Abstract
Background
Optimal parameters of rTMS for antidepressant efficacy in general, or within patients, have not been adequately delineated.
Methods
Using a double-blind, sham-controlled, cross-over design, 22 adult patients with treatment refractory major depression (n=9; bipolar disorder, depressed phase) were randomized to active rTMS (20-Hz or 1-Hz) or sham rTMS conditions and given 5 rTMS treatments per week for two weeks. Repetitive TMS was administered at 100% of motor threshold for 1600 pulses over the left prefrontal cortex using a figure-eight coil. Patients initially randomized to sham rTMS were then exposed to two weeks of active rTMS with each frequency under blinded conditions. Those who received active 20-Hz and 1-Hz rTMS were crossed over to the opposite frequency for two weeks. Improvement in Hamilton Depression ratings were assessed after each two-week treatment phase. PET imaging was used to evaluate the patient’s baseline absolute regional cerebral activity (blood flow and metabolism) as potential predictor of clinical response.
Results
Changes in depression severity on 1-Hz and 20-Hz rTMS were inversely correlated. PET scans with baseline hypoperfusion (but not hypometabolism) were associated with better improvement on 20-Hz rTMS as predicted.
Limitations
The magnitude of the clinical change with either frequency at 100% motor threshold was not robust, and larger studies with higher intensities of rTMS for longer durations of time should be explored.
Conclusions
High and low frequency rTMS exerts differential effects on depressed mood within individual subjects. The brain activity predictors and correlates of an optimal antidepressant response to rTMS remain to be better defined.
Keywords: repetitive transcranial magnetic stimulation, depression, positron emission tomography, regional cerebral blood flow, hypoperfusion, hyperperfusion
I. INTRODUCTION
Several recent meta-analyses have suggested the efficacy of active repetitive transcranial magnetic stimulation (rTMS) over the left prefrontal cortex compared with sham control procedures in the treatment of acute depression (Burt et al., 2002; Kozel et al., 2002; Martin et al., 2003). However, some recent controlled studies have failed to observe differences from controls (Nahas et al., 2003; Hoppner et al., 2003; Loo et al., 2003a; Mosimann et al., 2004; Hausmann et al., 2004; Poulet et al., 2004).
There is suggestive evidence that treatment involving more robust rTMS parameters (such as higher intensities of stimulation for longer periods of time) are more likely to yield positive effects compared with studies with less robust procedures (George et al., 2000; Padberg et al., 2002; Rossini et al., 2005; O’Reardon et al., 2007).
The optimal frequency and location of rTMS remain largely unresolved factors in general, as do the optimal parameters for a given patient in particular (George et al., 2000). The studies that combined high frequency rTMS over the left prefrontal cortex, with low frequency rTMS over the right prefrontal cortex show significantly greater effects than sham in two studies (Garcia-Toro et al., 2006; Fitzgerald et al., 2006); but not another (Conca et al., 2002).
In the study of Kimbrell et al. (1999) in patients with major depression, opposite effects of 1- and 20-Hz stimulation (at 80% of MT) were observed in individual patients, i.e., patients who improved on one frequency tended to worsen on the other frequency (r = –0.797; p < 0.0004). We also found that 1- and 20-Hz rTMS exert opposite effects on regional brain activity lasting 48 hours after ten treatments (Speer et al., 2000); blood flow increased in a widespread fashion with 20 Hz and decreased to a lesser extent with 1-Hz rTMS. As predicted, those with lower levels of baseline metabolism on positron emission tomography (PET) tended to show better response to 20-Hz rTMS, whereas those with higher levels showed better response to 1 Hz (Kimbrell et al., 1999).
Given the data showing that high frequency rTMS increases regional cerebral blood flow (rCBF) and low frequency rTMS decreases it (Speer et al., 2000; Loo et al., 2003b), and that patients show differential frequency-dependent antidepressant responsiveness (Kimbrell et al., 1999), we sought to further examine possible regional cerebral predictors of improvement in depressed mood in response to higher intensity rTMS, i.e., 100% of MT. We predicted that depressed patients with low baseline regional cerebral activity measured by PET would experience better antidepressant response to high frequency (20-Hz) rTMS, while those with high baseline activity would respond better to low frequency (1-Hz) rTMS.
