Abstract
The brain contains a number of distinct regions that share expression of dopamine (DA) and its requisite biosynthetic machinery, but otherwise encompass a diverse array of features and functions. Across the vertebrate family, the olfactory bulb (OB) contains the major DA system in the forebrain. OB DA cells are primarily periglomerular interneurons that define the glomerular structures in which they receive innervation from olfactory receptor neurons as well as mitral and tufted cells, the primary OB output neurons. The OB DA cells are necessary for both discrimination and the dynamic range over which odorant sensory information can be detected. In the embryo, OB DA neurons are derived from the ventricular area of the evaginating telencephalon, the dorsal lateral ganglionic eminence, and the septum. However, most OB DA interneurons are generated post-natally and continue to be produced throughout adult life from neural stem cells in the subventricular zone of the lateral ventricle and rostral migratory stream. Adult born OB DA neurons are capable of integrating into existing circuits and do not appear to degenerate in Parkinson’s disease. Several genes have been identified that regulate the differentiation of OB DA interneurons from neural stem cells. These include transcription factors that modify the expression of tyrosine hydroxylase, the first enzyme in the DA biosynthetic pathway and a reliable marker of the DA phenotype. Elucidation of the molecular genetic pathways of OB DA differentiation may advance the development of strategies to treat neurological disease.
Index: AP-1; Aromatic amino acid decarboxylase (AADC); Beta-galactosidase (LacZ); Cortex; CREB; D2 receptor; Dlx; Dopamine (DA); Dopamine receptor; Dorsal lateral ganglionic eminence (dLGE); Er81; FosB; Gamma amino butyric acid (GABA); Glomerulus; Glutamic acid decarboxylase (GAD); Glutamatergic; Granule cell; Green fluorescent protein (GFP); Gsh2; Immediate early gene (IEG); Meis2; Migration; Mitral cells; Neuroblast; Neurogenesis; Nurr1; NGFI-B; Odor deprivation; Olfactory bulb (OB); Olfactory receptor neuron; Pax6; Periglomerular neuron Post-natal neurogenesis; Rostral migratory stream (RMS); Septum; Stem cell; Striatum; Subventricular zone (SVZ); Telencephalon; Transit amplifying cell; Tufted cell; Tyrosine hydroxylase (TH); Zic 1,3
Introduction
The dopaminergic (DA) neuronal systems of the brain exhibit substantial diversity. All DA neurons express the requisite enzymes for dopamine biosynthesis, but there are regional differences in the morphology and co-expression of other neuroactive substances, as well as the capacity for regeneration and the susceptibility to neurodegenerative diseases. For example, substantia nigra DA neurons co-express glutamate and CCK, and have long projections into the striatum that are essential for control of movement. These midbrain DA neurons also selectively degenerate in Parkinson’s Disease (PD).1 By contrast, olfactory bulb (OB) DA neurons co-express GABA, and have short axonal projections that remain within the main OB that are necessary for processing of odorant sensory information from olfactory receptor neurons.2–4 Furthermore, the OB DA neurons are continuously generated through out the lifespan of the adult5–7 and do not degenerate in PD.8 The molecular and genetic mechanisms responsible for the common DA phenotype (that is, the production of dopamine) as well as the wide variety of associated features remain an area of intensive study.
This chapter will focus primarily on the anatomical, molecular genetic and physiological characteristics of the OB DA neurons. These neurons are the major endogenous DA-producing system in the forebrain.9, 10 The OB DA neurons are a subgroup of a diverse population of interneurons in the OB that have been intensively studied in an effort to understand the mechanisms regulating neurogenesis and the generation of neuronal diversity.11–13 The OB DA neurons are an integral component of circuitry that serves as a powerful model for neural network learning, memory consolidation and behavioral plasticity.14–16 Much of the information presented in this chapter is derived from studies with the rodent OB (specifically, the mouse and rat), but a growing number of studies have revealed that the data derived from rodent studies extend into primates, including humans.17
Anatomy and function of OB DA neurons
In some vertebrate species, including monkeys and humans, DA-producing cells are found in forebrain regions such as the striatum.18, 19 However, the main OB contains the major forebrain DA system common to all vertebrates.20 Thus, this chapter will focus primarily on the OB DA neurons.
Approximately 5% of neurons in the main OB are DA interneurons. They show a distinct laminar distribution that is limited primarily to the glomerular layer.21 Most OB DA cells are small, periglomerular (PG) interneurons (about 5–10 μm in diameter), although some are larger external tufted cells (about 10–15 μm in diameter; Fig. 1).2, 20, 22 Several studies indicate that 10%–16% of all PG neurons are DA cells.23–25 Glomeruli are distinctive spheroid neuropil structures (50–150 μm in diameter in rodents) that are defined by a layer of PG and glial cells.20 These structures serve as the initial processing center of sensory information from the olfactory receptor neurons. The neuropil within the glomeruli is composed of the axon terminals from the olfactory receptor neurons, the apical dendrites from mitral and tufted (M/T) projection neurons, dendritic processes from OB juxtaglomerular neurons (including the DA cells), and terminals from centrifugal innervation of both basal forebrain cholinergic neurons and dorsal raphe cell serotoninergic projections.26–28
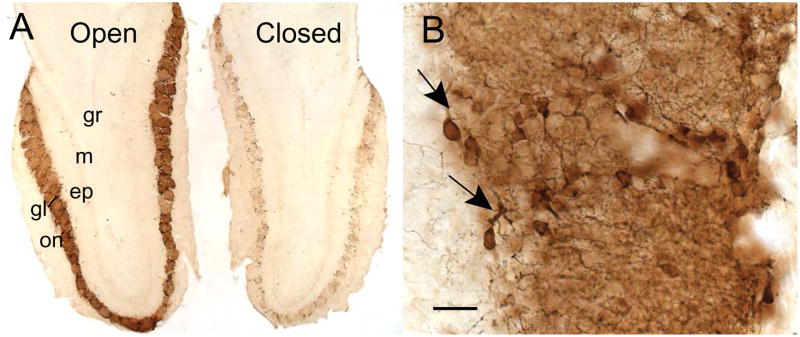
Figure 1.
Laminar distribution of TH-immunoreactive dopamine neurons in a horizontal section of olfactory bulbs taken from an adult mouse with unilateral naris closure. (A) A low magnification image shows the normal distribution of PG DA neurons in the glomerular layer of the OB contralateral to naris closure (open). The OB ipsilateral to the closure (closed) displays a drastic reduction in the number of TH-immunoreactive cells and processes. (B) A higher magnification micrograph illustrates the processes (arrows) of the PG DA neurons entering the glomeruli. Bar = 200 μm in A, and 20 μm in B. Abbreviations: epl, external plexiform layer; gl, glomerular layer; gr, granule cell layer; m, mitral cell layer; on, olfactory nerve layer.
Within the glomeruli, OB DA interneurons receive region-specific axo-dendritic innervation from the axon terminals of the olfactory receptor nerve fibers, and make dendro-dendritic contacts with the apical dendrites of OB M/T cells (Fig. 2).29, 30 These synaptic connections are distinct from other groups of PG interneurons. For example, both calretinin- and calbindin-expressing interneurons, which do not co-express DA, only make dendro-dendritic contacts with the M/T cells within the OB glomeruli.23, 31, 32 This heterogeneity in synaptic organization within the glomeruli suggests that OB DA interneurons have a function in the processing of olfactory sensory information distinct from the other sub-groups of OB interneurons.
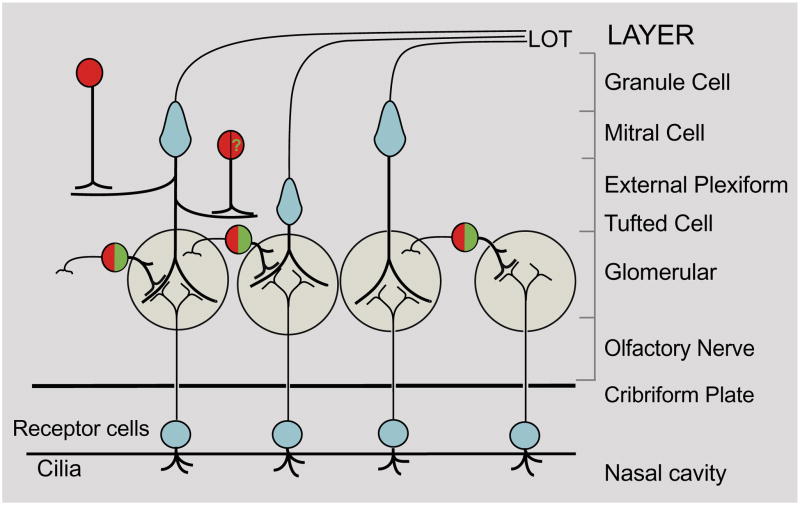
Figure 2.
Schematic representation of selected synaptic connections in the olfactory system. Axons from olfactory receptor neurons (small blue circles) make axo-dendritic synapses with apical dendrites of the mitral cells (large blue cells), tufted cells (small blue cell), and processes of PG cells, including the DA neurons (green). Axons from glutamatergic mitral/tufted cells are the primary output neurons of the OB through the lateral olfactory tract (LOT). DA interneurons are stimulated by both olfactory receptor neurons and mitral/tufted neurons. Mitral/tufted neurons also make dendro-dendritic synapses with granule cell interneurons. Both DA PG and granule cells express GABA (red). A population of granule cells in the mitral cell layer (red cell with green “?”) express GABA and TH mRNA, but not TH protein.
Across all vertebrate species, OB DA neurons are readily identified by the expression of tyrosine hydroxylase (TH), the first enzyme in the DA biosynthetic pathway.33, 34 The OB does not contain noradrenergic (NE)-producing neurons, but centrifugal NE afferents from the locus ceruleus to the OB also express TH.2, 35 However, TH expression in the NE terminations is very low and does not complicate analysis of OB DA neuronal function.2
In contrast to TH, expression of aromatic amino acid decarboxylase (AADC), the second and last enzyme in the DA biosynthetic pathway, exhibits cross species variation. For example, AADC is readily observable in the rat OB, but it is detectable at only low levels in the mouse OB.36 Other markers of functional DA cells, such as dopamine transporter (DAT) and both D1 and D2 DA receptors, are expressed at either low or variable levels.37–40 Thus, TH expression is considered the most reliable marker of OB DA neurons.
TH expression in OB PG interneurons is dependent on afferent synaptic activity in the olfactory receptor neurons.2, 21, 22, 41 Both TH mRNA and protein expression are dramatically down-regulated in the OB by perturbations that compromise either odorant access to the olfactory epithelium or cyclic nucleotide-gated channel function in the olfactory receptor cells (Fig. 1).22, 42 Studies in which animals were subjected to odor deprivation by either naris occlusion, chemical or surgical deafferentation have shown that the loss of TH is concomitant with a loss of detectable DA2, 43 as well as a dramatic increase in D2 receptor expression.44 As discussed below, the activity dependence of TH expression and DA production is likely critical for both odorant identification and detection of odorant intensity.
Almost all OB DA interneurons also co-express GABA.23, 31, 45 GABA, the major inhibitory neurotransmitter in brain, is found in about 55% of the interneurons in the glomerular layer and almost all interneurons in the granule cell layer.23, 25, 31 OB GABAergic interneurons are typically sub-divided by the co-expression of other neuroactive substances such as DA, calbindin, calretinin and CCK.31, 46 Activation of DA receptors are reported to modulate the response of GABA receptors within the same cell.47 These results suggest that the co-release of DA with GABA may modify the response of both the olfactory receptor neurons and M/T cells to the inhibitory effects of GABA.
OB DA interneurons are a necessary element in the processing of afferent sensory information from the olfactory epithelium (Fig. 3). Within the glomeruli, the axons of glutamatergic olfactory receptor neurons provide excitatory input to both M/T and PG neurons, including DA cells.48–50 Glutamate released from M/T cells is also excitatory on DA neurons and other PG cells.51 Stimulation of PG GABAergic interneurons results in the release of GABA which inhibits both olfactory receptor and M/T neurons 52–55 as well as other PG neurons.56, 57 OB DA interneurons also release dopamine that acts pre-synaptically on D2 receptors to modulate the release of glutamate from olfactory receptor neurons.51, 58, 59 Although somewhat controversial, several studies have reported that M/T neurons are also pre-synaptically inhibited by DA through D2 receptors.60–62 Together, GABA and DA modify the output of sensory information from the OB by directly modulating the excitation of both the olfactory receptor and M/T neurons.
