Abstract
Background
Suggested intervals for postpolypectomy surveillance colonoscopy are currently based upon the adenoma findings from the most recent exam.
Objective
Determine the risk of clinically significant adenoma recurrence based upon the results of two prior colonoscopies.
Design
Prospective cohort
Setting
Academic and private centers in North America
Patients
Subjects in an adenoma chemoprevention trial in which all subjects had ≥ 1 adenoma at the time of a complete colonoscopy at entry. For this analysis, we included only subjects whose qualifying adenoma was their first. All subjects then had second and third study colonoscopy at roughly 3-year intervals.
Measurements
Proportion of patients developing high-risk findings at the third study colonoscopy: either at least one advanced (≥1cm or advanced histology) adenoma or multiple (≥ 3) adenomas.
Results
Fifty-eight of 564 subjects (10%) had high-risk findings at the third study exam. If the second exam showed high-risk findings, then results from the first exam added no significant information about the probability of detecting high-risk findings on the third exam (18.2% if the first exam had high-risk findings vs. 20% if the first exam had low-risk findings (P=0.78). If the second exam showed no adenomas, then the results from the first exam added significant information about the probability of detecting high-risk findings on the third exam (12.3% if the first exam had high-risk findings vs. 4.9% if the first exam had low-risk findings (P=0.015)
Limitations
This observational study cannot specifically examine adenoma recurrence risk at intervals suggested for low risk adenoma patients (e.g. 5 vs. 10 years).
Conclusions
Information from two prior exams may help identify low-risk populations that benefit little from intense surveillance. Surveillance guidelines might be tailored in selected subjects to use information from two prior exams, not just the most recent one.
Funding
Dr Robertson’s work is supported by a VA HSR&D Career Development Award. The parent study was supported by grant R01 CA 59005 from the National Institutes of Health.
Introduction
Surveillance colonoscopy for individuals with a prior colorectal adenoma is widely practiced in the United States (U.S.) and consumes large amounts of physician time and resources. In a recent national survey using the Clinical Outcomes Research Initiative database, 22% of all colonoscopy was performed for surveillance in those with prior colon polyps or cancer(1).
Practice guidelines have been developed to assist clinicians in determining the appropriate follow-up interval for patients detected with adenomas (2–5). The U.S. Multisociety Task Force on Colorectal Cancer and the American Cancer Society recommend a 3 year follow up interval for individuals with high-risk adenoma findings such as an advanced adenoma or multiple (> 3) adenomas. The recommendation for those with low-risk findings (e.g. one or two small adenomas without villous features) is a 5 to 10 year follow-up interval, but criteria for choosing between a 5 or 10 year interval are not clear.
Despite such guidelines, clinical practice varies widely. In a large survey, most endoscopists erred on the side of repeating examinations earlier than the recommended interval (6). Most survey participants said that published clinical evidence was the most important factor influencing their practice and not simply the guidelines.
More extensive information about adenoma recurrence risk could better inform surveillance practice decisions. To date, most studies have focused on how findings from one recent colonoscopy predict findings on the next exam. Data pooled from 8 large North American studies suggest that size of the largest adenoma and the numbers of adenomas on the most recent colonoscopy are important predictors of adenoma recurrence on the next exam. However, the utility of findings from multiple, prior colonoscopies in predicting risk of adenoma recurrence has not been reported. A risk profile based upon multiple examinations might better delineate high and low risk patients as compared to a single exam. For example, patients repeatedly found to have large or multiple adenomas on successive exams might compose a higher risk pool than those with adenomas on one exam, but none on the next.
We recently completed a large adenoma chemoprevention trial in which subjects underwent a baseline examination and were then followed through two surveillance colonoscopy cycles. Using trial data, we estimated the risk of adenoma recurrence on a third exam based upon the results of two prior colonoscopies. We also examined whether such risk estimates would assist in determining whether the patient’s next colonoscopy should be sooner or later.
Methods
Overview
The current analysis is a longitudinal follow up study of adenoma recurrence in a population of individuals that participated in a completed adenoma chemoprevention trial. We have previously reported the essential features, design and findings of the Aspirin Folate Polyp Prevention Study (7, 8) that forms the basis for the current analyses. This randomized clinical trial had a 3×2 factorial design, comparing 81 mg/day and 325 mg/day aspirin with placebo and comparing 1 mg/day folic acid with placebo. Institutional Review Board approval to perform the trial and collect the information that is reported herein was obtained from all participating centers in each study.