II. METHODS
Subjects
Twenty-two highly treatment-resistant depressed patients meeting DSM-IV criteria for either bipolar illness (n = 9) or unipolar major depression (n = 13) were included. Patients gave oral and written informed consent for the rTMS and associated brain imaging studies, and other procedures involved. The study was approved by both the Radiation Safety Committee of the National Institutes of Health and the Institutional Review Board of the NIMH. Two patients dropped out of the study prior to rTMS randomization.
Patients were randomized to receive 10 daily sessions of rTMS (5/week) over the left prefrontal cortex with either active (1-Hz or 20-Hz) or sham rTMS. Those receiving active rTMS were crossed over to the opposite frequency in the second two weeks to evaluate response within individuals. Those receiving sham rTMS first were then exposed to both of the other rTMS frequencies for two weeks ( 20-Hz rTMS; 1-Hz). After patients were exposed to both active frequencies, they were allowed to enter a continuation phase (at the rTMS frequency to which they had responded the best) for treatment confirmation and optimization. The rTMS was then tapered and pharmacological treatment begun prior to discharge. Two patients received only one frequency rTMS and declined further rTMS phases. Patients remained off of all psychotropic medication throughout the rTMS study except for three of the bipolar patients who were maintained on their prophylactic medication (one patient on valproate, one on carbamazepine, and one on T4 replacement).
Ratings
The Hamilton Rating Scale for Depression (HAM-D, 28-item expanded version) (Hamilton, 1960) was administered by highly-trained research assistants at: (1) baseline; (2) the end of weeks 1 and 2 of each rTMS treatment phase; and (3) on the day of the PET procedure. The raters and all other ward staff were blind to treatment assignment. The change from baseline until after two weeks of each phase was evaluated by paired t and response to high and low frequency rTMS using Pearson’s correlation coefficient (r).
rTMS
Repetitive transcranial magnetic stimulation was applied over the left prefrontal cortex at 100% of MT, as previously described in a subgroup of ten patients (Speer et al., 2000). Following a magnetic resonance imaging (MRI) scan to exclude structural abnormalities, MT was assessed by placing the coil over the left primary motor area and establishing the minimum amount of stimulator output necessary to cause visible movement of the flexed right thumb following at least five out of 10 single pulses.
One-Hz rTMS was given in a continuous train of 1600 pulses over 26 minutes 40 seconds. Twenty-Hz stimulation was administered with two seconds on and 28 seconds off, 40 times, for a total of 1600 stimulations per 20-minute session. The sham condition was administered at 20-Hz with one wing of the figure-eight coil in contact with the scalp and at a 45° angle with respect to the head.
18-Fluorodeoxyglucose (FDG) Imaging Procedures
Patients underwent baseline PET imaging after two medication-free weeks with FDG-PET (n = 13) and/or H215O water for assessment of blood flow (n = 19).
FDG imaging methods and analysis have been previously described in detail (Kimbrell et al., 1999; Ketter et al., 2001; Kimbrell et al., 2002a). Five mCi of FDG were infused over 1 minute followed by a 30-minute uptake period. The subjects’ eyes were patched, ambient noise and light were minimized, and subjects performed an auditory continuous performance task (CPT) to reduce variability in attention (Cohen et al., 1988). Head movement was restricted with individually molded thermoplastic masks.
To examine associations of baseline metabolic abnormalities with response to rTMS at different frequencies, images were produced at each patient’s deviation from the appropriate idealized age and gender control (Ketter et al., 1995; Willis et al., 1997). The resulting difference images were maintained in a format that preserved both hypometabolic (negative) and hypermetabolic (positive) deviations from normal.
Measures of global metabolic deviation from ideal were calculated by taking the mean of these images over the entire grey matter region in the brain so that each subject was categorized as globally hypometabolic or hypermetabolic. With the hypothesis that a subject with baseline hypometabolism would respond best to 20-Hz rTMS and a subject with hypermetabolism best to 1 Hz, these phases for these individuals were considered optimally “matched”.
In a second regional analysis, the difference images were correlated voxel-wise in SPM95 (Friston et al., 1991a) with the changes in HAM-D, producing a regional Z-map corresponding to raw probabilities of a Pearson correlation coefficient of zero. To correct for multiple comparisons, these Z-maps were then submitted to cluster analysis (Friston et al., 1994) with a Z-threshold of 1.96 (two-tailed p = 0.05) and a cluster probability threshold of < 0.05 as previously described (Kimbrell et al., 1999).
