Abstract
Longitudinal chromatic aberration (LCA) causes short wavelengths to be focused in front of long wavelengths. This chromatic signal is evidently used to guide ocular accommodation. We asked whether chick eyes exposed to static gratings simulating the chromatic effects of myopic or hyperopic defocus would “compensate” for the simulated defocus.
We alternately exposed one eye of each chick to a sine-wave grating (5 or 2 cycle/deg) simulating myopic defocus (“MY defocus”: image focused in front of retina; hence, red contrast higher than blue) and the other eye to a grating of the same spatial frequency simulating hyperopic defocus (“HY defocus”: blue contrast higher than red). The chicks were placed in a drum with one eye covered, with one grating, and then switched to another drum with the other eye covered, with the other grating. To minimize the effects of altered eye-growth on image contrast, we studied only the earliest responses: first, we measured changes in choroidal thickness 45 min to 1 hour after one 15-min episode in the drum, then we measured glycosaminoglycans (GAG) synthesis in sclera and choroid (by the incorporation of labeled sulfate in tissue culture) after a day of four 30-min episodes in the drum.
The eyes compensated in the appropriate directions: The choroids of the eyes exposed to the HY simulation showed significantly more thinning (less thickening) over the course of the experiment than the choroids of the eyes exposed to the MY simulation (all groups mean: −17 μm; 5 c/d groups: −24 μm; paired t-test (one-tailed): p=0.0006). The rate of scleral GAG synthesis in the eye exposed to the HY simulation was significantly greater than in the eye exposed to the MY simulation (HY/MY ratio = 1.20; one sample t-test (one-tailed): p=0.015). There was no significant interaction between the sign of the simulated defocus and either the spatial frequency or the presence of a +3 D lens used to compensate for the 33 cm distance of the drum.
Although previous work has shown that chromatic cues to defocus are not essential for lens-compensation, in that chicks can compensate in monochromatic light, our evidence implies that the eye may be able to infer whether the eye is myopic or hyperopic from the different chromatic contrasts that result from different signs of defocus.
Keywords: emmetropization, myopia, choroid, sclera, hyperopia, ocular length, choroidal thickness, chromatic, color
Introduction
Emmetropization is an active process that uses visual cues to match the eye length with the focal length of the eye’s optics. How the eye discerns the sign of defocus has been a question that has resisted resolution for many years. One possibility is that a color signal from longitudinal chromatic aberration (LCA) could be used to help detect the sign of defocus of the eye (Fincham, 1951; Flitcroft, 1990), as it does in accommodation (Kruger, Mathews, Aggarwala & Sanchez, 1993; Kruger, Mathews, Aggarwala, Yager & Kruger, 1995). However, eyes can compensate for spectacle lenses in monochromatic light (Rohrer, Schaeffel & Zrenner, 1992; Rucker & Wallman, 2008; Schaeffel & Howland, 1991; Wildsoet, Howland, Falconer & Dick, 1993). This shows that LCA is not necessary for lens compensation; we now test whether it is sufficient.
LCA affects the contrast transmitted by the different cone types. As a result of LCA, the shorter wavelengths of the incident light are refracted more than the longer wavelengths by the cornea and lens, producing an image in which the shorter wavelengths (blue) are focused closer to the lens than the longer wavelengths (red). This difference in refraction with wavelength affects the contrast of the retinal image differentially for the different cone types (Marimont & Wandell, 1994; Rucker & Osorio, 2008). In a well focused image the focal plane lies between the optima for the middle-wavelength-sensitive cones (M-cones) and the long-wavelength-sensitive cones (L-cones). Short-wavelength light which stimulates the short-wavelength-sensitive cones (S-cones) tends to be somewhat out of focus; with myopic defocus (focal plane in front of the photoreceptors) this defocus of short-wavelengths is exaggerated. Conversely, if the retinal image is focused hyperopically (focal plane behind the photoreceptors) then short wavelengths will be more in focus than long wavelengths, and so the short-wavelength component of the retinal image will have higher contrast than the long-wavelength component.
1.1 Criteria for an emmetropization signal from LCA
The first criterion for the existence of an emmetropization signal from LCA is that the eye can detect the effects of LCA. Several investigators have demonstrated changes in eye length that correspond to changes in focus caused by illumination with different wavelengths. In fish (blue acara) eyes, the naso-temporal diameter was larger when they were exposed to red light compared to when they were exposed to blue light (Kröger & Wagner, 1996). In birds, chicks kept in red (615 nm) or blue (430 nm) monochromatic illumination for two days developed a relative myopic or hyperopic shift, respectively (Seidemann & Schaeffel, 2002); similar results were found by Rucker & Wallman (2008). Clearly the eye can detect the effects of LCA.
A second criterion for the existence of an emmetropization signal from LCA is that several different cone types must contribute to the response because a chromatic response from LCA requires comparing the responses of two or more cone types. Early experiments (Kröger & Wagner, 1996; Rohrer et al., 1992) suggested that long-wavelength sensitive cones (L-cones) or double cones (D-cones) contribute to lens compensation, but ultra-violet sensitive cones (UV-cones) do not (Rohrer et al., 1992). Rucker & Wallman (2008) subsequently showed that in addition to an L- or D-cone contribution, these short-wavelength sensitive (S-cones) can also contribute to the emmetropization response. At low illumination levels the form of the emmetropization response in chicks depends on cone type. If S-cones were stimulated, defocus was compensated mostly by adjusting the rate of ocular elongation, whereas if L-cones or D-cones were stimulated it was the choroidal thickness that changed (Rucker & Wallman, 2008). These experiments confirm that more than one cone type participates in detecting the effects of LCA and compensating for lens-induced defocus.
1.2 Effect of LCA on the retinal image of a black/white grating
The effects of LCA on the image of a black and white square-wave grating pattern are shown in Figure 1. With hyperopic defocus (Figure 1: top row) the red and green components of the image are focused further behind the retina than the blue components, and hence the image of these components will be more blurred. Therefore, if the eye views a black/white edge, then the red and green components of the edge will be blurred relative to the blue (Figure 1A). If the pattern is repetitive as in a square wave, the blurred wavelengths will reach neither the maximum nor the minimum brightness of the focused wavelengths, resulting in lower modulation or contrast (Figure 1B). Thus, there will be a lower amplitude of red in the bright bars and a higher amplitude of red in the dark bars. In a sine-wave grating the effect of defocus is only to reduce contrast (Figure 1C). Therefore, if the image is defocused hyperopically (behind the photoreceptors) the amplitude of the blue component will be higher than the green or red component. The reverse is true with myopic defocus (Figure 1D).
Figure 1.