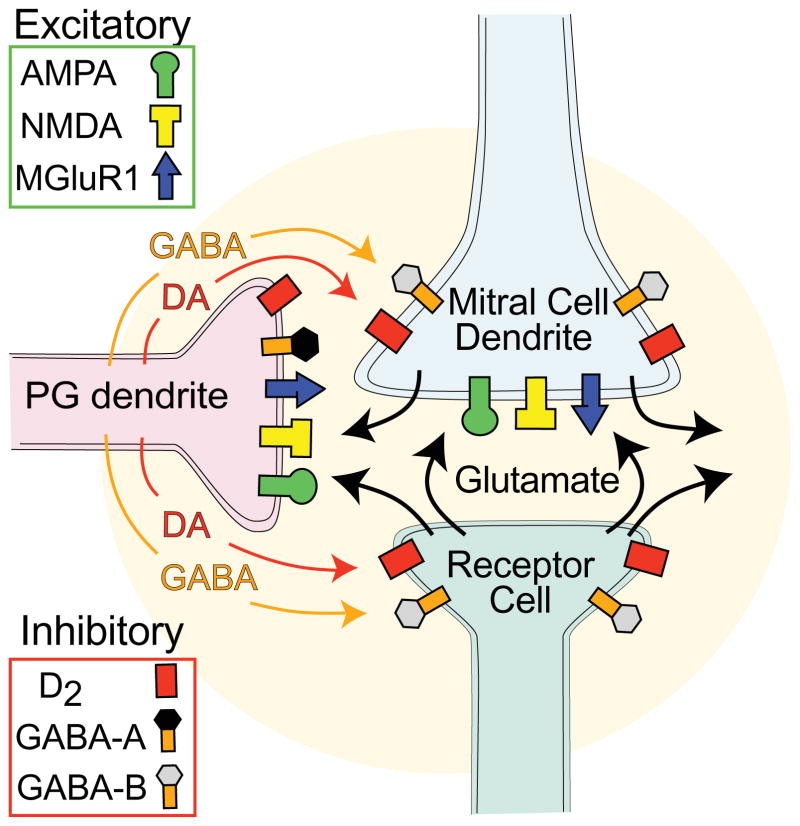
Figure 3.
Schematic representation of selected neurotransmitters and their cognate receptors in OB glomeruli. Olfactory receptor axon terminals release glutamate that excite mitral/tufted and PG cells through AMPA, NMDA and mGluR1 receptors. PG DA neurons release both DA and GABA that inhibit both olfactory receptor and mitral/tufted neurons through D2 and GABA-B receptors. GABA can also inhibit PG neurons through GABA-A receptors.
The activity dependent expression of TH suggests that DA is essential for the regulation of odorant information processing in response to either high or low levels of afferent odor-induced synaptic activity. When odorant access to the OE is prevented by naris occlusion, the M/T cell responses to odor stimulation show enhanced sensitivity.3, 4 The finding that expression of the isoforms of the GABA biosynthetic enzyme, glutamic acid decarboxylase, are not activity dependent suggests that this enhanced M/T cell sensitivity is likely the result of diminished DA-mediated inhibition.63, 64 Furthermore, on restoration of sensory input following prolonged odorant sensory deprivation, M/T neurons show impaired discrimination of individual odorants.4 Thus, the OB DA system is critical for both discrimination and the dynamic range over which odorant sensory information can be relayed from olfactory receptor neurons to other brain regions.
OB DA neurogenesis
Embryonically (E)-derived OB interneurons are predominantly generated from progenitor cells located in the subventricular zone (SVZ) of the dorsal lateral ganglionic eminence (dLGE) beginning at about E14 in the mouse (Fig. 4).65 The dLGE is a proliferative zone in the developing telencephalon that is defined by the expression of transcription factor proteins such as Pax6, Gsh2, Er81, and Dlx-1,2,5,6.66, 67 Immature OB interneurons tangentially migrate from the dLGE to the developing OB and then radially migrate to their final glomerular or granule cell layer positions.7, 68, 69
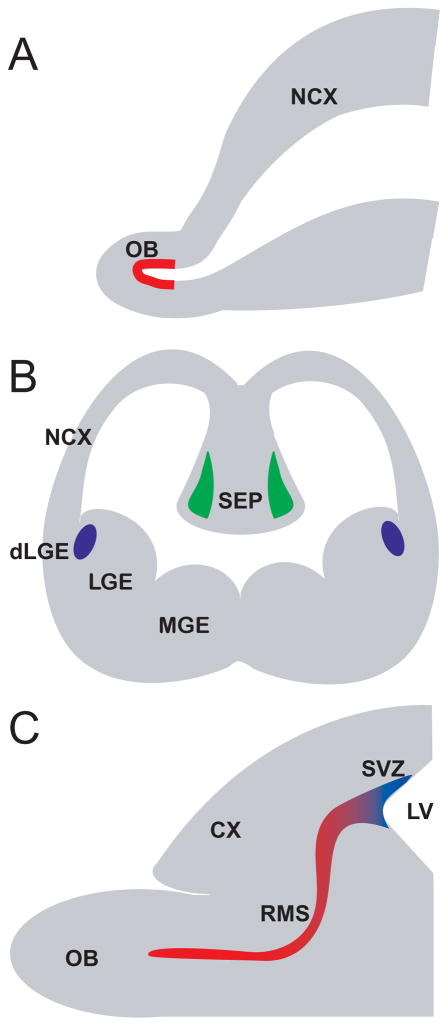
Figure 4.
Schematic representation of embryonic and post-natal origins of OB DA neurons. (A) The earliest reported origin of DA cells are derived from stem cells in the evaginating telencephalon (red layer), at approximately E13.5 in the mouse. (B) Mid-embryonically derived OB DA neurons originate from the dorsal lateral ganglionic eminence (blue regions) and the medial septum (green regions), at about E16.5 in the mouse. (C) Post-natally and adult derived OB DA neurons are generated from progenitors in the subventricular zone of the lateral ventricle. These progenitors migrate to the OB through the rostral migratory stream, which is also a putative source for some OB DA neurons. Abbreviations: CX, cortex; dLGE, dorsal lateral ganglionic eminence; LGE, lateral ganglionic eminence; MGE, medial ganglionic eminence; NCX, neocortex; OB, olfactory bulb; RMS, rostral migratory stream; SVZ, subventricular zone.
Although the dLGE is considered the primary source of embryonic OB DA interneurons, additional sites of origin have been proposed. A recent study suggested that precursor cells localized to the ventricular layer of the evaginating telencephalon may also contribute neurons to the embryonic OB, including DA interneurons (Fig. 4).70 These OB neural stem cells have molecular features distinct from OB progenitors originating from the dLGE. A second alternative embryonic origin may be the medial septum (Fig. 4).71 Neural progenitors in the medial septum also have molecular features distinct from the dLGE, including the expression of the Zic1 and Zic3 transcription factor proteins. The consequences of these alternative embryonic origins and their distinct molecular features are not clear, but the OB DA neurons derived form these alternative origins may have functional properties that differ from those cells of the dLGE lineage.
Although the generation of OB DA interneurons is initiated during mid-embryonic development, the majority of these interneurons are born during late embryonic and neonatal time periods.65, 72, 73 These late-embryonic and post-natal neurons are generated in the rostral migratory stream (RMS) and subventricular zone (SVZ) of the lateral ventricle, which is believed to be, in part, a remnant of the embryonic LGE. Nearly all of the transcription factors that define the embryonic dLGE are also expressed in the post-natal SVZ.66, 67, 74–79 In the mouse, neurogenesis of OB interneurons peaks between E18 and post-natal (P) day 5.65 These late embryonic and post-natally generated neurons migrate tangentially through the RMS before moving radially to their final positions in the granule and glomerular layers of the OB. Although their proliferation rate decreases after P5, neurogenesis of OB interneurons, including DA cells, continues throughout the lifetime of the adult, including humans.17, 80
The predominant hypothesis is that late-embryonic and post-natally generated OB interneurons, including DA cells, are derived from slowly dividing neural stem cells located in SVZ and RMS (for a comprehensive review, see refs. 5, 81–84). These neural stem cells have several features attributed to astrocytes, such as the expression of GFAP, but they can also be cultured in the presence of EGF to generate both neurons and glia. In both the RMS and SVZ, these slowly dividing stem cells produce transit amplifying cells that express markers such as NG2 and Olig2. The transit amplifying cells give rise to migrating neuroblasts, which can be identified by the expression of such genes as PSA-NCAM, doublecortin and neuron-specific type III-tubulin (TuJ1). These neuroblasts (precursor cells) tangentially migrate through the SVZ and RMS in chains that are enclosed in tubes formed by transit amplifying cells, slowly dividing neural stem cells and glia. It has been estimated that approximately 30,000 per day progenitors enter the adult OB, but only a small percentage of these cells mature and differentiate into functional OB neurons.
There is a growing consensus that progenitors for specific OB interneuron subtypes, such as DA cells, are generated in distinct regions within the SVZ and RMS. Consistent with this idea is the observation that the location of stem cells in the SVZ and RMS reflect different embryonic origins.85 For example, the majority of cells lining the lateral ventricle are derived from the Gsh2 expressing regions in the LGE, whereas the ventral and dorsal regions of the SVZ contain stem cells derived from the Nkx2.1 expressing region of the MGE and the Emx1 expressing regions of the embryonic cortex, respectively. There is a general agreement that the majority of post-natally derived OB DA neurons are generated from the more dorsal neurogenic regions in the SVZ.85, 86 However, there is controversy as to whether there is a preferential rostral-caudal origin of OB DA cells. Some studies have suggested that PG interneurons, including DA neurons, are preferentially derived from stem cells in the RMS.16 By contrast, other studies have suggested that OB DA cells are generated from stem cells within a long neurogenic region that includes the dorsal SVZ and subcallosal zone.86 Generation of neuronal diversity may also show temporal-dependence, suggesting that distinct PG interneuron sub-types are produced during different developmental windows.24 Current controversies surrounding the spatial and temporal origins of OB neurons may result from the use of different experimental techniques, but there is a clear consensus that stem cells within the neurogenic SVZ and RMS regions are not a homogenous population.
Identification of the specific origin for DA progenitors in both the embryonic and post-natal animal is also complicated by the fact that there are no known markers specific to DA cells prior to terminal differentiation. Both requisite biosynthetic enzyme proteins for DA production, TH and AADC, are expressed only in the differentiated neurons in the glomerular layer and not in migrating immature DA precursor cells.36 The transcription factor Pax6, Er81, Meis2 and Dlx-1,2,5,6 proteins are co-expressed in both the immature and terminally differentiated neurons.16, 66, 75, 77, 87–92 However, these transcription factor proteins are expressed in other OB interneuron sub-types that do not express TH and, thus, these proteins do not specifically label DA precursor cells.
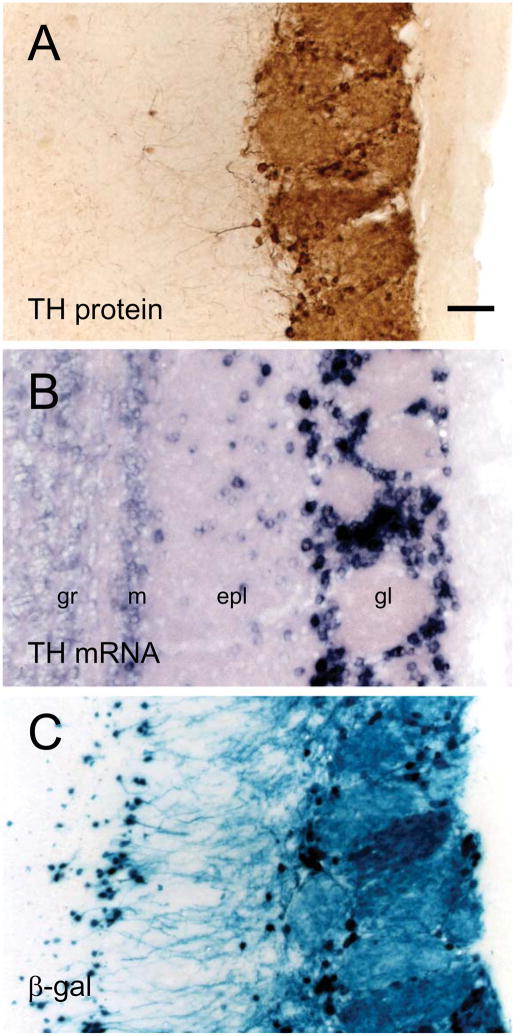
Although TH protein is expressed only in the glomerular layer (Fig. 5A), several studies have shown that the upstream gene regulatory region of TH is transcriptionally active in areas outside of the glomerular layer. TH mRNA is expressed in the superficial granule cell layer, even though TH protein is not detectable in this layer (Fig. 5B).88, 93 Transgenic mice containing either GFP or LacZ reporter genes under the control of either 9kb or 4.5kb of the TH upstream gene regulatory region also exhibited transgene expression in the superficial granule cell layer as well as in the RMS (Fig. 5C).93–95 Together, these studies suggest that there are spatially dependent translational regulatory mechanisms that limit the expression of TH protein, and consequently DA biosynthesis, to the OB glomerular layer. It is possible that the cells in the superficial granule cell layer which contain TH promoter activity, but lack TH protein, are immature DA neurons. However, these cells do not appear to migrate and express NeuN, a marker of terminally differentiated neurons.88
Figure 5.