H215O PET Imaging During the Resting State
In contrast to the 18FDG scans which used the CPT, H215O scans were performed while subjects lay on the scanner bed with eyes closed and ears unplugged in a quiet, darkened environment. They were instructed to monitor their mood state over the subsequent several minutes in an attempt to provide more consistency and specificity of cognitive processing during this resting state.
Each subject underwent five scans (averaged together for statistical analysis) separated by 10–12 min, obtained after bolus intravenous injection of 10 mCi of H215O water for assessment of rCBF as described by Speer et al (2000). The atlas of Talairach and Tournoux (1988) was used to identify the brain regions where blood flow deviation from normal was associated with response.
III. RESULTS
A. Differential improvement in depression as a function of rTMS frequency
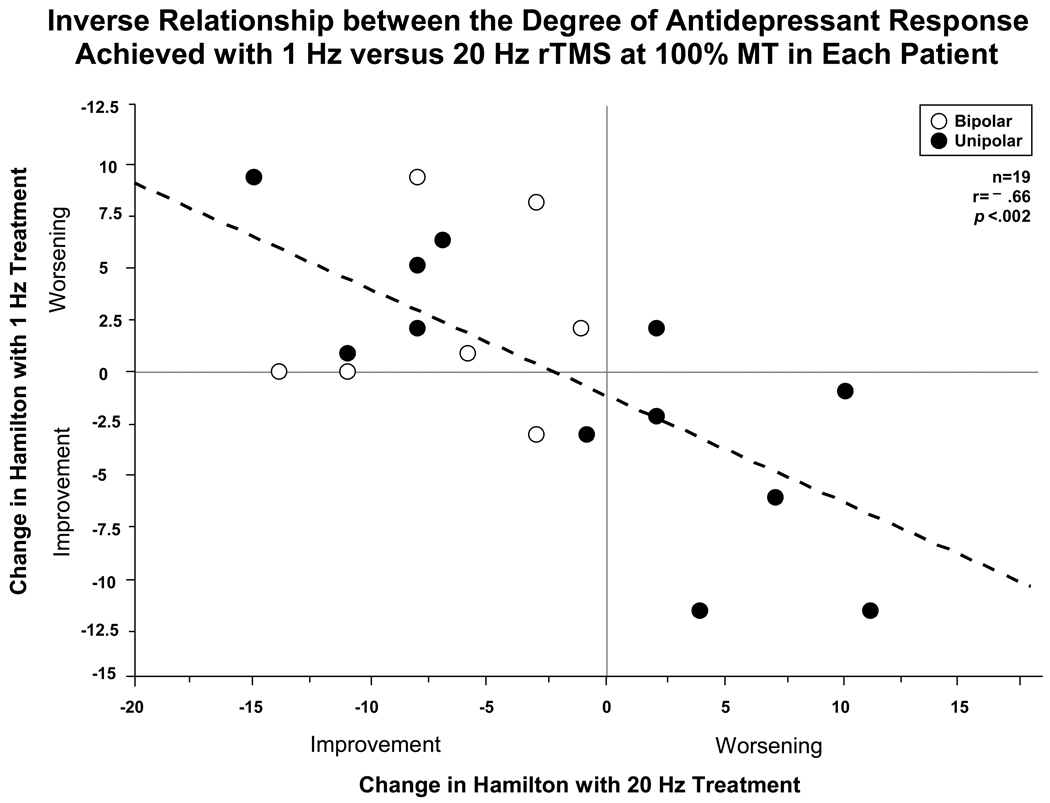
Within the 19 patients who received both high and low frequency active rTMS, a strong inverse relationship to the degree of antidepressant response was noted (r = –0.66, n = 19, p < 0.002) as illustrated in fig. 1. Those who improved on one frequency tended to worsen on the other. The 12 unipolar patients showed a relatively equal distribution of those improving on 1 versus 20-Hz and deteriorating on the other rTMS frequency, whereas the 7 bipolar depressed patients improved on 20-Hz. There was no effect of rTMS order and demographic and clinical illness characteristics revealed no striking correlates of response to 20- versus 1-Hz rTMS.
Figure 1.