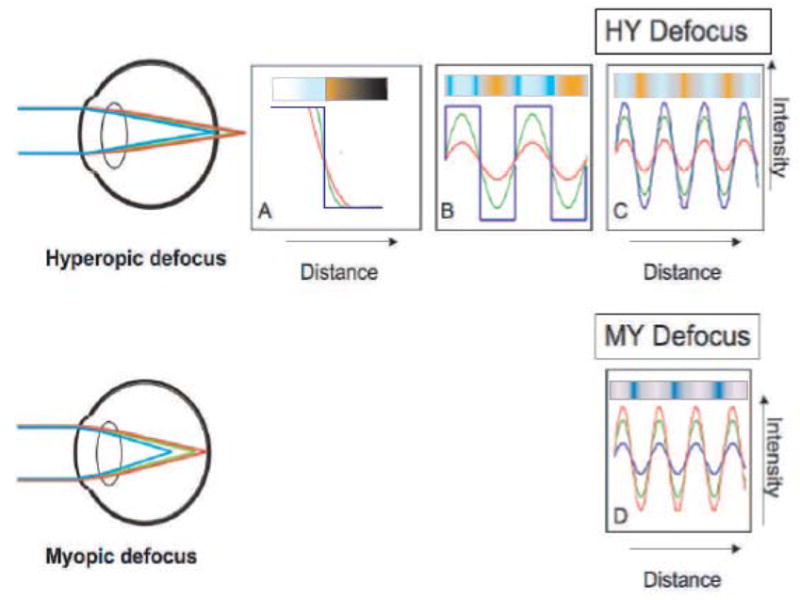
Effect of LCA on defocus and contrast. Top Row: If the eye is focused on short wavelengths, longer wavelengths would be focused behind the retina and would produce a blurred retinal image. A: A black/white edge will therefore be better defined by the short wavelengths. B: If the pattern is a square-wave, then the longer-wavelengths may never reach maximum or minimum intensity. C: If the pattern is a sine-wave, in which the only change with defocus is a change in contrast, with hyperopic defocus the short-wavelength component will have a higher contrast than the longer-wavelength components (HY defocus). Bottom row: The reverse is true for myopic defocus (MY defocus).
In this experiment we have simulated the effects of defocus caused by LCA with sine-wave gratings to determine if a chromatic signal from LCA can drive an emmetropization response. Image simulations of this sort have been shown to drive reflex accommodation in the predicted direction (Kruger, et al., 1995; Lee, Stark, Cohen & Kruger, 1999; Stark, Lee, Kruger, Rucker, & Ying, 2002; Rucker & Kruger, 2004). Some of this work has been presented previously at ARVO 2007.
2.0 Methods
2.1 Animals and measurements
White leghorn chicks (Gallus gallus domesticus, Cornell K strain; Cornell University, Ithaca, NY) were acquired as eggs. Upon hatching, the chicks were raised in a 14 hour light, 10 hour dark cycle, with a continuous supply of food and water. The experiments were performed on chicks 7–14 days old. The experimental eye was fitted with a Velcro ring, which was glued to the feathers around the eye. Lenses and patches were attached to this ring using matching Velcro. At the start and end of the period of exposure, A-scan ultrasound biometry was performed on anesthetized birds. Care and use of the animals adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
2.2 Drum conditions
During the experiment, chicks (two at a time) were free roaming in a raised 24-cm-diameter enclosure with transparent walls, which was rotated by a motor at a velocity of 30 deg/s (direction switched every 30 s). This enclosure was positioned in the center of a 60-cm-diameter drum. The drum walls were papered with sine-wave gratings that simulated the chromatic effects of LCA with defocus (as described below). The clear plastic lid of the drum was covered with a translucent diffuser that allowed light to enter but prevented the chicks from seeing out. Drums were illuminated (500 lux) with daylight light bulbs (Solux) that provided the full color spectrum of natural daylight (4700K) with a beam spread of 36 degrees. During exposure to the simulations one eye was exposed at a time, the other eye was patched. Chicks were otherwise kept in the dark in a sound- and lightproof chamber (61×81 cm).
2.3 Simulations
To test the hypothesis that a color signal from chromatic aberration can control eye growth we created simulations of the blurring effects of chromatic aberration on a black and white sine wave by adjusting the contrast of the red and blue components. Hyperopic and myopic defocus was simulated by gratings like the ones shown in Figure 1. To create the simulations the sine waves were created on a computer monitor using Matlab and then printed on a HP Designjet 500 series printer with high resolution (1200 × 600 dpi). The output of the printer was calibrated to ensure a linear printed output range.
To ensure the validity of the simulations the spectral reflection of each of the gratings was analyzed. Spectral reflectance (380 nm - 700 nm) of each grating was analyzed at 0.12 mm intervals (for the 5 c/d grating), or 0.25 mm intervals (for the 2 c/d grating), across multiple cycles of the printed pattern with an Ocean Optics XR2000 spectral radiometer using a 200 μm quartz fiber with an integration time of 0.5 secs. The spectral radiometer averages ten traces for each measurement. The spectral reflections from the simulations were then used to calculate the cone contrasts of the retinal image.
2.4 Calculation of cone contrast
Our intent was to create gratings that had contrasts for each cone type similar to what would be the case for black-and-white gratings defocused in either the myopic or hyperopic direction. Because the inks and illuminant had broad spectral distributions, we obtained the excitation of each cone type by multiplying, at each wavelength, the reflectance of the peak and trough of each grating by (a) the modulation transfer function for the spatial frequency of the grating (Avila, 2008) and (b) the spectral sensitivity of each cone type after absorption by the cone oil droplets (Rucker & Wallman, 2008). The contrast is expressed as Michelson contrast. In these calculations, we used the modulation transfer function described by Avila (2008) for a 2 mm pupil, with defocus induced by LCA in the chick eye (Mandelman & Sivak, 1983). A pupil diameter of 2.0–2.8 mm is typical of birds 7 to 15 days old (Avila, 2008). The through-focus calculations were done in OSLO EDU (edition 6.3.1) for Ross Steggles 308 chicks by Natalia Avila and Sally McFadden. Calculated cone contrasts were similar (3% difference) if the MTF described by Coletta, Marcos, Wildsoet, & Troilo (2003) for a 4 mm diameter pupil, 5 week old, White Leghorn chick, was used instead of the function described by Avila (2008). As mentioned above, when one views an object, some wavelengths are defocused hyperopically, others myopically and some wavelength is exactly in focus. Because we did not know what wavelength this would be in our experiment, we calculated the cone-contrasts at two extreme wavelengths to ensure that the relative cone-contrasts of our gratings (that is, that the L-cone contrast would be higher than the M-cone contrast, or vice versa) would be maintained across small changes in focus.
