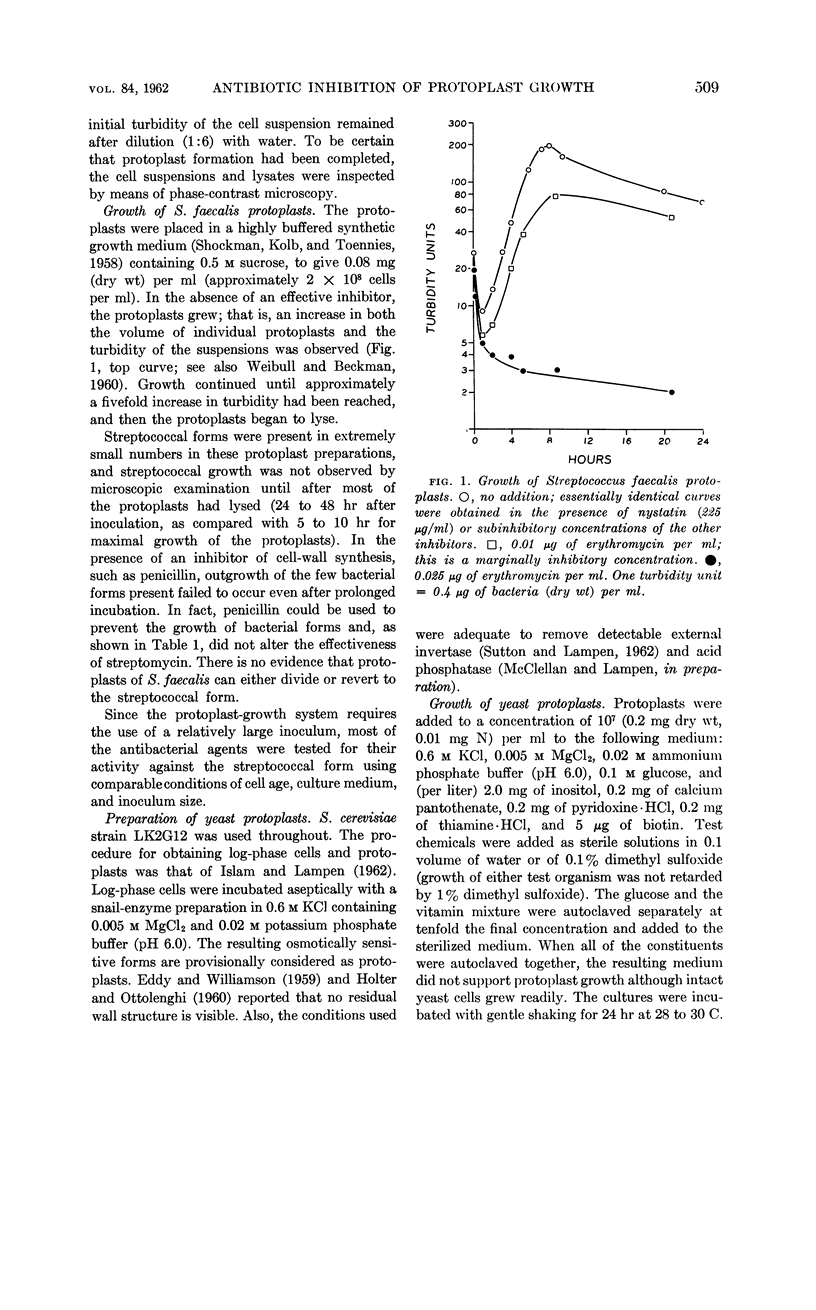
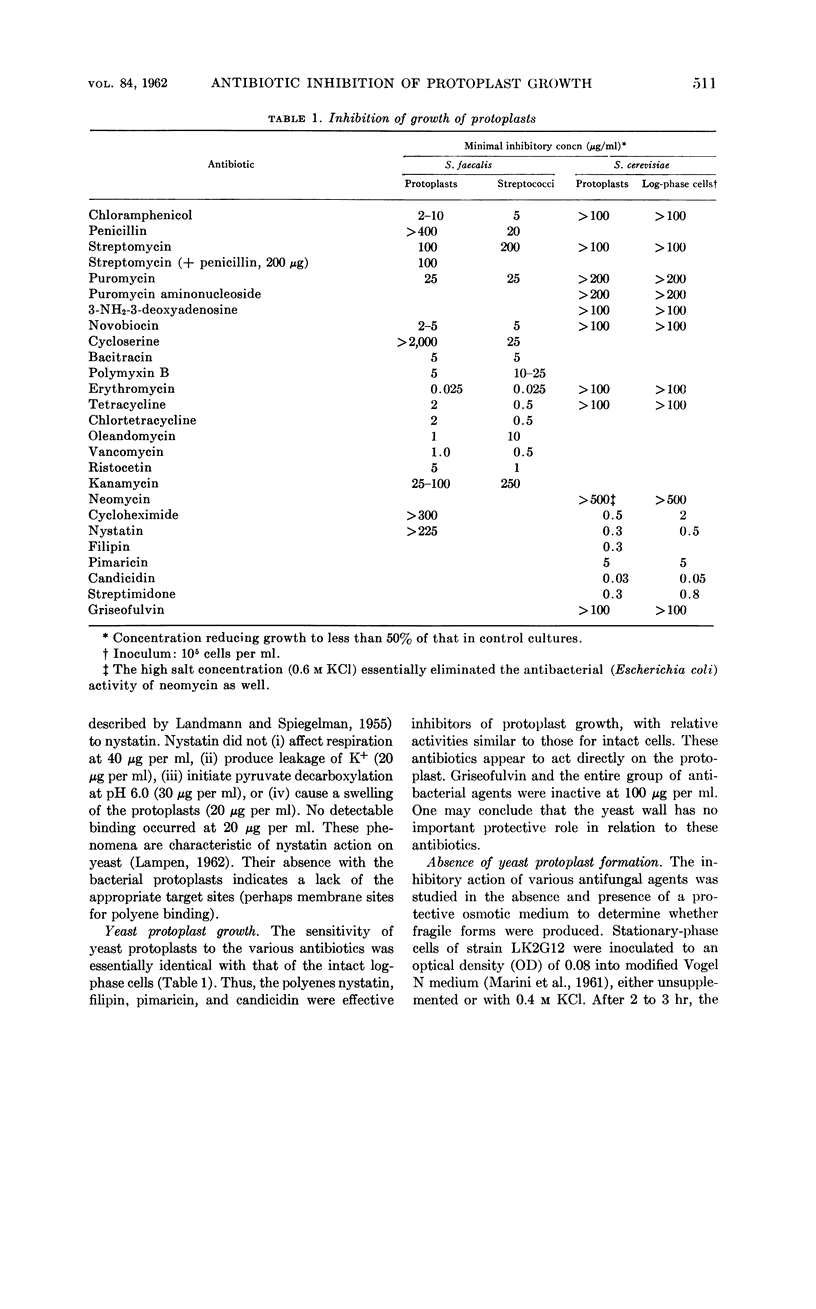
Abstract
Shockman, Gerald D. (Temple University School of Medicine, Philadelphia, Pa.) and J. Oliver Lampen. Inhibition by antibiotics of the growth of bacterial and yeast protoplasts. J. Bacteriol. 84:508–512. 1962.—The characteristics and requirements for growth of bacterial (Streptococcus faecalis) and yeast (Saccharomyces cerevisiae) protoplasts were established and the effect of a variety of antibacterial and antifungal antibiotics determined. A clear differentiation was obtained between such inhibitors of bacterial cell wall synthesis as penicillin and cycloserine, which did not prevent protoplast growth, and all others, antibacterial and antifungal, which inhibited protoplasts and intact organisms at the same range of concentration. Novobiocin, previously reported to inhibit bacterial wall synthesis, was also effective against a reaction(s) essential to the growth of S. faecalis protoplasts. The antibacterial action of streptomycin, neomycin, and kanamycin was essentially eliminated by the high salt concentration needed to maintain the protoplasts. Removal of the cell wall did not significantly increase antibiotic susceptibility of a resistant species. Protoplasts of Bacillus megaterium were insensitive to the antifungal agent, nystatin, and did not bind it to any detectable degree. Thus, the yeast or bacterial cell wall does not appear to play a major role in determining relative antibiotic susceptibility by masking internal sensitive target sites. A variety of antifungal antibiotics tested on the growth of log-phase yeast cells failed to produce osmotically fragile forms.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROCK T. D., BROCK M. L. Effect of novobiocin on permeability of Escherichia coli. Arch Biochem Biophys. 1959 Nov;85:176–185. doi: 10.1016/0003-9861(59)90461-8. [DOI] [PubMed] [Google Scholar]
