Abstract
Rationale
Matrix Gla protein (MGP) is a calcification inhibitor, which binds and inhibits bone morphogenetic protein (BMP)-2 and -4.
Objective
The objective was to determine if MGP also binds other proteins, which could interfere with its function.
Methods and Results
We transfected bovine aortic endothelial cells with N-terminally FLAG-tagged MGP, and used immunoprecipitation and LC-MS/MS-analysis to identify MGP-binding proteins. Heat shock protein 70 (HSP70), a stress-induced protein expressed in atherosclerotic lesions and soluble in serum, was identified as a novel MGP-binding protein. The interaction between MGP and HSP70 was confirmed by co-immunoprecipitation and chemical crosslinking, and blocked the interaction between MGP and BMP-4. In endothelial cells, HSP70 enhanced BMP-4-induced proliferation and tube formation, and in calcifying vascular cells (CVC), HSP70 enhanced BMP-induced calcium deposition. In addition, HSP70 mediated the procalcific effect of interleukin (IL)-6 on CVC. In apolipoprotein E null mice, a model for atherosclerosis, levels of BMP-4, HSP70, MGP and IL-6 were elevated in the aortic wall. Levels of BMP-4, HSP70 and IL-6 were also elevated in serum, and anti-HSP70 antibodies diminished its procalcific effect on CVC.
Conclusion
HSP70 binds MGP and enhances BMP activity, thereby functioning as a potential link between cellular stress, inflammation and BMP-signaling.
Keywords: Heat shock protein 70, Matrix Gla protein, Bone morphogenetic protein, Protein interaction
INTRODUCTION
Matrix Gla protein (MGP) is best known as an inhibitor of arterial calcification 1. It is a secreted protein expressed in the vascular endothelium and media 2-4 that binds and inhibits the activity of bone morphogenetic protein (BMP)-2 and -4 1, 5. MGP prevents BMP-2-induced mineralization in multipotent C3H10T1/2 cells and calcifying vascular cells (CVC) in vitro 1, 6, but it also prevents BMP-4-activity in endothelial cells in vitro, and pulmonary vascular development in vivo 5, 7. Recently, we showed that the ability of MGP to bind BMP-4 depends on the presence of the proline-64 (Pro64) residue and gamma-carboxylated glutamate residues (so-called Gla-residues) in the MGP protein 8. It is not known, however, if MGP is able to bind additional proteins.
The heat shock proteins (HSP) were initially identified as intracellular proteins, which facilitate protein refolding, chaperone proteins and increase during stress to provide cellular protection 9, 10. However, it is now known that HSPs, in particular HSP70 (also referred to as HSP72 and HSPA1A), are released to the extracellular milieu and circulation in response to stressful stimuli, where they exhibit potent immunomodulatory effects 11-13. The heat shock proteins have also been implicated in atherosclerosis 10, 14. In humans, high levels of HSP70 appear to have atheroprotective effects 14, and antibodies to HSP70 have been reported increased in patients with vascular disease 10. However, in apolipoprotein E null (Apoe-/-) mice, where tissues under stress are more easily defined since the stress-inducible form of HSP70 is not constitutively expressed, HSP70 levels correlate with lesion severity 15. Overexpression of HSP70 are found in several cell types in the lesions including endothelial cells and smooth muscle cells 10, 15 but the precise role performed by HSP70 remains unclear.
In this study, we identified HSP70 as a MGP binding protein and hypothesized that this interaction would affect BMP-signaling due to MGP’s role as a BMP-inhibitor. Our results showed that HSP70 enhanced the effect of BMP-4 on endothelial and medial vascular cells, and mediated a procalcific effect of interleukin-6 (IL-6), an inflammatory cytokine 16, in vitro. Together with BMP-4 and MGP, HSP70 was detected at increased levels in the aortas and sera of fat-fed apolipoprotein E null (Apoe-/-) mice, a model for atherosclerosis, and neutralization of HSP70 in serum decreased its procalcific effect. The results suggest that HSP70 may function as a potential link between cellular stress, inflammation and BMP signaling.
METHODS
Cell Culture and Transfection Assays
Bovine aortic endothelial cells (BAEC) were cultured and transfected as previously described 5. Luciferase assays were performed as described previously and normalized to Renilla luciferase 5. Human aortic endothelial cells (HAEC) were cultured and transiently transfected with siRNA as described previously 5. Conditioned media from HEK293 cells transfected with N-FLG-MGP construct were prepared as previously described 8. Bovine CVC were isolated and cultured as previously described 6. Transfection of CVC with siRNA were performed as previously described 17 using the Amaxa Nucleofector® and the human AoSMC Nucleofector® kit. The siRNAs for human and bovine HSP70 were obtained from Applied Biosystems (Foster City, CA) as part of Taqman® Gene Expression Assays. Recombinant human BMP-2, BMP-4 and IL-6 (R&D Systems, Minneapolis, MN), recombinant HSP70 and anti-HSP70 antibodies (Stressgen, Ann Arbor, MI), and conditioned media with N-FLAG-MGP were added at the time of transfection or plating.
Apoe-/- mice
Wild type and Apoe-/- mice on C57BL/6J background (Jackson Laboratory, Bar Harbor ME) were fed a standard chow or a high-fat/high-cholesterol diet (Western diet) (Research Diets, #D12079B) for 16 weeks, starting at 8-10 weeks of age. Aortas and sera were collected for analysis. All procedures were conducted in accordance with the animal care guidelines set by the University of California, Los Angeles.
Vector Constructions
See online supplement for the BMP-responsive reporter gene (BRE-Luc) and the expression constructs for FLAG-tagged and mutated human MGP-proteins.
LC-MS/MS
BAEC were transfected with an expression construct for N-FLAG-MGP or empty control vector. Cells lysates were prepared 24 hours after transfection, and immunoprecipitated with anti-FLAG antibodies. The immunoprecipitated complexes were analyzed by SDS-PAGE gels and stained with SYPRO Ruby. In-gel digestion of proteins was performed as previously described 18, 19 (see online supplement). Protein identification was accomplished by LC-ESI-MS/MS using an LTQ linear ion trap mass spectrometer (Thermo Electron) with a dedicated Surveyor pump system equipped with a reversed phase HPLC column (75 μm × 10 cm, BioBasic C18, 5 μm particle size, New Objective, Woburn, MA) as described in detail in the online supplement. Spectra were acquired in data-dependent mode and searched against the human database using SEQUEST. All proteins were identified on the basis of 2 or more peptides. Criteria for positive identification include: Xcorr values of >3.0 (+1), >4 (+2), and >5 (+3); >2 sequenced peptides; and deltaCN >0.1. All spectra used for identification were manually inspected to ensure that the most abundant peaks were assigned.
Immunoprecipitation, Immunoblotting and Crosslinking
Co-immunoprecipitation, immunoblotting and crosslinking were perfomed using previously described methods 5, 8 (see online supplement for details).
Immunohistochemistry and ELISA
Immunohistochemistry were performed as described previously 7, 17 using antibodies to BMP-4 (R&D Systems), HSP70 (Stressgen), MGP 5, pSMAD1/5/8 (Cell Signaling Technology, Danvers, MA), pSMAD2/3 (Cell Signaling) and total SMAD (Santa Cruz Biotechnology, Santa Cruz, CA). ELISA for BMP-4 was from R&D Systems.
Proliferation and Tube Formation Assays
Cell proliferation was determined by 3H-thymidine incorporation, and tube formation by Matrigel assays (see online supplement for details).
Alkaline Phosphatase (ALP) Assay and Quantification of Calcium Deposition
ALP activity and calcium deposition were quantified as previously described 6, 8.
Statistical Analysis
Data were analyzed for statistical significance by two-way analysis of variance with post hoc Tukey’s analysis using the GraphPad Instat® 3.0 software (GraphPad Software, San Diego, CA). P-values less than 0.05 were considered significant. All experiments were repeated a minimum of three times.