Patterns of both TH protein and TH mRNA expression as well as TH/LacZ reporter gene activity in the adult OB. (A) TH protein immunoreactivity is restricted to the glomerular layer. (B) High level expression of TH mRNA is seen both in the glomerular layer and in cells scattered in the external plexiform layer. Lightly-labeled cells are found in the mitral and superficial granule cell layers. (C) An X-gal stained section reveals expression of the LacZ reporter gene under the control of the 9kb upstream TH gene regulatory region. X-gal activity can be detected in the same layers as the TH mRNA. Bar = 50 μm. Abbreviations: epl, external plexiform layer; gl, glomerular layer; gr, superficial granule cell layer; m, mitral cell layer.
Although the OB is the major DA system in the forebrain, there is TH gene activity in other regions. In mice, TH mRNA and reporter gene expression under the control of the TH promoter has been detected in both the cortex and striatum.96 The human and primate striata contain a small number of projection neurons that express TH protein.18, 19 Although the origin of these cells with TH gene activity is not presently known, it is interesting to note that some cortical interneurons and striatal projection neurons are also derived from the LGE.97, 98 The functional role of these non-OB neurons with either TH mRNA and/or TH protein in the forebrain remains to be determined.
Molecular genetic mechanisms of OB DA neuron differentiation
The underlying molecular genetic pathway of midbrain DA neuron differentiation is well established. Briefly, midbrain DA neurons originate in the ventral mes-diencephalic neuroepithelium where Sonic hedgehog (Shh) and FGF8 signaling pathways cooperatively interact. This interaction between Shh and FGF8 initiates expression of the transcription factors Otx1, Nkx2.2 and Sox2 in neuroblasts. Midbrain neural progenitor cells develop from these neuroblasts and express transcription factors such as Lmx1a, Msx1 and Ngn2. The committed midbrain DA neuronal precursor cells express AADC and the transcription factors Lmx1b, and En1. Subsequently, terminal differentiation of midbrain DA neurons occurs with the expression of genes such as TH, VMAT2, DAT and the transcription factors Nurr1 and Pitx3.
By contrast, the molecular genetic pathways that regulate OB DA differentiation are not well defined. A significant challenge associated with defining the molecular genetic pathways necessary for OB DA neurons is that there is no single spatial and temporal origin specific to these neurons. As discussed above, there is evidence for multiple embryonic origins of these neurons, and the origin of post-natally derived neurons within either the RMS or SVZ is ambiguous. Furthermore, it is not clear whether these various origins have either the same, partially overlapping or unique molecular genetic pathways for differentiation of OB DA neurons.
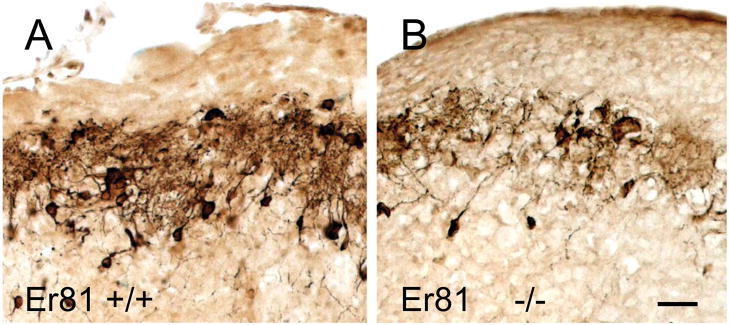
Despite the ambiguity surrounding their spatial and temporal origins, several genes involved in the differentiation of OB DA neurons have been identified and are summarized in Tables 1–3. One such gene, Er81, is expressed in both the embryonic dLGE and post-natal SVZ, RMS and OB.66, 77 Almost all OB TH immunoreactive cells also contain Er81, and TH expression is drastically reduced in Er81 deficient mice (Fig. 6). Like TH, Er81 expression levels are also dependent on afferent synaptic activity of olfactory receptor neurons.77 However, Er81 is not specific for DA differentiation since it also expressed in some OB interneurons that do not contain TH, such as calretinin containing neurons.90
Table 1.
| Potential Key Regulators of OB DA Interneuron Differentiation | |
|---|---|
| AP-1 | Heterodimer formed by members of the Fos and Jun basic-leucine zipper transcription factors; several Fos and Jun family members are expressed in the OB and the expression of some members is dependent on olfactory neuron afferent synaptic activity (Liu 1999); mutation of AP-1 binding site in TH proximal promoter eliminates reporter gene expression in the OB of transgenic mice;94 OB expression of FosB and JunD is activity dependent and both proteins in OB cell lysates bind an AP-1 binding site in the TH promoter.117 |
| CREB | Basic-leucine zipper transcription factor; CREB is expressed in the post-natal OB granule and glomerular layers;117 mutation of an evolutionarily conserved CRE binding site in TH proximal promoter eliminates reporter gene expression in the OB of transgenic mice.116 |
| Dlx-family | Homeodomain transcription factors; Dlx1,2,5,6 are expressed in the LGE and post-natal SVZ, RMS and OB; 66, 74–76, 93, 142–144 Dlx2 is expressed in transit amplifying cells and migrating neuroblasts within the SVZ and RMS;91 mice lacking Dlx1 or Dlx2 have a modest or strong reduction of OB TH+ cells, respectively, whereas mice lacking both Dlx1 and Dlx2 have a near total loss of OB TH+ cells;145, 146 mice lacking Dlx5 have a strong reduction in the number of OB TH+ cells;75 TH+ cells in the OB overlap with reporter gene expression driven by a Dlx5/6 gene regulatory fragment.90, 92 |
| Er81 | ETS-DNA binding domain transcription factor is expressed in the dLGE, SVZ, RMS and OB;66, 77 Er81 is co-expressed with TH in periglomerular interneurons;77 Er81 expression in the OB is activity dependent;77 there is a major loss of TH+ cells in homozygous Er81 mutant mice (Cave and Baker, unpublished). |
| Gsh2 | Homeodomain transcription factor protein; expressed in the embryonic LGE and post- natal SVZ, RMS and OB67, 79, 147; mice lacking Gsh2 have a large loss of OB TH+ cells. |
| Meis2 | TALE homeodomain protein; Meis2 is expressed in the embryonic LGE as well as the post-natal SVZ, RMS and OB;90 Meis2 is co-expressed in OB TH+ cells.90 |
| Nurr1/NGFI-B | Homologous orphan nuclear receptor transcription factors; both proteins are expressed in the superficial granule cell and glomerular layers of OB in a synaptic activity-dependent manner;117 TH proximal promoter contains a functional Nurr1 binding response element.110, 111 |
| Pax6 | Paired box and homeodomain transcription factor; Pax6 is expressed in the embryonic dLGE and post-natal SVZ, RMS and OB;78, 87–89 nearly all TH+ cells in OB also co- express Pax6 and there is an almost total loss of OB TH+ cell in mice heterozygous for Pax6 Sey mutation.87 |
| Zic1,3 | Zinc finger transcription factors; Zic1,3 are expressed in the embryonic septum as well as the glomerular and granule cell layers of the post-natal OB;71 mice lacking both Zic1 and Zic3 have a significant loss of OB interneurons, including TH+ cells.71 |
Table 3.
| Regulators of OB DA Differentiation Through Olfactory Neuron Innervation of the OB | |
|---|---|
| Arx | Homeodomain transcription factor; Arx is expressed in the embryonic LGE as well as the post-natal SVZ, RMS and OB;128 mice lacking Arx have a loss of olfactory neuron innervation of the OB and a substantial decrease of TH+ cells in the OB.128, 184 |
| Dlx5 | Homeodomain transcription factor; Dlx5 is expressed in the LGE, SVZ, OB as well as olfactory epithelium and olfactory placode;185 in mice lacking Dlx5, olfactory receptor neurons fail to properly innervate the OB and there is a strong reduction in the number of OB TH+ cells.185 |
| CNG2 | Transmembrane cyclic AMP gated channel; CNG2 (OCNC1) is expressed in the olfactory epithelium and is required for signal transduction in olfactory receptor neurons; loss of CNG2 results in abnormal pruning of olfactory receptor neuron fibers, as well as a block of afferent olfactory receptor neuron synaptic activity in the OB which dramatically reduces TH expression.42 |
| Zic1,3 | Zinc finger transcription factors; olfactory receptor neurons fail to properly innervate the OB in mice lacking both Zic1 and Zic3. 71 |
Figure 6.
TH immunoreactivity in wild-type and Er81-mutant mice. (A) TH immunoreactivity in the OB glomerular layer of a wild-type mouse. (B) Homozygous mutation of the Er81 gene drastically reduces TH immnoreactivity. Bar = 20 μm.
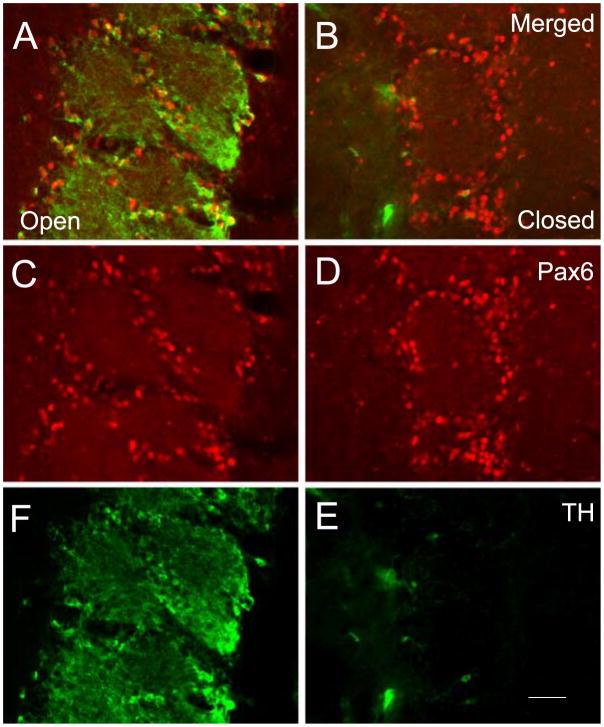
The transcription factor Pax6 is also critical for OB DA differentiation. The Pax6Sey mutation is embryonic lethal when homozygous, but heterozygous Pax6Sey mutant mice are viable and have an almost total loss of TH expression in the OB. In wild-type mice, nearly all OB DA cells co-express TH and Pax6.87 However, a significant fraction of Pax6 immunoreactive cells lack TH expression, suggesting that Pax6 is not specific to OB DA neurons. Also, Pax6 expression in the OB is not dependent on afferent synaptic activity of the olfactory receptor neurons (Fig. 7). The molecular genetic mechanism by which Pax6 regulates differentiation of OB DA neurons is unclear, in part, because the Pax6 gene encodes at least three different DNA-binding protein isoforms that each have a unique consensus target DNA binding sequence.99–103 The relevant Pax6 isoforms and the target genes of these isoforms necessary for OB DA differentiation have not been identified. Furthermore, Pax6 has been reported to influence neuronal progenitor migration and proliferation.104–107 These non-specific, general neurogenic functions of Pax6 complicate analysis of specific contributions to OB DA differentiation.
Figure 7.
