Abstract
MGCD0103, an orally available class I histone deacetylase (HDAC) inhibitor, was examined for pre-clinical activity in chronic lymphocytic leukaemia (CLL). A phase II clinical trial was performed, starting at a dose of 85 mg/day, three times per week. Dose escalation to 110 mg or the addition of rituximab was permitted in patients without a response after 2 or more cycles. MGCD0103 demonstrated pre-clinical activity against CLL cells with a LC50 (concentration lethal to 50%) of 0.23 μM and increased acetylation of the HDAC class I specific target histone H3. Twenty-one patients received a median of 2 cycles of MGCD0103 (range, 0–12). All patients had previously received fludarabine, 33% were fludarabine refractory, and 71% had del(11q22.3) or del(17p13.1). No responses according to the National Cancer Institutes 1996 criteria were observed. Three patients received 110 mg and 4 patients received concomitant rituximab, with no improvement in response. Grade 3–4 toxicity consisted of infections, thrombocytopenia, anemia, diarrhea, and fatigue. HDAC inhibition was observed in 6 out of 9 patients on day 8. Limited activity was observed with single agent MGCD0103 in high risk patients with CLL. Future investigations in CLL should focus on broad HDAC inhibition, combination strategies, and approaches to diminish constitutional symptoms associated with this class of drugs.
Keywords: Chronic lymphocytic leukemia, MGCD0103, histone deacetylase inhibitors, epigenetics
INTRODUCTION
Historically, loss of tumour suppressor genes and genomic silencing via DNA mutation or deletion have been thought to contribute to tumorigenesis by permitting apoptotic escape, sustained growth, limitless replication, immunological evasion, and metastasis of the malignant cell. However, epigenetic modifications that favour transcriptionally repressive chromatin are also common in neoplastic transformation, particularly in B-cell malignancies.(Baur, et al 1999, Costello, et al 2000, Koduru, et al 1995) CpG island promoter methylation and post-translational modifications of histone proteins alter chromatin conformation, favouring transcriptional repression and genomic silencing. Eukaryotic DNA is condensed 10,000-fold via the nucleosome, a histone octamer consisting of a histone H3 and H4 tetramer and two histone H2A and H2B dimers. Post-translational modifications of histone proteins including histone acetylation are critical to transcriptional regulation of genes. Histone acetylation and histone deacyetylation regulated by histone acetyltransferases and histone deacetylases (HDACs) leads to either transcriptionally active hyperacetylated chromatin or transcriptionally repressive hypoacetylated chromatin, respectively. Four classes of HDACs remove acetyl groups from lysine residues in the N-terminal tails of core histones in protein repressor and chromatin remodeling complexes including HDACs 1, 2, 3 and 8 (class I isotypes located within the nucleus); HDACs 4, 5, 6, 7, 9, and 10 (class II isotypes which shuttle between the cytoplasm and the nucleus), Sirt 1 to 7 (class III istoypes), and HDAC 11 (class IV isotype).(de Ruijter, et al 2003) While genomic deletions and mutations irreversibly alter the sequence of a gene, histone modifications can be readily targeted by therapies that inhibit histone deacetylation. In addition to nuclear modification of histone proteins, several of the class II HDAC enzymes can also alter acetylation on cytoplasmic proteins. Given the robust number of proteins targeted by HDAC isotypes, agents that target HDAC enzymes represent a novel target for anti-cancer therapy.
Several studies have demonstrated that HDAC inhibitors, including depsipeptide (a potent inhibitor of class I HDAC enzymes), MS-275 (a selective class I HDAC inhibitor), and valproic acid (a non-selective HDAC inhibitor), can alter histone modifications in chronic lymphocytic leukemia (CLL) and lead to selective cytotoxicity of CLL cells.(Aron, et al 2003, Byrd, et al 2005, Byrd, et al 1999) Depsipeptide has led to reductions in peripheral blood lymphocyte counts in patients with fludarabine-refractory CLL(Byrd, et al 2005), a dose-dependent increase in acetylation of total histone H4,(Aron, et al 2003) and inhibition of global HDAC activity.(Byrd, et al 1999) Depsipeptide-induced apoptosis in CLL appears to occur through activation of caspase 3 and caspase 8, with minimal alteration in caspase 9 activity.(Aron, et al 2003) Therefore, the HDAC inhibitor depsipeptide utilizes the tumour necrosis factor-receptor pathway of apoptosis to activate caspase 8, which leads to recruitment of caspase 3 and cleavage of poly(ADP-ribose)polymerase (PARP). The observation that depsipeptide operates via a caspase 8-mediated process is significant, as this pathway is not activated by other agents currently used in the treatment of CLL, which more frequently activate the mitochondrial/caspase 9-dependent pathway of apoptosis.(Aron, et al 2003, Byrd, et al 1999, Genini, et al 2000)
MGCD0103 is an orally available, aminophenylbenzamide small molecule HDAC inhibitor that selectively targets class I (HDAC isotypes 1, 2, and 3) and class IV (HDAC isotype 11) enzymes. In pre-clinical testing, intermittent dosing schedules have led to sustained dose-dependent growth inhibition in a variety of human cancer cell lines and implanted tumors in mice.(Bonfils, et al 2008, Fournel, et al 2008) Previously conducted phase I trials of MGCD0103 in acute myeloid leukemia (AML), myelodysplastic syndromes, and solid tumours have evaluated three times per week dosing schedules with dose levels ranging from 12.5 – 80 mg/m2/day every 21 days.(Garcia-Manero, et al 2008a, Siu, et al 2008) Maximum tolerated doses were 60 mg/m2 and 45 mg/m2/day in AML and solid tumours, respectively,(Garcia-Manero, et al 2008a, Siu, et al 2008) with dose-limiting toxicities consisting of fatigue, nausea, vomiting, diarrhoea and dehydration. Using fixed MGCD0103 dosing, the recommended phase 2 doses were 110 mg in AML and 85 mg in solid tumours.