Patients
All subjects at entry had at least one histologically documented large-bowel adenoma removed and had undergone a complete (i.e. to the cecum) colonoscopic examination, which constituted the qualifying “baseline colonoscopy” with the attending endoscopist attesting that no known colorectal polyps remained. A protocol-driven surveillance colonoscopy was performed in all patients about 3 years after the baseline colonoscopy at which time all visualized polyps were removed. The timing of the next exam (either 3 or 5 years) was left to the discretion of the endoscopist. At both the 3-year protocol colonoscopy and subsequent colonoscopy the location and size of all polyps removed from the bowel were noted. A single study pathologist reviewed all tissue specimens.
Our interest for the present analyses was the occurrence of high-risk findings among patients diagnosed with their first lifetime adenoma(s) at the baseline colonoscopy who then had a second and third colonoscopy within the trial. To assure more complete knowledge of each subject’s entire adenoma history, we excluded those who reported having an adenoma detected before their baseline colonoscopy. We also excluded those found with cancer at or before the second colonoscopy since they would not be expected to follow routine surveillance patterns. In all, 564 of the 1121 initially randomized subjects met our criteria for inclusion in the current analyses (Figure 1). In total, 557 were excluded, many (n=340) because they had an adenoma before their baseline study colonoscopy. There were a small number of subjects (n=29) that either died or did not complete a second study colonoscopy. Of the subjects completing the second colonoscopy, 188 were not followed through completion of the third colonoscopy; these patients were younger and less likely to be Caucasian than the 564 included in our analyses (see on line table 1).
Figure 1.
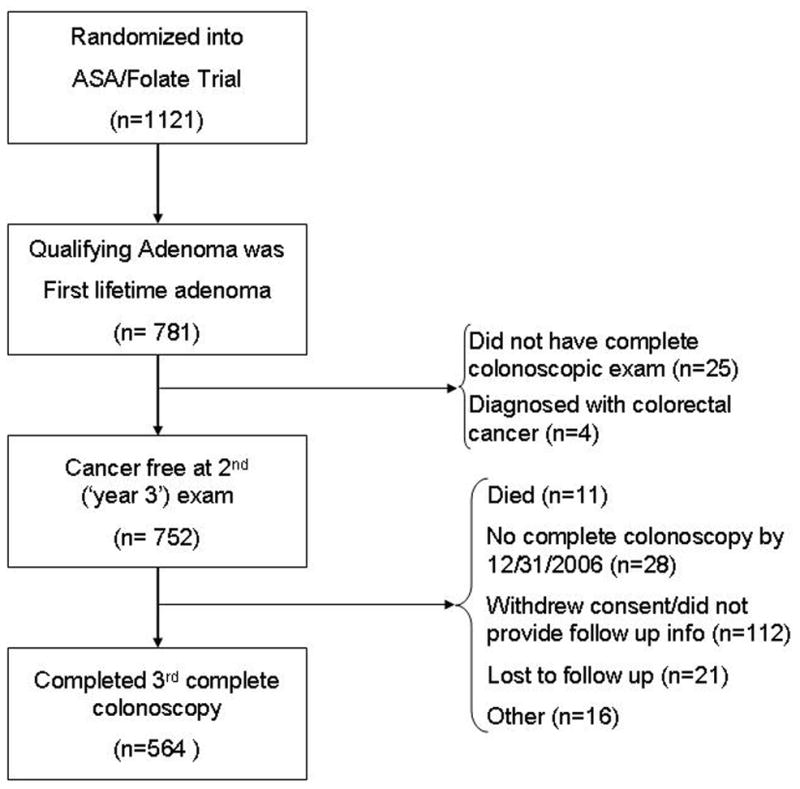
Composition of analytic cohort relative to those randomized into the parent ASA/Folate trial
Table 1.
Proportion of patients with High-Risk Findings at Third Colonoscopy According to Baseline Characteristics
| Baseline Characteristic | n | high risk findings @ 3rd exam (n=58) | % high risk findings @ 3rd exam |
|---|---|---|---|
| Age | |||
| <40 | 11 | 0 | 0% |
| 40–49 | 107 | 10 | 9% |
| 50–59 | 235 | 24 | 10% |
| 60–69 | 156 | 19 | 12% |
| 70+ | 55 | 5 | 9% |
| Gender | |||
| Male | 329 | 37 | 11% |
| Female | 235 | 21 | 9% |
| Race | |||
| Caucasian | 492 | 55 | 11% |
| African American | 22 | 1 | 5% |
| Other | 50 | 2 | 4% |
| Family History Colorectal Cancer | |||
| No | 284 | 23 | 8% |
| Yes | 191 | 24 | 13 |
| Unknown | 89 | 11 | 12% |
| Smoking History* | |||
| Never | 250 | 22 | 9% |
| Former | 235 | 32 | 14% |
| Current | 74 | 4 | 5% |
5 subjects missing smoking history
Outcomes
We defined ‘high-risk findings’ to be those used in current adenoma surveillance guidelines (≥3 adenomas or at least one advanced adenoma)(5). We used the commonly accepted definition of ‘advanced adenoma’(9) as one that was ≥1cm or had tubulovillous or villous histological features or high grade dysplasia.