To do this the focal plane of the eye was set at either 590 nm or 440 nm and the subsequent defocus over the visible spectrum was calculated. Two functions were then produced: one that described the MTF (λ) over the visible spectrum with focus at 590 nm and one that described the MTF (λ) with focus at 440 nm. These functions were then used to calculate cone contrast as described above.
Table 1 shows the cone contrasts (including the defocusing effects of LCA) produced by viewing the simulations. The grating simulating HY defocus had greater modulation in blue than in red for both focal planes (590 and 440 nm), whereas the grating simulating MY defocus had greater modulation in red than in blue (Table 1). The contrast of the middle-wavelength component of the grating was similar for both gratings (HY: 0.38; MY: 0.43) and the mean reflectance for both gratings was the same.
Table 1.
Cone contrast produced by the printed grating simulations. Cone contrast is calculated for the 5 c/d grating simulations used in the main experiment, and for the control experiment in which the mean color balance was changed, and includes the estimated effect of defocusing caused by LCA.
| Main experiment | Cone Contrasts | ||||||
|---|---|---|---|---|---|---|---|
| Focal plane (nm) | Condition | Spatial Frequency | L | M | S | UV | D |
| 590 | MY defocus | 5 | 0.56 | 0.38 | 0.26 | 0.23 | 0.49 |
| 440 | MY defocus | 5 | 0.54 | 0.34 | 0.23 | 0.24 | 0.39 |
| 590 | HY defocus | 5 | 0.30 | 0.43 | 0.53 | 0.69 | 0.37 |
| 440 | HY defocus | 5 | 0.42 | 0.43 | 0.57 | 0.67 | 0.44 |
| Color Balance Control Experiment | Cone Contrasts | ||||||
| Focal plane (nm) | Condition | Spatial Frequency | L | M | S | UV | D |
| 590 | MY defocus | 5 | 0.82 | 0.61 | 0.42 | 0.47 | 0.74 |
| 440 | MY defocus | 5 | 0.78 | 0.56 | 0.39 | 0.38 | 0.62 |
| 590 | HY defocus | 5 | 0.40 | 0.51 | 0.57 | 0.84 | 0.46 |
| 440 | HY defocus | 5 | 0.40 | 0.51 | 0.60 | 0.77 | 0.47 |
2.5 Spatial frequencies
LCA affects the contrast of long-, middle- and short-wavelength components of the retinal image differently at different spatial frequencies. Since the effects of LCA are more pronounced with high spatial frequencies (Marimont & Wandell, 1994; Rucker & Osorio, 2008), it might be expected that the chromatic signal from LCA will be more pronounced with the 5 c/d grating than with the 2 c/d grating. Although a 5 c/d grating is close to the resolution limit of the young chick (Schmid & Wildsoet, 1997; Jarvis, Abeyeshinghe, McMahon, Wathes, 2009) there is evidence that 5 c/d can maintain emmetropia more effectively than lower frequencies (Avila, 2008), though others find that episodes of 4.3 c/deg is less effective than lower frequencies in countering the effects of form deprivation (Schmid & Wildsoet, 1998). For these reasons two spatial frequencies were tested: 5 c/deg and 2 c/deg. Depending on the location of the chick within the central enclosure the spatial frequency experienced varied from 3.8–5.9 c/deg for the 5 c/deg grating and from 1.4–2.1 c/deg for the 2 c/deg grating.
2.6 Lenses
Because the nearness of the drum walls (33 cm, −3.00 D) some of the chicks were fitted with +3.00 D defocusing lenses on both eyes to neutralize this possible defocus.
2.7 Controlling for differences in the mean color of the gratings
Although we attempted to make the average luminance and chromaticity of the two gratings identical, when the gratings were analyzed with a spectral radiometer, and the cone excitations were calculated, we found that there was a slight difference in mean cone excitation. In the grating that simulated hyperopic defocus the excitation of the long-wavelength sensitive cone was slightly lower, and the excitation of the short-wavelength sensitive cone was slightly higher than in the grating that simulated myopic defocus, i.e., the HY grating was slightly bluer than the MY grating. To control for the possibility that our results were distorted by this difference, we ran the experiment under a second (control) condition in which we deliberately reversed the color balance, thus making the HY grating less blue than the MY grating, and the MY grating less red than the HY grating. We achieved this by superimposing a daffodil yellow (Lee 310) filter between the light source and the drum for the HY grating, and a cyan (Lee 4315) filter between the light source and the drum for the MY grating.
2.8 Procedures for measuring the change in choroidal thickness
Because in a hyperopically defocused eye the S-cone contrast is relatively higher than in a myopically defocused eye, if our simulation of myopic defocus (lower S-cone contrast) caused the eye to compensate by becoming hyperopic, this change in the optics of the eye would reverse the cone contrasts produced by our simulation. To avoid this complication, we measured the ocular changes over as short a time as was possible, to approach an open-loop measurement. Thus we measured the choroidal thickness forty five minutes to an hour after brief exposures to the gratings, and we measured the scleral growth changes after less than a day of several brief exposures to the gratings. As diagrammed in Figure 2, one eye was exposed to the HY grating with the other eye patched, and then the patch was switched, and the uncovered eye was exposed to the MY grating for 15 min. The eyes were exposed either with or without a +3.00 D lens. Because our gratings only simulated a difference in defocus of around +1.5 D, only small, initial compensatory changes in choroidal thickness were anticipated. Hence, inter-ocular comparisons were made to avoid the inter-bird variability. After the 15-min exposures of each eye, chicks were kept in the dark for 45 min to one hour before being re-measured by ultrasound biometry.
Figure 2.