As a result of the compelling evidence supporting the role of epigenetic silencing in CLL and the feasibility of intermittent dosing in patients with AML and solid tumours, a multi-centre phase II trial of MGCD103 was conducted in patients with relapsed and refractory CLL to determine the overall response rate. In addition, in patients not initially responding to MGCD0103, combination therapy with rituximab and MGCD0103 was explored with the therapeutic rationale that MGCD0103 and rituximab induce apoptosis through different pathways and have non-overlapping toxicities. While HDAC inhibitors induce apoptosis in CLL cells through activation of caspase 8 and 3(Aron, et al 2003), rituximab-induced CLL apoptosis occurs through the caspase 9 effector pathway.(Byrd, et al 2002) In addition, declines in Mcl-1 following rituximab therapy may further sensitize CLL cells to MGCD0103 cytotoxicity.(Byrd, et al 2002)
METHODS
Preclinical studies
In vitro assessment of MGCD0103 effects on CLL patient cells
Peripheral blood was obtained from patients with a confirmed diagnosis of CLL, following written informed consent. Leukemic B-cells were negatively selected using RosetteSep reagent (Stem Cell Technologies, Vancouver, BC). Cells were incubated at 37°C and 5% CO2 in RPMI 1640 medium with 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin, and 2mM L-glutamine (Sigma, St. Louis, MO). MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assays and immunoblots were performed as described(Aron, et al 2003) andLC50 (concentration lethal to 50%) was calculated using Prism (GraphPad Software, San Diego, CA). Antibodies including acetylated tubulin (Sigma) acetylated H3 (Upstate, Lake Placid, NY), and GAPDH (Chemicon, Temecula, CA).
Phase II clinical trial, patients and methods
Patient Eligibility
Patients 18 years of age or older with histologically confirmed CLL,(Cheson, et al 1996) relapsed or refractory after at least one prior nucleoside-analog containing therapy (unless nucleoside analogs were contraindicated) and requiring treatment according to National Cancer Institutes (NCI) criteria(Cheson, et al 1996) were enrolled. Patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, a total bilirubin ≤ 1.5 × the upper limit of normal (ULN), an aspartate aminotransferase (AST) and alanine aminotransferase (ALT) ≤ 2.5 × ULN, and a serum creatinine ≤ 1.5 × ULN. The institutional review boards of all participating centres approved the trial and all patients provided written informed consent as per institutional guidelines and in accordance with the Declaration of Helsinki.
Trial Design and Dose Modifications
Patients received MGCD0103 at a starting dose of 85 mg three times per week (TIW) for four weeks. Twenty-eight days defined a cycle. Dose escalation to 110 mg TIW was permitted beginning with cycle 2 in patients who failed to achieve a complete response (CR) and who had no grade 2 or higher adverse events. In patients without evidence of response after dose escalation to 110 mg (unless dose escalation was contra-indicated due to toxicity), rituximab was administered. Rituximab dosing started at 100 mg over 4 h on day 1, followed by 375 mg/m2 days 3, 5, and then three times a week for a maximum of 12 doses. Therapy was continued until disease progression or unacceptable toxicity. Anti-emetic, anti-diarrhoeal, and hematopoietic growth factor support were provided at the discretion of the treating physician.
In patients with grade 3 non-hematological toxicity, MGCD0103 was withheld until improvement of the toxicity to ≤ grade 1. For subsequent cycles, dose reduction by either one (60mg) or two (40mg) dose levels was required for the first and second events, respectively. Dose reduction below 40 mg was not permitted and grade 4 non-hematological toxicity necessitated study removal. In patients with pre-treatment platelet count > 75 × 109/l and absolute neutrophil count (ANC) > 2.0 × 109/l, the development of grade 4 cytopenias persisting for more than 7 days required cessation of MGCD0103 until hematological recovery defined as ≥ 75% of baseline or ≤ grade 1. At resumption of therapy, patients were dose-reduced by one dose level to either 60 or 40 mg of MGCD0103. In patients with baseline pre-treatment platelet counts ≤ 75 × 109/l or ANC ≤ 2.0 × 109/l, cytopenias < 75% of baseline led to cessation of MGCD0103 therapy until recovery to ≥ 75% of baseline or ≤ grade 1. Treatment resumed at the next lower dose level, but dose reductions below 40 mg required study removal.
Toxicity and Response Evaluations
Complete blood counts, serum chemistries, and liver function tests were monitored weekly during the first two cycles of therapy and with dose escalation to 110 mg or the addition of rituximab. Starting with cycle 3, blood counts, chemistries, and liver function tests were assessed on days 1 and 15. Twelve lead electrocardiograms were performed pre-treatment; prior to dosing, 1- and 2-h post-dose on day 1 of cycles 1–2; and prior to dosing for cycles 3 and beyond. Response was assessed according to the revised NCI Working Group Criteria(Cheson, et al 1996) after every cycle, with bone marrow biopsy repeated to confirm CR or after every 4 cycles of therapy. Hematological and non-hematological toxicity was graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf).