Statistical Analysis
We calculated the proportion of patients with high-risk findings on the third exam stratified by findings from the two prior colonoscopies. 95% confidence intervals were calculated by the binomial exact test. Findings on each exam were categorized as follows:
no adenoma = no adenomas detected
low-risk findings = one or two small (< 1cm) tubular adenomas detected
high-risk findings = at least one advanced adenoma or cancer detected or multiple (≥3) adenomas of any size detected
To be eligible for the clinical trial, all subjects had at least one adenoma detected on their baseline exam thus; the category of ‘no adenomas’ only pertains to the results on the second or third exam.
Chi squared testing was used to assess for between group differences in proportions.
We used logistic regression to estimate the relative odds of being detected with high-risk findings on the third exam associated with the results obtained on the first two examinations while adjusting for age, sex, study center, treatment assignment, smoking status and family history of colorectal cancer. Smoking and family history were obtained by a patient study entry questionnaire. The referent group for this analysis was the highest risk profile (high-risk finding on both the first and second exams). All calculations were performed with SAS version 9(SAS Institute, Cary NC).
Funding
Dr Robertson’s work is supported by a VA HSR&D Career Development Award. The parent study was supported by grant R01 CA 59005 from the National Institutes of Health. Funding agencies had no role in the conduct of the study, in the collection, analysis or interpretation of the data.
Results
Among the analyzed group of 564 patients who had a baseline and two subsequent colonoscopic examinations, the mean (+/− sd) follow up time between the baseline and second examination was 37.4 (±3.1) months and between the second and third examination was 47.6 (± 14.3) months. The follow up time between the second exam and third exams was longer in those with no adenomas detected at the second exam (51 mos) as compared to those with advanced or multiple adenomas on the second exam (39.5 mos P<.001). At the third colonoscopy, 58 subjects (10.3%; 95% CI= 7.9%, 13.1%) had high-risk findings. The findings were high risk in 36 subjects because of advanced adenoma detection, 14 because of multiplicity and the remaining 8 met definition for both. The proportion of patients with high-risk findings did not differ greatly according to their demographic characteristics, smoking or family history of colorectal cancer (Table 1).
Findings on the second colonoscopy were strongly associated with findings on the third (final) colonoscopy (Figure 2). Of the 84 subjects with high-risk findings on the second exam, 16 (19.0%; 95 %=CI 11.3% – 29.1%) had high-risk findings on the third exam, while only 25 (7.7%; 95%=CI 5.0% – 11.1%) of the 326 subjects with no adenomas on the second exam, had high-risk findings on the third exam (P=.002). The absolute risk difference was 11.4% (95% CI= 2%–20%). Those with low risk findings on the second exam (n=154) had a probability for high risk findings on the third exam that was intermediate between those two groups (11%; 95 % CI= 6.5% – 17.1%). The absolute risk difference for those with high risk findings on the second exam compared to those with low risk findings on the second exam was 8.0% (95% CI= −2%–18%)
Figure 2.
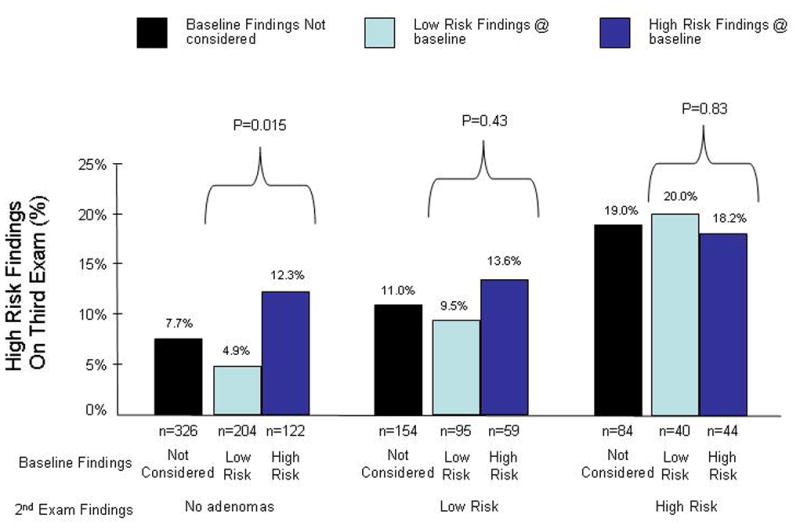
Absolute risk of advanced or multiple adenoma detection on the third colonoscopy stratified on the findings from the first 2 colonoscopies or when only considering the second colonoscopy.