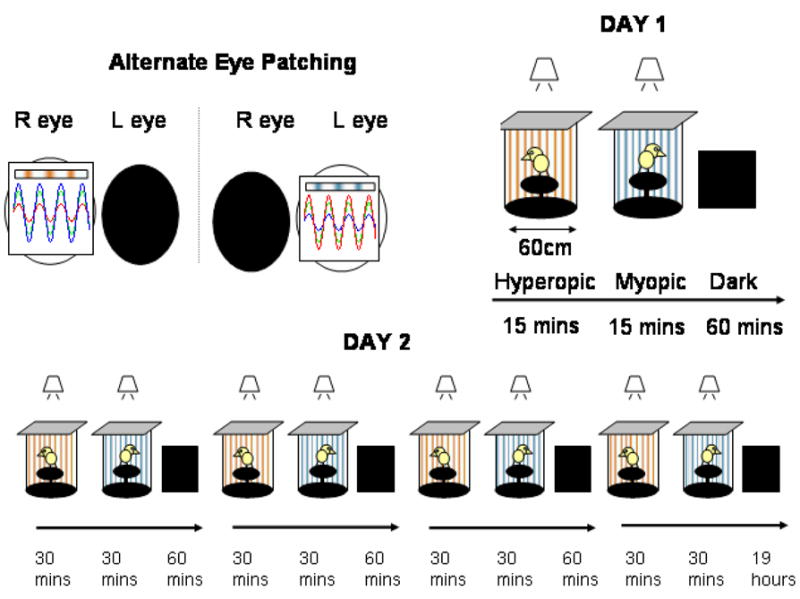
Method: Chicks were kept in drums that had printed simulations of HY defocus or MY defocus papered on the walls. (Top left:as viewed with the chick facing the observer) Each eye was exposed briefly to either the HY simulation or MY simulation, during exposures the other eye was patched.
Day 1: (Top right) Each eye was exposed briefly to either the HY simulation or MY simulation for 15 minutes. After the exposures the chicks were kept in the dark for 45 min to one hour. Choroidal thickness was measured at the beginning and end of the procedure.
Day 2: (Bottom) Each eye was exposed briefly to either the HY simulation or MY simulation for 30 min, four times a day. The chick was kept in the dark at other times. During exposures the other eye was patched. GAG synthesis was measured after 19 hours of darkness.
2.9 Procedures for measurement of GAG synthesis
We measured scleral proteoglycan synthesis (GAG) as an indicator of eye growth, and choroidal GAG synthesis as a correlate of changes in choroidal thickness. Changes in scleral extracellular matrix are associated with changes in the rate of growth of the eye during experimentally induced myopia. Increased eye growth in chicks either as a result of form deprivation or negative-lens-induced defocus is associated with increased glycosaminoglycan synthesis in the posterior pole of the sclera (Nickla, Wildsoet & Wallman, 1997; Rada, McFarland, Cornuet & Hassell, 1992). The increase in eye growth in response to wearing negative lenses is associated with a thinning of the choroid and a commensurate decrease in glycosaminoglycans in the choroid. Similarly, thickening of the choroids in response to positive lens defocus is associated with an increase in GAG synthesis in the choroid (Marzani & Wallman, 1997; Nickla et al., 1997).
On Day 2 chicks were given a 30 min exposure to each eye, with the other eye patched, four times for a total of 2 hours in one day, and were otherwise kept in the dark (Figure 2). Nineteen hours after the final exposure birds were euthanized with an overdose of intraperitoneal sodium pentobarbital. After eyes were removed and bisected along the ora serrata, 7 mm diameter punches were taken from the posterior part of the eye and kept in cold Carbon Dioxide Independent Medium supplemented with glutamine (Gibco, Carlsbad, CA) during dissection of the choroids and scleras. The method used to assay the glycosaminoglycan synthesis was similar to that used by Nickla et al., (1997). Choroidal and scleral punches were incubated in L-15 (Millipore Corporation, Phillipsburg, NJ) in separate wells with Na235SO4 (40 υCi/ml) for 3 hours. After radioactive pulsing, tissues were frozen at −20°C until samples were processed. Tissues were digested overnight at 57°C in 0.06% of Proteinase-K (protease type XXVIII, Sigma in 10 mM EDTA, 0.1 M phosphate, pH = 6.5), then centrifuged for 15 min. GAGs were precipitated by the addition of 0.5 % of cetylpyridinium chloride and chondroitin sulfate (1mg/ml in distilled water) in 2 mM Na2SO4; samples were incubated overnight at 37°C. The precipitate was captured by vacuum filtration on Whatman GF/F filters wetted with 4 ml of 0.05 M NaCl with 0.1% cetylpyridinium chloride. After the sample was added the filters were rinsed 3 times with 4 ml of the same solution. Radioactivity was measured by liquid scintillation counting in 20 ml of cocktail (CytoScint, Fisher, Atlanta, GA.)
2.10 Statistical analysis
The relative change in choroidal thickness was defined as the change, from before the exposure to 45 min to 1 hour after the exposure, in choroidal thickness in the eye exposed to HY minus the equivalent change in choroidal thickness in the eye exposed to MY:
(ΔHY-ΔMY) = [Δ eye exposed to HY defocus] minus [Δ eye exposed to MY defocus]
The relative change in choroidal thickness was compared to zero with a paired t-test (one-tailed). The effects of different spatial frequencies and lens powers on the relative change (ΔHY-ΔMY) were compared with ANOVA.
GAG synthesis was analyzed by taking the ratio of the counts in the eye that was exposed to the HY grating to the counts in the eye that was exposed to the MY grating:
Log Ratio (HY/MY) = Log ([counts in eye exposed to HY] divided by [counts in eye exposed to MY]). The log ratio (HY/MY) of GAG synthesis was compared with 0 (one-sample t-test; one-tailed).
Because our measures of GAG synthesis were ratios, when comparing different conditions we wanted to avoid the asymmetry of averaging ratios (a ratio of 5 having more weight than the inverse ratio of 0.2). The asymmetry could make it appear that one treatment was more effective than another. To avoid this asymmetry we first converted the ratios to logs, took the average, and then found the anti-log of the mean of the log ratios. Comparison of the effects of different spatial frequencies and lens powers on the log ratio (HY/MY) were compared with ANOVA.
3.0 Results
Our principal result is that the eye altered its growth as would be predicted if it normally used a chromatic signal resulting from LCA to compensate for defocus. There was choroidal thinning (or less thickening) and an increase in scleral GAG synthesis in response to the condition that simulated the focal plane lying behind the photoreceptors (HY condition), when compared to the condition that simulated the focal plane lying in front of the photoreceptors (MY condition), as shown in Table 2 and Figure 3.
Table 2.