RESULTS
Identification of HSP70 as a MGP-binding protein
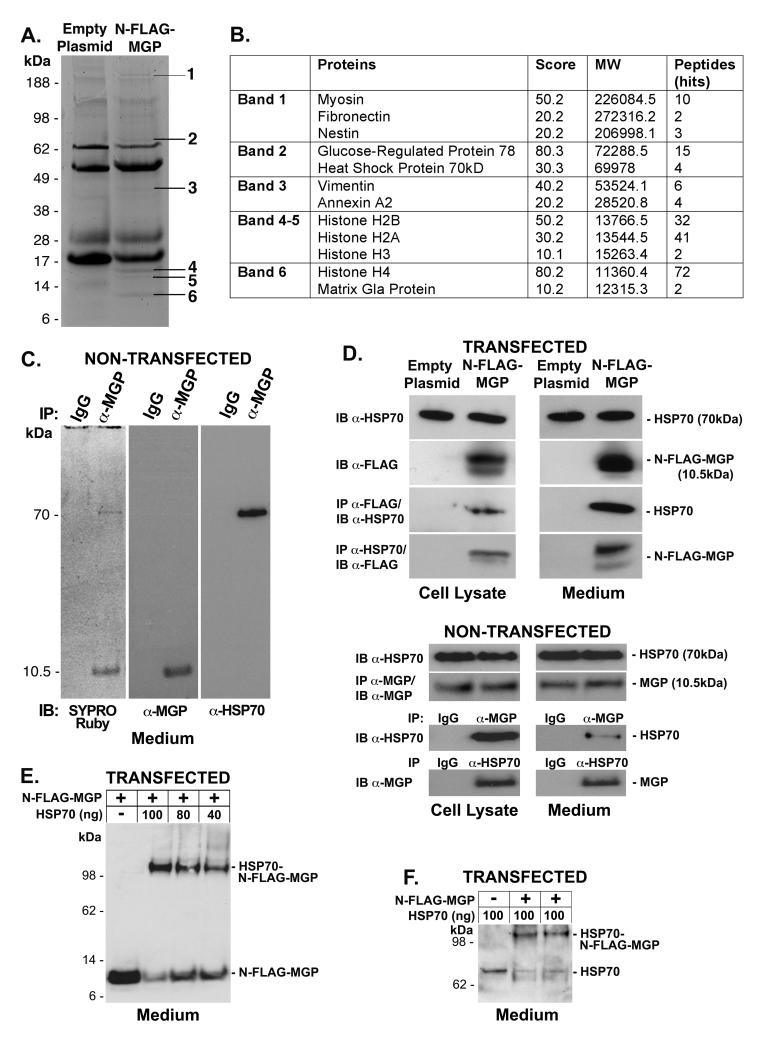
To identify MGP-binding proteins, we transfected BAEC with an expression construct for N-FLAG-MGP or an empty control vector, and collected cell lysates and media 24 hours after transfection.. The FLAG-tagged MGP was used because it was easier to perform immunoprecipitation and immunoblotting with anti-FLAG antibodies; previous studies showed no functional effect of the FLAG-tag 5, 8. We first studied cell lysates since we previously found MGP closely associated with the cells 20, suggesting that extracellular MGP functions close to the cell surface. The immunoprecipitated complexes were analyzed by SDS-PAGE gels and stained with SYPRO Ruby. Six bands, detected at 11, 15, 40, 70 and 200 kDa, were unique for samples from cells expressing N-FLAG-MGP (Fig. 1A). These bands were extracted and tryptic peptides were analyzed by LC-MS/MS. The data-dependent spectra were compared to bovine, mouse and human databases. The proteins that were identified in all three databases as highly identical proteins are listed in figure 1B. Of the proteins identified in this initial screen, we further pursued HSP70 since it has been strongly associated with vascular disease 10, 14, and shown to be active extracellularly 11-13.
Figure 1. HSP70 binds to MGP.
The N-FLAG-MGP expression construct of empty vector was overexpressed in BAEC. Cell lysates were used for immunoprecipitation using anti-FLAG antibodies.
(A) SYPRO Ruby stained gels showed 6 bands from the immunoprecipitated N-FLAG-MGP complex that was not present in the control complex.
(B) Proteins present in the different bands were identified by LC-MS/MS-analysis of tryptic digests; HSP70 was present in band 2.
(C) Ten ml of BAEC-conditioned medium was immunoprecipitated (IP) using anti-MGP antibodies or control IgG and analyzed by SDS-PAGE. The gel was stained with SYPRO Ruby protein stain, or analyzed by immunoblotting (IB) using anti-MGP or anti-HSP70 antibodies.
(D) The interaction between MGP and HSP70 was examined in cell lysates or medium from cells transfected with empty vector of N-FLAG-MGP by IP followed by IB using anti-HSP70 and anti-FLAG antibodies (top), or in cell lysates and medium from non-transfected cells using anti-HSP70 and anti-MGP antibodies. In the case of non-transfected cells, non-specific immunoglobulins (IgG) were used for control IP.
(E, F) The interaction between the MGP and HSP70 in medium from cells transfected with N-FLAG-MGP was examined by chemical crosslinking followed by immunoblotting using anti-FLAG (E) or anti-HSP70 antibodies (F).
In the next step, we tested whether the interaction was truly between extracellular MGP and HSP70 and if it could be detected in the medium from non-transfected cells. Ten ml of BAEC-conditioned medium was immunoprecipitated using anti-MGP antibodies or control IgG, and analyzed by SDS-PAGE gels. SYPRO Ruby protein stain revealed two bands, which corresponded in size to HSP70 (70 kDa) and MGP (10.5 kDa) (Fig. 1C), and reacted with anti-HSP70 and anti-MGP antibodies, respectively (Fig. 1C). This supports an interaction between extracellular HSP70 and MGP.
To further confirm the interaction between HSP70 and MGP, lysates and media from non-transfected cells and cells transfected with N-FLAG-MGP were co-immunoprecipited with anti-MPG and anti-FLAG antibodies, respectively, and analyzed by immunoblotting using anti-HSP70 antibodies. Conversely, anti-HSP70 immunoprecipitates were analyzed by immunoblotting with anti-MGP or anti-FLAG antibodies. In all cases, MGP and HSP70 co-immunoprecipitated (Fig. 1D).
Finally, we performed crosslinking experiments using medium from BAEC transfected with N-FLAG-MGP. HSP70 (60 - 100 ng) was added to 80 μl of serum free medium containing N-FLAG-MGP (approximately 50 ng/ml) 5, and cross-linked with DSS. The complexes were analyzed by immunoblotting using anti-FLAG or anti-HSP70 antibodies. Crosslinking of HSP70 and N-FLAG-MGP resulted in the formation of a complex that migrated at approximately 100 kDa on immunoblotting using both anti-FLAG (Fig. 1E) and anti-HSP70 antibodies (Fig. 1F). N-FLAG-MGP and HSP70 migrated at approximately 10 and 70 kDa, respectively. Although no additional bands were detected between HSP70 and the HSP70-N-FLAG-MGP complex, the change in migration suggested that more than one MGP bound to HSP70. Our previous crosslinking studies suggested that a dimer of MGP bound to a dimer of BMP-4 8 (see also Fig. 2B, lane 4, middle panel). Thus, the change in migration of HSP70 after crosslinking could be accounted for by one or two dimers of MGP. Taken together, the results strongly supported an interaction between HSP70 and MGP.
