, Hillig T, Pilegaard H, Richter EA, Wojtaszewski JF, Kiens B. Higher skeletal muscle alpha2AMPK activation and lower energy charge and fat oxidation in men than in women during submaximal exercise. J.Physiol. 2006;574:125–138. doi: 10.1113/jphysiol.2006.108720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Roepstorff C, Vistisen B, Donsmark M, Nielsen JN, Galbo H, Green KA, Hardie DG, Wojtaszewski JF, Richter EA, Kiens B. Regulation of hormone-sensitive lipase activity and Ser563 and Ser565 phosphorylation in human skeletal muscle during exercise. J.Physiol. 2004;560:551–562. doi: 10.1113/jphysiol.2004.066480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rogers CJ, Colbert LH, Greiner JW, Perkins SN, Hursting SD. Physical activity and cancer prevention : pathways and targets for intervention. Sports Med. 2008;38:271–296. doi: 10.2165/00007256-200838040-00002. [DOI] [PubMed] [Google Scholar]
- 164.Rose AJ, Bisiani B, Kiens B, Richter EA. Skeletal muscle eEF2 and 4EBP1 phosphorylation during endurance exercise is dependent on intensity and muscle fiber type. Am.J.Physiol. 2008 doi: 10.1152/ajpregu.90806.2008. Accepted for publication. [DOI] [PubMed] [Google Scholar]
- 165.Rose AJ, Broholm C, Kiillerich K, Finn SG, Proud CG, Rider MH, Richter EA, Kiens B. Exercise rapidly increases eukaryotic elongation factor 2 phosphorylation in skeletal muscle of men. J.Physiol. 2005;569:223–228. doi: 10.1113/jphysiol.2005.097154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rose AJ, Richter EA. Skeletal muscle glucose uptake during exercise: how is it regulated? Physiology.(Bethesda.) 2005;20:260–270. doi: 10.1152/physiol.00012.2005. [DOI] [PubMed] [Google Scholar]
- 167.Ruderman N, Prentki M. AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nat.Rev.Drug Discov. 2004;3:340–351. doi: 10.1038/nrd1344. [DOI] [PubMed] [Google Scholar]
- 168.Ruderman NB, Keller C, Richard AM, Saha AK, Luo Z, Xiang X, Giralt M, Ritov VB, Menshikova EV, Kelley DE, Hidalgo J, Pedersen BK, Kelly M. Interleukin-6 regulation of AMP-activated protein kinase. Potential role in the systemic response to exercise and prevention of the metabolic syndrome. Diabetes. 2006;55 Suppl 2:S48–S54. doi: 10.2337/db06-s007. [DOI] [PubMed] [Google Scholar]
- 169.Ruderman NB, Saha AK, Vavvas D, Kurowski T, Laybutt DR, Schmitz-Peiffer C, Biden T, Kraegen EW. Malonyl CoA as a metabolic switch and a regulator of insulin sensitivity. Adv.Exp.Med.Biol. 1998;441:263–270. doi: 10.1007/978-1-4899-1928-1_24. [DOI] [PubMed] [Google Scholar]
- 170.Ruderman NB, Shulman G. The metabolic syndrome. In: Jamieson J, L D, editors. Textbook of Endocrinology. Philadelphia: Elsevier; 2006. pp. 1149–1166. [Google Scholar]
- 171.Saha AK, Schwarsin AJ, Roduit R, Masse F, Kaushik V, Tornheim K, Prentki M, Ruderman NB. Activation of malonyl-CoA decarboxylase in rat skeletal muscle by contraction and the AMP-activated protein kinase activator 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside. J Biol.Chem. 2000;275:24279–24283. doi: 10.1074/jbc.C000291200. [DOI] [PubMed] [Google Scholar]
- 172.Sahlin K. Muscle carnitine metabolism during incremental dynamic exercise in humans. Acta Physiol Scand. 1990;138:259–262. doi: 10.1111/j.1748-1716.1990.tb08845.x. [DOI] [PubMed] [Google Scholar]
- 173.Sakamoto K, Goransson O, Hardie DG, Alessi DR. Activity of LKB1 and AMPK-related kinases in skeletal muscle: effects of contraction, phenformin, and AICAR. Am J Physiol Endocrinol Metab. 2004;287:E310–E317. doi: 10.1152/ajpendo.00074.2004. [DOI] [PubMed] [Google Scholar]