Change in choroidal thickness, ratio of GAG synthesis in the choroid, and ratio of GAG synthesis in the sclera, when the eyes are exposed to the HY and MY simulations at 5 c/d and 2 c/d, and with the color balance changed. The mean ratio for scleral and choroidal GAG synthesis is the mean of the log ratios for the individual birds converted from log values.
| Δ Choroid Thickness (μm) |
GAG Ratio Choroid |
GAG Ratio Sclera |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spatial Frequency c/d | Experiment | Number | Lens (D) | HY | MY | (ΔHY-ΔMY) | Number | Mean Ratio | Mean Log Ratio | Number | Mean Ratio (10^ Log Ratio) | Mean Log ratio |
| 5 | Main | 12 | None | −18 | 8 | −26 | 16 | 0.81 | −0.090 | 14 | 1.25 | 0.098 |
| 5 | Main | 27 | 3 | 14 | 36 | −22 | 14 | 0.91 | −0.039 | 14 | 0.89 | −0.051 |
| 5 | Color Balance | 16 | None | 8 | 30 | −22 | 22 | 1.11 | 0.047 | 22 | 1.26 | 0.099 |
| 2 | Main | 7 | None | 0 | −6 | 6 | 14 | 0.93 | −0.034 | 14 | 1.25 | 0.099 |
| 2 | Main | 24 | 3 | 16 | 26 | −9 | 14 | 0.99 | −0.006 | 15 | 1.35 | 0.131 |
| Mean | 86 | 8 | 25 | −17 | 80 | 0.96 | −0.02 | 79 | 1.20 | 0.08 | ||
Figure 3.
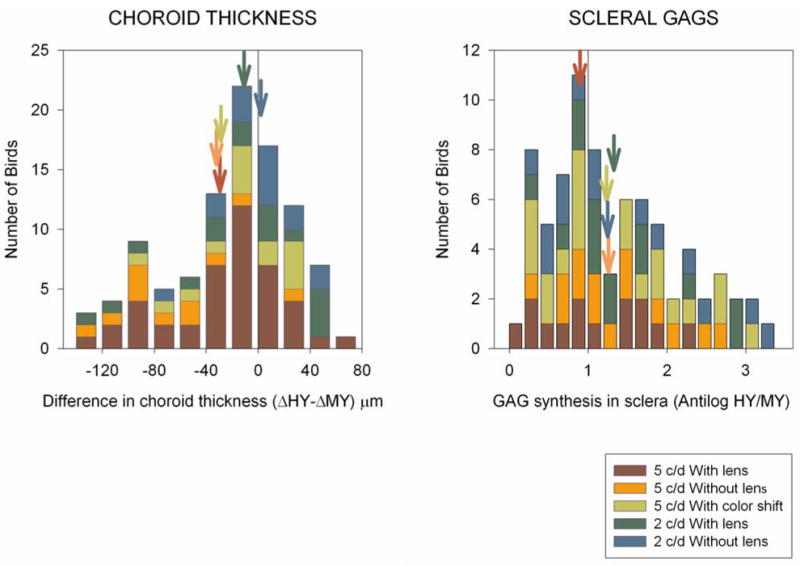
Left Panel: histogram of the relative change in choroidal thickness between the eye exposed to the HY simulation and the eye exposed to the MY simulation. Bars to the left of the zero line indicate that the choroid thinned more in the eye exposed to the HY simulation than they did in the eye exposed to the MY simulation.
Right Panel: Histogram of the HY/MY ratio of GAG synthesis in the sclera. Bars to the right of the line of HY/MY ratio equals 1 showed an increased rate of GAG synthesis in the eye exposed to HY relative to the GAG synthesis in the eye exposed to MY. Means of the individual conditions are indicated by arrows of the appropriate color. Mean ratio (HY/MY) GAG synthesis is calculated as the anti-log of the mean of the individual log ratios (HY/MY). The mean ratio (HY/MY) when all conditions are included is 1.20 when calculated in this way, if the mean ratio was calculated as the mean of the ratios and not the anti-log of the mean log ratios, then the mean ratio (HY/MY) would have been 1.51.
3.1 Choroidal thickness
There was more thinning (or less thickening) of the choroids of the eyes exposed to the HY simulation than there was of the choroids of eyes exposed to the MY simulation. Across all groups there was an increase of 25 μm in the mean choroidal change in the eye exposed to MY, compared to 8 μm in the eye exposed to HY (paired t-test (one tailed): p=0.0006). In Figure 3 the majority of the birds lie to the left of the zero line indicating that there was more thinning of the choroids in the eye exposed to the HY simulation relative to eyes exposed to the MY simulation.
The relative change in choroidal thickness (ΔHY-ΔMY) was not significantly affected by lens power or spatial frequency (ANOVA: p=0.58; p=0.07), nor was there a significant interaction between spatial frequency and the presence of the lens (ANOVA: p=0.45).
3.2 Scleral GAG synthesis
The HY condition produced a 20 % increase in GAG synthesis relative to the GAG synthesis in the eye exposed to the MY condition (Figure 3). In Figure 3 the majority of the birds lie to the right of the line indicating a ratio of greater than 1 (the anti-log of the log ratio HY/MY=0). The log ratio (HY/MY) was significantly different to zero (one sample t-test [one-tailed]: p = 0.015), indicating that the rate of GAG synthesis was higher in the eye exposed to the HY simulation than in the eye exposed to the MY simulation. There was no effect of lens power (ANOVA: p = 0.44) or spatial frequency (ANOVA: p = 0.23), nor any significant interaction between spatial frequency or presence of the lens (ANOVA: p = 0.24).
3.3 Relationship between choroidal thinning and scleral GAG synthesis
Since choroidal thinning is typically associated with an increase in axial length when the eye is exposed to hyperopic defocus, we tested to see if there was an association between these factors in response to viewing the simulations. When the grating was 5 c/d and the HY condition was compared to the MY condition, birds in which the choroid thinned more with HY tended to be those in which scleral GAGs increased more with HY (Fisher exact test p < 0.05). Out of twenty one birds for which we had both sets of data, fifteen birds showed greater choroidal thinning in response to the HY simulation, and eleven of these also showed more scleral GAG synthesis in response to the HY simulation.
3.4 Choroidal GAG synthesis
The HY condition simulating focus behind the retina failed to produce a decrease in choroidal GAG synthesis relative to the GAG synthesis in the eye exposed to the MY condition. The log GAG ratio (HY/MY) was not significantly different to zero (one-sample t-test [two-tailed]: p = 0.28).
3.5 Results for individual spatial frequency and lens conditions
With the 5 c/d stimulus gratings (Figure 4) there appeared to be more choroidal thinning in the eye exposed to HY grating relative to the eye exposed to the MY grating, both with and without the + 3.00 D lens ([ΔHY-ΔMY] no lens: −26 μm; with lens: −22 μm). However, the increase in scleral GAG synthesis with the HY condition was only found without the lens (mean HY/MY ratio=1.25).
Figure 4.

Effect of spatial frequency and presence of spectacle lens. In the choroidal thickness graph, bars below the zero difference line indicate greater choroidal thinning in response to the HY simulations than to the MY simulations. In the GAG synthesis graphs, bars above the ratio HY/MY =1 line indicate greater GAG synthesis in the eye exposed to the HY simulation relative to the MY simulation.