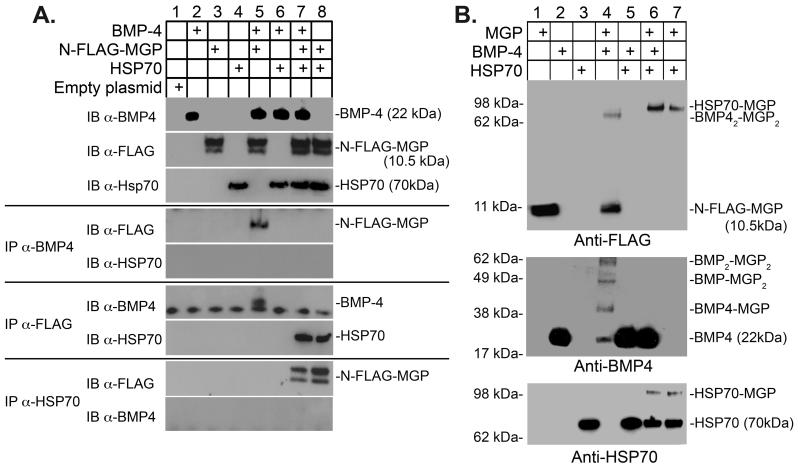
Figure 2. Binding of HSP70 to MGP blocks the interaction between MGP and BMP-4.
(A) BMP-4 (10 ng), HSP70 (100 ng) and conditioned medium containing N-FLAG-MGP (80 μl; approximately 50 ng/ml) were combined as indicated in the top panel, and the presence of the respective protein was confirmed by immunoblotting (IB) (top 3 blots). Interactions between the proteins were analyzed by immunoprecipitation (IP) followed by immunoblotting with antibodies as indicated.
(B) BMP-4 (10 ng), HSP70 (100 ng) and conditioned medium containing N-FLAG-MGP (80 μl; approximately 50 ng/ml) were combined as indicated in the top panel. Interactions were analyzed using chemical crosslinking followed by immunoblotting with antibodies as indicated.
Binding of HSP70 to MGP blocked its binding to BMP-4
Others and we have previously shown that MGP binds and inhibits BMP-2 and -4 1, 5, 8. To determine if HSP70 interfered with the binding between BMP-4 and MGP, we performed co-immunoprecipitation experiments. We added BMP-4, HSP70 or both to conditioned media containing N-FLAG-MGP (Fig. 2A, lanes 1-4), and immunoprecipitated with anti-FLAG, anti-HSP70 or anti-BMP-4 antibodies. The precipitated complexes were then analyzed by immunoblotting; if one of the antibodies was used for immunoprecipitation, the other two antibodies were used for immunoblotting. The results revealed that co-immunoprecipitation of N-FLAG-MGP and BMP-4 occurred when HSP70 was absent (Fig. 2A, lane 5). Addition of HSP70 abolished the co-immunoprecipitation of MGP and BMP-4 (Fig. 2A, lane 7). However, co-immunoprecipitation of N-FLAG-MGP and HSP70 occurred no matter if BMP-4 was present or not (Fig. 2A, lanes 7 and 8), suggesting that HSP-70 blocked the binding between BMP-4 and MGP. No interaction was seen between BMP-4 and HSP70 (Fig. 2A, lanes 6 and 9).
To validate our results, we performed crosslinking experiments. BMP-4, HSP70 or both were added to serum-free conditioned media containing N-FLAG-MGP (Fig. 2B, lanes 1-3), and crosslinked. Immunoblotting with anti-FLAG-antibodies revealed that incubation of BMP-4 and MGP led to the formation of the previously reported 62 kDa complex 8 (Fig. 2B, lane 4, top panel), corresponding to a BMP-4 dimer bound to a MGP dimer. Incubation of HSP70 and MGP led to the formation of a 100 kDa complex as before (Fig. 2B, lane 7, top panel). Incubation of all three proteins led to the formation of 100 kDa complex only (Fig. 2B, lane 6, top panel), suggesting that MGP preferentially bound to HSP70. Immunoblotting with anti-BMP-4 antibodies only detected complexes of BMP-4 and MGP (Fig. 2B, lane 4, middle panel). Addition of HSP70 abolished the formation of BMP4-MGP complexes (Fig. 2B, lane 6, middle panel). No crosslinked complexes of BMP-4 and HSP70 were detected (Fig. 2B, lane 5, middle panel). Immunoblotting with anti-HSP70 antibodies also detected the 100 kDa complex of crosslinked HSP70 and MGP no matter if BMP-4 was present or not (Fig. 2B, lanes 6-7, bottom panel). Taken together, the results supported that the binding between BMP-4 and MGP was abolished by HSP70.
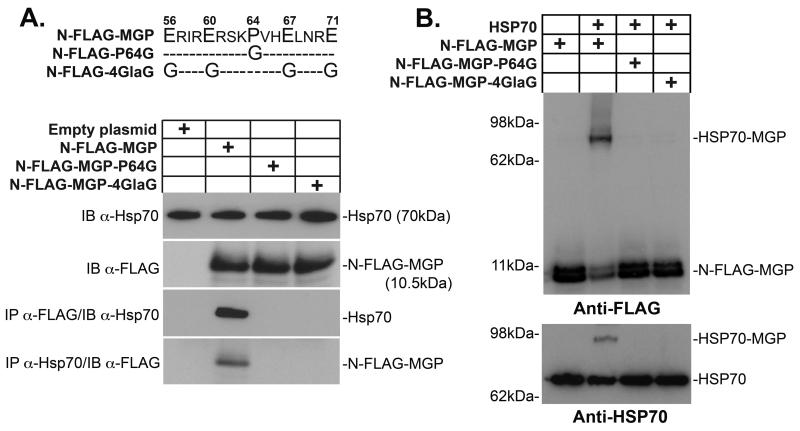
Pro64 and Gla residues were required for the binding between HSP70 and MGP
We previously showed that an intact Pro64 and at least one Gla residue on each side in multi-Gla domain of MGP was critical for the binding between BMP-4 and MGP 8. To determine if these residues were also essential for the binding between MGP and HSP70, we used two mutant versions of N-FLAG-MGP that are unable to bind BMP-4. N-FLAG-MGP-P64G contained a glycine instead of proline in position 64, and N-FLAG-MGP-4GlaG contained 4 glycine residues instead of 4 glutamate/Gla residues 8 (Fig. 3A, top). HSP70 was added to control medium or conditioned media containing N-FLAG-MGP, -MGP-P64G or -MGP-4GlaG, and co-immunoprecipitated with anti-FLAG or anti-HSP70 antibodies. Anti-FLAG immunoprecipitates were analyzed by immunoblotting using anti-HSP70 antibodies, and vice versa. No co-immunoprecipitation was detected for N-FLAG-MGP-P64G or -MGP-4GlaG (Fig. 3A), suggesting that Pro64 and Gla residues were essential for the interaction between HSP70 and MGP.
Figure 3. Binding of HSP70 to MGP requires Pro64 and Gla-residues.
(A) HSP70 (100 ng) was added to conditioned media (80 μl; approximately 50 ng/ml) containing N-FLAG-MGP, -MGP-P64G or -MGP-4GlaG. Interactions were analyzed by immunoprecipitation (IP) followed by immunoblotting (IB) using antibodies as indicated.
(B) HSP70 (100 ng) was added to the same conditioned media as in (A), and interactions were analyzed by chemical crosslinking followed by immunoblotting using anti-HSP70 and anti-FLAG antibodies as indicated.
We also performed crosslinking experiment to validate the results. HSP70 was added to conditioned media containing N-FLAG-MGP, -MGP-P64G or -MGP-4GlaG and crosslinked with DSS. The crosslinked complexes were analyzed by immunoblotting using anti-FLAG or anti-HSP70 antibodies. No crosslinking was detected between the mutant N-FLAG-MGP proteins and HSP70 (Fig. 3B), which supported that the Pro64 and Gla residues were required for binding between HSP70 and MGP. Thus, HSP70 likely binds to the same MGP domain as BMP-4 thereby blocking the binding between MGP and BMP-4.