- 174.Sakamoto K, Goransson O, Hardie DG, Alessi DR. Activity of LKB1 and AMPK-related kinases in skeletal muscle: effects of contraction, phenformin, and AICAR. Am.J.Physiol Endocrinol.Metab. 2004;287:E310–E317. doi: 10.1152/ajpendo.00074.2004. [DOI] [PubMed] [Google Scholar]
- 175.Sakamoto K, McCarthy A, Smith D, Green KA, Grahame HD, Ashworth A, Alessi DR. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J. 2005;24:1810–1820. doi: 10.1038/sj.emboj.7600667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem.J. 2007;403:139–148. doi: 10.1042/BJ20061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sano H, Kane S, Sano E, Miinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J.Biol.Chem. 2003;278:14599–14602. doi: 10.1074/jbc.C300063200. [DOI] [PubMed] [Google Scholar]
- 178.Schiaffino S, Sandri M, Murgia M. Activity-dependent signaling pathways controlling muscle diversity and plasticity. Physiology.(Bethesda.) 2007;22:269–278. doi: 10.1152/physiol.00009.2007. [DOI] [PubMed] [Google Scholar]
- 179.Simard-Lefort N, St-Amand E, Morasse S, Cote CH, Marette A. The {alpha} subunit of AMPK is essential for submaximal contraction-mediated glucose transport in skeletal muscle in vitro {alpha} Am J Physiol Endocrinol Metab. 2008 doi: 10.1152/ajpendo.90362.2008. [DOI] [PubMed] [Google Scholar]
- 180.Song XM, Fiedler M, Galuska D, Ryder JW, Fernstrom M, Chibalin AV, Wallberg-Henriksson H, Zierath JR. 5-Aminoimidazole-4-carboxamide ribonucleoside treatment improves glucose homeostasis in insulin-resistant diabetic (ob/ob) mice. Diabetologia. 2002;45:56–65. doi: 10.1007/s125-002-8245-8. [DOI] [PubMed] [Google Scholar]
- 181.Sriwijitkamol A, Coletta DK, Wajcberg E, Balbontin GB, Reyna SM, Barrientes J, Eagan PA, Jenkinson CP, Cersosimo E, DeFronzo RA, Sakamoto K, Musi N. Effect of acute exercise on AMPK signaling in skeletal muscle of subjects with type 2 diabetes: a time-course and dose-response study. Diabetes. 2007;56:836–848. doi: 10.2337/db06-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Steffensen CH, Roepstorff C, Madsen M, Kiens B. Myocellular Triacylglycerol Breakdown in Females but not in Males During Exercise. Am J Physiol Endocrinol Metab. 2002;282:E634–E642. doi: 10.1152/ajpendo.00078.2001. [DOI] [PubMed] [Google Scholar]
- 183.Steinberg GR, Smith AC, Van Denderen BJ, Chen Z, Murthy S, Campbell DJ, Heigenhauser GJ, Dyck DJ, Kemp BE. AMP-activated protein kinase is not down-regulated in human skeletal muscle of obese females. J Clin Endocrinol Metab. 2004;89:4575–4580. doi: 10.1210/jc.2004-0308. [DOI] [PubMed] [Google Scholar]
- 184.Steinberg GR, Watt MJ, McGee SL, Chan S, Hargreaves M, Febbraio MA, Stapleton D, Kemp BE. Reduced glycogen availability is associated with increased AMPKalpha2 activity, nuclear AMPKalpha2 protein abundance, and GLUT4 mRNA expression in contracting human skeletal muscle. Appl.Physiol Nutr.Metab. 2006;31:302–312. doi: 10.1139/h06-003. [DOI] [PubMed] [Google Scholar]
- 185.Stephens TJ, Chen ZP, Canny BJ, Michell BJ, Kemp BE, McConell GK. Progressive increase in human skeletal muscle AMPKalpha2 activity and ACC phosphorylation during exercise. Am J Physiol Endocrinol Metab. 2002;282:E688–E694. doi: 10.1152/ajpendo.00101.2001. [DOI] [PubMed] [Google Scholar]
- 186.Suter M, Riek U, Tuerk R, Schlattner U, Wallimann T, Neumann D. Dissecting the role of 5'-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J Biol.Chem. 2006;281:32207–32216. doi: 10.1074/jbc.M606357200. [DOI] [PubMed] [Google Scholar]