Although the 5 c/d and 2 c/d gratings were not significantly different, at 2 c/d the compensatory changes seemed less robust (Figure 4). There were no significant changes in choroid thickness, regardless of lens (−9 and 6 μm, with and without the lens, respectively). There was an increase in scleral GAG synthesis in response to the HY simulation with and without a +3.00 D lens, giving a mean HY/MY ratio of 1.35 with the lens, and 1.25 without the lens. And there was little difference in choroidal GAG synthesis (HY/MY ratio 0.93 and 0.99; with and without the lens respectively).
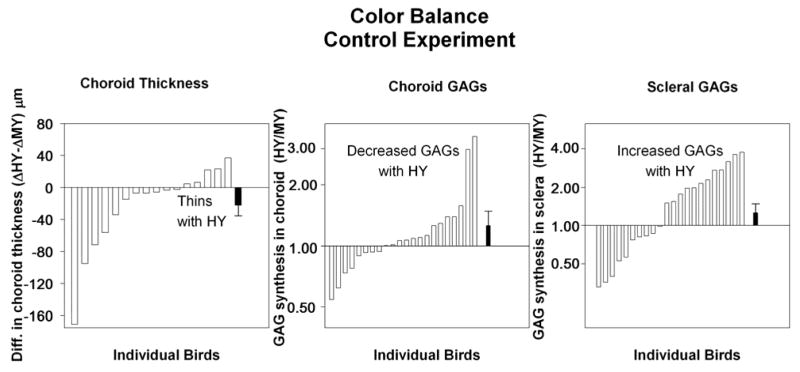
3.6 Results for color balance control condition
The differences we report are not due to the slight difference in overall tint of the gratings (Figure 5). Both the choroidal thinning and the scleral GAG ratio were essentially the same as in the main experiment. There was relative choroidal thinning (ΔHY-ΔMY) of 22 μm and a scleral GAG ratio (HY/MY) of 1.26. Animals exposed with the overall color changed were not significantly different from those in the groups without the color change.
Figure 5.
Effect of altering color balance of the gratings. Conventions as in Figure 4.
3.7 The effects of binocular positive lens wear
We had some birds wear +3.00 D lenses over both eyes, in case the nearness of the cylinder walls imposed hyperopic defocus. The results show that these lenses may have caused myopic defocus in some eyes, in that the majority of the choroids thickened when the birds were wearing the binocular +3.00 D lenses, and the lens-wearing birds had 16–32 μm thicker choroids in both the eyes in the HY and MY conditions than the non-lens-wearing birds. These results suggest that the lens-wearing birds experienced some myopic defocus when wearing the lenses, although this did not diminish the effect of the simulated defocus.
4.0 Discussion
The results presented here show that by manipulating the contrast of the red, green and blue components of a sine-wave grating, the eyes of chicks viewing these gratings respond as though they had been presented with hyperopic or myopic defocus. Specifically, in the eye that was exposed to the simulation of hyperopic defocus, the choroid thinned more (or thickened less) and scleral GAGs increased relative to the eye exposed to the simulation of myopic defocus. These results therefore imply that longitudinal chromatic aberration provides a cue sufficient for the eye to discern the sign of defocus and grow in a compensatory direction.
Three questions need to be answered. First, because light of different wavelengths is focused by the eyes at different planes, and eyes are known to change their refraction in response to such colored illuminants (Kröger & Wagner, 1996; Rucker & Wallman, 2008; Seidemann & Schaeffel, 2002), might our results be a consequence of differences in the average color of the different gratings, rather than the contrasts of the different chromatic components? We are confident this is not the case because there was no difference in the compensatory responses whether the average color of the grating simulating hyperopic defocus (the HY grating) was slightly bluer (as in the main experiment) or slightly redder (as in the control experiment) than the grating simulating myopic defocus (the MY grating).
Second, because the birds were exposed to the gratings in an enclosed space, might the strength of the compensation depend on whether the walls of the drum caused hyperopic defocus, which was superimposed on the defocus simulated by the gratings? We suspect this was not a major problem because those chicks wearing +3 D lenses over both eyes compensated just as much as those without lenses, even though their choroids thickened about 28 μm more than the chicks without the lenses (Table 2).
Third, might the degree of compensation depend on the spatial frequency of the gratings used to simulate defocus? As can be seen from Fig. 1A, if the spatial frequency is low enough, the red and blue contrasts would be the same, regardless of the defocus caused by LCA. Also, at higher spatial frequencies (above 20 c/d) only wavelengths very close to the focal plane will be seen, wavelengths further away will be too blurred to be detected (Marimont & Wandell, 1994; Rucker & Osorio, 2008). In fact the human eye will only have the full trichromatic range of color vision for spatial frequencies below 6 c/deg with a 3 mm pupil (Marimont & Wandell, 1994). The optimal range of spatial frequency for a chromatic signal from LCA is therefore around 2-6 c/deg, where there is trichromatic vision and a substantial change in contrast over a range of defocus. Not surprisingly then, in this experiment the changes in choroidal thickness and scleral GAG synthesis were more obvious with the 5 c/d grating than with the 2 c/d grating, although the chick eye responded to both gratings.
How might the eye use the LCA to infer the sign of defocus? Flitcroft (1990) modeled the chromatic signal from LCA as the rate of change of the modulation transfer function with respect to defocus. He showed, using the known properties of color-opponent neurons, that either the red/green or blue/yellow opponent neurons would signal the defocus across a moderate range of hyperopic and myopic defocus, with the blue/yellow neurons having a slightly larger range of defocus and being more sensitive, despite the paucity of blue cones, as well as having its position of optimal focus 0.75 D in the myopic direction.