Pax6 and TH expression in the glomerular layer of an adult mouse with unilateral naris closure. Expression of Pax6 and TH is red and green, respectively. As shown in (A, C and F), almost all neurons with perikaryal TH immunofluorescence also contain Pax6 in the OB contralateral to naris closure, although there are several cells with Pax6 that lack TH. As shown (B, D and E), Pax6 immunofluorescence in the OB ipsilateral to the naris closure is unchanged even though only a few cells express TH. Bar = 100 μm.
The immediate early gene (IEG) family is likely to be essential for mediating the synaptic activity-dependent expression of TH in OB DA precursor cells. The homologous IEG family members Nurr1 and NGFI-B are orphan nuclear receptor transcription factors, which are expressed in the OB in a synaptic activity-dependent manner. Nurr1, but not NGFI-B, is also expressed in the midbrain.108, 109 However, there is no evidence that either midbrain TH or Nurr1 expression is activity dependent. Nurr1 can also modulate TH gene expression through binding sites in the TH proximal promoter.110, 111 In Nurr1 deficient mice, TH expression is absent in the midbrain, but still present in the OB as a likely consequence of NGFI-B functional redundancy.108, 112, 113
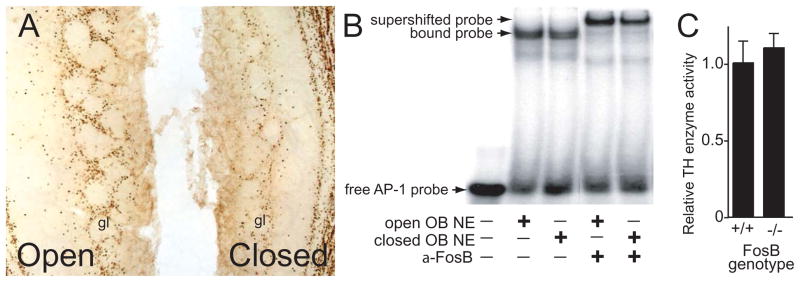
The TH proximal promoter also contains evolutionarily conserved binding sites for the IEG basic-leucine zipper (bZip) transcription factor proteins CREB and AP-1 (the latter is a heterodimer formed by members of the Fos and Jun protein families).114, 115 In vivo mouse studies have shown that mutation of either the AP-1 or CREB binding site in the TH proximal promoter can disrupt reporter gene expression under the control of the 9kb TH promoter in the OB.94, 116 However, there are several IEG bZip proteins expressed in the OB glomerular layer in a synaptic activity dependent manner that can bind these consensus sites.117 Thus, like Nurr1 and NGFI-B, there is likely to be redundancy in the regulation of TH expression by bZip IEGs. For example, expression of the bZip FosB protein in the OB glomerular layer is activity dependent and FosB can bind the AP-1 binding site, 117 but TH immunoreactivity and enzymatic activity are normal in mice lacking FosB (Fig. 8).
Figure 8.
Functional redundancy of FosB in the regulation of TH within the OB. (A) Immunohistochemistry in an adult mouse with unilateral naris closure reveals that FosB expression in the glomerular layer (gl) is dependent on olfactory neuron afferent synaptic activity (cf. open versus closed). (B) FosB antibody super-shift electromobility gel-shift assays reveal that FosB is present in OB nuclear extracts (OB NE) and can bind a probe containing the AP-1 binding site in the TH proximal gene promoter. The FosB supershift with OB NE ipsilateral to unilateral naris closure (closed) presumably results from residual FosB expression. (C) Relative TH enzyme activity in the OB is not significantly different in mice lacking FosB relative to wild-type mice.
There is a dearth of knowledge regarding the membrane channels and receptors as well as their cognate intra-cellular signaling pathways in the DA progenitor cells that mediate DA differentiation in response to afferent synaptic activity. Studies with primary cultures of OB and forebrain organotypic slice cultures indicate that L-type calcium channels are critical for activity dependent expression of TH.118, 119 It is tempting to speculate that the activation of calcium channels induces IEG expression, and consequently TH expression, through well established calcium second messenger signaling pathways.120 Forebrain slice cultures have also suggested that OB TH expression is modulated by GABA (unpublished observation). As stated above, a majority of the PG interneurons are GABAergic and the DA interneurons also contain GABA-A receptors. GABA plays well documented roles in regulating proliferation, migration and gene expression in neural progenitors in both the SVZ and hippocampus.24, 121–126 It is possible that the modulation of TH expression by GABA is necessary for the terminal differentiation of DA progenitor cells.
There are also genes that modulate the OB DA phenotype through either general aspects of neurogenesis (Table 2) or olfactory receptor neuron function (Table 3), rather than specifically regulating OB DA differentiation. For example, the loss of either Notch1 or Arx impairs proliferation and migration of OB interneuron progenitors.127, 128 Alternatively, the loss of Dlx5 disrupts olfactory receptor neuron innervation of the OB,75 and mutations in the cyclic nucleotide gated channel 2 (CNG2) gene blocks signal transduction in olfactory receptor neurons.129 Thus, the regulation of OB DA neuron differentiation is complex and requires the convergence of diverse molecular genetic pathways.
Table 2.
| Non-specific Regulators of OB Interneuron Differentiation | |
|---|---|
| Arx | Homeodomain transcription factor; Arx is expressed in the embryonic LGE as well as the post-natal SVZ, RMS and OB,128, 148 mice lacking Arx have impaired SVZ-progenitor proliferation and migration as well as a substantial loss of TH+ cells in the OB.128 |
| Dcx | Microtubule-associated phosphoprotein; migrating neuroblasts in the post-natal SVZ, RMS and OB express Dcx,149–151 knock down of Dcx diminishes migration of SVZ- derived neuroblasts.152 |
| Ephrins | Ephrins are membrane-bound ligands for Eph receptor tyrosine kinases; ephrins A2, B2 and B3 and Eph receptors A4, A7 and B1–3 are expressed in the post-natal SVZ and RMS where they regulate progenitor proliferation and migration.153, 154 |
| Mash1 | Basic-helix-loop-helix transcription factor; Mash1 is expressed in the embryonic LGE76, 155–157 as well as the post-natal SVZ and RMS;158 transit amplifying precursor cells express Mash1, and loss of Mash1 substantially reduces the number of TH+ cells in the OB 158. |
| Myst4 | Histone acetyltransferase; Myst4 is expressed in the embryonic LGE and post-natal SVZ;159 Myst4 is critical for progenitor proliferation, and mice lacking Myst4 have a progressive loss of TH+ cells in the OB.159 |
| Notch1 | Transmembrane receptor that proteolytically releases a transcription co-activator upon ligand binding; Notch1 and its ligands Jagged1 and Delta1 as well as its downstream target gene Hes5 are expressed in the post-natal SVZ and RMS160–164; Notch1 is critical for proliferation of SVZ progenitors, and over-expression of activated Notch1 drastically reduces the number of SVZ-derived migrating progenitors.127 |
| Olig2 | Basic helix-loop-helix transcription factor; Olig2 is expressed in the transit amplifying precursor cell;16, 165 positive regulator of oligodendritic cell fates and negative regulator of neuronal lineages in precursor cells.16 |
| PK2 | Cysteine-rich secreted protein; PK2 and its receptors are expressed in complementary patterns within embryonic and post-natal OB where PK2 acts as a chemoattractant for migrating SVZ-derived progenitors;166 periglomerular layer is indiscernible or malformed in mice lacking PK2.166 |
| PSA-NCAM | Polysialylated neural cell adhesion molecule; PSA-NCAM is expressed in migrating neuroblasts and is critical for tangential migration in the SVZ and RMS.167–170 |
| Reelin | Secreted glycoprotein that promotes shift from tangential to radial migration of SVZ- derived progenitors in the OB;171 in the OB, Reelin is highly expressed in the olfactory nerve layer, mitral cell layer and in a descending gradient through the granule cell layer;171 the Reelin receptor ApoER2 is strongly expressed in the RMS.171 |
| Shh | Secreted signaling protein; Shh is expressed in the slowly dividing neural stem cell and the transit amplifying cell (type B and C cells, respectively) in the post-natal SVZ, and Shh is critical for maintenance and proliferation of these cells.172–174 |
| Slit1, 2 | Secreted ligand proteins that bind Robo receptors; Slit1,2 are expressed in both embryonic and post-natal septum whereas the Robo1,2 receptors are expressed in the post-natal SVZ and RMS;175–178 Slit proteins are chemorepellants that guide migrating SVZ-derived progenitors.175, 178–180 |
| Tenascin-R | Extracellular matrix glycoprotein that promotes radial migration of progenitor cells by initiating detachment of tangentially migrating SVZ-derived neuroblasts;181 in the post- natal OB, Tenascin-R is expressed in the granule cell and internal plexiform layer, and is dependent on olfactory receptor neuron synaptic activity.181 |
| Vax1 | Homeodomain transcription factor; Vax1 is expressed in the embryonic LGE 182 as well as the post-natal SVZ and RMS;183 loss of Vax1 results in disorganization of the RMS and impaired OB interneuron progenitor migration.183 |
Expression and function of forebrain DA receptors
Dopamine acts through five receptor variants, D1 – D5, that are expressed in distinct and partially overlapping patterns within the forebrain (for an extensive review, see refs. 130, 131). D1, D2 and D5 receptors are widely expressed in the striatum, limbic system and OB as well as the pre-frontal, pre-motor, cingulate and entorhinal cortices. D5 receptor levels are notably lower than either the D1 or D2 receptors in most regions. Both D3 and D4 receptor expression is largely limited to the limbic system, although D4 receptors are also highly expressed in the frontal cortex.
Forebrain neurons expressing DA receptors are innervated primarily by midbrain DA cell groups. The mesostriatal DA projections from the substantia nigra, ventral tegmentum and retrorubral nucleus (area A9, A10 and A8, respectively) innervate several regions within the striatum as part of the neural circuitry that controls movement.132 As stated above, loss of the substantia nigra DA neurons and their associated projections is the hallmark of Parkinson’s Disease (PD). In addition to the mesostriatal system, the mesolimbic/mesocortical DA projections that originate largely from the ventral tegmentum (area A10) innervate limbic system regions that include the hippocampus and amygdala as well as cortical regions that include the cingulate and pre-frontal cortex.133 These mesolimbic/mesocortical DA projections have been implicated in several neurological conditions, including drug addiction (reward and reinforcement mechanisms)134 and schizophrenia,135 as well as learning and memory.136
Prospective directions for OB DA neurobiology
The mechanisms for OB DA differentiation may be important for advancing cell-replacement therapeutic strategies to treat neurodegenerative disorders, such as PD. OB DA neurons have several advantageous properties that include a capacity to readily integrate into pre-existing circuitry11 and a resistance to degeneration in PD.8 Emerging cell-transplant therapeutic strategies use replacement DA neurons generated from stem cells (either embryonic or adult-derived), but efficient production of functional replacement DA neurons remains elusive.137–139 Also, DA production alone is not sufficient, and other neuronal properties are also critical, to generate cells suitable for transplant.140, 141 Thus, it is important to not only delineate the various molecular genetic pathways that afford DA production, but also the pathways that generate the diverse array of features and functions of DA neurons in the brain. Elucidation of these diverse pathways may enable the engineering of replacement neurons that incorporate the unique, advantageous properties of OB DA neurons in order to improve the clinical effectiveness of replacement cells.