- 187.Suwa M, Nakano H, Radak Z, Kumagai S. Endurance exercise increases the SIRT1 and peroxisome proliferator-activated receptor [gamma] coactivator-1[alpha] protein expressions in rat skeletal muscle. Metabolism. 2008;57:986–998. doi: 10.1016/j.metabol.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 188.Suzuki K, Koike T. Resveratrol abolishes resistance to axonal degeneration in slow Wallerian degeneration (WldS) mice: activation of SIRT2, an NAD-dependent tubulin deacetylase. Biochem.Biophys.Res.Commun. 2007;359:665–671. doi: 10.1016/j.bbrc.2007.05.164. [DOI] [PubMed] [Google Scholar]
- 189.Takekoshi K, Fukuhara M, Quin Z, Nissato S, Isobe K, Kawakami Y, Ohmori H. Long-term exercise stimulates adenosine monophosphate-activated protein kinase activity and subunit expression in rat visceral adipose tissue and liver. Metabolism. 2006;55:1122–1128. doi: 10.1016/j.metabol.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 190.Taylor EB, An D, Kramer HF, Yu H, Fujii NL, Roeckl KS, Bowles N, Hirshman MF, Xie J, Feener EP, Goodyear LJ. Discovery of TBC1D1 as an insulin-, AICAR-, and contraction-stimulated signaling nexus in mouse skeletal muscle. J Biol.Chem. 2008;283:9787–9796. doi: 10.1074/jbc.M708839200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Thompson AM, Church TS, Janssen I, Katzmarzyk PT, Earnest CP, Blair SN. Cardiorespiratory fitness as a predictor of cancer mortality among men with pre-diabetes and diabetes. Diabetes Care. 2008;31:764–769. doi: 10.2337/dc07-1648. [DOI] [PubMed] [Google Scholar]
- 192.Thomson DM, Porter BB, Tall JH, Kim HJ, Barrow JR, Winder WW. Skeletal muscle and heart LKB1 deficiency causes decreased voluntary running and reduced muscle mitochondrial marker enzyme expression in mice. Am.J.Physiol Endocrinol.Metab. 2007;292:E196–E202. doi: 10.1152/ajpendo.00366.2006. [DOI] [PubMed] [Google Scholar]
- 193.Thomson DM, Porter BB, Tall JH, Kim HJ, Barrow JR, Winder WW. Skeletal muscle and heart LKB1 deficiency causes decreased voluntary running and reduced muscle mitochondrial marker enzyme expression in mice. Am J Physiol Endocrinol Metab. 2007;292:E196–E202. doi: 10.1152/ajpendo.00366.2006. [DOI] [PubMed] [Google Scholar]
- 194.Treebak JT, Birk JB, Rose AJ, Kiens B, Richter EA, Wojtaszewski JF. AS160 phosphorylation is associated with activation of {alpha}2{beta}2{gamma}1 but not {alpha}2{beta}2{gamma}3 AMPK trimeric complex in skeletal muscle during exercise in humans. Am.J.Physiol Endocrinol.Metab. 2006 doi: 10.1152/ajpendo.00380.2006. [DOI] [PubMed] [Google Scholar]
- 195.Treebak JT, Birk JB, Rose AJ, Kiens B, Richter EA, Wojtaszewski JF. AS160 phosphorylation is associated with activation of alpha2beta2gamma1- but not alpha2beta2gamma3-AMPK trimeric complex in skeletal muscle during exercise in humans. Am J Physiol Endocrinol Metab. 2007;292:E715–E722. doi: 10.1152/ajpendo.00380.2006. [DOI] [PubMed] [Google Scholar]
- 196.Treebak JT, Glund S, Deshmukh A, Klein DK, Long YC, Jensen TE, Jorgensen SB, Viollet B, Andersson L, Neumann D, Wallimann T, Richter EA, Chibalin AV, Zierath JR, Wojtaszewski JF. AMPK-Mediated AS160 Phosphorylation in Skeletal Muscle Is Dependent on AMPK Catalytic and Regulatory Subunits. Diabetes. 2006;55:2051–2058. doi: 10.2337/db06-0175. [DOI] [PubMed] [Google Scholar]
- 197.Tullson PC, Bangsbo J, Hellsten Y, Richter EA. IMP metabolism in human skeletal muscle after exhaustive exercise. J.Appl.Physiol. 1995;78:146–152. doi: 10.1152/jappl.1995.78.1.146. [DOI] [PubMed] [Google Scholar]