Pharmacodynamic assays
Peripheral blood evaluations of whole cell HDAC enzyme activity and cytokine analysis were assessed pre-treatment, on cycle 1 day 8, and at completion of protocol therapy. Bone marrow aspirations were collected pre-treatment, on cycle 1 day 8, and at end of study therapy were used to qualify changes in HDAC activity over time. Whole cell HDAC enzyme assays were performed as previously described.(Bonfils, et al 2008, Siu, et al 2008) Plasma levels of interleukin 6 (IL-6) were determined using an enzyme-linked immunosorbent assay kit from eBioscience San Diego, CA).
Statistical methods
This study was a multi-institutional single-arm phase II study designed to evaluate the overall response rate (ORR) with MGCD0103 in patients with relapsed or refractory CLL. The study was designed according to Simon’s two-stage design, targeting a true response probability of ≥ 20%, with null hypothesis that the true response rate was < 5 %. The study had a type 1 error rate of 5% and power of 90%. According to the study design, study closure was required if fewer than 2 responses were observed in the first 21 patients. If sufficient responses were observed in stage 1, a total study enrollment of 41 patients was planned, with observation of 5 or more responses considered worthy of further evaluation.
RESULTS
Preclinical Results
MGCD0103 mediates in vitro cytoxicity against CLL cells
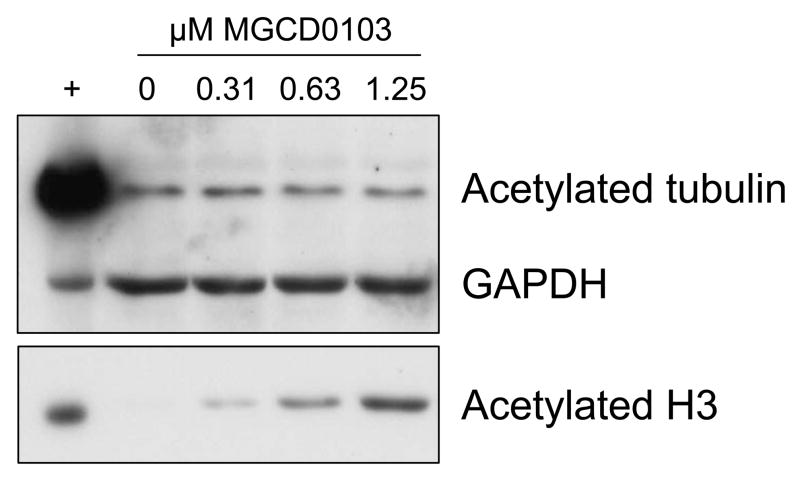
CLL cells from untreated patients (n=8) were incubated for 72 hours with or without various concentrations of MGCD0103, and viability was assessed by MTT assay. Under these conditions, the LC50 was 0.23 μM (95% confidence interval 0.17–0.31) relative to time-matched controls. To assess acetylation of known HDAC class I and II targets, CLL patient cells (n=4) were treated with MGCD0103 at several concentrations. Lysates were prepared after a 16-h incubation, prior to the time when cell death is observed by annexin/PI flow cytometry data (data not shown). By immunoblot analysis, MGCD0103 treatment induced acetylation of the HDAC class I substrate histone H3 but not the class II target tubulin (Figure 1). These data confirm that at the doses examined, MGCD0103 causes class I HDAC target hyperacetylation followed by cell death.
Figure 1.
CLL patient cells were incubated alone or with various concentrations of MGCD0103 for 16 h. Lysates were analyzed for acetylation of tubulin and histone H3 by standard immunoblot (one representative sample shown). Lysate from 697 cells treated with vorinostat (5 μM, 16 h) was included as a positive control.
Phase II clinical trial in CLL
Patient characteristics
Twenty-one patients completed a median of 2 cycles of therapy (range 0–12). Three patients were dose escalated to 110 mg and 4 patients received rituximab beginning in cycle 4 (range, 2–8 doses) for lack of response to single agent MGCD0103. In the twenty-one patients, the median age was 63 (range, 48–80) years and 20 patients had Rai stage 3–4 disease (Table 1). Most patients were heavily pre-treated, receiving a median of 5 prior therapies (range, 1–13) and 13 (62%) patients failed to respond to the prior treatment regimen. No patients were previously transplanted. All 21 patients had received fludarabine, and seven (33%) patients failed to respond to their last fludarabine-containing regimen. The majority of patients had adverse cytogenetics, with 15 (71%) patients with either del(11q22.3) or del(17p13.1), and 3 patients with both deletion 11q and 17p (Table 1).
Table 1.