Depletion of HSP70 did not affect expression and secretion of MGP
Although HSP70 is known to act as a chaperone protein 21, we were unable to detect an effect on expression and secretion of MGP from a depletion of HSP70 (online supplement, Suppl. Fig. I).
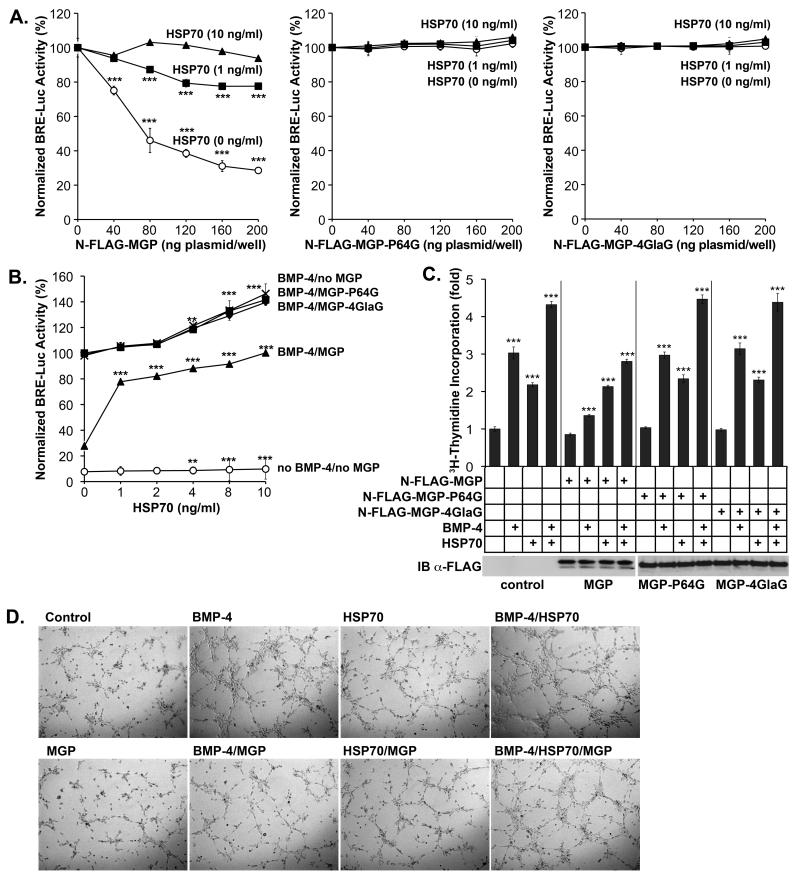
Extracellular HSP70 abolished the inhibitory effect of MGP on BMP-4 activity
We have previously shown that MGP inhibits BMP activity in vascular cells 5, 6. To determine if HSP70 interfered with BMP-signaling, we co-transfected BAEC with a BMP-responsive luciferase reporter gene (BRE-luc) and increasing amounts of N-FLAG-MGP construct (0-200 ng plasmid/well). The cells were treated with BMP-4 alone (40 ng/ml), or in combination with HSP70 (1 or 10 ng /ml) for 24 hours. Luciferase assays showed that the BMP-4 activation of BRE-Luc was inhibited by MGP, but that 1 ng/ml of HSP70 partially recovered and 10 ng /ml of HSP70 fully recovered BMP-4 activity (Fig. 4A). HSP70 had no significant effect on endogenous MGP-expression (data not shown). We repeated the experiments using the N-FLAG-MGP-P64G and -MGP-4GlaG vectors, but the BMP-4 activity was unaffected by both the increased MGP levels and the added HSP70 (Fig. 4A). We also co-transfected BAEC with BRE-Luc and the N-FLAG-MGP construct or empty vector (200 ng/well), and treated with BMP-4 (40 ng/ml) in combination with increasing amounts of HSP70 (0 - 10 ng/ml) for 24 hours. This revealed that HSP70 mildly enhanced BMP signaling in absence or presence of exogenous BMP-4 (Fig. 4B), but significantly recovered BMP-4 activity in presence of MGP (Fig. 4B). Again, N-FLAG-MGP-P64G and -MGP-4GlaG had no effect on BMP-4 activity and were unaffected by HSP70 (Fig. 4B). The levels of the FLAG-tagged MGP proteins were similar as determined by immunoblotting (Fig. 4C). Together, the results support that both the inhibition by MGP and stimulation by HSP70 of BMP-4 activity depend on a MGP protein that is able to bind BMP-4 and HSP70.
Figure 4. Extracellular HSP70 enhances BMP-4 activity and endothelial activation.
(A) BAEC were co-transfected with the BRE-luc luciferase reporter gene and increasing amounts of N-FLAG-MPG, -MGP-P64G or –MGP-4GlaG construct, and treated with BMP-4 (40 ng/ml) with or without HSP70 (1 or 10 ng/ml). BMP-4 activity was determined by luciferase assays.
(B) BAEC were transfected with the BRE-luc reporter gene and the N-FLAG-MPG, -MGP-P64G, –MGP-4GlaG construct or empty vector, and treated with increasing levels of HSP70 with or without BMP-4 (40 ng/ml). BMP-activity was determined by luciferase assays.
(C) BAEC were transfected with the N-FLAG-MPG, -MGP-P64G or –MGP-4GlaG construct or empty vector, and treated with BMP-4 and/or HSP70 as indicated. Cell proliferation was determined by 3H-thymidine incorporation (C) and tube formation was determined on Matrigel™ (online supplement, Suppl. Fig. II).
Extracellular HSP70 enhanced BMP-4-induced activation of endothelial cells
BMP-4 is known to stimulate EC proliferation and vascular formation 22, and we therefore determined the effect of MGP and HSP70 on BMP-4-induced EC proliferation. We transfected BAEC with empty vector, N-FLAG-MGP, -MGP-P64G or -MGP-4GlaG and treated with BMP-4 (40 ng/ml) in absence of presence of HSP70 (10 ng/ml). The expression of the respective MGP proteins was similar (Fig. 4C). 3H-Thymidine incorporation revealed that BMP-4 increased proliferation, and that N-FLAG-MGP inhibited this effect (Fig. 4C). However, HSP70 enhanced proliferation in absence and presence of exogenous BMP-4, and counteracted the effect of MGP (Fig. 4C). N-FLAG-MGP-P64G and –MGP-4GlaG were indistinguishable from empty vector (Fig. 4C).
To determine the effect of MGP and HSP70 on tube formation induced by BMP-4, we cultured BAEC on Matrigel™ in conditioned media from BAEC transfected with empty vector, N-FLAG-MGP, -MGP-P64G or -MGP-4GlaG (MGP levels approximately 40 ng/ml), and treated with BMP-4 (40 ng/ml) in absence or presence of HSP70 (10 ng/ml). The results showed that BMP-4 increased and MGP decreased tube formation (Fig. 4D). Similar to proliferation, HSP70 enhanced tube formation and counteracted the effect of MGP (Fig. 4D). Together, the results suggested that HSP70 enhanced BMP-4 activity in EC. Again, the mutated MGP proteins had no effect on BMP-4 activity (online supplement, Suppl. Fig. II).