Inverse relationship (r = −0.66) between the degree of antidepressant response (change in HAM-D) achieved with 1-Hz versus 20-Hz rTMS at 100% MT in each patient. Those improving on 20-Hz rTMS tended to worsen on 1-Hz rTMS, while those who improved on 1-Hz tended to worsen with 20-Hz, suggesting a clear frequency “preference” for each patient.
In the third active phase, patients were allowed to revisit the frequency of rTMS to which they had previously responded best. This was followed by attempts at response optimization during a continuation phase. During this average period of 6.1 weeks, and average number of treatments of 26.1 ± 11.8 (range 7–42), additional degrees of clinical improvement were not consistently observed. Baseline HAM-D ratings averaged 30.3 ± 5.9 at baseline, increased to 32.6 ± 9.8 on 1-Hz and decreased to 27.2 ± 4.6 on 20-Hz and increased to 31.8 ± 9.5 on sham. However, at the end of the continuation phase on 1-Hz, HAM-D rating averaged 21.6 ± 10.8 and on 20-Hz averaged 29.6 ± 5.1.
B. Association of response to baseline PET activity
Given the large individual difference in responsivity to high versus low frequency rTMS within different patients, and the large variations in baseline activity on PET in both unipolar and bipolar patients, we sought to re-examine the possibility of predicting better responsivity based on patterns of cerebral activity assessed at baseline with PET procedures (Kimbrell et al., 1999). We first examined the data in the 13 patients who participated in both the FDG and H215O PET studies to determine whether they were “matched” to their predicted optimal rTMS frequency (i.e., hypoactive with 20-Hz and hyperactive with 1-Hz). Significantly greater improvement occurred during “matched” than “unmatched” phases as evaluated by baseline perfusion, but not metabolism.
In order to examine the predictive relationships in the larger group of all patients who had a baseline H215O scan and a subsequent active rTMS phase, we compared the 11 patients hypoperfused at baseline (fig. 2, left side) with the eight who were hyperperfused at baseline (fig. 2, right side). Depressed patients with baseline presentations of hypoperfusion showed greater improvement (–4.5 ± 2.5 HAM-D points) when they received 20-Hz rTMS (matched), whereas they showed an exacerbation of their depressive mood (+2.8 ± 1.5 points) when treated with 1-Hz rTMS (p < 0.01), which would be expected to further decrease their baseline hypoperfusion (i.e., a mismatch).
Figure 2.
Using all 19 patients who had PET scans, baseline cerebral hypoperfusion (n = 11) predicted clinical response to 20-Hz rTMS and worsening on 1-Hz rTMS (left side). Baseline hyperperfusion (n = 8) did not show such a differential response to 1- vs. 20-Hz rTMS (right side). Those randomized to sham rTMS worsened slightly (middle).
In contrast, in the eight patients who were hyperperfused at baseline (fig. 2, right side), the degree of improvement was not significantly different, as predicted. However, when examining all patients when “matched” to the expected frequency, the improvement on the HAM-D was still greater (-3.7 ± 2.5) than when they were mismatched (0.7 ± 1.6; p < 0.04).
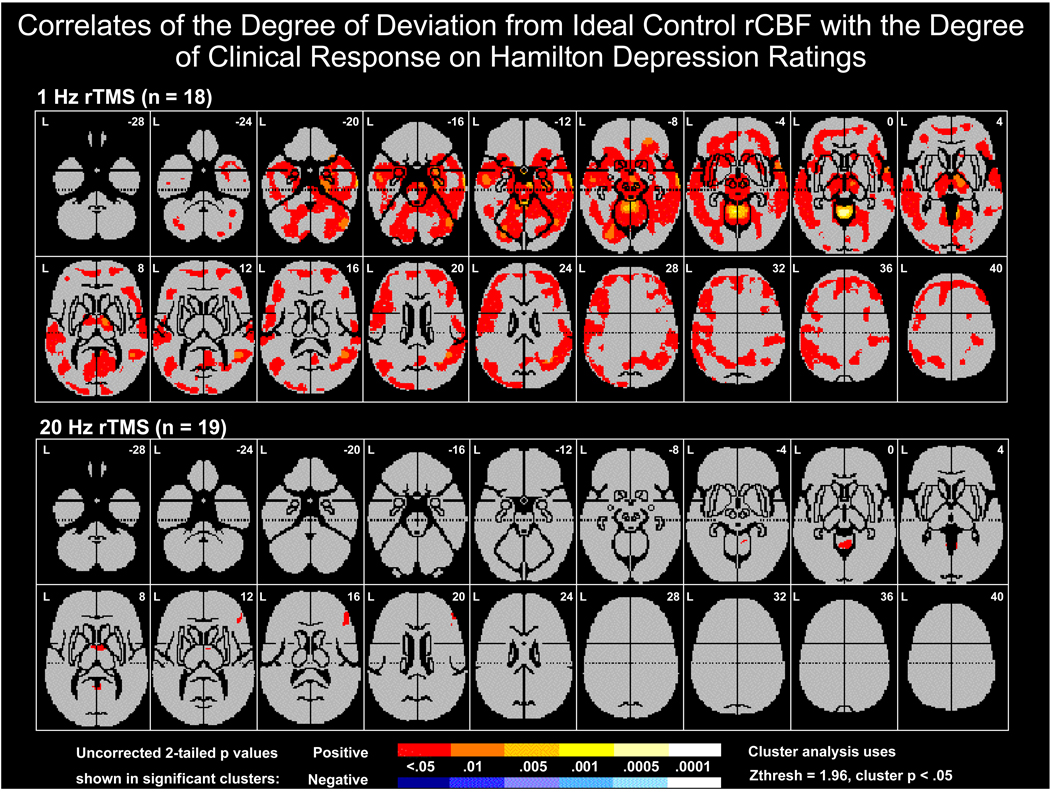