Patient Characteristics
| Characteristic | All patients (n=21) | % |
|---|---|---|
| Median Age, years (range) | 63 (48–80) | |
| Male:Female | 14:7 | |
| Caucasian | 20 | 95 |
| African American | 1 | 5 |
| Median time in months from diagnosis(range) | 100.6 (31.3–187.5) | |
| Median Number of prior therapies (range) | 5 (1–13) | |
| Refractory to last therapy | 13 | 62 |
| Prior fludarabine | 21 | 100 |
| Fludarabine refractory | 7 | 33 |
| Rai Stage | ||
| Missing | 1 | 5 |
| 3 | 3 | 14 |
| 4 | 17 | 81 |
| Adenopathy | ||
| ≥ 5 cm | 4 | 19 |
| ≥ 10 cm | 0 | 0 |
| Splenomegaly | 7 | 33 |
| Blood | ||
| WBC (109/l) | ||
| Median | 30.6 | |
| Range | 1.1–347.8 | |
| Absolute lymphocyte count (109/l) | ||
| Median | 27.5 | |
| Range | 0.41–331 | |
| Hemoglobin (g/l) | ||
| Median | 101 | |
| Range | 79–151 | |
| Platelets (109/l) | ||
| Median | 49 | |
| Range | 10–220 | |
| Cytogenetic abnormalities | ||
| None | 1 | 5 |
| del(13q) | 2 | 10 |
| del(11q22.3) | 9 | 43 |
| del(17p13.1) | 9 | 43 |
| Complex | 2 | 10 |
| Diploid | 1 | 5 |
Response
In the cohort of 21 patients, there were no complete or partial responses. Twenty patients had stable disease, and no improvement in response occurred with either MGCD0103 dose escalation or the addition of rituximab. Median pre- and post-treatment white blood cell, absolute lymphocyte, and platelet counts were 30.6 × 109/l and 34 × 109/l (white blood cells); 27.5 × 109/l and 31.3 × 109/l (absolute lymphocyte count); and 49 × 109/l and 38 × 109/l) (platelets), respectively. Four patients who received 5, 2, 2, and 1 cycles of MGCD0103 had 73.4%, 36.7%, 93.9%, and 55.4% declines, respectively, in absolute lymphocyte counts. All of these patients stopped therapy due to toxicity including infection, diarrhea, fatigue, and nausea. In addition, four patients with stable disease were also able to continue MGCD0103 for 5, 7, 9 and 12 cycles, respectively.
Toxicity
Fifty-nine MGCD0103 cycles were completed. Six patients required dose reduction by one dose level to 60 mg three times a week, 1 patient required two dose reductions to 40 mg three times a week, and 16 patients had dosing delays primarily due to gastrointestinal symptoms (nausea, vomiting, and diarrhoea), fatigue, anorexia, infections, or thrombocytopenia. There were no treatment-related deaths.
Grade 3–4 hematological events included thrombocytopenia (29%), anemia (29%), and neutropenia (5%, Table 2). Non-hematological grade 3 or 4 adverse events were uncommon (Table 2), with infection (39%), febrile neutropenia (20%), diarrhoea (10%), fatigue (10%), and abdominal pain (5%) occurring most frequently. The majority of the grade 1–2 adverse events were also gastrointestinal, constitutional, or infectious, including diarrhoea (n=16), nausea (n=16), non-neutropenic infections (n=13), anorexia (n=10), vomiting (n=7), rash (n=10), fatigue (n=7), abdominal pain (n=6), weight loss (n=5), headache (n=5), and oedema (n=5). With respect to cardiac complications, no evidence of QT prolongation was observed. Only three patients experienced cardiac events. One patient, with a history of pulmonary hypertension that was probably secondary to bronchiectasis, developed grade 3 right ventricular failure with pleural effusions and oedema that was thought to be related to the pre-existing pulmonary disease. A second patient with a history of hypertension developed a grade 1 asymptomatic pericardial effusion on echocardiogram that resolved after discontinuation of the study drug and a third patient developed grade 2 ventricular tachycardia and grade 2 bradycardia in the setting of grade 4 influenza and bacterial meningitis.
Table 2.
Grade 3–4 Adverse events
| Toxicity | Number of patients with toxicity (%) | ||
|---|---|---|---|
| Grade 3 | Grade 4 | Grade 5 | |
| Haematological | |||
| Thrombocytopenia | 2 (10) | 4 (19) | 0 |
| Anaemia | 6 (29) | 0 | 0 |
| Neutropenia | 0 | 1 (5) | 0 |
| Leukopenia | 1 (5) | 0 | 0 |
| Non-Haematological | |||
| Constitutional | |||
| Fatigue | 1 (5) | 1 (5) | 0 |
| Myalgias | 1 | 0 | 0 |
| Cardiac | |||
| Oedema | 1 (5) | 0 | 0 |
| Hypertension | 1 (5) | 0 | 0 |
| Right ventricular failure | 1 (5) | 0 | 0 |
| Coagulation | |||
| Deep venous thrombosis | 1 (5) | 0 | 0 |
| Gastrointestinal | |||
| Abdominal pain | 1 (5) | 0 | 0 |
| Diarrhea | 2 (10) | 0 | 0 |
| Infectious | |||
| Febrile Neutropenia | 2 (10) | 1 (5) | 1 (5) |
| Infection without neutropenia | 5 (24) | 2 (10) | 1 (5) |
| Metabolic | |||
| Hypokalaemia | 1 (5) | 0 | 0 |
| Neurological | |||
| Hallucinations | 1 (5) | 0 | 0 |
| Pulmonary | |||
| Acute respiratory distress | 0 | 1 (5) | 0 |
Infections were common, with 2 deaths attributed to pneumonia. Reported grade 3–5 infections were febrile neutropenia (n=4), pneumonia (n=3), influenza (n=2), meningitis (n=1), cellulitis (n=1), and methicillin-resistant Stapholoccocus epidermidis bacteremia (n=1). Grade 1–2 infections included pneumonia (n=3), urinary tract infections (n=3), sinusitis (n=2), otitis externa (n=1), oral candidasis (n=1), orchitis/epididymitis (n=1), cellulitis (n=1), and folliculitis (n=1).