- 198.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. n.Engl.J.Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 199.UKPDS. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 200.Unger RH. Lipotoxic diseases. Annu.Rev.Med. 2002;53:319–336. doi: 10.1146/annurev.med.53.082901.104057. [DOI] [PubMed] [Google Scholar]
- 201.van Hall G, Steensberg A, Sacchetti M, Fischer C, Keller C, Schjerling P, Hiscock N, Møller K, Saltin B, Febbraio MA, Pedersen BK. Interleukin-6 stimulates lipolysis and fat oxidation in humans. J.Clin.Endocrinol.Metab. 2003 doi: 10.1210/jc.2002-021687. In press. [DOI] [PubMed] [Google Scholar]
- 202.van Loon LJ, Greenhaff PL, Constantin-Teodosiu D, Saris WH, Wagenmakers AJ. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J.Physiol. 2001;536:295–304. doi: 10.1111/j.1469-7793.2001.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Villena JA, Viollet B, Andreelli F, Kahn A, Vaulont S, Sul HS. Induced adiposity and adipocyte hypertrophy in mice lacking the AMP-activated protein kinase-alpha2 subunit. Diabetes. 2004;53:2242–2249. doi: 10.2337/diabetes.53.9.2242. [DOI] [PubMed] [Google Scholar]
- 204.Viollet B, Andreelli F, Jorgensen SB, Perrin C, Geloen A, Flamez D, Mu J, Lenzner C, Baud O, Bennoun M, Gomas E, Nicolas G, Wojtaszewski JF, Kahn A, Carling D, Schuit FC, Birnbaum MJ, Richter EA, Burcelin R, Vaulont S. The AMP-activated protein kinase alpha2 catalytic subunit controls whole-body insulin sensitivity. J Clin.Invest. 2003;111:91–98. doi: 10.1172/JCI16567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Viollet B, Foretz M, Guigas B, Horman S, Dentin R, Bertrand L, Hue L, Andreelli F. Activation of AMP-activated protein kinase in the liver: a new strategy for the management of metabolic hepatic disorders. J.Physiol. 2006;574:41–53. doi: 10.1113/jphysiol.2006.108506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Vissing K, McGee SL, Roepstorff C, Schjerling P, Hargreaves M, Kiens B. Effect of sex differences on human MEF2 regulation during endurance exercise. Am J Physiol Endocrinol Metab. 2008;294:E408–E415. doi: 10.1152/ajpendo.00403.2007. [DOI] [PubMed] [Google Scholar]
- 207.Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO. Interleukin-6-deficient mice develop mature-onset obesity. Nat.Med. 2002;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- 208.Watt MJ, Holmes AG, Pinnamaneni SK, Garnham AP, Steinberg GR, Kemp BE, Febbraio MA. Regulation of HSL serine phosphorylation in skeletal muscle and adipose tissue. Am J Physiol Endocrinol Metab. 2006;290:E500–E508. doi: 10.1152/ajpendo.00361.2005. [DOI] [PubMed] [Google Scholar]
- 209.Williams T, Brenman JE. LKB1 and AMPK in cell polarity and division. Trends Cell Biol. 2008;18:193–198. doi: 10.1016/j.tcb.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 210.Winder WW, Arogyasami J, Barton RJ, Elayan IM, Verhs PR. Muscle malonyl-CoA decreases during exercise. J.Appl.Physiol. 1989;67:2230–2233. doi: 10.1152/jappl.1989.67.6.2230. [DOI] [PubMed] [Google Scholar]
- 211.Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl.Physiol. 2000;88:2219–2226. doi: 10.1152/jappl.2000.88.6.2219. [DOI] [PubMed] [Google Scholar]
- 212.Witczak CA, Fujii N, Hirshman MF, Goodyear LJ. Ca2+/calmodulin-dependent protein kinase kinase-alpha regulates skeletal muscle glucose uptake independent of AMP-activated protein kinase and Akt activation. Diabetes. 2007;56:1403–1409. doi: 10.2337/db06-1230. [DOI] [PubMed] [Google Scholar]
- 213.Wojtaszewski JF, Jorgensen SB, Hellsten Y, Hardie DG, Richter EA. Glycogen-dependent effects of 5-aminoimidazole-4-carboxamide (AICA)-riboside on AMP-activated protein kinase and glycogen synthase activities in rat skeletal muscle. Diabetes. 2002;51:284–292. doi: 10.2337/diabetes.51.2.284. [DOI] [PubMed] [Google Scholar]