- BROWN R., HAZEN E. L. Present knowledge of nystatin, an antifungal antibiotic. Trans N Y Acad Sci. 1957 Mar;19(5):447–456. [PubMed] [Google Scholar]
- EDDY A. A., WILLIAMSON D. H. Formation of aberrant cell walls and of spores by the growing yeast protoplast. Nature. 1959 Apr 18;183(4668):1101–1104. doi: 10.1038/1831101a0. [DOI] [PubMed] [Google Scholar]
- HOLTER H., OTTOLENGHI P. Observations on yeast protoplasts. C R Trav Lab Carlsberg. 1960;31:409–422. [PubMed] [Google Scholar]
- HUPPERT M., MACPHERSON D. A., CAZIN J. Pathogenesis of Candida albicans infection following antibiotic therapy. I. The effect of antibiotics on the growth of Candida albicans. J Bacteriol. 1953 Feb;65(2):171–176. doi: 10.1128/jb.65.2.171-176.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMPEN J. O., MORGAN E. R., SLOCUM A., ARNOW P. Absorption of nystatin by microorganisms. J Bacteriol. 1959 Aug;78:282–289. doi: 10.1128/jb.78.2.282-289.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landman O. E., Spiegelman S. ENZYME FORMATION IN PROTOPLASTS OF BACILLUS MEGATERIUM. Proc Natl Acad Sci U S A. 1955 Oct 15;41(10):698–704. doi: 10.1073/pnas.41.10.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARINI F., ARNOW P., LAMPEN J. O. The effect of monovalent cations on the inhibition of yeast metabolism by nystatin. J Gen Microbiol. 1961 Jan;24:51–62. doi: 10.1099/00221287-24-1-51. [DOI] [PubMed] [Google Scholar]