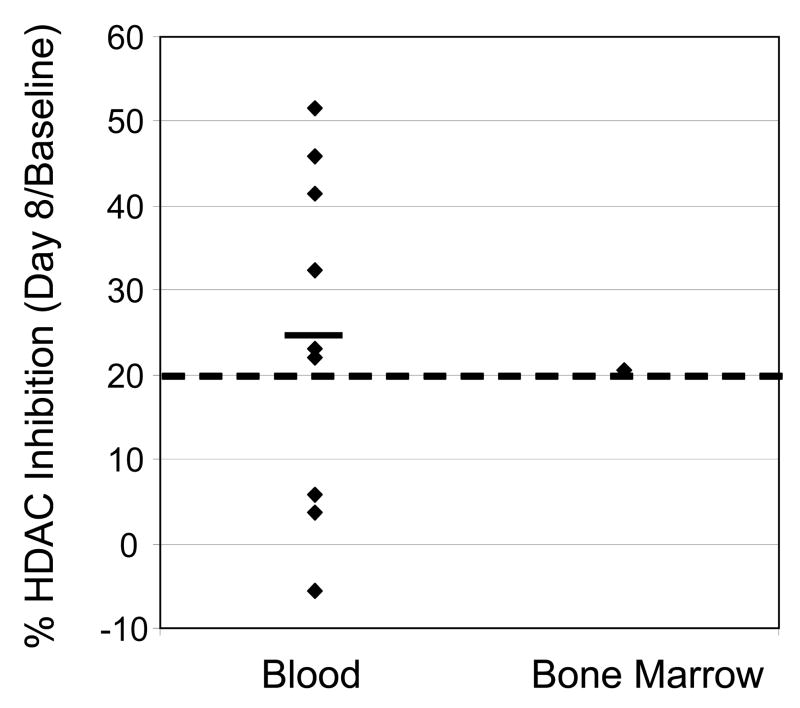
Pharmacodynamic studies
Bone marrow basal HDAC activity levels were comparable to those in peripheral white blood cells in the same patients (Table 3). The mean change in HDAC enzyme inhibition in 9 patients with pre-treatment and post-treatment (day 8) samples available was 24.5% (range −5.5% to 51.5%, Figure 2). In 6 of these 9 patients, HDAC inhibition greater than 20% was observed relative to pre-treatment values (Figure 2). In 21 patients, pre-treatment IL-6 plasma levels varied (ranging from < 1ng/l to 155.8 ng/l). IL-6 levels failed to correlate with fatigue; however, in 5 of 6 patients with grades 3–4 fever, IL-6 levels were increased 10-fold compared to pre-treatment levels.
Table 3.
Pre-treatment levels of HDAC enzyme activity in peripheral blood mononuclear cells compared to bone marrow cells from patients receiving MGCD0103 as measured by μM of deacetylated product released.
| Patient | Baseline HDAC activity (μM deacetylated product released) | |
|---|---|---|
| Peripheral blood mononuclear cells | Bone Marrow Cells | |
| 1 | 60.1 | 53.1 |
| 2 | 84.5 | NE |
| 3 | 75.8 | 77.1 |
| 4 | 71.4 | 60.0 |
| 5 | 43.1 | 45.6 |
| 6 | 75.6 | 81.9 |
| 7 | 47.6 | NE |
| 8 | 52.9 | 54.2 |
| 9 | 49.6 | 52.4 |
| 10 | 48.3 | NE |
| 11 | 96.1 | NE |
| 12 | 57.8 | 52.5 |
NE: not evaluable
Figure 2.
Percentage of HDAC Inhibition in Peripheral White Blood Cells or in Bone Marrow from CLL Patients After MGCD0103 Treatment (Day 8). Each dot represents a patient. Black line: average. Dotted line: 20% inhibition.
DISCUSSION
Preclinical evidence supporting the efficacy of histone deacetylase inhibitors in CLL in vitro has been published by several groups.(Aron, et al 2003, Bokelmann and Mahlknecht 2008, Byrd, et al 2005, Lucas, et al 2004, Zhang, et al 2004) In a clinical trial with depsipeptide,(Byrd, et al 2005) a class I specific HDAC inhibitor, modest evidence of clinical efficacy was demonstrated, but problematic fatigue and cardiac toxicity has limited its further development. Due to this toxicity, alternative class I HDAC inhibitors, including MGCD0103, are under evaluation in CLL. In the current study, pre-clinical activity in CLL cells was demonstrated, with loss of tumor cell viability and hyperacetylation of histone H3 after MGCD0103 exposure. This pre-clinical efficacy justified the pursuit of the herein described multi-center phase II trial of MGCD0103 in patients with relapsed and refractory CLL. Unfortunately in this phase II trial, limited single agent efficacy was observed, with stable disease in 20 of 21 patients after 0–12 cycles of MGCD0103. Prolonged administration for 5 or more cycles, dose escalation to 110 mg, and the addition of rituximab failed to improve MGCD0103’s activity. Collectively, this study, together with the previous trial of the more potent class I HDAC inhibitor depsipeptide (Byrd, et al 2005), suggests that alternative strategies using HDAC inhibitors in patients with CLL will be required including pursuit of non-selective or broad HDAC isotype inhibition or combination strategies based upon pre-clinical synergy studies with other novel targeted therapies.