References
- 1.Hornykiewicz O. Parkinson’s disease: from brain homogenate to treatment. Fed Proc. 1973 Feb;32(2):183–190. [PubMed] [Google Scholar]
- 2.Baker H, Kawano T, Margolis FL, Joh TH. Transneuronal regulation of tyrosine hydroxylase expression in olfactory bulb of mouse and rat. J Neurosci. 1983;3:69–78. doi: 10.1523/JNEUROSCI.03-01-00069.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson DA, Sullivan RM. The D2 antagonist spiperone mimics the effects of olfactory deprivation on mitral/tufted cell odor response patterns. J Neurosci. 1995;15(8):5574–5581. doi: 10.1523/JNEUROSCI.15-08-05574.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson DA, Wood JG. Functional consequences of unilateral olfactory deprivation: time course and age sensitivity. Neurosci. 1992;49:183–192. doi: 10.1016/0306-4522(92)90086-h. [DOI] [PubMed] [Google Scholar]
- 5.Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006 Mar;7(3):179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- 6.Lledo PM, Saghatelyan A, Lemasson M. Inhibitory interneurons in the olfactory bulb: from development to function. Neuroscientist. 2004 Aug;10(4):292–303. doi: 10.1177/1073858404263460. [DOI] [PubMed] [Google Scholar]
- 7.Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- 8.Huisman E, Uylings HB, Hoogland PV. A 100% increase of dopaminergic cells in the olfactory bulb may explain hyposmia in Parkinson’s disease. Mov Disord. 2004 Jun;19(6):687–692. doi: 10.1002/mds.10713. [DOI] [PubMed] [Google Scholar]
- 9.Halasz N, Hokfelt T, Ljungdahl A, Johansson O, Goldstein M. Dopamine neurons in the olfactory bulb. Adv Biochem Psychopharmacol. 1977;16:169–177. [PubMed] [Google Scholar]
- 10.Lidbrink P, Jonsson G, Fuxe K. Selective reserpine-resistant accumulation of catecholamines in central dopamine neurones after DOPA administration. Brain Res. 1974 Mar 8;67(3):439–456. doi: 10.1016/0006-8993(74)90493-4. [DOI] [PubMed] [Google Scholar]
- 11.Lledo PM, Saghatelyan A. Integrating new neurons into the adult olfactory bulb: joining the network, life-death decisions, and the effects of sensory experience. Trends Neurosci. 2005 May;28(5):248–254. doi: 10.1016/j.tins.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Marin O, Rubenstein JL. A long, remarkable journey: tangential migration in the telencephalon. Nat Rev Neurosci. 2001 Nov;2(11):780–790. doi: 10.1038/35097509. [DOI] [PubMed] [Google Scholar]
- 13.Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006 Sep;7(9):687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- 14.Gheusi G, Cremer H, McLean H, Chazal G, Vincent JD, Lledo PM. Importance of newly generated neurons in the adult olfactory bulb for odor discrimination. Proc Natl Acad Sci U S A. 2000 Feb 15;97(4):1823–1828. doi: 10.1073/pnas.97.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gheusi G, Lledo PM. Control of early events in olfactory processing by adult neurogenesis. Chem Senses. 2007 May;32(4):397–409. doi: 10.1093/chemse/bjm012. [DOI] [PubMed] [Google Scholar]
- 16.Hack MA, Saghatelyan A, de Chevigny A, et al. Neuronal fate determinants of adult olfactory bulb neurogenesis. Nat Neurosci. 2005 Jul;8(7):865–872. doi: 10.1038/nn1479. [DOI] [PubMed] [Google Scholar]
- 17.Ortega-Perez I, Murray K, Lledo PM. The how and why of adult neurogenesis. J Mol Histol. 2007 Jun 29; doi: 10.1007/s10735-007-9114-5. [DOI] [PubMed] [Google Scholar]
- 18.Cossette M, Lecomte F, Parent A. Morphology and distribution of dopaminergic neurons intrinsic to the human striatum. J Chem Neuroanat. 2005 Jan;29(1):1–11. doi: 10.1016/j.jchemneu.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Cossette M, Levesque D, Parent A. Neurochemical characterization of dopaminergic neurons in human striatum. Parkinsonism Relat Disord. 2005 Aug;11(5):277–286. doi: 10.1016/j.parkreldis.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Farbman AI. Cell Biology of Olfaction. New York: Cambridge University Press; 1992. [Google Scholar]
- 21.Baker H, Farbman AI. Olfactory afferent regulation of the dopamine phenotype in the fetal rat olfactory system. Neurosci. 1993;52:115–134. doi: 10.1016/0306-4522(93)90187-k. [DOI] [PubMed] [Google Scholar]
- 22.Baker H, Morel K, Stone DM, Maruniak JA. Adult naris closure profoundly reduces tyrosine hydroxylase expression in mouse olfactory bulb. Brain Res. 1993;614:109–116. doi: 10.1016/0006-8993(93)91023-l. [DOI] [PubMed] [Google Scholar]
- 23.Kosaka K, Aika Y, Toida K, et al. Chemically defined neuron groups and their subpopulations in the glomerular layer of the rat main olfactory bulb. Neurosci Res. 1995 Aug;23(1):73–88. [PubMed] [Google Scholar]
- 24.De Marchis S, Bovetti S, Carletti B, et al. Generation of distinct types of periglomerular olfactory bulb interneurons during development and in adult mice: implication for intrinsic properties of the subventricular zone progenitor population. J Neurosci. 2007 Jan 17;27(3):657–664. doi: 10.1523/JNEUROSCI.2870-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parrish-Aungst S, Shipley MT, Erdelyi F, Szabo G, Puche AC. Quantitative analysis of neuronal diversity in the mouse olfactory bulb. J Comp Neurol. 2007 Feb 20;501(6):825–836. doi: 10.1002/cne.21205. [DOI] [PubMed] [Google Scholar]
- 26.Macrides F, Davis BJ, Youngs WM, Nadi NS, Margolis FL. Cholinergic and catecholaminergic afferents to the olfactory bulb in the hamster: a neuroanatomical, biochemical, and histochemical investigation. J Comp Neurol. 1981 Dec 10;203(3):495–514. doi: 10.1002/cne.902030311. [DOI] [PubMed] [Google Scholar]
- 27.McLean JH, Shipley MT. Serotonergic afferents to the rat olfactory bulb: II. Changes in fiber distribution during development. J Neurosci. 1987 Oct;7(10):3029–3039. doi: 10.1523/JNEUROSCI.07-10-03029.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLean JH, Shipley MT. Serotonergic afferents to the rat olfactory bulb: I. Origins and laminar specificity of serotonergic inputs in the adult rat. J Neurosci. 1987 Oct;7(10):3016–3028. doi: 10.1523/JNEUROSCI.07-10-03016.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kasowski HJ, Kim H, Greer CA. Compartmental organization of the olfactory bulb glomerulus. J Comp Neurol. 1999 May 3;407(2):261–274. [PubMed] [Google Scholar]
- 30.White EL. Synaptic organization of the mammalian olfactory glomerulus: new findings including an intraspecific variation. Brain Res. 1973 Oct 12;60(2):299–313. doi: 10.1016/0006-8993(73)90792-0. [DOI] [PubMed] [Google Scholar]
- 31.Kosaka K, Toida K, Aika Y, Kosaka T. How simple is the organization of the olfactory glomerulus?: the heterogeneity of so-called periglomerular cells. Neurosci Res. 1998;30(2):101–110. doi: 10.1016/s0168-0102(98)00002-9. [DOI] [PubMed] [Google Scholar]
- 32.Toida K, Kosaka K, Aika Y, Kosaka T. Chemically defined neuron groups and their subpopulations in the glomerular layer of the rat main olfactory bulb--IV. Intraglomerular synapses of tyrosine hydroxylase-immunoreactive neurons. Neuroscience. 2000;101(1):11–17. doi: 10.1016/s0306-4522(00)00356-0. [DOI] [PubMed] [Google Scholar]
- 33.Nagatsu T, Levitt M, Udenfriend S. Tyrosine hydroxylase: The initial step in norepinephrine biosynthesis. J Biol Chem. 1964;239:2910–2917. [PubMed] [Google Scholar]
- 34.Joh TH, Geghman C, Reis DJ. Immunochemical demonstration of increased accumulation of TH protein in sympathetic ganglia and adrenal medulla elicited by reserpine. J Neurochem. 1973;39:342–348. doi: 10.1073/pnas.70.10.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLean JH, Shipley MT. Postnatal development of the noradrenergic projection from locus coeruleus to the olfactory bulb in the rat. J Comp Neurol. 1991 Feb 15;304(3):467–477. doi: 10.1002/cne.903040310. [DOI] [PubMed] [Google Scholar]
- 36.Baker H, Abate C, Szabo A, Joh TH. Species specific distribution of aromatic 1-amino acid decarboxylase in rodent adrenal gland, cerebellum and olfactory bulb. J Comp Neurol. 1991;305:119–129. doi: 10.1002/cne.903050111. [DOI] [PubMed] [Google Scholar]
- 37.Cerruti C, Walther DM, Kuhar MJ, Uhl GR. Dopamine transporter mRNA expression is intense in rat midbrain neurons and modest outside midbrain. Brain Res Mol Brain Res. 1993 Apr;18(1–2):181–186. doi: 10.1016/0169-328x(93)90187-t. [DOI] [PubMed] [Google Scholar]
- 38.Coronas V, Srivastava LK, Liang JJ, Jourdan F, Moyse E. Identification and localization of dopamine receptor subtypes in rat olfactory mucosa and bulb: a combined in situ hybridization and ligand binding radioautographic approach. J Chem Neuroanat. 1997 May;12(4):243–257. doi: 10.1016/s0891-0618(97)00215-9. [DOI] [PubMed] [Google Scholar]
- 39.Koster NL, Norman AB, Richtand NM, et al. Olfactory receptor neurons express D2 dopamine receptors. J Comp Neurol. 1999 Sep 6;411(4):666–673. doi: 10.1002/(sici)1096-9861(19990906)411:4<666::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 40.Meador-Woodruff JH, Mansour A, Bunzow JR, Van Tol HH, Watson SJ, Jr, Civelli O. Distribution of D2 dopamine receptor mRNA in rat brain. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7625–7628. doi: 10.1073/pnas.86.19.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baker H. Unilateral, neonatal olfactory deprivation alters tyrosine hydroxylase expression but not aromatic amino acid decarboxylase or GABA immunoreactivity. Neurosci. 1990;36:761–771. doi: 10.1016/0306-4522(90)90018-y. [DOI] [PubMed] [Google Scholar]
- 42.Baker H, Cummings DM, Munger SD, et al. Targeted deletion of a cyclic nucleotide-gated channel subunit (OCNC1): Biochemical and morphological consequences in adult mice. J Neurosci. 1999;19:9313–9321. doi: 10.1523/JNEUROSCI.19-21-09313.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Philpot BD, Men D, McCarty R, Brunjes PC. Activity-dependent regulation of dopamine content in the olfactory bulbs of naris-occluded rats. Neuroscience. 1998;85(3):969–977. doi: 10.1016/s0306-4522(97)00667-2. [DOI] [PubMed] [Google Scholar]
- 44.Guthrie KM, Pullara JM, Marshall JF, Leon M. Olfactory deprivation increases dopamine D2 receptor density in the rat olfactory bulb. Synapse. 1991;8:61–70. doi: 10.1002/syn.890080109. [DOI] [PubMed] [Google Scholar]
- 45.Gall CM, Hendry SCH, Seroogy KB, Jones EG, Haycock JW. Evidence for coexistence of GABA and dopamine in neurons of the rat olfactory bulb. J Comp Neurol. 1987;266:307–318. doi: 10.1002/cne.902660302. [DOI] [PubMed] [Google Scholar]
- 46.Waclaw RR, Allen ZJ, 2nd, Bell SM, et al. The zinc finger transcription factor Sp8 regulates the generation and diversity of olfactory bulb interneurons. Neuron. 2006 Feb 16;49(4):503–516. doi: 10.1016/j.neuron.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 47.Brunig I, Sommer M, Hatt H, Bormann J. Dopamine receptor subtypes modulate olfactory bulb gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A. 1999 Mar 2;96(5):2456–2460. doi: 10.1073/pnas.96.5.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aroniadou-Anderjaska V, Ennis M, Shipley MT. Glomerular synaptic responses to olfactory nerve input in rat olfactory bulb slices. Neuroscience. 1997;79(2):425–434. doi: 10.1016/s0306-4522(96)00706-3. [DOI] [PubMed] [Google Scholar]