In a second more discrete regional analysis the degree of baseline hyperperfusion was significantly correlated with the predicted better degrees of responsivity to 1-Hz rTMS in a number of regions, including: bilateral dorsolateral prefrontal cortex, ventrolateral prefrontal cortex, medial and lateral temporal lobe, and thalamus; midbrain regions encompassing red nucleus and substantia nigra; cerebellum; and right amygdala, and large areas of frontal to occipital cortices However, the magnitude of baseline hypoperfusion (from idealized control) in any specific cortical area lacked substantial relationships to the magnitude of clinical improvement on 20-Hz rTMS. The relationships of the degree of responsivity to 1-Hz rTMS to FDG measures of regional hyper- metabolism were not as striking as those of rCBF.
IV. DISCUSSION
A. Differential antidepressant response as a function of rTMS frequency
The clinical response results in this study at 100% of MT replicate and extend previous observations at 80% of MT (Kimbrell et al., 1999) of differential responsivity to high vs. low rTMS frequencies within individual depressed subjects. There was a striking inverse relationship between degree of change on 1- versus on 20-Hz rTMS within these 19 individuals (r = –0.66, p < .002). The overall magnitude of the clinical changes at 100% of MT in this study were not, however, greater than those at 80% MT in the first study (Kimbrell et al., 1999). A third completed study that used rTMS at higher intensity (110% of MT) and for an additional third week of treatment has shown a larger magnitude of clinical improvement (Speer et al., 2007, unpublished data).
B. Relationship of baseline rCBF to degree of response and to 1- versus 20-Hz rTMS
In this study, the dichotomization of baseline levels of neural activity compared with age and gender-matched ideal controls, as assessed with H215O blood flow (but not by FDG metabolism) on PET, offer some support for the hypothesized better outcome when a patient with baseline cerebral activity was matched to the rTMS frequency that changes that pattern toward normal. As predicted, those patients with baseline hypoperfusion showed a significant improvement on 20-Hz rTMS and some deterioration on 1 Hz (fig. 2). This would be expected if the altered levels of baseline cerebral perfusion reflected a primary pathophysiological process associated with depression that needed to be reversed or ameliorated, as opposed to a compensatory or adaptive change that would need to be further enhanced.
Conversely, one would predict a better response to 1-Hz rTMS in those with a global baseline pattern of hyperperfusion. Although this was not seen when the eight patients dichotomized as being hyperperfused were given 1- vs. 20-Hz rTMS (fig. 2), when all of the patients who received 1 Hz were considered in the regional correlational analysis, there was a very striking predicted relationship. The degree of antidepressant response to 1-Hz rTMS was widely correlated with the degree of hyperperfusion in many brain areas. These areas most prominently included: the cerebellum, which has previously been reported to be hyperactive in both unipolar and bipolar depressed patients; the amygdala; and other midbrain and cortical areas (Ketter et al., 2001; 2002b).