Although four patients did demonstrate reductions lymphocyte count that could be construed as clinical benefit, constitutional symptoms associated with MGCD0103 were significant and frequently led to cessation of therapy. Despite the prolonged pharmacokinetic and pharmacodynamic half-life of MGCD0103 when compared to other HDAC inhibitors(Bonfils, et al 2008), which permits three times a week rather than daily oral dosing,(Garcia-Manero, et al 2008a, Siu, et al 2008) dose escalation and re-treatment with MGCD0103 in this trial were often prohibited by these side effects including nausea, vomiting, anorexia, diarrhoea, and severe fatigue. Such toxicities are similarly found with other class I HDAC inhibitors including depsipeptide in patients with CLL (Byrd, et al 2005) and in previous MGCD0103 phase I and II trials in AML, Hodgkin lymphoma, non-Hodgkin lymphoma, and solid tumours.(Bociek, et al 2008, Byrd, et al 2005, Crump, et al 2008, Duvic, et al 2007, Garcia-Manero, et al 2008a, Garcia-Manero, et al 2008b, Siu, et al 2008) In these trials, maximal or sustained HDAC inhibition with optimal steady state dose levels may not have been achievable due to toxicity, suggesting that alternative prolonged dosing schedules of HDAC inhibitors may enhance the clinical activity and HDAC enzyme inhibition with these agents in patients with CLL. In the current trial, the majority of patients could only tolerate up to 2 cycles of MGCD0103; however, four patients remained on study for 5–12 cycles, with no additional efficacy observed despite prolonged dosing in these 4 patients. In pharmacodynamic evaluations in patient-derived peripheral blood or bone marrow mononuclear cells in 6 of 9 patients with available samples on this trial, greater than 20% inhibition of HDAC activity was observed. It remains unclear if the 20% threshold is sufficient for therapeutic efficacy and further evaluation is warranted. Therefore, despite intermittent three times a week dosing of MGCD0103 that permitted several patients to remain on study for 5 or more cycles and preliminary evidence of HDAC inhibition, efficacy with this agent was limited in CLL, suggesting that either combination strategies with class I HDAC inhibitors or use of non-selective HDAC inhibitors may be necessary to fully appreciate the benefit of this class of agents in patients with relapsed CLL. Additionally, efforts to understand and effectively eliminate the constitutional symptoms observed with class I specific HDAC inhibitors in CLL will be important for prolonged therapy to be feasible.
The lack of response with MGCD0103 as a single agent in CLL raises the question of how to continue development of HDAC inhibitors in CLL. Combination strategies with HDAC inhibitors are currently in development in other hematological malignancies including combinations of HDAC inhibitors and DNA methyltransferase inhibitors,(Garcia-Manero, et al 2007) cell cycle regulatory agents (i.e. flavopiridol),(Grant, et al 2008) anti-apoptotic agents,(Inoue, et al 2007) bortezomib,(Badros, et al 2007, Heider, et al 2006) and conventional chemotherapeutic agents.(Hurwitz, et al 2008) These combination strategies may synergize with respect to inhibition of HDAC enzyme activity, ultimately permitting the use of less frequent and smaller doses of HDAC inhibitors which may not only improve the clinical efficacy of these agents but also limit cumulative toxicity. While consideration of HDAC inhibitor combinations with flavopiridol are reasonable given the clinical activity of this agent in CLL,(Byrd, et al 2007) alternative agents, such as bortezomib or the hypomethylating agent decitabine, are less attractive due to the lack of single agent activity.(Blum, et al 2008) In a subset of patients on this trial, the addition of rituximab to MGCD0103 was well tolerated, and this may also be incorporated into future combination approaches. Alternatively, pursuit of altered dosing schedules of isotype selective HDAC inhibitors or use of broad class I and II or class III HDAC inhibitors may also represent options for further study in CLL.
Acknowledgments
This study was supported by MethylGene Inc., which provided clinical research costs and MGCD0103 to the participating institutions. K.A. Blum is supported by National Cancer Institute grant K23 CA109004-01A1. J.C. Byrd and D.M. Lucas are supported by CA101956, the Leukemia and Lymphoma Society, and the D. Warren Brown Foundation.
Footnotes
Conflict of Interest Disclosure: J. Dumochel, M. Drouin, and R.E. Martell are employees of Methylgene. There are no relevant financial conflicts of interest to disclose for any of the investigators participating in this trial.
References
- Aron JL, Parthun MR, Marcucci G, Kitada S, Mone AP, Davis M, Shen T, Murphy T, Wickham J, Kanakry C, Lucas D, Reed J, Grever M, Byrd JC. Depsipeptide ( FR901228) induces histone acetylation and inhibition of histone deacetylase in chronic lymphocytic leukemia cells concurrent with activation of caspase 8-mediated apoptosis and down-regulation of c-FLIP protein. Blood. 2003;102:652–658. doi: 10.1182/blood-2002-12-3794. [DOI] [PubMed] [Google Scholar]