- 49.Berkowicz DA, Trombley PQ, Shepherd GM. Evidence for glutamate as the olfactory receptor cell neurotransmitter. J Neurophysiol. 1994 Jun;71(6):2557–2561. doi: 10.1152/jn.1994.71.6.2557. [DOI] [PubMed] [Google Scholar]
- 50.Ennis M, Zimmer LA, Shipley MT. Olfactory nerve stimulation activates rat mitral cells via NMDA and non- NMDA receptors in vitro [see comments] Neuroreport. 1996;7(5):989–992. doi: 10.1097/00001756-199604100-00007. [DOI] [PubMed] [Google Scholar]
- 51.Ennis M, Zhou FM, Ciombor KJ, et al. Dopamine D2 receptor-mediated presynaptic inhibition of olfactory nerve terminals. J Neurophysiol. 2001 Dec;86(6):2986–2997. doi: 10.1152/jn.2001.86.6.2986. [DOI] [PubMed] [Google Scholar]
- 52.Aroniadou-Anderjaska V, Zhou FM, Priest CA, Ennis M, Shipley MT. Tonic and synaptically evoked presynaptic inhibition of sensory input to the rat olfactory bulb via GABA(B) heteroreceptors. J Neurophysiol. 2000 Sep;84(3):1194–1203. doi: 10.1152/jn.2000.84.3.1194. [DOI] [PubMed] [Google Scholar]
- 53.Keller A, Yagodin S, Aroniadou-Anderjaska V, et al. Functional organization of rat olfactory bulb glomeruli revealed by optical imaging. J Neurosci. 1998;18(7):2602–2612. doi: 10.1523/JNEUROSCI.18-07-02602.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murphy GJ, Glickfeld LL, Balsen Z, Isaacson JS. Sensory neuron signaling to the brain: properties of transmitter release from olfactory nerve terminals. J Neurosci. 2004 Mar 24;24(12):3023–3030. doi: 10.1523/JNEUROSCI.5745-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palouzier-Paulignan B, Duchamp-Viret P, Hardy AB, Duchamp A. GABA(B) receptor-mediated inhibition of mitral/tufted cell activity in the rat olfactory bulb: a whole-cell patch-clamp study in vitro. Neuroscience. 2002;111(2):241–250. doi: 10.1016/s0306-4522(02)00003-9. [DOI] [PubMed] [Google Scholar]
- 56.Murphy GJ, Darcy DP, Isaacson JS. Intraglomerular inhibition: signaling mechanisms of an olfactory microcircuit. Nat Neurosci. 2005 Mar;8(3):354–364. doi: 10.1038/nn1403. [DOI] [PubMed] [Google Scholar]
- 57.Smith TC, Jahr CE. Self-inhibition of olfactory bulb neurons. Nat Neurosci. 2002 Aug;5(8):760–766. doi: 10.1038/nn882. [DOI] [PubMed] [Google Scholar]
- 58.Berkowicz DA, Trombley PQ. Dopaminergic modulation at the olfactory nerve synapse. Brain Res. 2000 Feb 7;855(1):90–99. doi: 10.1016/s0006-8993(99)02342-2. [DOI] [PubMed] [Google Scholar]
- 59.Hsia AY, Vincent JD, Lledo PM. Dopamine depresses synaptic inputs into the olfactory bulb. J Neurophysiol. 1999 Aug;82(2):1082–1085. doi: 10.1152/jn.1999.82.2.1082. [DOI] [PubMed] [Google Scholar]
- 60.Davila NG, Blakemore LJ, Trombley PQ. Dopamine modulates synaptic transmission between rat olfactory bulb neurons in culture. J Neurophysiol. 2003 Jul;90(1):395–404. doi: 10.1152/jn.01058.2002. [DOI] [PubMed] [Google Scholar]
- 61.Gutierrez-Mecinas M, Crespo C, Blasco-Ibanez JM, et al. Distribution of D2 dopamine receptor in the olfactory glomeruli of the rat olfactory bulb. Eur J Neurosci. 2005 Sep;22(6):1357–1367. doi: 10.1111/j.1460-9568.2005.04328.x. [DOI] [PubMed] [Google Scholar]
- 62.Davison IG, Boyd JD, Delaney KR. Dopamine inhibits mitral/tufted--> granule cell synapses in the frog olfactory bulb. J Neurosci. 2004 Sep 15;24(37):8057–8067. doi: 10.1523/JNEUROSCI.2138-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baker H, Towle AC, Margolis FL. Differential afferent regulation of the dopamine and gabaergic systems of the mouse main olfactory bulb. Brain Res. 1988;450:69–80. doi: 10.1016/0006-8993(88)91545-4. [DOI] [PubMed] [Google Scholar]
- 64.Stone DM, Grillo M, Margolis FL, Joh TH, Baker H. Differential effect of functional olfactory bulb deafferentation on tyrosine hydroxylase and glutamic acid decarboxylase messenger RNA levels in rodent juxtaglomerular neurons. J Comp Neurol. 1991;311:223–233. doi: 10.1002/cne.903110205. [DOI] [PubMed] [Google Scholar]
- 65.Hinds JW. Autoradiographic study of histogenesis in the mouse olfactory bulb. I. Time of origin of neurons and neuroglia. J Comp Neurol. 1968;134(3):287–304. doi: 10.1002/cne.901340304. [DOI] [PubMed] [Google Scholar]
- 66.Stenman J, Toresson H, Campbell K. Identification of two distinct progenitor populations in the lateral ganglionic eminence: implications for striatal and olfactory bulb neurogenesis. J Neurosci. 2003 Jan 1;23(1):167–174. doi: 10.1523/JNEUROSCI.23-01-00167.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yun K, Garel S, Fischman S, Rubenstein JL. Patterning of the lateral ganglionic eminence by the Gsh1 and Gsh2 homeobox genes regulates striatal and olfactory bulb histogenesis and the growth of axons through the basal ganglia. J Comp Neurol. 2003 Jun 23;461(2):151–165. doi: 10.1002/cne.10685. [DOI] [PubMed] [Google Scholar]
- 68.Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996 Feb 16;271(5251):978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- 69.Luskin MB, Zigova T, Soteres BJ, Stewart RR. Neuronal progenitor cells derived from the anterior subventricular zone of the neonatal rat forebrain continue to proliferate in vitro and express a neuronal phenotype. Mol and Cell Neurosci. 1997;8:351–366. doi: 10.1006/mcne.1996.0592. [DOI] [PubMed] [Google Scholar]
- 70.Vergano-Vera E, Yusta-Boyo MJ, de Castro F, Bernad A, de Pablo F, Vicario-Abejon C. Generation of GABAergic and dopaminergic interneurons from endogenous embryonic olfactory bulb precursor cells. Development. 2006 Nov;133(21):4367–4379. doi: 10.1242/dev.02601. [DOI] [PubMed] [Google Scholar]
- 71.Inoue T, Ota M, Ogawa M, Mikoshiba K, Aruga J. Zic1 and Zic3 regulate medial forebrain development through expansion of neuronal progenitors. J Neurosci. 2007 May 16;27(20):5461–5473. doi: 10.1523/JNEUROSCI.4046-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Altman J, Das GD. Autoradiographic and histological studies of postnatal neurogenesis. I. A longitudinal investigation of the kinetics, migration and transformation of cells incorporating tritiated thymidine in neonate rats, with special reference to postnatal neurogenesis in some brain regions. J Comp Neurol. 1966;126(3):337–389. doi: 10.1002/cne.901260302. [DOI] [PubMed] [Google Scholar]
- 73.Bayer SA. [3H]Thymidine-radiographic studies of neurogenesis in the rat olfactory bulb. Exp Brain Res. 1983;50:329–340. doi: 10.1007/BF00239197. [DOI] [PubMed] [Google Scholar]
- 74.Bulfone A, Kim HJ, Puelles L, Porteus MH, Grippo JF, Rubenstein JL. The mouse Dlx-2 (Tes-1) gene is expressed in spatially restricted domains of the forebrain, face and limbs in midgestation mouse embryos. Mech Dev. 1993 Mar;40(3):129–140. doi: 10.1016/0925-4773(93)90071-5. [DOI] [PubMed] [Google Scholar]
- 75.Long JE, Garel S, Depew MJ, Tobet S, Rubenstein JL. DLX5 regulates development of peripheral and central components of the olfactory system. J Neurosci. 2003 Jan 15;23(2):568–578. doi: 10.1523/JNEUROSCI.23-02-00568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Porteus MH, Bulfone A, Liu JK, Puelles L, Lo LC, Rubenstein JL. DLX-2, MASH-1, and MAP-2 expression and bromodeoxyuridine incorporation define molecularly distinct cell populations in the embryonic mouse forebrain. J Neurosci. 1994 Nov;14(11 Pt 1):6370–6383. doi: 10.1523/JNEUROSCI.14-11-06370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saino-Saito S, Cave JW, Akiba Y, et al. ER81 and CaMKIV identify anatomically and phenotypically defined subsets of mouse olfactory bulb interneurons. J Comp Neurol. 2007 Jun 1;502(4):485–496. doi: 10.1002/cne.21293. [DOI] [PubMed] [Google Scholar]
- 78.Stoykova A, Gruss P. Roles of Pax-genes in developing and adult brain as suggested by expression patterns. J Neurosci. 1994 Mar;14(3 Pt 2):1395–1412. doi: 10.1523/JNEUROSCI.14-03-01395.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Toresson H, Campbell K. A role for Gsh1 in the developing striatum and olfactory bulb of Gsh2 mutant mice. Development. 2001 Dec;128(23):4769–4780. doi: 10.1242/dev.128.23.4769. [DOI] [PubMed] [Google Scholar]
- 80.Curtis MA, Kam M, Nannmark U, et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007 Mar 2;315(5816):1243–1249. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- 81.Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003 Nov;6(11):1127–1134. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- 82.Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003 Oct;13(5):543–550. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 83.Merkle FT, Alvarez-Buylla A. Neural stem cells in mammalian development. Curr Opin Cell Biol. 2006 Dec;18(6):704–709. doi: 10.1016/j.ceb.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 84.Luskin MB, Coskun V. The progenitor cells of the embryonic telencephalon and the neonatal anterior subventricular zone differentially regulate their cell cycle. Chem Senses. 2002 Jul;27(6):577–580. doi: 10.1093/chemse/27.6.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Young KM, Fogarty M, Kessaris N, Richardson WD. Subventricular zone stem cells are heterogeneous with respect to their embryonic origins and neurogenic fates in the adult olfactory bulb. J Neurosci. 2007 Aug 1;27(31):8286–8296. doi: 10.1523/JNEUROSCI.0476-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007 Jul 20;317(5836):381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- 87.Dellovade TL, Pfaff DW, Schwanzel-Fukuda M. Olfactory bulb development is altered in small-eye (Sey) mice. J Comp Neurol. 1998 Dec 21;402(3):402–418. [PubMed] [Google Scholar]
- 88.Kohwi M, Osumi N, Rubenstein JL, Alvarez-Buylla A. Pax6 is required for making specific subpopulations of granule and periglomerular neurons in the olfactory bulb. J Neurosci. 2005 Jul 27;25(30):6997–7003. doi: 10.1523/JNEUROSCI.1435-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stoykova A, Fritsch R, Walther C, Gruss P. Forebrain patterning defects in Small eye mutant mice. Development. 1996 Nov;122(11):3453–3465. doi: 10.1242/dev.122.11.3453. [DOI] [PubMed] [Google Scholar]
- 90.Allen ZJ, 2nd, Waclaw RR, Colbert MC, Campbell K. Molecular identity of olfactory bulb interneurons: transcriptional codes of periglomerular neuron subtypes. J Mol Histol. 2007 Jul 12; doi: 10.1007/s10735-007-9115-4. [DOI] [PubMed] [Google Scholar]
- 91.Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002 Dec 19;36(6):1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- 92.Kohwi M, Petryniak MA, Long JE, et al. A subpopulation of olfactory bulb GABAergic interneurons is derived from Emx1- and Dlx5/6-expressing progenitors. J Neurosci. 2007 Jun 27;27(26):6878–6891. doi: 10.1523/JNEUROSCI.0254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Saino-Saito S, Sasaki H, Volpe BT, Kobayashi K, Berlin R, Baker H. Differentiation of the dopaminergic phenotype in the olfactory system of neonatal and adult mice. J Comp Neurol. 2004 Nov 22;479(4):389–398. doi: 10.1002/cne.20320. [DOI] [PubMed] [Google Scholar]