In contrast, we did not observe the predicted relationship of regions of decreased rCBF to the degree of response to 20-Hz rTMS as might have been expected from the dichotomized distinction of hypoperfusion in seen in fig. 2. In a larger series of subjects, Dunn et al. (2006) found marked dissociations in regional measures of blood flow versus metabolism in patients with unipolar depression, but not in bipolar patients or normal volunteers where flow and metabolism continued to be tightly coupled. This could, in part, account for the better prediction of response with blood flow in this study as opposed to metabolism in the Kimbrell et al. (1999) study. Whether PET assessments will eventually prove to be clinically useful in the prediction of response to rTMS remains for further study in larger groups of unipolar and bipolar depressed patients. Moreover, since blood flow appeared to be a better correlate of response to rTMS than metabolism in this study, it at least raises the possibility that the more affordable and widely available SPECT scan techniques compared with PET could be used for this purpose. However, Garcia-Toro et al. (2006) found no extra advantages of focusing their high-frequency left and low-frequency right rTMS on brain regions identified by SPECT.
C. Limitations
Given that the clinical extensions of rTMS treatment in the continuation phase at 100% of MT did not robustly enhance therapeutic efficacy, one could wonder whether the driving of the baseline abnormalities in rCBF toward or beyond normal with either very low (1-Hz) or very high (20-Hz) frequency rTMS is an optimal strategy, and perhaps a more intermediate frequency of 10-Hz might be more effective, as utilized in many recent studies comparing rTMS with ECT (Post and Speer, 2007). Moreover, in the largest study to date, O’Reardon et al. (2007) found robust effect of 10-Hz rTMS of left prefrontal cortex at 120% of MT.
Jin and colleagues (2005) reported optimal effects of rTMS in patients with schizophrenia when the frequency was matched to patient’s individual alpha frequency (very close to an average of 10-Hz) and showed lesser degrees of response to high or low frequency rTMS, or sham rTMS. Other studies will have to address whether this midrange 10-Hz frequency is more optimal for the treatment of depression than either the high and low frequencies chosen for study here in an effort to maximize their differential clinical and physiological effects.
Although this study provides a strong replication for the initial observations that individual depressed patients respond differentially to high- versus low-frequency rTMS administered over the left dorsolateral prefrontal cortex, how these observations may eventually be linked to the clinical therapeutics of depression is not yet clear and needs further study, perhaps using higher intensities for long durations of treatment. The results of this study also provide only limited support for the hypothesized predictive relationships. Low baseline brain activity (i.e., as measured with H215O blood flow, but not by FDG metabolism) was linked to a better response with 20-Hz rTMS. There were also many of the hypothesized regional correlates of relative hyperperfusion to degree of antidepressant response to 1-Hz rTMS.
However, the magnitude of the clinical change and the ability to sustain or increase therapeutic effects with continuation of the best high or low frequency for a given patient (at least at 100% of MT) was not as clinically robust as one might have hoped. How baseline brain activity may predict differential response to rTMS will require much larger studies of more homogeneous groups of patients before there can be clinical applications of these findings. Nevertheless, this study does add the rTMS frequency dimension within different patients to the many other parameters that have been linked to more successful acute rTMS outcome, such as area, focality, intensity, and numbers of stimulations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Burt T, Lisanby SH, Sackeim HA. Neuropsychiatric applications of transcranial magnetic stimulation: a meta analysis, Int. J. Neuropsychopharmacol. 2002;5:73–103. doi: 10.1017/S1461145702002791. [DOI] [PubMed] [Google Scholar]