- Badros A, Philip S, Niesvizky R, Goloubeva O, Harris C, Zweibel J, Wright J, Burger A, Grant S, Baer MR, Egorin MJ. Phase I Trial of Suberoylanilide Hydroxamic Acid (SAHA) + Bortezomib (Bort) in Relapsed Multiple Myeloma (MM) Patients (pts) Blood (American Society of Hematology Annual Meeting Abstracts) 2007;110:1168. (abstr) [Google Scholar]
- Baur AS, Shaw P, Burri N, Delacretaz F, Bosman F, Chaubert P. Frequent methylation silencing of p15/INK4b (MTS2)and p16/INK4a (MTS1) in B-cell and T-cell lymphomas. Blood. 1999;94:1773–1781. [PubMed] [Google Scholar]
- Blum KA, Liu Z, Lucas DM, Baiocchi R, Lin TS, Benson D, Devine SM, Jones J, Andritsos L, Flynn J, Cheng P, Xie Z, Marcucci G, Chan KK, Grever MR, Byrd JC. A Phase I Evaluation of Low Dose Decitabine Targeting DNA Hypermethylation in Patients with Chronic Lymphocytic Leukemia (CLL) and Non-Hodgkin’s Lymphoma (NHL): Dose-Limiting Myelosuppression without Evidence of Hypomethylation. Blood (American Society of Hematology Annual Meeting Abstracts) 2008;112:3169. (abstr) [Google Scholar]
- Bociek R, Kuruvilla J, Pro B, Wedgewood A, Li Z, Drouin M, Patterson T, Ward R, Martell RE, Younes A. Isotype-selective histone deacetylase inhibitor MGCD0103 demonstrates clinical activity and safety in patients with relapsed/refractory classical Hodgkin lymphoma (HL) Journal of Clinical Oncology (Proceedings of the American Society of Clinical Oncology) 2008;26:8507. (abstr) [Google Scholar]
- Bokelmann I, Mahlknecht U. Valproic acid sensitizes chronic lymphocytic leukemia cells to apoptosis and restores the balance between pro- and antiapoptotic proteins. Molecular Medicine. 2008;14:20–27. doi: 10.2119/2007-00084.Bokelmann. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfils C, Kalita A, Dubay M, Siu LL, Carducci MA, Reid G, Martell RE, Besterman JM, Li Z. Evaluation of the pharmacodynamic effects of MGCD0103 from preclinical models to human using a novel HDAC enzyme assay. Clinical Cancer Research. 2008;14:3441–3449. doi: 10.1158/1078-0432.CCR-07-4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd JC, Shinn C, Ravi R, Willis CR, Waslenenko JK, Flinn IW, Dawson NA, Grever MR. Depsipeptide ( FR901228): a novel therapeutic agent with selective, in vitro activity against human B-cell chronic lymphocytic leukemia cells. Blood. 1999;94:1401–1408. [PubMed] [Google Scholar]
- Byrd JC, Kitada S, Flinn IW, Aron JL, Pearson M, Lucas D, Reed JC. The mechanism of tumor cell clearance by rituximab in vivo in patients with B-cell chronic lymphocytic leukemia: evidence of caspase activation and apoptosis induction. Blood. 2002;99:1038–1043. doi: 10.1182/blood.v99.3.1038. [DOI] [PubMed] [Google Scholar]
- Byrd JC, Marcucci G, Parthun MR, Xiao JJ, Klisovic RB, Moran M, Lin TS, Liu S, Sklenar AR, Davis ME, Lucas DM, Fischer B, Shank R, Tejaswi SL, Binkley P, Wright J, Chan KK, Grever MR. A phase 1 and pharmacodynamic study of depsipeptide (FK228) in chronic lymphocytic leukemia and acute myeloid leukemia. Blood. 2005;105:959–967. doi: 10.1182/blood-2004-05-1693. [DOI] [PubMed] [Google Scholar]
- Byrd JC, Lin TS, Dalton JT, Wu D, Phelps MA, Fischer B, Moran M, Blum KA, Rovin B, Brooker-McEldowney M, Broering S, Schaaf LJ, Johnson AJ, Lucas DM, Heerema NA, Lozanski G, Young DC, Suarez JR, Colevas AD, Grever MR. Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood. 2007;109:399–404. doi: 10.1182/blood-2006-05-020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheson BD, Bennett JM, Grever M, Kay N, Keating M, O’Brien S, Rai K. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]
- Costello JF, Fruhwald MC, Smiraglia DJ, Rush L, Robertson G, Gao X, Wright F, Feramisco J, Peltomaki P, Lang J, Schuller D, Yu L, Bloomfield C, Caligiuri M, Yates A, Nishikawa R, Su Huang H, Petrelli N, Zhang X, O’Dorisio M, Held W, Cavenee W, Plass C. Aberrant CpG-island methylation has non-random and tumor-type-specific patterns. Nature Genetics. 2000;25:132–138. doi: 10.1038/72785. [DOI] [PubMed] [Google Scholar]
- Crump M, Andreadis C, Assouline S, Rizzeria D, Wedgewood A, McLaughlin P, Laille E, Li Z, Martell RE, Younes A. Treatment of relapsed or refractory non-Hodgkin lymphoma with the oral isotype-selective histone deacetylase inhibitor MGCD0103: Interim results from a phase II study. Journal of Clinical Oncology (Proceedings of the American Society of Clinical Oncology) 2008;26:8528. (abstr) [Google Scholar]
- de Ruijter AJM, van Gennip AH, Caron HN, Kemp S, Van Kuilenburg A. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochemical Journal. 2003;370:737–749. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvic M, Olsen EA, Breneman D, Pacheco TR, Parker S, Vonderheid EC, Ricker JL, Rizvi S, Chen C, Boileau K, Cooley P, Geskin LJ. Vorinostat (Suberoylanilide Hydroxamic Acid, SAHA) Provides Prolonged Safety and Clinical Benefit to Patients with Advanced Cutaneous T-Cell Lymphoma (CTCL) Blood (American Society of Hematology Annual Meeting Abstracts) 2007;110:2582. (abstr) [Google Scholar]