- Maass E. A., Johnson M. J. PENICILLIN UPTAKE BY BACTERIAL CELLS. J Bacteriol. 1949 Apr;57(4):415–422. doi: 10.1128/jb.57.4.415-422.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean I. W., Schwab J. L., Hillegas A. B., Schlingman A. S. SUSCEPTIBILITY OF MICRO-ORGANISMS TO CHLORAMPHENICOL (CHLOROMYCETIN). J Clin Invest. 1949 Sep;28(5 Pt 1):953–963. doi: 10.1172/JCI102185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHOCKMAN G. D., KOLB J. J., TOENNIES G. Relations between bacterial cell wall synthesis, growth phase, and autolysis. J Biol Chem. 1958 Feb;230(2):961–977. [PubMed] [Google Scholar]
- SHOCKMAN G. D. Reversal of cycloserine inhibition by D-alanine. Proc Soc Exp Biol Med. 1959 Aug-Sep;101:693–695. doi: 10.3181/00379727-101-25064. [DOI] [PubMed] [Google Scholar]
- STROMINGER J. L. Biosynthesis of bacterial cell walls. Fed Proc. 1962 Jan-Feb;21:134–143. [PubMed] [Google Scholar]
- STROMINGER J. L., THRENN R. H. The optical configuration of the alanine residues in a uridine nucleotide and in the cell wall of Staphylococcus aureus. Biochim Biophys Acta. 1959 May;33(1):280–281. doi: 10.1016/0006-3002(59)90538-4. [DOI] [PubMed] [Google Scholar]
- SUTTON D. D., LAMPEN J. O. Localization of sucrose and maltose fermenting systems in Saccharomyces cerevisiae. Biochim Biophys Acta. 1962 Jan 29;56:303–312. doi: 10.1016/0006-3002(62)90567-x. [DOI] [PubMed] [Google Scholar]
- Shockman G. D., Conover M. J., Kolb J. J., Phillips P. M., Riley L. S., Toennies G. LYSIS OF STREPTOCOCCUS FAECALIS. J Bacteriol. 1961 Jan;81(1):36–43. doi: 10.1128/jb.81.1.36-43.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Conover M. J., Kolb J. J., Riley L. S., Toennies G. NUTRITIONAL REQUIREMENTS FOR BACTERIAL CELL WALL SYNTHESIS. J Bacteriol. 1961 Jan;81(1):44–50. doi: 10.1128/jb.81.1.44-50.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIBULL C., BECKMAN H. Growth of bacterial L forms and bacterial protoplasts. J Bacteriol. 1960 May;79:638–649. doi: 10.1128/jb.79.5.638-649.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]