- Cohen RM, Semple WE, Gross M, Holcomb HH, Dowling MS, Nordahl TE. Functional localization of sustained attention: Comparison to sensory stimulation in the absence of instruction. Neuropsychiatry Neuropsychol. Behav. Neurol. 1988;1:3–20. [Google Scholar]
- Conca A, Di Pauli J, Beraus W, Hausmann A, Peschina W, Schneider H, Konig P, Hinterhuber H. Combining high and low frequencies in rTMS antidepressive treatment: preliminary results. Hum. Psychopharmacol. 2002;17:353–356. doi: 10.1002/hup.422. [DOI] [PubMed] [Google Scholar]
- Dunn RT, Willis MW, Benson BE, Repella JD, Kimbrell TA, Ketter TA, Speer AM, Osuch EA, Post RM. Preliminary findings of uncoupling of flow and metabolism in unipolar compared with bipolar affective illness and normal controls. Psychiatry Res. 2005;140:181–198. doi: 10.1016/j.pscychresns.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Benitez J, De Castella A, Daskalakis ZJ, Brown TL, Kulkarni J. A randomized, controlled trial of sequential bilateral repetitive transcranial magnetic stimulation for treatment-resistant depression. Am. J. Psychiatry. 2006;163:88–94. doi: 10.1176/appi.ajp.163.1.88. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Comparing functional (PET) images: the assessment of significant change. J. Cereb. Blood Flow Metab. 1991a;11:690–699. doi: 10.1038/jcbfm.1991.122. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activation using their spatial extent. Human Brain Mapping. 1994;1:210–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Garcia-Toro M, Salva J, Daumal J, Andres J, Romera M, Lafau O, Echevarria M, Mestre M, Bosch C, Collado C, Ibarra O, Agu`irre I. High (20-Hz) and low (1-Hz) frequency transcranial magnetic stimulation as adjuvant treatment in medication-resistant depression. Psychiatry Res. 2006;146:53–57. doi: 10.1016/j.pscychresns.2004.08.005. [DOI] [PubMed] [Google Scholar]
- George MS, Nahas Z, Molloy M, Speer AM, Oliver NC, Li XB, Arana GW, Risch SC, Ballenger JC. A controlled trial of daily left prefrontal cortex TMS for treating depression. Biol. Psychiatry. 2000;48:962–970. doi: 10.1016/s0006-3223(00)01048-9. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;12:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppner J, Schulz M, Irmisch G, Mau R, Schlafke D, Richter J. Antidepressant efficacy of two different rTMS procedures High frequency over left versus low frequency over right prefrontal cortex compared with sham stimulation. Eur. Arch. Psychiatry Clin. Neurosci. 2003;253:103–109. doi: 10.1007/s00406-003-0416-7. [DOI] [PubMed] [Google Scholar]
- Jin Y, Potkin SG, Kemp AS, Huerta ST, Alva G, Thai TM, Carreon D, Bunney WE., Jr Therapeutic effects of individualized alpha frequency transcranial magnetic stimulation (aTMS) on the negative symptoms of schizophrenia. Schizophr. Bull. 2006;32:556–561. doi: 10.1093/schbul/sbj020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketter TA, George MS, Kimbrell TA, Stein RM, Willis MW, Little JT, Frye MA, Cora-Locatelli G, Benson BE, Herscovitch P, Post RM. Assessment of PET data in individual patients with mood disorders. ACNP Abstracts. 1995:230. [Google Scholar]
- Ketter TA, Kimbrell TA, George MS, Dunn RT, Speer AM, Benson BE, Willis MW, Danielson A, Frye MA, Herscovitch P, Post RM. Effects of mood and subtype on cerebral glucose metabolism in treatment-resistant bipolar disorder. Biol. Psychiatry. 2001;49:97–109. doi: 10.1016/s0006-3223(00)00975-6. [DOI] [PubMed] [Google Scholar]
- Ketter TA, Wang PW. Predictors of treatment response in bipolar disorders: evidence from clinical and brain imaging studies. J. Clin. Psychiatry. 2002b;63 Suppl. 3:21–25. [PubMed] [Google Scholar]
- Kimbrell TA, Little JT, Dunn RT, Frye MA, Greenberg BD, Wassermann EM, Repella JD, Danielson AL, Willis MW, Benson BE, Speer AM, Osuch E, George MS, Post RM. Frequency dependence of antidepressant response to left prefrontal repetitive transcranial magnetic stimulation (rTMS) as a function of baseline cerebral glucose metabolism. Biol. Psychiatry. 1999;46:1603–1613. doi: 10.1016/s0006-3223(99)00195-x. [DOI] [PubMed] [Google Scholar]