- Fournel M, Bonfils C, Hou Y, Yan PT, Trachy-Bourget MC, Kalita A, Liu J, Lu AH, Zhou NZ, Robert MF, Gillespie J, Wang JJ, Ste-Croix H, Rahil J, Lefebvre S, Moradei O, Delorme D, Macleod AR, Besterman JM, Li Z. MGCD0103, a novel isotype-selective histone deacetylase inhibitor, has broad spectrum antitumor activity in vitro and in vivo. Molecular Cancer Therapeutics. 2008;7:759–768. doi: 10.1158/1535-7163.MCT-07-2026. [DOI] [PubMed] [Google Scholar]
- Garcia-Manero G, Yang A, Klimek V, Luger S, Newsome W, Berman N, Patterson T, Maroun C, Li Z, Ward R, Martell RE. Phase I/II study of the novel oral isotype-selective histone deacetylase (HDAC) inhibitor MGCD0103 in combination with azacitidine in patients with high risk myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML) Journal of Clinical Oncology (Proceedings of the American Society of Clinical Oncology) 2007;25:7062. (abstr) [Google Scholar]
- Garcia-Manero G, Assouline S, Cortes J, Estrov Z, Kantarjian H, Yang H, Newsome WM, Miller WH, Jr, Rousseau C, Kalita A, Bonfils C, Dubay M, Patterson TA, Li Z, Besterman JM, Reid G, Laille E, Martell RE, Minden M. Phase 1 study of the oral isotype specific histone deacetylase inhibitor MGCD0103 in leukemia. Blood. 2008a;112:981–989. doi: 10.1182/blood-2007-10-115873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Manero G, Yang H, Bueso-Ramos C, Ferrajoli A, Cortes J, Wierda WG, Faderl S, Koller C, Morris G, Rosner G, Loboda A, Fantin VR, Randolph SS, Hardwick JS, Reilly JF, Chen C, Ricker JL, Secrist JP, Richon VM, Frankel SR, Kantarjian HM. Phase 1 study of the histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid [SAHA]) in patients with advanced leukemias and myelodysplastic syndromes. Blood. 2008b;111:1060–1066. doi: 10.1182/blood-2007-06-098061. [DOI] [PubMed] [Google Scholar]
- Genini D, Adachi S, Chao Q, Rose D, Carrera C, Cottam H, Carson D, Leoni L. Deoxyadenosine analogs induce programmed cell death in chronic lymphocytic leukemia cells by damaging the DNA and by directly affecting the mitochondria. Blood. 2000;96:3537–3543. [PubMed] [Google Scholar]
- Grant S, Kolla S, Sirulnik LA, Shapiro G, Supko J, Cooper B, Perkins E, Ramakrishnan V, Espinoza-Delgado I, Tombes MB, Roberts JD. Phase I Trial of Vorinostat (SAHA) in Combination with Alvocidib (Flavopiridol) in Patients with Relapsed, Refractory or (Selected) Poor Prognosis Acute Leukemia or Refractory Anemia with Excess Blasts-2 (RAEB-2) Blood (American Society of Hematology Annual Meeting Abstracts) 2008;112:2986. (abstr) [Google Scholar]
- Heider U, Kaiser M, Zavrski I, Sterz J, Jakob C, Fleissner C, Hecht M, Kleeberg L, Braun C, Possinger K, Sezer O. Synergistic Interaction of the Histone Deacetylase Inhibitor SAHA with the Proteasome Inhibitor Bortezomib in Mantle Cell Lymphoma. Blood (American Society of Hematology Annual Meeting Abstracts) 2006;108:2509. (abstr) [Google Scholar]
- Hurwitz H, Nelson B, O’Dwyer P, Chiorean E, Gabrail N, Li Z, Laille E, Drouin M, Rothenberg M, Chan E. Phase I/II: The oral isotype-selective HDAC inhibitor MGCD0103 in combination with gemcitabine (Gem) in patients (pts) with refractory solid tumors. Journal of Clinical Oncology (Proceedings of the American Society of Clinical Oncology) 2008;26:4625. (abstr) [Google Scholar]
- Inoue S, Riley J, Gant TW, Dyer MJ, Cohen GM. Apoptosis induced by histone deacetylase inhibitors in leukemic cells is mediated by Bim and Noxa. Leukemia. 2007;21:1773–1782. doi: 10.1038/sj.leu.2404760. [DOI] [PubMed] [Google Scholar]
- Koduru PRK, Zariwala M, Soni M, Gong J, Xiong Y, Broome J. Deletion of cyclin-dependent kinase 4 inhibitor genes p15 and p15 in non-Hodgkin’s lymphoma. Blood. 1995;86:2900–2905. [PubMed] [Google Scholar]
- Lucas DM, Davis ME, Parthun MR, Mone AP, Kitada S, Cunningham KD, Flax EL, Wickham J, Reed JC, Byrd JC, Grever MR. The histone deacetylase inhibitor MS-275 induces caspase-dependent apoptosis in B-cell chronic lymphocytic leukemia cells. Leukemia. 2004;18:1207–1214. doi: 10.1038/sj.leu.2403388. [DOI] [PubMed] [Google Scholar]
- Siu LL, Pili R, Duran I, Messersmith WA, Chen EX, Sullivan R, MacLean M, King S, Brown S, Reid GK, Li Z, Kalita AM, Laille EJ, Besterman JM, Martell RE, Carducci MA. Phase I study of MGCD0103 given as a three-times-per-week oral dose in patients with advanced solid tumors. Journal of Clinical Oncology. 2008;26:1940–1947. doi: 10.1200/JCO.2007.14.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Freitas MA, Wickham J, Parthun MR, Klisovic MI, Marcucci G, Byrd JC. Differential expression of histone post-translational modifications in acute myeloid and chronic lymphocytic leukemia determined by high-pressure liquid chromatography and mass spectrometry. Journal of the American Society of Mass Spectrometry. 2004;15:77–86. doi: 10.1016/j.jasms.2003.10.001. [DOI] [PubMed] [Google Scholar]