Among the 326 subjects who had no adenomas on their second examination, the results of the baseline examination strongly predicted the outcome of their third examination (Figure 2). The 122 patients with a high-risk baseline exam and negative second exam were significantly more likely to have high-risk findings on the third exam (12.3%; 95% CI= 7.1% – 19.5%) than the 204 patients with a low-risk baseline exam and negative second exam (4.9%; 95% CI= 2.4% – 8.9%) (P=0.015). The absolute risk difference between these two group was 7.4%(95% CI= 0.8%–13.9%).
There were 154 patients who had low risk adenomas found at the second exam; for them the results of the baseline examination appeared to provide little, if any, discrimination in risk (Figure 2). For example, among those with a high risk finding on the baseline exam followed by a low risk second exam, the probability of a high risk finding on the third exam was 13.6% (95% CI=6.0% – 25.0%), compared to a probability of 9.5% (95% CI=4.4% – 17.2%) for those with a low risk finding on both the baseline and second exams (P= 0.43). The absolute risk difference between these two group was 4.0% (95% CI= −6.4%–14.6%).
Among the 84 patients who had high-risk findings on their second examination, the results from the baseline colonoscopy examination were not associated with the results of the third examination (Figure 2). Of the 44 patients in this group who had high-risk findings on the baseline exam, the probability of high-risk findings on the third exam was nearly identical (18.2%; 95% CI=8.2% – 32.7%) to that for the 40 subjects who did not have high-risk findings on the baseline exam (20.0%; 95% CI= 9.1% – 35.7%) (P=0.78).
Results of the multivariate model closely approximated the unadjusted findings. Subjects with the lowest risk profile on the first two examinations (i.e. low-risk first exam and no adenoma second exam) were significantly less likely to develop high-risk findings on the third exam compared to those with two consecutive high-risk exams (OR= 0.15; 95% CI 0.05, 0.51).
Two of the 58 subjects with high-risk findings on the third exam had either high grade dysplasia (one) or cancer (one). In each of these two cases the subject had an initial exam with high-risk findings and then a second exam with low risk findings prior to the exam showing high grade dysplasia or cancer.
Based on the results of the first two exams, the lowest risk group was also the largest (35.9% of all subjects). We estimate that 20 colonoscopic examinations would be needed to detect one individual with high-risk findings on their next exam (Table 2). In contrast, high-risk findings on a third exam would be detected in roughly one of every 5 subjects with high-risk findings on both their first and second exams.
Table 2.
Absolute and relative risk for high risk findings at third exam based on findings from two prior examinationsa
| Adenoma Findings @ baseline | Adenoma Findings @ 2nd exam | Mean follow up time (mos) 2nd–3rd exam (SD) | n (%) in strata (a) |
n with high risk findings on 3rd exam (b) |
Absolute risk (95% CI) for advanced or multiple on 3rd exam (c=b/a) |
Number Needed to Scope to identify clinically significant findings on 3rd exam (d=1/c) |
|---|---|---|---|---|---|---|
| High Risk | High Risk | 42 (16) | 44 (7.8) | 8 | 18.2% (8.2, 32.7) |
5.5 |
| High Risk | Low Risk | 44 (13) | 59(10.5) | 8 | 13.6% (6.0, 25.0) |
7.4 |
| High Risk | No adenomas | 51 (13) | 122(21.6) | 15 | 12.3% (7.0, 19.5) |
8.1 |
| Low Risk | High Risk | 37 (13) | 40(7.1) | 8 | 20.0% (9.0, 35.6) |
5 |
| Low Risk | Low Risk | 44 (12) | 95(16.8) | 9 | 9.5% (4.4, 17.2) |
10 |
| Low Risk | No adenomas | 51 (14) | 204(36.2) | 10 | 4.9% (2.4, 8.8) |
20.4 |
The risk difference between those with no adenomas at 2nd exam and high risk vs low risk findings at baseline was 0.07 (95% CI 0.01, 0.14)
The risk difference between those with low risk adenomas at 2nd exam and high risk vs low risk findings at baseline was 0.04 (95% CI −0.06, 0.15)
The risk difference between those with high risk adenomas at 2nd exam and high risk vs low risk findings at baseline was −0.02 (95% CI −0.19, 0.15)
When we considered advanced adenoma on the third exam as the outcome, results were broadly similar to that reported for high risk findings above (see online table 2). For example, for the largest group of subjects with no adenomas on the second exam (n=326), results of the baseline examination significantly influenced risk for the finding of advanced adenoma on the third exam. The 122 patients with a high-risk baseline exam and negative second exam were significantly more likely to have an advanced adenoma on the third exam (11.5 %; 95% CI= 1.3%–6.9%) than the 204 patients with a low-risk baseline exam and negative second exam (3.4%; 95% CI=6.4%–18.5%) (P=0.004). The absolute risk difference between these two groups was 8.0% (95% CI = 1.9%–14.2%).