Extracellular HSP70 enhanced BMP-2 and BMP-4-induced cell condensation and osteogenic differentiation in CVC
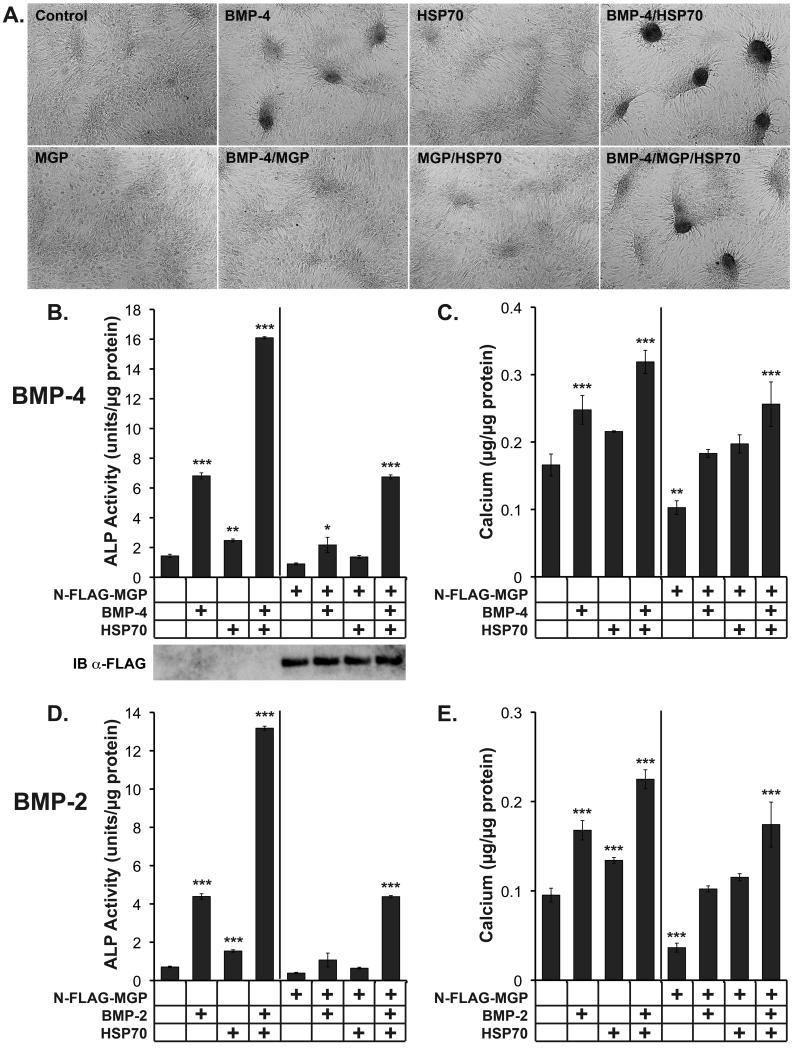
MGP inhibits formation of cell condensation and mineralization induced by BMP-2 and -4 in CVC, a well-established in vitro model for vascular calcification 1, 6. To determine if HSP70 blocked the activity of MGP in CVC, we treated CVC with BMP-4 (50 ng/ml) together with HSP70 (50 ng/ml) and/or conditioned medium containing N-FLAG-MGP (approximately 50 ng/ml, visualized by immunoblotting with anti-FLAG-antibodies, Fig. 5B) for 2 or 8 days. After 2 days, BMP-4 induced formation of condensations (Fig. 5A), and activity of alkaline phosphatase (ALP), an early osteogenic marker (Fig. 5B). After 8 days, BMP-4 increased mineralization, a late osteogenic marker (Fig. 5C). N-FLAG-MGP alone inhibited all three parameters (Fig. 5A-C), whereas HSP70 alone had a mildly stimulating effect, likely due to enhancement of endogenously expressed BMP 6 (Fig. 5A-C). When BMP-4 was added together with MGP, the stimulating effect of BMP-4 was inhibited. However, addition of HSP70 further enhanced the BMP-4 effect on condensation, ALP activity and mineralization (Fig. 5A-C), and if added together with N-FLAG-MGP, it neutralized the inhibitory effect of MGP (Fig. 5A-C).
Figure 5. Extracellular HSP70 enhances BMP-induced condensation formation, ALP activity and mineralization in CVC.
CVC were treated with BMP-4 (50 ng/ml) or BMP-2 (300 ng/ml), HSP70 (50 ng/ml) and conditioned medium from BAEC transfected with the N-FLAG-MGP (approximately 50 ng/ml) construct or empty vector as indicated for 2 or 8 days. After 2 days, formation of condensations was examined (A), and ALP activity was determined (B, D). After 8 days, accumulation of calcium was determined (C, E).
Asterisks indicate statistically significant differences compared to control (empty plasmid). *, p<0.05; **, p < 0.01; ***, p < 0.001; Tukey’s test.
Since MGP is also known to inhibit BMP-2 6, 20, we determined if replacing BMP-4 with BMP-2 (300 ng/ml) would give similar results. The higher concentration of BMP-2 was due to lower bioactivity per ng compared to BMP-4. Indeed, condensation formation (not shown), ALP activity and mineralization results were similar to those obtained with BMP-4 (Fig. 5D,E). We also replaced N-FLAG-MGP with -MGP-P64G or -MGP-4GlaG and examined condensation formation induced by BMP-4 (online supplement, Suppl. Fig III), and ALP activity and mineralization induced by BMP-2 and BMP-4 (online supplement, Suppl. Fig. IV). The results showed that the mutated MGP proteins had no effect on BMP-activity. Altogether, the results supported that HSP70 enhanced both BMP-2 and -4 signaling by diminishing the inhibitory effect of MGP.
IL-6-induced condensation and osteogenic differentiation in CVC is mediated by HSP70
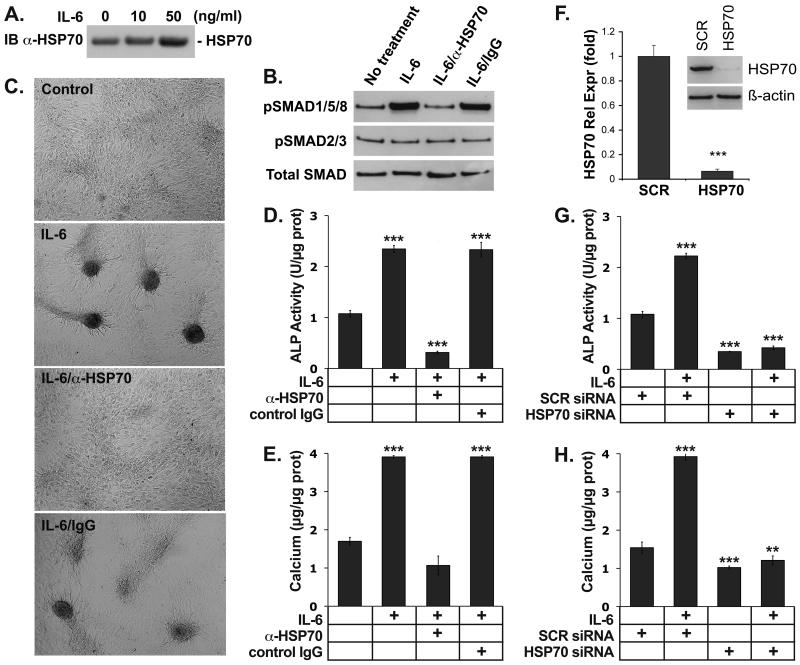
IL-6 is an inflammatory cytokine, which is known to induce mineralization in CVC 23 and expression of HSP70 in other cells 24. To determine if IL-6 induced HSP70 in CVC, the cells were treated with IL-6 (10 or 50 ng/ml) for 24 hours. The results showed that IL-6 increased media levels of HSP70 in media as determined by immunoblotting (Fig. 6A). We then examined if IL-6 and HSP70 affected BMP-signaling in CVC, and if HSP70 mediated the IL-6 effect on condensation formation and osteogenic differentiation. The CVC were treated with IL-6 (50 ng/ml) in absence or presence of neutralizing anti-HSP70 antibodies (300 ng/ml) or control IgG. BMP-signaling was determined after 12 hours by immunoblotting for phosphorylated (p)SMAD1/5/8, and compared to pSMAD2/3 (known to mediate transforming growth factor (TGF)-ß signaling) and total SMAD proteins. Condensation formation and ALP activity were determined after 2 days, and calcium accumulation after 8 days. The results showed that IL-6 promoted BMP-signaling as well as the other parameters and that the anti-HSP70 antibodies abolished these effects (Fig. 6B-E). It suggested that HSP70 mediated the enhanced BMP-signaling and the procalcific effect of IL-6. To confirm the procalcific effect, we depleted the CVC of HSP70 using HSP70 siRNA, which decreased the HSP70 level to less than 10% compared to scrambled control siRNA (Fig. 6E). The cells were treated with IL-6 (50 ng/ml) starting 24 hours after transfection, and ALP activity and calcium accumulation was determined after 2 and 8 days, respectively. The depletion of HSP70 abolished the stimulating effect of IL-6 on both ALP-activity and mineralization (Fig. 6F,G), suggesting that HSP70 may mediate procalcific effects of IL-6 in the artery wall.