- 94.Baker H, Liu N, Chun HS, et al. Phenotypic differentiation during migration of dopaminergic progenitor cells to the olfactory bulb. J Neurosci. 2001;21(21):8505–8513. doi: 10.1523/JNEUROSCI.21-21-08505.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schimmel JJ, Crews L, Roffler-Tarlov S, Chikaraishi DM. 4.5 kb of the rat tyrosine hydroxylase 5′ flanking sequence directs tissue specific expression during development and contains consensus sites for multiple transcription factors. Brain Res Mol Brain Res. 1999;74(1–2):1–14. doi: 10.1016/s0169-328x(99)00234-x. [DOI] [PubMed] [Google Scholar]
- 96.Baker H, Kobayashi K, Okano H, Saino-Saito S. Cortical and striatal expression of tyrosine hydroxylase mRNA in neonatal and adult mice. Cell Mol Neurobiol. 2003 Oct;23(4–5):507–518. doi: 10.1023/A:1025015928129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Anderson SA, Marin O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001 Feb;128(3):353–363. doi: 10.1242/dev.128.3.353. [DOI] [PubMed] [Google Scholar]
- 98.Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 2001 Oct;128(19):3759–3771. doi: 10.1242/dev.128.19.3759. [DOI] [PubMed] [Google Scholar]
- 99.Chi N, Epstein JA. Getting your Pax straight: Pax proteins in development and disease. Trends Genet. 2002 Jan;18(1):41–47. doi: 10.1016/s0168-9525(01)02594-x. [DOI] [PubMed] [Google Scholar]
- 100.Czerny T, Busslinger M. DNA-binding and transactivation properties of Pax-6: three amino acids in the paired domain are responsible for the different sequence recognition of Pax-6 and BSAP (Pax-5) Mol Cell Biol. 1995 May;15(5):2858–2871. doi: 10.1128/mcb.15.5.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Epstein JA, Glaser T, Cai J, Jepeal L, Walton DS, Maas RL. Two independent and interactive DNA-binding subdomains of the Pax6 paired domain are regulated by alternative splicing. Genes Dev. 1994 Sep 1;8(17):2022–2034. doi: 10.1101/gad.8.17.2022. [DOI] [PubMed] [Google Scholar]
- 102.Kozmik Z, Czerny T, Busslinger M. Alternatively spliced insertions in the paired domain restrict the DNA sequence specificity of Pax6 and Pax8. Embo J. 1997 Nov 17;16(22):6793–6803. doi: 10.1093/emboj/16.22.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mishra R, Gorlov IP, Chao LY, Singh S, Saunders GF. PAX6, paired domain influences sequence recognition by the homeodomain. J Biol Chem. 2002 Dec 20;277(51):49488–49494. doi: 10.1074/jbc.M206478200. [DOI] [PubMed] [Google Scholar]
- 104.Caric D, Gooday D, Hill RE, McConnell SK, Price DJ. Determination of the migratory capacity of embryonic cortical cells lacking the transcription factor Pax-6. Development. 1997 Dec;124(24):5087–5096. doi: 10.1242/dev.124.24.5087. [DOI] [PubMed] [Google Scholar]
- 105.Haubst N, Berger J, Radjendirane V, et al. Molecular dissection of Pax6 function: the specific roles of the paired domain and homeodomain in brain development. Development. 2004 Dec;131(24):6131–6140. doi: 10.1242/dev.01524. [DOI] [PubMed] [Google Scholar]
- 106.Quinn JC, Molinek M, Martynoga BS, et al. Pax6 controls cerebral cortical cell number by regulating exit from the cell cycle and specifies cortical cell identity by a cell autonomous mechanism. Dev Biol. 2007 Feb 1;302(1):50–65. doi: 10.1016/j.ydbio.2006.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Talamillo A, Quinn JC, Collinson JM, et al. Pax6 regulates regional development and neuronal migration in the cerebral cortex. Dev Biol. 2003 Mar 1;255(1):151–163. doi: 10.1016/s0012-1606(02)00046-5. [DOI] [PubMed] [Google Scholar]
- 108.Liu N, Baker H. Activity-dependent Nurr1 and NGFI-B gene expression in adult mouse olfactory bulb. Neuroreport. 1999;10(4):747–751. doi: 10.1097/00001756-199903170-00016. [DOI] [PubMed] [Google Scholar]
- 109.Zetterstrom RH, Williams R, Perlmann T, Olson L. Cellular expression of the immediate early transcription factors Nurr1 and NGFI-B suggests a gene regulatory role in several brain regions including the nigrostriatal dopamine system. Brain Res Mol Brain Res. 1996 Sep 5;41(1–2):111–120. doi: 10.1016/0169-328x(96)00074-5. [DOI] [PubMed] [Google Scholar]
- 110.Kim KS, Kim CH, Hwang DY, et al. Orphan nuclear receptor Nurr1 directly transactivates the promoter activity of the tyrosine hydroxylase gene in a cell-specific manner. J Neurochem. 2003 May;85(3):622–634. doi: 10.1046/j.1471-4159.2003.01671.x. [DOI] [PubMed] [Google Scholar]
- 111.Sakurada K, Ohshima-Sakurada M, Palmer TD, Gage FH. Nurr1, an orphan nuclear receptor, is a transcriptional activator of endogenous tyrosine hydroxylase in neural progenitor cells derived from the adult brain. Development. 1999 Sep;126(18):4017–4026. doi: 10.1242/dev.126.18.4017. [DOI] [PubMed] [Google Scholar]
- 112.Le W, Conneely OM, Zou L, et al. Selective agenesis of mesencephalic dopaminergic neurons in Nurr1-deficient mice. Exp Neurol. 1999 Oct;159(2):451–458. doi: 10.1006/exnr.1999.7191. [DOI] [PubMed] [Google Scholar]
- 113.Zetterstrom RH, Solomin L, Jansson L, Olson L, Hoffer BJ, Perlmann T. Dopamine neuron agenesis in Nurr1-deficient mice. Science. 1997;276:248–250. doi: 10.1126/science.276.5310.248. [DOI] [PubMed] [Google Scholar]
- 114.Fung BP, Yoon SO, Chikaraishi DM. Sequences that direct rat tyrosine hydroxylase gene expression. J Neurochem. 1992 Jun;58(6):2044–2052. doi: 10.1111/j.1471-4159.1992.tb10945.x. [DOI] [PubMed] [Google Scholar]
- 115.Nagamoto-Combs K, Piech KM, Best JA, Sun B, Tank AW. Tyrosine hydroxylase gene promoter activity is regulated by both cyclic AMP-responsive element and AP1 sites following calcium influx. Evidence for cyclic amp-responsive element binding protein-independent regulation. J Biol Chem. 1997 Feb 28;272(9):6051–6058. doi: 10.1074/jbc.272.9.6051. [DOI] [PubMed] [Google Scholar]
- 116.Trocme C, Sarkis C, Hermel JM, et al. CRE and TRE sequences of the rat tyrosine hydroxylase promoter are required for TH basal expression in adult mice but not in the embryo. Eur J Neurosci. 1998;10(2):508–521. doi: 10.1046/j.1460-9568.1998.00059.x. [DOI] [PubMed] [Google Scholar]
- 117.Liu N, Cigola E, Tinti C, et al. Unique regulation of immediate early gene and tyrosine hydroxylase expression in the odor-deprived mouse olfactory bulb. J Biol Chem. 1999;274:3042–3047. doi: 10.1074/jbc.274.5.3042. [DOI] [PubMed] [Google Scholar]
- 118.Akiba Y, Sasaki H, Saino-Saito S, Baker H. Temporal and spatial disparity in cFOS expression and dopamine phenotypic differentiation in the neonatal mouse olfactory bulb. Neurochem Res. 2007 Apr-May;32(4–5):625–634. doi: 10.1007/s11064-006-9134-7. [DOI] [PubMed] [Google Scholar]
- 119.Cigola E, Volpe BT, Lee JW, Franzen L, Baker H. Tyrosine hydroxylase expression in primary cultures of olfactory bulb: role of L-type calcium channels. J Neurosci. 1998;18:7638–7649. doi: 10.1523/JNEUROSCI.18-19-07638.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.West AE, Chen WG, Dalva MB, et al. Calcium regulation of neuronal gene expression. Proc Natl Acad Sci U S A. 2001 Sep 25;98(20):11024–11031. doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Behar TN, Smith SV, Kennedy RT, McKenzie JM, Maric I, Barker JL. GABA(B) receptors mediate motility signals for migrating embryonic cortical cells. Cereb Cortex. 2001 Aug;11(8):744–753. doi: 10.1093/cercor/11.8.744. [DOI] [PubMed] [Google Scholar]
- 122.Bolteus AJ, Bordey A. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. J Neurosci. 2004 Sep 1;24(35):7623–7631. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cancedda L, Fiumelli H, Chen K, Poo MM. Excitatory GABA action is essential for morphological maturation of cortical neurons in vivo. J Neurosci. 2007 May 9;27(19):5224–5235. doi: 10.1523/JNEUROSCI.5169-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Carleton A, Petreanu LT, Lansford R, Alvarez-Buylla A, Lledo PM. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci. 2003 May;6(5):507–518. doi: 10.1038/nn1048. [DOI] [PubMed] [Google Scholar]
- 125.Gascon E, Dayer AG, Sauvain MO, et al. GABA regulates dendritic growth by stabilizing lamellipodia in newly generated interneurons of the olfactory bulb. J Neurosci. 2006 Dec 13;26(50):12956–12966. doi: 10.1523/JNEUROSCI.4508-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ge S, Pradhan DA, Ming GL, Song H. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 2007 Jan;30(1):1–8. doi: 10.1016/j.tins.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 127.Chambers CB, Peng Y, Nguyen H, Gaiano N, Fishell G, Nye JS. Spatiotemporal selectivity of response to Notch1 signals in mammalian forebrain precursors. Development. 2001 Mar;128(5):689–702. doi: 10.1242/dev.128.5.689. [DOI] [PubMed] [Google Scholar]
- 128.Yoshihara S, Omichi K, Yanazawa M, Kitamura K, Yoshihara Y. Arx homeobox gene is essential for development of mouse olfactory system. Development. 2005 Feb;132(4):751–762. doi: 10.1242/dev.01619. [DOI] [PubMed] [Google Scholar]
- 129.Baker H, Cummings DM, Munger SD, et al. Targeted deletion of a cyclic nucleotide-gated channel subunit (OCNC1): biochemical and morphological consequences in adult mice. J Neurosci. 1999 Nov 1;19(21):9313–9321. doi: 10.1523/JNEUROSCI.19-21-09313.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998 Jan;78(1):189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 131.Mansour A, Watson SJ., Jr . Dopamine receptor expression in the central nervous system. In: Bloom F, Kupfer D, editors. Pyschopharmacology: The fourth generation of progress. New York City: Raven Press, Ltd; 1995. pp. 207–219. [Google Scholar]
- 132.Mink J. Basal Ganglia. In: Zigmond M, Bloom F, Landis S, Roberts J, Squire L, editors. Fundamental neuroscience. New York: Academic Press; 1999. pp. 951–972. [Google Scholar]
- 133.Fallon J, Loughlin S. Substantia nigra. In: Paxinos G, editor. The rat nervous system. New York: Academic Press; 1995. pp. 215–237. [Google Scholar]
- 134.Nestler EJ. Molecular mechanisms of drug addiction. Neuropharmacology. 2004;47 (Suppl 1):24–32. doi: 10.1016/j.neuropharm.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 135.Gray JA, Joseph MH, Hemsley DR, et al. The role of mesolimbic dopaminergic and retrohippocampal afferents to the nucleus accumbens in latent inhibition: implications for schizophrenia. Behav Brain Res. 1995 Nov;71(1–2):19–31. doi: 10.1016/0166-4328(95)00154-9. [DOI] [PubMed] [Google Scholar]
- 136.Pezze MA, Feldon J. Mesolimbic dopaminergic pathways in fear conditioning. Prog Neurobiol. 2004 Dec;74(5):301–320. doi: 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 137.Goldman S. Stem and progenitor cell-based therapy of the human central nervous system. Nat Biotechnol. 2005 Jul;23(7):862–871. doi: 10.1038/nbt1119. [DOI] [PubMed] [Google Scholar]
- 138.Lindvall O, Kokaia Z. Stem cells for the treatment of neurological disorders. Nature. 2006 Jun 29;441(7097):1094–1096. doi: 10.1038/nature04960. [DOI] [PubMed] [Google Scholar]
- 139.Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders-how to make it work. Nat Med. 2004 Jul;10 (Suppl):S42–50. doi: 10.1038/nm1064. [DOI] [PubMed] [Google Scholar]