- 214.Wojtaszewski JF, MacDonald C, Nielsen JN, Hellsten Y, Hardie DG, Kemp BE, Kiens B, Richter EA. Regulation of 5'AMP-activated protein kinase activity and substrate utilization in exercising human skeletal muscle. Am J Physiol Endocrinol Metab. 2003;284:E813–E822. doi: 10.1152/ajpendo.00436.2002. [DOI] [PubMed] [Google Scholar]
- 215.Wojtaszewski JF, MacDonald C, Nielsen JN, Hellsten Y, Hardie GD, Kemp BE, Kiens B, Richter EA. Regulation of 5'AMP-activated protein kinase activity and substrate utilization in exercising human skeletal muscle. Am J Physiol Endocrinol Metab. 2002 doi: 10.1152/ajpendo.00436.2002. [DOI] [PubMed] [Google Scholar]
- 216.Wojtaszewski JF, Mourtzakis M, Hillig T, Saltin B, Pilegaard H. Dissociation of AMPK activity and ACCbeta phosphorylation in human muscle during prolonged exercise. Biochem.Biophys.Res.Commun. 2002;298:309–316. doi: 10.1016/s0006-291x(02)02465-8. [DOI] [PubMed] [Google Scholar]
- 217.Wojtaszewski JF, Nielsen JN, Richter EA. Invited Review: Effect of acute exercise on insulin signaling and action in humans. J Appl.Physiol. 2002;93:384–392. doi: 10.1152/japplphysiol.00043.2002. [DOI] [PubMed] [Google Scholar]
- 218.Wojtaszewski JF, Nielsen P, Hansen BF, Richter EA, Kiens B. Isoform-specific and exercise intensity-dependent activation of 5'-AMP-activated protein kinase in human skeletal muscle. J Physiol. 2000;528(Pt 1):221–226. doi: 10.1111/j.1469-7793.2000.t01-1-00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wojtaszewski JF, Richter EA. Effects of acute exercise and training on insulin action and sensitivity: focus on molecular mechanisms in muscle. Essays Biochem. 2006;42:31–46. doi: 10.1042/bse0420031. [DOI] [PubMed] [Google Scholar]
- 220.Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 221.Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr.Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 222.Xiao B, Heath R, Saiu P, Leiper FC, Leone P, Jing C, Walker PA, Haire L, Eccleston JF, Davis CT, Martin SR, Carling D, Gamblin SJ. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature. 2007;449:496–500. doi: 10.1038/nature06161. [DOI] [PubMed] [Google Scholar]
- 223.Xie M, Zhang D, Dyck JR, Li Y, Zhang H, Morishima M, Mann DL, Taffet GE, Baldini A, Khoury DS, Schneider MD. A pivotal role for endogenous TGF-beta-activated kinase-1 in the LKB1/AMP-activated protein kinase energy-sensor pathway. Proc.Natl.Acad.Sci.U.S.A. 2006;103:17378–17383. doi: 10.1073/pnas.0604708103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Yu X, McCorkle S, Wang M, Lee Y, Li J, Saha AK, Unger RH, Ruderman NB. Leptinomimetic effects of the AMP kinase activator AICAR in leptin-resistant rats: prevention of diabetes and ectopic lipid deposition. Diabetologia. 2004;47:2012–2021. doi: 10.1007/s00125-004-1570-9. [DOI] [PubMed] [Google Scholar]
- 225.Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, Cohen RA. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006;55:2180–2191. doi: 10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]
- 226.Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc.Natl.Acad.Sci.U.S.A. 2002;99:15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zorzano A, Balon T, Garetto L, Goodman M, Ruderman N. Muscle alpha-aminoisobutyric acid transport after exercise: enhhanced stimulation by insulin. Am.J.Physiol. 1985;248:E546–E552. doi: 10.1152/ajpendo.1985.248.5.E546. [DOI] [PubMed] [Google Scholar]