Figure 6. HSP70 mediates the formation of condensations, ALP and mineralization induced by IL-6 in CVC.
(A) CVC were treated with IL-6 (10 or 50 ng/ml), and HSP70 in the media was determined by immunoblotting after 24 hours.
(B-E) CVC were treated with IL-6 (50 ng/ml) alone or with anti-HSP70 antibodies or control IgG (300 ng/ml). After 12 hours, activation of SMAD1/5/8 was determined by pSMAD1/5/8 immunoblotting and compared to pSMAD2/3 and total SMAD (B). After 2 days, formation of condensations was examined (C), and ALP activity was determined (D). After 8 days, accumulation of calcium was determined (E).
(F-H) CVC were transfected with siRNA to HSP70 or scrambled control IgG. The decrease in HSP70 expression was confirmed by real-time PCR and immunoblotting after 24 hours (E). The CVC were treated with IL-6 (50 ng/ml) for up to 8 days. ALP activity and calcium accumulation were determined after 2 and 8 days, respectively (F, G).
Asterisks indicate statistically significant differences compared to control (no treatment). *, p<0.05; ***, p < 0.001; Tukey’s test.
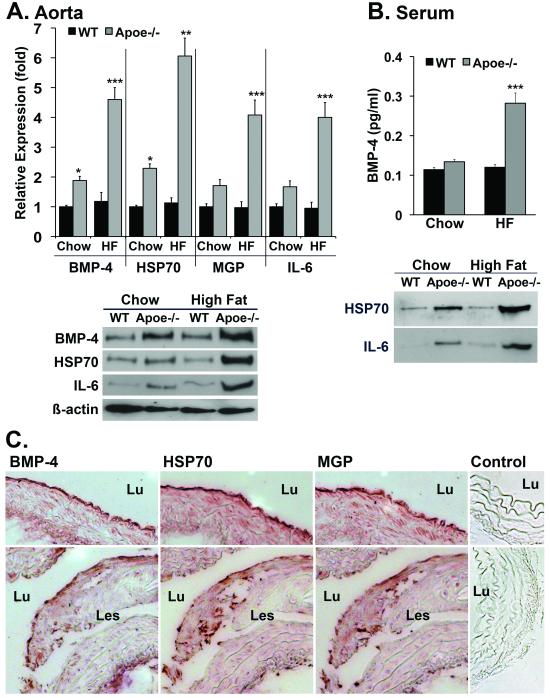
Increased levels of BMP-4, HSP70 and MGP increased in Apoe-/- mice
High levels of HSP70 were previously described in atherosclerotic lesions in Apoe-/- mice 15. To compare vascular expression of HSP70 to that of BMP-4, MGP and IL-6, aortas and sera were collected from wild type and Apoe-/-mice fed regular chow or a high fat diet for 16 weeks. Aortic expression of BMP-4, HSP70, MGP and IL-6 was elevated in both chow- and fat-fed Apoe-/- mice as determined by real-time PCR and immunoblotting (Fig. 7A) (we were unable to perform immunoblotting for MGP in aortic extract). Serum levels of BMP-4, HSP70 and IL-6 were also elevated as determined by ELISA and immunoblotting (Fig. 7B). Analysis of the Apoe-/- aortas by immunohistochemistry revealed that the expression of all three proteins was strongest in proximity of the endothelium in chow-fed Apoe-/- mice (Fig. 7C, top panels), whereas expression was found both close to the endothelium and in atherosclerotic lesions in fat-fed Apoe-/- mice (Fig. 7C, bottom panels). We also found that staining for pSMAD1/5/8 was increased in areas positive for HSP70, supporting an increase in BMP-signaling (online supplement, Suppl. Fig. V).
Figure 7. Increased levels of BMP-4, HSP70 and MGP increased in Apoe-/- mice.
(A, B) Wild type (WT) and Apoe-/- mice were fed chow or a high-fat diet for 16 weeks. Aortic expression of BMP-4, HSP70, MGP and IL-6 was determined by real-time PCR and immunoblotting (A), and serum levels of BMP-4, HSP70 and IL-6 were determined by ELISA and immunoblotting (B).
(C) Immunohistochemistry for BMP-4, HSP70, MGP and control (secondary antibody only) in aorta from chow-fed Apoe-/- mice (top panels), and for fat-fed Apoe-/- mice (bottom panels). Lu, lumen; Les, lesion.
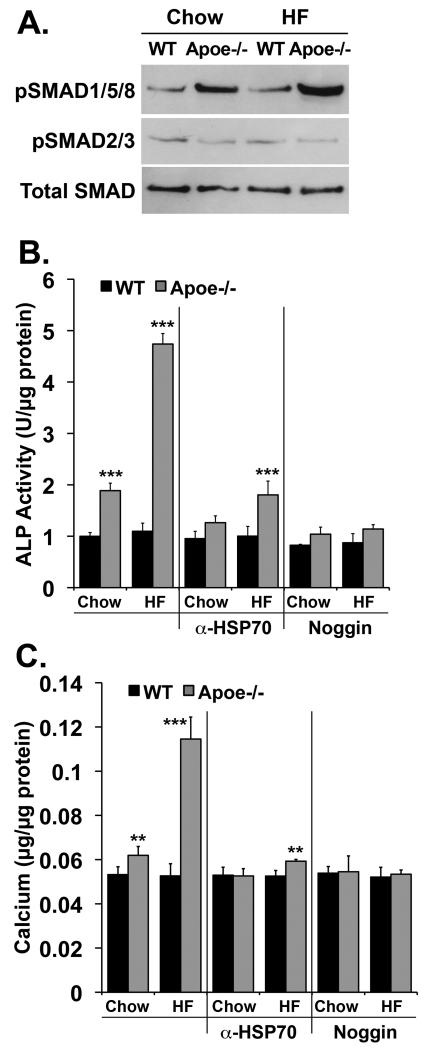
We then determined the effect of sera from wild type and Apoe-/- mice on chow or high-fat diet on BMP-signaling and calcification in CVC. BMP-signaling was examined after 6 hours incubation with 20% mouse serum alone using pSMAD1/5/8-immunoblotting. ALP activity and calcium deposition were determined after 2 and 8 days incubation, respectively, with 20% serum alone or in combination with neutralizing antibodies to HSP70 antibodies (300 ng/ml) or the BMP-inhibitor Noggin (300 ng/ml). BMP-signaling, ALP activity and calcium deposition were all stimulated in CVC incubated with serum from Apoe-/- mice, especially fat-fed ones (Fig. 8A-C). However, anti-HSP70 antibodies and Noggin diminished the procalcific effect of the mouse sera (Fig. 8B,C), suggesting that HSP70 and BMP both contribute to vascular calcification.
Figure 8. Enhanced procalcific effect of sera from fat-fed Apoe-/- mice.
(A) CVC were treated for 6 hours with 20% serum from WT and Apoe-/- mice fed chow or a high-fat diet. Activation of SMAD1/5/8 was determined by pSMAD1/5/8 immunoblotting and compared to pSMAD2/3 and total SMAD.