Discussion
Using the results from a first and second colonoscopy we determined the probability of high-risk findings on a third colonoscopy to examine whether such information would assist with surveillance recommendations. Risk varied markedly depending upon the findings on the prior endoscopies. Patients with advanced or multiple adenomas at both the first and second colonoscopy had a relatively high likelihood (18.2%) of having high-risk findings at the third exam. Conversely, relatively few subjects (5.0%) with low risk findings on both of their initial two examinations had high-risk results at their third colonoscopy. Our findings suggest that a longer follow up interval may be appropriate for this latter and much larger group.
To identify other relevant work informing risk of adenoma recurrence in those with a personal history of adenomas we utilized two comprehensive systematic reviews on this topic both published in 2006(5, 10). We also looked for the most recent studies by searching Pubmed (MESH terms ‘colonoscopy’ and ‘adenoma’) limited to the last 3 years. Studies have typically determined predictors of adenoma recurrence based on a single prior examination (5). These investigations have differed both in design and endoscopic technique (e.g. colonoscopy; sigmoidoscopy). This work has recently and extensively been reviewed (5) and from it, a few clear patterns emerge. In randomized controlled trials utilizing colonoscopy, the presence of multiple adenomas (≥3) at baseline has been associated with subsequent risk of both all adenomas (11–14) and advanced adenomas (11, 14). In a meta-analysis (10), adenoma multiplicity (≥3 vs. 1–2 adenomas) was, the strongest predictor of subsequent advanced adenoma formation (pooled relative risk = 2.5; 95% CI 1.1–6.0). Adenoma size (≥ 1cm) also appears to be an important predictor of adenoma(11, 13) and advanced adenoma recurrence(13), but the association (at least in the randomized controlled trials) seems less pronounced than that observed for multiplicity. The strongest evidence for an association between adenoma histology at baseline and subsequent risk for neoplasia comes from studies using sigmoidoscopy. The presence of villous adenomas in the rectum at baseline was associated with a 5-fold risk of subsequent colorectal cancer(15).
While risk factors for adenoma recurrence on the next examination are fairly well defined, the clinical utility of these factors in stratifying risk is less clear. One study assessed risk for adenoma recurrence based on a single most recent exam and used the same definitions of high and low risk findings that we used here(16). The probability of advanced adenoma recurrence was 5% in the group at low baseline risk and 9% in those at high risk. The authors concluded that relying only on findings from the most recent exam had limited predictive ability. Our study, stratifying individuals based on the results of two complete examinations provides more informative risk estimates, ranging from 4.9% in the lowest risk population (low risk: no adenomas) to 18.2% in the highest risk population (high risk: high risk).
In two randomized controlled trials of adenoma chemoprevention (13, 14), a reported history of polyps (specific information on adenoma status was not known) prior to the baseline examination was associated with an increased risk for advanced neoplasia at surveillance. Also, a single report specifically addressed the significance of a normal ‘interim’ colonoscopy between a first and third lifetime colonoscopy(17). That study was smaller (n=204) and with shorter follow up (median 4.5 years) than ours and most of the ‘interim’ exams were presumably driven by symptoms rather than routine surveillance. Nevertheless, the results were generally consistent with ours in that only 15% of patients with a normal interim exam had subsequent neoplasia compared to 40% of those with neoplasia on the interim exam. Long term follow up of adenoma patients in the Polyp Prevention Trial indicates that individuals with low risk findings on one examination, with no adenomas at a follow up examination are at low risk (2.8%) for advanced adenomas on their subsequent colonoscopy(18). However in that trial, 97% of subjects had 3 total exams within four years—a schedule of surveillance colonoscopy that was more frequent than in our trial or in current clinical practice. Follow up of subjects from a sigmoidoscopy based screening trial where some subjects had an adenoma detected at baseline and then a series of subsequent colonoscopies has also been summarized(19). As in our trial, those with a baseline exam with no advanced adenomas followed by a second exam without any adenomas were the lowest risk group for advanced adenoma recurrence on the third exam.