- Kimbrell TA, Dunn RT, George MS, Danielson AL, Willis MW, Repella JD, Benson BE, Herscovitch P, Post RM, Wassermann EM. Left prefrontal-repetitive transcranial magnetic stimulation (rTMS) and regional cerebral glucose metabolism in normal volunteers. Psychiatry Res. Neuroimaging. 2002a;115:101–113. doi: 10.1016/s0925-4927(02)00041-0. [DOI] [PubMed] [Google Scholar]
- Kozel FA, George MS. Meta-analysis of left prefrontal repetitive transcranial magnetic stimulation (rTMS) to treat depression. J. Psychiatr. Pract. 2002;8:270–275. doi: 10.1097/00131746-200209000-00003. [DOI] [PubMed] [Google Scholar]
- Loo CK, Mitchell PB, Croker VM, Malhi GS, Wen W, Gandevia SC, Sachdev PS. Double-blind controlled investigation of bilateral prefrontal transcranial magnetic stimulation for the treatment of resistant major depression. Psychol. Med. 2003a;33:33–40. doi: 10.1017/s0033291702006839. [DOI] [PubMed] [Google Scholar]
- Loo CK, Sachdev PS, Haindl W, Wen W, Mitchell PB, Croker VM, Malhi GS. High (15 Hz) and low (1 Hz) frequency transcranial magnetic stimulation have different acute effects on regional cerebral blood flow in depressed patients. Psychol. Med. 2003b;33:997–1006. doi: 10.1017/s0033291703007955. [DOI] [PubMed] [Google Scholar]
- Martin JL, Barbanoj MJ, Schlaepfer TE, Thompson E, Perez V, Kulisevsky J. Repetitive transcranial magnetic stimulation for the treatment of depression. Systematic review and meta-analysis. Br. J. Psychiatry. 2003;182:480–491. doi: 10.1192/bjp.182.6.480. [DOI] [PubMed] [Google Scholar]
- Mosimann UP, Schmitt W, Greenberg BD, Kosel M, Muri RM, Berkhoff M, Hess CW, Nahas Z, Kozel FA, Li X, Anderson B, George MS. Left prefrontal transcranial magnetic stimulation (TMS) treatment of depression in bipolar affective disorder: a pilot study of acute safety and efficacy. Bipolar Disord. 2003;5:40–47. doi: 10.1034/j.1399-5618.2003.00011.x. [DOI] [PubMed] [Google Scholar]
- O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, McDonald WM, O’Reardon D, Fitzgerald PB, Loo C, Fitzgerald PB, Demitrack MA, George MS, Sackeim HA, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol. Psychiatry. 2007;62:1208–1216. doi: 10.1016/j.biopsych.2007.01.018. 2007. [DOI] [PubMed] [Google Scholar]
- Padberg F, Zwanzger P, Keck ME, Kathmann N, Mikhaiel P, Ella R, Rupprecht P, Thoma H, Hampel H, Toschi N, Moller HJ. Repetitive transcranial magnetic stimulation (rTMS) in major depression: relation between efficacy and stimulation intensity. Neuropsychopharmacology. 2002;27:638–645. doi: 10.1016/S0893-133X(02)00338-X. [DOI] [PubMed] [Google Scholar]
- Post RM, Speer AM. Repetitive Transcranial Magnetic Stimulation and Related Somatic Therapies: Prospects for the Future. In: George MS, Belmaker RH, editors. Transcranial Magnetic Stimulation in Clinical Psychiatry. Washington, DC: American Psychiatric Publishing, Inc; 2007. pp. 225–255. [Google Scholar]
- Poulet E, Brunelin J, Boeuve C, Lerond J, D’Amato T, Dalery J, Saoud M. Repetitive transcranial magnetic stimulation does not potentiate antidepressant treatment. Eur. Psychiatry. 2004;19:382–383. doi: 10.1016/j.eurpsy.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Rossini D, Lucca A, Zanardi R, Magri L, Smeraldi E. Transcranial magnetic stimulation in treatment-resistant depressed patients: A double-blind, placebo-controlled trial. Psychiatry Res. 2005;137:1–10. doi: 10.1016/j.psychres.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Speer AM, Kimbrell TA, Wassermann EM, Repella D, Willis MW, Herscovitch P, Post RM. Opposite effects of high and low frequency rTMS on regional brain activity in depressed patients. Biol. Psychiatry. 2000;48:1133–1141. doi: 10.1016/s0006-3223(00)01065-9. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain: Three-Dimensional Proportional System: An Approach to Cerebral Imaging. New York: Thieme; 1988. [Google Scholar]
- Willis MW, Ketter TA, Kimbrell TA, Danielson AL, Benson BE, Little JT, Post RM. Differential changes in cerebral metabolism with age in mood disorder subtypes. Biol. Psychiatry. 1997;41:19S. [Google Scholar]