(B, C) CVC were treated for up to 8 days with 20% serum from WT and Apoe-/- mice fed chow or a high-fat diet. Anti-HSP70 (300 ng/ml) or Noggin (300 ng/ml) were added where indicated. ALP activity (B) and calcium deposition (C) were determined after 2 and 8 days, respectively. Asterisks indicate statistically significant differences compared to control (chow-fed WT). *, p<0.05; **, p < 0.01, ***, p < 0.001; Tukey’s test.
DISCUSSION
In this study, we provide evidence that HSP70 directly interacts with MGP, a BMP-inhibitor. The interaction is dependent on the presence of Proline-64 and Gla-residues in the MGP-protein, and blocks the interaction between BMP-4 and MGP thereby enhancing BMP-4 signaling. The enhancement of BMP signaling explains the effect of HSP70 on proliferation and tube formation in EC and on mineralization in CVC in vitro.
We initially used cell lysates to identify the interaction between MGP and HSP70 since we had observed that MGP accumulates close to cells 20 and speculated that it functioned close to the cell membrane. We confirmed that the interaction occurred in cell lysate and media from non-transfected cells using antibodies to native MGP, which supports that the interaction is physiological rather than secondary to overexpression of FLAG-tagged MGP. It also supports that the HSP70-MGP interaction occurs extracellularly, and may be more important than the intracellular interaction based on the lack of effect on MGP secretion of HSP70 depletion. Interestingly, HSP70 is known to bind cell surface receptors such as LOX-1 in endothelial cells 25, which may be affected by the HSP70-MGP interaction.
Even though HSP70 has been identified as a chaperone protein able to bind several proteins, and may have a higher likelihood for non-specific interactions, it is unlikely that the interaction between HSP70 and MPG is non-specific. Mutation of one amino acid (Pro64) or the Gla-residues, the same residues required for interaction between MGP and BMP-4 8, abolished the HSP70-MGP interaction. Furthermore, the HSP70-MGP interaction blocked the BMP-4-MGP interaction. If HSP70 were generally “sticky”, HSP70 would have been more likely to bind the BMP-4-MGP protein complex, and we would have seen the presence of all three proteins in one complex. In addition, the mutated MGP proteins are unaffected by HSP70 in cell assays, further supporting specificity in the interaction.
We used two vascular cell models to study the cellular effects of altered BMP-activity, aortic endothelial cells and CVC. In both cases, the results showed that the BMP-activity was enhanced by HSP70. In the endothelial cells, HSP70 enhanced BMP-4-induced cell proliferation and tube formation, whereas in CVC, it enhanced ALP activity and calcium accumulation, which strongly supports that HSP70 is a regulator of BMP-activity. Expression of BMP-2 and -4 are induced in endothelial cells by oscillatory shear stress and inflammatory mediators, and appears to have a pro-atherogenic and pro-inflammatory effect in vitro 4, 26, 27. However, BMP-2 and -4 also stimulate the expression of MGP 5, 17, and the same stimuli that induce BMPs also induce BMP-inhibitors 4, making it difficult to predict whether BMP or BMP-inhibitors ultimately will dominate. Thus depending on the context, HSP70 may initially enhance BMP-signaling, and then increase BMP-inhibition as a secondary effect.
HSP70 is known to be induced by inflammatory mediators 28, and is expressed by multiple cell types during atherogenesis 10, 15. In humans, it is believed to be atheroprotective although the mechanism is not yet fully understood 14. In Apoe-/- mice, a model for atherosclerosis, the HSP70 levels correlated with lesion severity 15 suggesting that HSP70 expression may be upregulated for protection and thus be increased due to the greater disease burden. Levels of circulating HSP70 antibodies have also been correlated with the severity of vascular disease and may have prognostic value 10. It is possible that the relative levels of HSP70 and anti-HSP70 antibodies affects the HSP70 effect.
Increased expression of IL-6, an inflammatory cytokine, is associated with atherosclerosis and other vascular disease 16, and IL-6 addition enhances vascular calcification in vitro 23. In our experiments, HSP70 mediated the stimulatory effect of IL-6 on mineralization, which was abolished by HSP70 depletion. These results are more consistent with HSP70 being an inflammatory mediator with the potential to accelerate vascular disease rather than having a protective effect. However, it is also possible that our in vitro model does not accurately reflect the in vivo situation. Overall, the importance of the balance between BMP and BMP-inhibitors, HSP70- and anti-HSP70-antibodies, and IL-6 and HSP70 in a given location makes it difficult to predict whether the ultimate effect will be pro- or anti-atherogenic for individual cells and for the organism, as a whole.
Our results showed expression of HSP70, BMP-4, MGP and IL-6 in proximity of the endothelium and in atherosclerotic lesions in Apoe-/- mice, which is consistent with previous reports 3, 4, 15 and makes protein interactions plausible. Since inhibition of both BMP-4 and HSP70 decreased the procalcific effect of sera from Apoe-/- mice in vitro, circulating BMP-4 and HSP70 may affect inflammatory reactions and vascular calcification in the vascular wall. We were unable to test the effect of serum MGP in our study due to lack of neutralizing antibodies. However, circulating MGP has been reported to have very little effect on vascular calcification 29.
Together, our results suggest that HSP70 binds MGP and enhances BMP activity, thereby functioning as a potential link between cellular stress, inflammation and BMP-signaling. However, whether this effect in the end is beneficial or detrimental may depend on the particular context.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING This work was funded in part by the NIH grants HL30568 and HL81397, and the American Heart Association (Western Affiliate).
NON-STANDARD ABBREVIATIONS AND ACRONYMS
- ALP
Alkaline phosphatase
- Apoe
Apolipoprotein E
- BAEC
Bovine aortic endothelial cells
- BMP
Bone morphogenetic protein
- BRE
BMP responsive element
- CVC
Calcifying vascular cells
- DSS
Disuccinimidyl suberate
- EC
Endothelial cells
- ELISA
Enzyme-linked immunosorbent assay
- ESI
Electrospray ionization
- FLAG
FLAG octapeptide (DYKDDDDK)
- Gla
gamma-carboxyglutamic acid
- HAEC
Human aortic endothelial cells
- HEK293 cells
Human embryonic kidney 293 cells
- HSP
Heat shock protein
- IB
Immunoblotting
- IgG
Immunoglobulin G
- IL
Interleukin
- IP
Immunoprecipitation
- LC
Liquid chromatography
- Les
Lesion
- LOX-1
Lectin-like oxidized low-density lipoprotein receptor-1
- Luc
Luciferase
- Lu
Lumen
- MGP
Matrix Gla protein
- MS
Mass spectrometry
- Pro
Proline
- pSMAD
Phosphorylated SMAD
- siRNA
small interfering ribonucleic acid
- SMAD
Homolog of the drosophila protein, mothers against decapentaplegic (MAD) and the C. elegans protein SMA
- TGF-ß
Transforming growth factor ß
Footnotes
DISCLOSURES None.