Our study has a number of important implications for adenoma surveillance. A substantial number of patients (about 15% in our trial) have high-risk findings on their second exam despite a prior colonoscopy with the intent to clear the colon. This group continues to be at high risk (approximately 19% in our study) for advanced or multiple adenomas on the next surveillance colonoscopy and this risk is not substantially modified by knowledge of the results of the baseline examination. Current guidelines recommend a 3-year interval for patients with high-risk findings (i.e. advanced or multiple adenomas) and our results support the approach of performing more intensive surveillance for this group.
Our study also has important implications for patients found with no adenomas on their surveillance examination. 58% of our subjects (all of whom had at least one adenoma at baseline) did not have an adenoma on the second colonoscopy. Knowledge of the results of the prior examination in such patients can help assess the probability of a high-risk finding on a third exam. For the large group (36% of our cohort) who only had low-risk findings at baseline and then no adenomas on the second exam, the probability of high-risk findings on the third exam was very small (5%). To put this absolute risk in better context, in a large US Veterans colonoscopy screening study (20) advanced or multiple adenomas were detected in 15.5% (484/3121) of screened participants; this is roughly 3 times the yield of the second surveillance exam in our lowest-risk population.
Likewise, a recent study (21) revealed that 4.0% (12/298) of US Veterans who had no neoplasia at a baseline colonoscopy developed advanced adenomas at a first follow up exam 3–5.5 years later. In a separate report studying a community based practice(22), a slightly lower risk (1.3%) for advanced adenomas were detected 5 years after a normal screening exam. These risks for advanced adenoma are similar to that of our lowest risk group (3.4%; appendix). Thus the risk of high risk findings in our lowest risk group resembles that of a screening population with a prior negative exam for whom a 10 year interval examination is recommended (23). The most recent consensus guidelines endorse a 5–10 year follow up interval in low-risk adenoma patients, but leave selection of the precise timing to the ‘clinicians judgment and patient’s preference’. Our results may provide clinicians a rationale to suggest a 10-year surveillance interval in this lowest risk group. Of course, other clinical factors (e.g. prep quality) may also determine the correct interval.
The strengths of our study include its relatively large size and the precision of our risk estimates. Assessment of outcome was prospective and a single study pathologist independently adjudicated the pathology results. In the original clinical trial, completeness of both clinical and endoscopic follow up was good, making it unlikely that important outcomes were missed. However, the original ASA folate trial began as a 3 year study but was later extended to a six year study. After the 3 year follow up a number of subjects either formally withdrew or did not provide subsequent colonoscopy data and thus could not be included in our current analyses (figure 1). Younger age and non-Caucasian race were both associated with dropout after the second exam and since both factors can influence adenoma recurrence (in opposite directions) we cannot exclude the possibility that our results may have been different had data from these subjects been available.
Interpretation of our results also requires consideration of several important aspects of the study design. Firstly, prevailing practice norms mandated that patients were seen with 3–5 year surveillance intervals, so we cannot know what percentage of subjects would have had clinically significant findings at a five or 10-year surveillance exam. However, in the absence of a formal surveillance trial, this type of data may be the best that will be available. Secondly, follow up of subjects was not uniform as in a trial. Subjects with no adenomas on the second exam had longer intervals prior to their third exam. Nonetheless, despite this slightly longer follow up interval fewer high risk findings were noted in this group— a finding that only serves to strengthen our main conclusion. Thirdly, our analysis was based on data from subjects participating in a randomized trial of adenoma chemoprevention recently identified with a first lifetime adenoma, and their risk profile may not match that of a more general population of adenoma patients. Lastly, low-dose aspirin in our trial modestly reduced adenoma recurrence (7); therefore our results may slightly underestimate the number and type of lesions that would be found in the absence of such supplementation. However, when we adjusted our analyses for study treatment assignment, the relative risk estimates (comparing the highest to lowest risk profiles) were essentially unchanged.