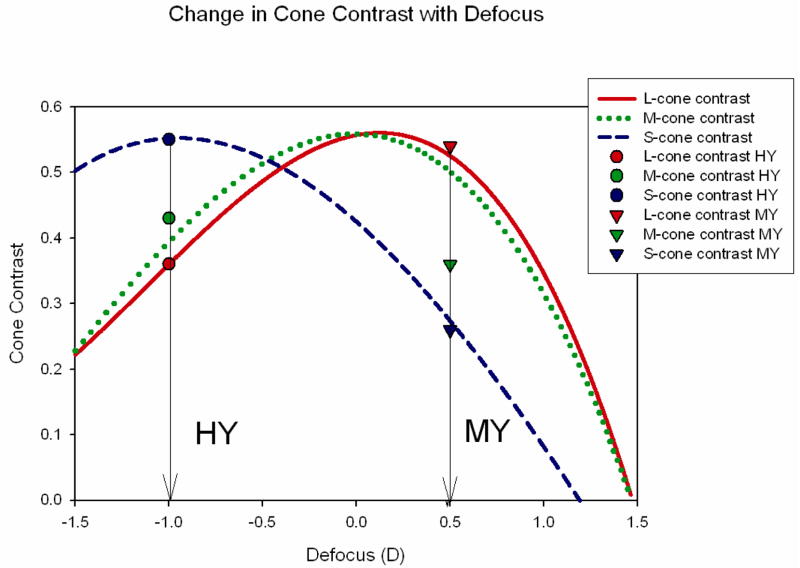
To approximately visualize the relationship of the different cones’ responses to our gratings, we modeled the human eye, using the methods of Flitcroft (1990) and of Rucker and Osorio (2008). As is evident from Figure 6, our construction of the two gratings was compatible with the cone contrasts for the S-cones and L-cones that would be experienced by an eye viewing defocused black-and-white gratings, although the fit was less good for the M-cones. [This may be because although the through-focus MTF’s of humans and chicks (Avila, 2008; Coletta et al., 2003) are similar, there is a difference of about 25 % at 500 nm that affects M-cone contrast.] One can see from these curves that the amount of defocus separating the two gratings was modest, 1.5 D. Consequently, it is not surprising that the degree of changes in choroidal thickness and scleral GAG synthesis was also modest. Furthermore, one can see that, had we allowed the experiment to continue until the eyes had substantially changed their refractive error in compensation for the simulated defocus, the actual defocus of the gratings would have overwhelmed the simulated defocus we produced.
Figure 6.
Cone contrasts as a function of defocus, showing fit of cone contrasts of simulation-gratings used in the main experiment. Curves are cone-contrasts of human eye obtained by multiplying spectral sensitivity of human cone-types, which includes effects of macular pigment and lens absorption (DeMarco, Pokorny & Smith, 1992) by the MTF at 3 cpd (3 mm pupil) degraded by defocus (Hopkins, 1955) with the degree of defocus caused by LCA at each wavelength as calculated by Thibos, et al. (1992), and with contrast expressed as Michelson contrast. The peak values of all curves are equal because the S-, M- and L-cones were calculated as being focused at 460, 530, and 555 nm, respectively, thus there being minimal defocus at those values for each cone type. Defocus values on abscissa assume eye is focused at 555 nm.
The curves shown in Figure 6 also suggest that from knowing the cone contrasts one could infer the magnitude as well as the sign of the defocus of a visual stimulus (in this case an achromatic grating). For example, the S- and L-cone-contrasts shown for our HY grating would only occur for the degree of defocus shown. Had the stimulus been higher or lower in contrast, both cone contrasts would shift up and down accordingly at that level of defocus, but these relative values of cone-contrasts would not occur at other levels of defocus. In the case of the MY grating, however, the relative cone-contrasts shown could occur for a range of defocus. Thus, the chromatic signal (the difference between the L- and S- cone-contrasts) would change more rapidly as a function of degree of defocus on the hyperopic side of emmetropia, but the luminance signal, which follows the L- and M-curves, would change more rapidly as a function of the degree of myopic defocus. Therefore, chromatic signals, as embodied by comparison of cone-contrasts, could yield more information about hyperopic defocus than about myopic defocus, but changes with defocus in the luminance signals could yield more information about myopic defocus. Whether the emmetropization mechanism makes use of these measures of defocus magnitude remains to be seen.
Figure 6 also makes clear that the range over which LCA would provide a useful signal for emmetropization is quite restricted to the region around emmetropia. At lower spatial frequencies the useful region would be expanded (Flitcroft, 1990), but at the expense of a smaller change in cone-contrast with each increment of defocus. At some point, the changes in contrast would be too small to be useful. Therefore, the finding that spectacle lenses are compensated over a range of at least ± 10 D (Irving, Sivak & Callender, 1992), argues that cues other than LCA are utilized.
If emmetropization proceeded until a certain balance between the various cone-contrasts was attained, anything that shifted the balance towards longer wavelengths might result in longer, and hence myopic, eyes. This might explain why in a population of color-normal individuals, those with greater red sensitivity tended to be myopic (Cernea & Constantin, 1977; Rucker & Kruger, 2006; Wienke, 1960), and why in two highly myopic subjects the L-cone pigments had their peak absorption at longer than normal wavelengths (Wagner-Schuman, Neitz & Neitz, 2008). Carrying this conjecture one step further, deficiencies in the red/green color mechanism might shift the emmetropization set-point in the hyperopic direction, and this might explain the finding that red/green-deficient children were less likely to be myopic (Qian, Chu, He, Sun, Zhou, Zhao, Hu, Hoffman, Dai, Qu & Pao, 2009). In conclusion, the results presented here provide the first direct evidence that chromatic signals like those produced by LCA provide a signal for emmetropization, resulting in choroidal and scleral compensatory responses in chicks. Because these signals would only be useful close to emmetropia, other signed cues to defocus must be present to explain the robust compensation observed over a wide range of myopic and hyperopic defocus.
Acknowledgments
We would like to thank Adriana Garzon for measuring the GAG synthesis for this experiment.