REFERENCES
- 1.Wallin R, Wajih N, Greenwood GT, Sane DC. Arterial calcification: a review of mechanisms, animal models, and the prospects for therapy. Med. Res. Rev. 2001;21:274–301. doi: 10.1002/med.1010. [DOI] [PubMed] [Google Scholar]
- 2.Shanahan CM, Cary NR, Salisbury JR, Proudfoot D, Weissberg PL, Edmonds ME. Medial localization of mineralization-regulating proteins in association with Monckeberg’s sclerosis: evidence for smooth muscle cell-mediated vascular calcification. Circulation. 1999;100:2168–2176. doi: 10.1161/01.cir.100.21.2168. [DOI] [PubMed] [Google Scholar]
- 3.Dhore CR, Cleutjens JP, Lutgens E, Cleutjens KB, Geusens PP, Kitslaar PJ, Tordoir JH, Spronk HM, Vermeer C, Daemen MJ. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. 2001;21:1998–2003. doi: 10.1161/hq1201.100229. [DOI] [PubMed] [Google Scholar]
- 4.Chang K, Weiss D, Suo J, Vega JD, Giddens D, Taylor WR, Jo H. Bone morphogenic protein antagonists are coexpressed with bone morphogenic protein 4 in endothelial cells exposed to unstable flow in vitro in mouse aortas and in human coronary arteries: role of bone morphogenic protein antagonists in inflammation and atherosclerosis. Circulation. 2007;116:1258–1266. doi: 10.1161/CIRCULATIONAHA.106.683227. [DOI] [PubMed] [Google Scholar]
- 5.Yao Y, Zebboudj AF, Shao E, Perez M, Bostrom K. Regulation of bone morphogenetic protein-4 by matrix GLA protein in vascular endothelial cells involves activin-like kinase receptor 1. J. Biol. Chem. 2006;281:33921–33930. doi: 10.1074/jbc.M604239200. [DOI] [PubMed] [Google Scholar]
- 6.Zebboudj AF, Shin V, Bostrom K. Matrix GLA protein and BMP-2 regulate osteoinduction in calcifying vascular cells. J. Cell. Biochem. 2003;90:756–765. doi: 10.1002/jcb.10669. [DOI] [PubMed] [Google Scholar]
- 7.Yao Y, Nowak S, Yochelis A, Garfinkel A, Bostrom KI. Matrix GLA Protein, an Inhibitory Morphogen in Pulmonary Vascular Development. J. Biol. Chem. 2007;282:30131–30142. doi: 10.1074/jbc.M704297200. [DOI] [PubMed] [Google Scholar]
- 8.Yao Y, Shahbazian A, Bostrom KI. Proline and gamma-carboxylated glutamate residues in matrix Gla protein are critical for binding of bone morphogenetic protein-4. Circ. Res. 2008;102:1065–1074. doi: 10.1161/CIRCRESAHA.107.166124. [DOI] [PubMed] [Google Scholar]
- 9.Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell. Mol. Life Sci. 2005;62:670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta TA, Greenman J, Ettelaie C, Venkatasubramaniam A, Chetter IC, McCollum PT. Heat shock proteins in vascular disease--a review. Eur. J. Vasc. Endovasc. Surg. 2005;29:395–402. doi: 10.1016/j.ejvs.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Calderwood SK, Theriault JR, Gong J. Message in a bottle: role of the 70-kDa heat shock protein family in anti-tumor immunity. Eur. J. Immunol. 2005;35:2518–2527. doi: 10.1002/eji.200535002. [DOI] [PubMed] [Google Scholar]
- 12.Johnson JD, Fleshner M. Releasing signals, secretory pathways, and immune function of endogenous extracellular heat shock protein 72. J. Leukoc. Biol. 2006;79:425–434. doi: 10.1189/jlb.0905523. [DOI] [PubMed] [Google Scholar]
- 13.Asea A. Initiation of the Immune Response by Extracellular Hsp72: Chaperokine Activity of Hsp72. Curr Immunol Rev. 2006;2:209–215. doi: 10.2174/157339506778018514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pockley AG, Calderwood SK, Multhoff G. The atheroprotective properties of Hsp70: a role for Hsp70-endothelial interactions? Cell Stress Chaperones. 2009 doi: 10.1007/s12192-009-0113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanwar RK, Kanwar JR, Wang D, Ormrod DJ, Krissansen GW. Temporal expression of heat shock proteins 60 and 70 at lesion-prone sites during atherogenesis in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2001;21:1991–1997. doi: 10.1161/hq1201.100263. [DOI] [PubMed] [Google Scholar]
- 16.Abeywardena MY, Leifert WR, Warnes KE, Varghese JN, Head RJ. Cardiovascular biology of interleukin-6. Curr. Pharm. Des. 2009;15:1809–1821. doi: 10.2174/138161209788186290. [DOI] [PubMed] [Google Scholar]
- 17.Yao Y, Zebboudj AF, Torres A, Shao E, Bostrom K. Activin-like kinase receptor 1 (ALK1) in atherosclerotic lesions and vascular mesenchymal cells. Cardiovasc. Res. 2007;74:279–289. doi: 10.1016/j.cardiores.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Edmondson RD, Vondriska TM, Biederman KJ, Zhang J, Jones RC, Zheng Y, Allen DL, Xiu JX, Cardwell EM, Pisano MR, Ping P. Protein kinase C epsilon signaling complexes include metabolism- and transcription/translation-related proteins: complimentary separation techniques with LC/MS/MS. Mol Cell Proteomics. 2002;1:421–433. doi: 10.1074/mcp.m100036-mcp200. [DOI] [PubMed] [Google Scholar]
- 19.Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 20.Zebboudj AF, Imura M, Bostrom K. Matrix GLA protein, a regulatory protein for bone morphogenetic protein-2. J. Biol. Chem. 2002;277:4388–4394. doi: 10.1074/jbc.M109683200. [DOI] [PubMed] [Google Scholar]
- 21.Morano KA. New tricks for an old dog: the evolving world of Hsp70. Ann. N. Y. Acad. Sci. 2007;1113:1–14. doi: 10.1196/annals.1391.018. [DOI] [PubMed] [Google Scholar]
- 22.Heinke J, Wehofsits L, Zhou Q, Zoeller C, Baar KM, Helbing T, Laib A, Augustin H, Bode C, Patterson C, Moser M. BMPER is an endothelial cell regulator and controls bone morphogenetic protein-4-dependent angiogenesis. Circ. Res. 2008;103:804–812. doi: 10.1161/CIRCRESAHA.108.178434. [DOI] [PubMed] [Google Scholar]
- 23.Abedin M, Lim J, Tang TB, Park D, Demer LL, Tintut Y. N-3 fatty acids inhibit vascular calcification via the p38-mitogen-activated protein kinase and peroxisome proliferator-activated receptor-gamma pathways. Circ. Res. 2006;98:727–729. doi: 10.1161/01.RES.0000216009.68958.e6. [DOI] [PubMed] [Google Scholar]
- 24.Febbraio MA, Steensberg A, Fischer CP, Keller C, Hiscock N, Pedersen BK. IL-6 activates HSP72 gene expression in human skeletal muscle. Biochem. Biophys. Res. Commun. 2002;296:1264–1266. doi: 10.1016/s0006-291x(02)02079-x. [DOI] [PubMed] [Google Scholar]
- 25.Theriault JR, Mambula SS, Sawamura T, Stevenson MA, Calderwood SK. Extracellular HSP70 binding to surface receptors present on antigen presenting cells and endothelial/epithelial cells. FEBS Lett. 2005;579:1951–1960. doi: 10.1016/j.febslet.2005.02.046. [DOI] [PubMed] [Google Scholar]
- 26.Csiszar A, Ahmad M, Smith KE, Labinskyy N, Gao Q, Kaley G, Edwards JG, Wolin MS, Ungvari Z. Bone morphogenetic protein-2 induces proinflammatory endothelial phenotype. Am. J. Pathol. 2006;168:629–638. doi: 10.2353/ajpath.2006.050284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorescu GP, Sykes M, Weiss D, Platt MO, Saha A, Hwang J, Boyd N, Boo YC, Vega JD, Taylor WR, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress stimulates an inflammatory response. J. Biol. Chem. 2003;278:31128–31135. doi: 10.1074/jbc.M300703200. [DOI] [PubMed] [Google Scholar]
- 28.Calderwood SK, Mambula SS, Gray PJ, Jr., Theriault JR. Extracellular heat shock proteins in cell signaling. FEBS Lett. 2007;581:3689–3694. doi: 10.1016/j.febslet.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 29.Murshed M, Schinke T, McKee MD, Karsenty G. Extracellular matrix mineralization is regulated locally; different roles of two gla-containing proteins. J. Cell Biol. 2004;165:625–630. doi: 10.1083/jcb.200402046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.