In summary, we determined the probability of high-risk findings on a third colonoscopy based on results from two prior colonoscopies. Our analysis indicates that using results from both examinations provide more refined risk estimates than the use of the most recent exam alone. Knowledge of prior adenoma history was most helpful for the large group of patients found with no adenomas on their second exam. Among patients who have had only one or two small tubular adenomas at a baseline exam, and then no adenomas on their first surveillance colonoscopy, the probability of high-risk findings at the next surveillance exam is comparable to that for patients with a negative screening examination; thus a 10-year follow-up colonoscopy schedule may be appropriate.
Acknowledgments
None of the authors have any financial interests or commercial affiliations which might be considered potential conflicts of interest.
Dr Robertson had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Protocol: For the Aspirin Folate Trial is available upon request through the Polyp Prevention Study Group
Statistical Code: Used for the analyses presented herein are available by contacting Dr Robertson (douglas.robertson@va.gov)
Data: The Polyp Prevention Study Group welcomes financially supported collaboration using the data from our chemoprevention trials. If interested please contact the group through Dr Robertson (douglas.robertson@va.gov)
Footnotes
This study was presented in part as an oral presentation at DDW, Los Angeles CA, May 2006
Addresses for Authors
Douglas J Robertson MD MPH, VA Medical Center, 215 N Main Street/Section of Gastroenterology (111E), White River Junction, VT 05009
Carol A. Burke MD, Department of Gastroenterology and Hepatology, Desk A30, 9500 Euclid Ave, Cleveland Ohio 44195
H Gilbert Welch MD MPH, VA Medical Center, 215 N Main Street, VA Outcomes Group(111B), White River Junction, VT 05009
Robert W. Haile, Dr.Ph, Department of Preventive Medicine, Genetic Epidemiology Program, Harlyne Norris Research Tower, 1450 Biggy Street, Room 1506, Mail Code: LG591 MC9603, Los Angeles, CA 90033
Robert S Sandler MD, Nina C. and John T. Sessions Distinguished Professor, Chief, Division of Gastroenterology and Hepatology, CB# 7555, 4157 Bioinformatics Building, University of North Carolina, Chapel Hill, NC 27599-7555
E. Robert Greenberg MD, Cancer Research and Biostatistics, 1780 Minor Ave. Suite 1900, Seattle, WA 98101-1468
Dennis J Ahnen MD, Denver VA Medical Center 111E, 1055 Clermont St, Denver CO 80220
Robert S. Bresalier MD, Department of Gastroenterology, Hepatology and Nutrition, The University of Texas MD Anderson Cancer Center, Unit 1466, 1515 Holcombe Boulevard, Houston, Texas 77030-4009
Richard I Rothstein MD, Dartmouth Hitchcock Medical Center, 1 Medical Center Drive, Section of Gastroenterology, Lebanon, NH 03756-1000
Bernard Cole, Ph.D, Professor of Statistics, Director, Statistics Program, Department of Mathematics and Statistics, College of Engineering and Mathematical Sciences University of Vermont Burlington, VT 05405
Leila A. Mott, MS, Section of Biostatistics & Epidemiology, Dartmouth Medical School, Evergreen Center, 46 Centerra Parkway, Suite 300, Rm 339 Lebanon, NH 03766
John A Baron MD, Section of Biostatistics & Epidemiology, Dartmouth Medical School, Evergreen Center, 46 Centerra Parkway, Suite 300, Rm 333 Lebanon, NH 03766
References
- 1.Lieberman DA, Holub J, Eisen G, Kraemer D, Morris CD. Utilization of colonoscopy in the United States: results from a national consortium. Gastrointest Endosc. 2005;62(6):875–83. doi: 10.1016/j.gie.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 2.Atkin WS, Saunders BP. Surveillance guidelines after removal of colorectal adenomatous polyps. Gut. 2002;51 Suppl 5:V6–9. doi: 10.1136/gut.51.suppl_5.v6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bond JH. Polyp guideline: diagnosis, treatment, and surveillance for patients with colorectal polyps. Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol. 2000;95(11):3053–63. doi: 10.1111/j.1572-0241.2000.03434.x. [DOI] [PubMed] [Google Scholar]