Supported by NIH Grant EY-02727 and RR-03060
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Avila NV. PhD Dissertation. University of Newcastle; 2008. Spatial frequency and eye growth in the developing chick. [Google Scholar]
- Cernea P, Constantin F. Color Discrimination in Ametropic Eyes. Annales D Oculistique. 1977;210(5):383–386. [Google Scholar]
- Coletta NJ, Marcos S, Wildsoet C, Troilo D. Double-pass measurement of retinal image quality in the chicken eye. Optom Vis Sci. 2003;80(1):50–57. doi: 10.1097/00006324-200301000-00008. [DOI] [PubMed] [Google Scholar]
- DeMarco P, Pokorny J, Smith VC. Full-spectrum cone sensitivity functions for X-chromosome-linked anomalous trichromats. Journal Optical Society America A. 1992;9(9):1465–1476. doi: 10.1364/josaa.9.001465. [DOI] [PubMed] [Google Scholar]
- Fincham EF. The accommodation reflex and its stimulus. Br J Ophthalmol. 1951;35(7):381–393. doi: 10.1136/bjo.35.7.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flitcroft DI. A neural and computational model for the chromatic control of accommodation. Vis Neurosci. 1990;5(6):547–555. doi: 10.1017/s0952523800000705. [DOI] [PubMed] [Google Scholar]
- Hopkins HH. The frequency response of a defocused optical system. Proceedings of the Royal Society A. 1955;231:91–103. [Google Scholar]
- Irving EL, Sivak JG, Callender MG. Refractive plasticity of the developing chick eye. Ophthalmic Physiol Opt. 1992;12(4):448–456. [PubMed] [Google Scholar]
- Jarvis JR, Abeyesinghe SM, et al. Measuring and modelling the spatial contrast sensitivity of the chicken (Gallus g. domesticus) Vision Res. 2009;49(11):1448–54. doi: 10.1016/j.visres.2009.02.019. [DOI] [PubMed] [Google Scholar]
- Kröger RH, Wagner HJ. The eye of the blue acara (Aequidens pulcher, Cichlidae) grows to compensate for defocus due to chromatic aberration. J Comp Physiol [A] 1996;179(6):837–842. doi: 10.1007/BF00207362. [DOI] [PubMed] [Google Scholar]
- Kruger PB, Mathews S, Aggarwala KR, Sanchez N. Chromatic aberration and ocular focus: Fincham revisited. Vision Res. 1993;33(10):1397–1411. doi: 10.1016/0042-6989(93)90046-y. [DOI] [PubMed] [Google Scholar]
- Kruger PB, Mathews S, Aggarwala KR, Yager D, Kruger ES. Accommodation responds to changing contrast of long, middle and short spectral-waveband components of the retinal image. Vision Res. 1995;35(17):2415–2429. doi: 10.1016/0042-6989(94)00316-5. [DOI] [PubMed] [Google Scholar]
- Lee JH, Stark LR, Cohen S, Kruger PB. Accommodation to static chromatic simulations of blurred retinal images. Ophthalmic Physiological Optics. 1999;19(3):223–235. doi: 10.1046/j.1475-1313.1999.00440.x. [DOI] [PubMed] [Google Scholar]
- Mandelman T, Sivak JG. Longitudinal chromatic aberration of the vertebrate eye. Vision Res. 1983;23(12):1555–1559. doi: 10.1016/0042-6989(83)90169-4. [DOI] [PubMed] [Google Scholar]
- Marimont DH, Wandell BA. Matching color images: the effects of axial chromatic aberration. J Opt Soc Am A. 1994;11(12):3113. [Google Scholar]
- Marzani D, Wallman J. Growth of the two layers of the chick sclera is modulated reciprocally by visual conditions. Invest Ophthalmol Vis Sci. 1997;38(9):1726–1739. [PubMed] [Google Scholar]
- Nickla DL, Wildsoet C, Wallman J. Compensation for spectacle lenses involves changes in proteoglycan synthesis in both the sclera and choroid. Curr Eye Res. 1997;16(4):320–326. doi: 10.1076/ceyr.16.4.320.10697. [DOI] [PubMed] [Google Scholar]
- Qian YS, Chu RY, He JC, Sun XH, Zhou XT, Zhao NQ, Hu DN, Hoffman MR, Dai JH, Qu XM, Pao KE. Incidence of myopia in high school students with and without red-green color vision deficiency. Invest Ophthalmol Vis Sci. 2009;50(4):1598–1605. doi: 10.1167/iovs.07-1362. [DOI] [PubMed] [Google Scholar]
- Rada JA, McFarland AL, Cornuet PK, Hassell JR. Proteoglycan synthesis by scleral chondrocytes is modulated by a vision dependent mechanism. Curr Eye Res. 1992;11(8):767–782. doi: 10.3109/02713689209000750. [DOI] [PubMed] [Google Scholar]
- Rohrer B, Schaeffel F, Zrenner E. Longitudinal chromatic aberration and emmetropization: results from the chicken eye. J Physiol. 1992;449:363–376. doi: 10.1113/jphysiol.1992.sp019090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker FJ, Kruger PB. Cone contributions to signals for accommodation and the relationship to refractive error. Vision Res. 2006;46(19):3079–3089. doi: 10.1016/j.visres.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Rucker FJ, Kruger PB. Accommodation responses to stimuli in cone contrast space. Vision Research. 2004;44(25):2931–2944. doi: 10.1016/j.visres.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Rucker FJ, Osorio D. The effects of longitudinal chromatic aberration and a shift in the peak of the middle-wavelength sensitive cone fundamental on cone contrast. Vision Res. 2008;48(19):1929–1939. doi: 10.1016/j.visres.2008.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker FJ, Wallman J. Cone signals for spectacle-lens compensation: Differential responses to short and long wavelengths. Vision Res. 2008;48(19):1980–1991. doi: 10.1016/j.visres.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffel F, Howland HC. Properties of feedback loops controlling eye growth and refractive state in the chicken. Vision Res. 1991;31(4):717–734. doi: 10.1016/0042-6989(91)90011-s. [DOI] [PubMed] [Google Scholar]
- Schmid KL, Wildsoet CF. Contrast and spatial-frequency requirements for emmetropization in chicks. Vision Res. 1997;37(15):2011–2021. doi: 10.1016/s0042-6989(97)00014-x. [DOI] [PubMed] [Google Scholar]
- Schmid KL, Wildsoet CF. Assessment of visual acuity and contrast sensitivity in the chick using an optokinetic nystagmus paradigm. Vision Res. 1998;38(17):2629–2634. doi: 10.1016/s0042-6989(97)00446-x. [DOI] [PubMed] [Google Scholar]
- Seidemann A, Schaeffel F. Effects of longitudinal chromatic aberration on accommodation and emmetropization. Vision Res. 2002;42(21):2409–2417. doi: 10.1016/s0042-6989(02)00262-6. [DOI] [PubMed] [Google Scholar]
- Stark LR, Lee RS, Kruger PB, Rucker FJ, Ying Fan H. Accommodation to simulations of defocus and chromatic aberration in the presence of chromatic misalignment. Vision Research. 2002;42(12):1485–1498. doi: 10.1016/s0042-6989(02)00074-3. [DOI] [PubMed] [Google Scholar]
- Thibos LN, Ye M, Zhang X, Bradley A. The chromatic eye: a new reduced-eye model of ocular chromatic aberration in humans. Applied Optics. 1992;31:3594–3600. doi: 10.1364/AO.31.003594. [DOI] [PubMed] [Google Scholar]
- Wagner-Schuman MM, Neitz MM, Neitz JJ. Slowing the progression of myopia in children [Abstract] Journal of Vision. 2008;890(17):90a. [Google Scholar]
- Wienke RE. Refractive error and the green/red ratio. J Opt Soc Am. 1960;50:341–342. doi: 10.1364/josa.50.000341. [DOI] [PubMed] [Google Scholar]
- Wildsoet CF, Howland HC, Falconer S, Dick K. Chromatic aberration and accommodation: their role in emmetropization in the chick. Vision Res. 1993;33(12):1593–1603. doi: 10.1016/0042-6989(93)90026-s. [DOI] [PubMed] [Google Scholar]