- 140.Lindvall O, Bjorklund A. Cell therapy in Parkinson’s disease. NeuroRx. 2004 Oct;1(4):382–393. doi: 10.1602/neurorx.1.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Winkler C, Kirik D, Bjorklund A. Cell transplantation in Parkinson’s disease: how can we make it work? Trends Neurosci. 2005 Feb;28(2):86–92. doi: 10.1016/j.tins.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 142.Saino-Saito S, Berlin R, Baker H. Dlx-1 and Dlx-2 expression in the adult mouse brain: relationship to dopaminergic phenotypic regulation. Journal of Comparative Neurology. 2003 Jun 16;461(1):18–30. doi: 10.1002/cne.10611. [DOI] [PubMed] [Google Scholar]
- 143.Eisenstat DD, Liu JK, Mione M, et al. DLX-1, DLX-2, and DLX-5 expression define distinct stages of basal forebrain differentiation. J Comp Neurol. 1999 Nov 15;414(2):217–237. doi: 10.1002/(sici)1096-9861(19991115)414:2<217::aid-cne6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 144.Liu JK, Ghattas I, Liu S, Chen S, Rubenstein JL. Dlx genes encode DNA-binding proteins that are expressed in an overlapping and sequential pattern during basal ganglia differentiation. Dev Dyn. 1997;210(4):498–512. doi: 10.1002/(SICI)1097-0177(199712)210:4<498::AID-AJA12>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 145.Bulfone A, Wang F, Hevner R, et al. An olfactory sensory map develops in the absence of normal projection neurons or GABAergic interneurons. Neuron. 1998 Dec;21(6):1273–1282. doi: 10.1016/s0896-6273(00)80647-9. [DOI] [PubMed] [Google Scholar]
- 146.Qiu M, Bulfone A, Martinez S, et al. Null mutation of Dlx-2 results in abnormal morphogenesis of proximal first and second branchial arch derivatives and abnormal differentiation in the forebrain. Genes Dev. 1995 Oct 15;9(20):2523–2538. doi: 10.1101/gad.9.20.2523. [DOI] [PubMed] [Google Scholar]
- 147.Corbin JG, Gaiano N, Machold RP, Langston A, Fishell G. The Gsh2 homeodomain gene controls multiple aspects of telencephalic development. Development. 2000;127(23):5007–5020. doi: 10.1242/dev.127.23.5007. [DOI] [PubMed] [Google Scholar]
- 148.Colombo E, Collombat P, Colasante G, et al. Inactivation of Arx, the murine ortholog of the X-linked lissencephaly with ambiguous genitalia gene, leads to severe disorganization of the ventral telencephalon with impaired neuronal migration and differentiation. J Neurosci. 2007 Apr 25;27(17):4786–4798. doi: 10.1523/JNEUROSCI.0417-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003 Dec 1;467(1):1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- 150.Gleeson JG, Lin PT, Flanagan LA, Walsh CA. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999 Jun;23(2):257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- 151.Nacher J, Crespo C, McEwen BS. Doublecortin expression in the adult rat telencephalon. Eur J Neurosci. 2001 Aug;14(4):629–644. doi: 10.1046/j.0953-816x.2001.01683.x. [DOI] [PubMed] [Google Scholar]
- 152.Ocbina PJ, Dizon ML, Shin L, Szele FG. Doublecortin is necessary for the migration of adult subventricular zone cells from neurospheres. Mol Cell Neurosci. 2006 Oct;33(2):126–135. doi: 10.1016/j.mcn.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 153.Conover JC, Doetsch F, Garcia-Verdugo JM, Gale NW, Yancopoulos GD, Alvarez-Buylla A. Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nat Neurosci. 2000 Nov;3(11):1091–1097. doi: 10.1038/80606. [DOI] [PubMed] [Google Scholar]
- 154.Holmberg J, Armulik A, Senti KA, et al. Ephrin-A2 reverse signaling negatively regulates neural progenitor proliferation and neurogenesis. Genes Dev. 2005 Feb 15;19(4):462–471. doi: 10.1101/gad.326905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Guillemot F, Joyner AL. Dynamic expression of the murine Achaete-Scute homologue Mash-1 in the developing nervous system. Mech Dev. 1993 Aug;42(3):171–185. doi: 10.1016/0925-4773(93)90006-j. [DOI] [PubMed] [Google Scholar]
- 156.Guillemot F, Lo LC, Johnson JE, Auerbach A, Anderson DJ, Joyner AL. Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell. 1993 Nov 5;75(3):463–476. doi: 10.1016/0092-8674(93)90381-y. [DOI] [PubMed] [Google Scholar]
- 157.Lo LC, Johnson JE, Wuenschell CW, Saito T, Anderson DJ. Mammalian achaete-scute homolog 1 is transiently expressed by spatially restricted subsets of early neuroepithelial and neural crest cells. Genes Dev. 1991 Sep;5(9):1524–1537. doi: 10.1101/gad.5.9.1524. [DOI] [PubMed] [Google Scholar]
- 158.Parras CM, Galli R, Britz O, et al. Mash1 specifies neurons and oligodendrocytes in the postnatal brain. Embo J. 2004 Nov 10;23(22):4495–4505. doi: 10.1038/sj.emboj.7600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Merson TD, Dixon MP, Collin C, et al. The transcriptional coactivator Querkopf controls adult neurogenesis. J Neurosci. 2006 Nov 1;26(44):11359–11370. doi: 10.1523/JNEUROSCI.2247-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Givogri MI, de Planell M, Galbiati F, et al. Notch signaling in astrocytes and neuroblasts of the adult subventricular zone in health and after cortical injury. Dev Neurosci. 2006;28(1–2):81–91. doi: 10.1159/000090755. [DOI] [PubMed] [Google Scholar]
- 161.Higuchi M, Kiyama H, Hayakawa T, Hamada Y, Tsujimoto Y. Differential expression of Notch1 and Notch2 in developing and adult mouse brain. Brain Res Mol Brain Res. 1995 Apr;29(2):263–272. doi: 10.1016/0169-328x(94)00257-f. [DOI] [PubMed] [Google Scholar]
- 162.Irvin DK, Nakano I, Paucar A, Kornblum HI. Patterns of Jagged1, Jagged2, Delta-like 1 and Delta-like 3 expression during late embryonic and postnatal brain development suggest multiple functional roles in progenitors and differentiated cells. J Neurosci Res. 2004 Feb 1;75(3):330–343. doi: 10.1002/jnr.10843. [DOI] [PubMed] [Google Scholar]
- 163.Irvin DK, Zurcher SD, Nguyen T, Weinmaster G, Kornblum HI. Expression patterns of Notch1, Notch2, and Notch3 suggest multiple functional roles for the Notch-DSL signaling system during brain development. J Comp Neurol. 2001 Jul 23;436(2):167–181. [PubMed] [Google Scholar]
- 164.Stump G, Durrer A, Klein AL, Lutolf S, Suter U, Taylor V. Notch1 and its ligands Delta-like and Jagged are expressed and active in distinct cell populations in the postnatal mouse brain. Mech Dev. 2002 Jun;114(1–2):153–159. doi: 10.1016/s0925-4773(02)00043-6. [DOI] [PubMed] [Google Scholar]
- 165.Hack MA, Sugimori M, Lundberg C, Nakafuku M, Gotz M. Regionalization and fate specification in neurospheres: the role of Olig2 and Pax6. Mol Cell Neurosci. 2004 Apr;25(4):664–678. doi: 10.1016/j.mcn.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 166.Ng KL, Li JD, Cheng MY, Leslie FM, Lee AG, Zhou QY. Dependence of olfactory bulb neurogenesis on prokineticin 2 signaling. Science. 2005 Jun 24;308(5730):1923–1927. doi: 10.1126/science.1112103. [DOI] [PubMed] [Google Scholar]
- 167.Cremer H, Lange R, Christoph A, et al. Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature. 1994 Feb 3;367(6462):455–459. doi: 10.1038/367455a0. [DOI] [PubMed] [Google Scholar]
- 168.Ono K, Tomasiewicz H, Magnuson T, Rutishauser U. N-CAM mutation inhibits tangential neuronal migration and is phenocopied by enzymatic removal of polysialic acid. Neuron. 1994 Sep;13(3):595–609. doi: 10.1016/0896-6273(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 169.Tomasiewicz H, Ono K, Yee D, et al. Genetic deletion of a neural cell adhesion molecule variant (N-CAM-180) produces distinct defects in the central nervous system. Neuron. 1993 Dec;11(6):1163–1174. doi: 10.1016/0896-6273(93)90228-j. [DOI] [PubMed] [Google Scholar]
- 170.Wichterle H, Garcia-Verdugo JM, Alvarez-Buylla A. Direct evidence for homotypic, glia-independent neuronal migration. Neuron. 1997 May;18(5):779–791. doi: 10.1016/s0896-6273(00)80317-7. [DOI] [PubMed] [Google Scholar]
- 171.Hack I, Bancila M, Loulier K, Carroll P, Cremer H. Reelin is a detachment signal in tangential chain-migration during postnatal neurogenesis. Nat Neurosci. 2002 Oct;5(10):939–945. doi: 10.1038/nn923. [DOI] [PubMed] [Google Scholar]
- 172.Balordi F, Fishell G. Hedgehog signaling in the subventricular zone is required for both the maintenance of stem cells and the migration of newborn neurons. J Neurosci. 2007 May 30;27(22):5936–5947. doi: 10.1523/JNEUROSCI.1040-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Machold R, Hayashi S, Rutlin M, et al. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003 Sep 11;39(6):937–950. doi: 10.1016/s0896-6273(03)00561-0. [DOI] [PubMed] [Google Scholar]
- 174.Palma V, Lim DA, Dahmane N, et al. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development. 2005 Jan;132(2):335–344. doi: 10.1242/dev.01567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hu H. Chemorepulsion of neuronal migration by Slit2 in the developing mammalian forebrain. Neuron. 1999 Aug;23(4):703–711. doi: 10.1016/s0896-6273(01)80029-5. [DOI] [PubMed] [Google Scholar]
- 176.Li HS, Chen JH, Wu W, et al. Vertebrate slit, a secreted ligand for the transmembrane protein roundabout, is a repellent for olfactory bulb axons. Cell. 1999 Mar 19;96(6):807–818. doi: 10.1016/s0092-8674(00)80591-7. [DOI] [PubMed] [Google Scholar]
- 177.Marillat V, Cases O, Nguyen-Ba-Charvet KT, Tessier-Lavigne M, Sotelo C, Chedotal A. Spatiotemporal expression patterns of slit and robo genes in the rat brain. J Comp Neurol. 2002 Jan 7;442(2):130–155. doi: 10.1002/cne.10068. [DOI] [PubMed] [Google Scholar]
- 178.Wu W, Wong K, Chen J, et al. Directional guidance of neuronal migration in the olfactory system by the protein Slit. Nature. 1999 Jul 22;400(6742):331–336. doi: 10.1038/22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chen JH, Wen L, Dupuis S, Wu JY, Rao Y. The N-terminal leucine-rich regions in Slit are sufficient to repel olfactory bulb axons and subventricular zone neurons. J Neurosci. 2001 Mar 1;21(5):1548–1556. doi: 10.1523/JNEUROSCI.21-05-01548.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sawamoto K, Wichterle H, Gonzalez-Perez O, et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006 Feb 3;311(5761):629–632. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- 181.Saghatelyan A, de Chevigny A, Schachner M, Lledo PM. Tenascin-R mediates activity-dependent recruitment of neuroblasts in the adult mouse forebrain. Nat Neurosci. 2004 Apr;7(4):347–356. doi: 10.1038/nn1211. [DOI] [PubMed] [Google Scholar]
- 182.Hallonet M, Hollemann T, Wehr R, et al. Vax1 is a novel homeobox-containing gene expressed in the developing anterior ventral forebrain. Development. 1998 Jul;125(14):2599–2610. doi: 10.1242/dev.125.14.2599. [DOI] [PubMed] [Google Scholar]
- 183.Soria JM, Taglialatela P, Gil-Perotin S, et al. Defective postnatal neurogenesis and disorganization of the rostral migratory stream in absence of the Vax1 homeobox gene. J Neurosci. 2004 Dec 8;24(49):11171–11181. doi: 10.1523/JNEUROSCI.3248-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Levy NS, Bakalyar HA, Reed RR. Signal transduction in olfactory neurons. J Steroid Biochem Mol Biol. 1991 Oct;39(4B):633–637. doi: 10.1016/0960-0760(91)90262-4. [DOI] [PubMed] [Google Scholar]
- 185.Long JE, Garel S, Alvarez-Dolado M, et al. Dlx-dependent and -independent regulation of olfactory bulb interneuron differentiation. J Neurosci. 2007 Mar 21;27(12):3230–3243. doi: 10.1523/JNEUROSCI.5265-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]