- 4.Davila RE, Rajan E, Baron TH, et al. ASGE guideline: colorectal cancer screening and surveillance. Gastrointest Endosc. 2006;63(4):546–57. doi: 10.1016/j.gie.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology. 2006;130(6):1872–85. doi: 10.1053/j.gastro.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Mysliwiec PA, Brown ML, Klabunde CN, Ransohoff DF. Are physicians doing too much colonoscopy? A national survey of colorectal surveillance after polypectomy. Ann Intern Med. 2004;141(4):264–71. doi: 10.7326/0003-4819-141-4-200408170-00006. [DOI] [PubMed] [Google Scholar]
- 7.Baron JA, Cole BF, Sandler RS, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348(10):891–9. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 8.Cole BF, Baron JA, Sandler RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. Jama. 2007;297(21):2351–9. doi: 10.1001/jama.297.21.2351. [DOI] [PubMed] [Google Scholar]
- 9.Schoen RE. Surveillance after positive and negative colonoscopy examinations: issues, yields, and use. Am J Gastroenterol. 2003;98(6):1237–46. doi: 10.1111/j.1572-0241.2003.07492.x. [DOI] [PubMed] [Google Scholar]
- 10.Saini SD, Kim HM, Schoenfeld P. Incidence of advanced adenomas at surveillance colonoscopy in patients with a personal history of colon adenomas: a meta-analysis and systematic review. Gastrointest Endosc. 2006;64(4):614–26. doi: 10.1016/j.gie.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 11.Winawer SJ, Zauber AG, O’Brien MJ, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. The National Polyp Study Workgroup. N Engl J Med. 1993;328(13):901–6. doi: 10.1056/NEJM199304013281301. [DOI] [PubMed] [Google Scholar]
- 12.van Stolk RU, Beck GJ, Baron JA, Haile R, Summers R. Adenoma characteristics at first colonoscopy as predictors of adenoma recurrence and characteristics at follow-up. The Polyp Prevention Study Group. Gastroenterology. 1998;115(1):13–8. doi: 10.1016/s0016-5085(98)70359-2. [DOI] [PubMed] [Google Scholar]
- 13.Martinez ME, Sampliner R, Marshall JR, Bhattacharyya AK, Reid ME, Alberts DS. Adenoma characteristics as risk factors for recurrence of advanced adenomas. Gastroenterology. 2001;120(5):1077–83. doi: 10.1053/gast.2001.23247. [DOI] [PubMed] [Google Scholar]
- 14.Bonithon-Kopp C, Piard F, Fenger C, et al. Colorectal adenoma characteristics as predictors of recurrence. Dis Colon Rectum. 2004;47(3):323–33. doi: 10.1007/s10350-003-0054-1. [DOI] [PubMed] [Google Scholar]
- 15.Atkin WS, Morson BC, Cuzick J. Long-term risk of colorectal cancer after excision of rectosigmoid adenomas. N Engl J Med. 1992;326(10):658–62. doi: 10.1056/NEJM199203053261002. [DOI] [PubMed] [Google Scholar]
- 16.Laiyemo AO, Murphy G, Albert PS, et al. Postpolypectomy colonoscopy surveillance guidelines: predictive accuracy for advanced adenoma at 4 years. Ann Intern Med. 2008;148(6):419–26. doi: 10.7326/0003-4819-148-6-200803180-00004. [DOI] [PubMed] [Google Scholar]
- 17.Blumberg D, Opelka FG, Hicks TC, Timmcke AE, Beck DE. Significance of a normal surveillance colonoscopy in patients with a history of adenomatous polyps. Dis Colon Rectum. 2000;43(8):1084–91. doi: 10.1007/BF02236554. discussion 1091–2. [DOI] [PubMed] [Google Scholar]
- 18.Laiyemo AO, Pinsky PF, Marcus PM, et al. Use and Yield of Surveillance Colonoscopy in the Continued Follow-Up Study of the Polyp Prevention Trial. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2008 doi: 10.1016/j.cgh.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paul FP, Robert ES, Joel LW, et al. The Yield of Surveillance Colonoscopy by Adenoma History and Time to Examination. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2009;7(1):86–92. doi: 10.1016/j.cgh.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343(3):162–8. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 21.Lieberman DA, Weiss DG, Harford WV, et al. Five-year colon surveillance after screening colonoscopy. Gastroenterology. 2007;133(4):1077–85. doi: 10.1053/j.gastro.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Imperiale TF, Glowinski EA, Lin-Cooper C, Larkin GN, Rogge JD, Ransohoff DF. Five-year risk of colorectal neoplasia after negative screening colonoscopy. N Engl J Med. 2008;359(12):1218–24. doi: 10.1056/NEJMoa0803597. [DOI] [PubMed] [Google Scholar]
- 23.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58(3):130–60. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]


