Abstract
Background
Women over the age of 30 are the main beneficiaries of improved cervical cancer screening with human papillomavirus (HPV) DNA testing. The role of vaccination against HPV types 16 and 18, recommended routinely in pre-adolescent girls, is unclear in this age group.
Objective
To assess the health and economic outcomes of HPV vaccination in older women participating in the U.S. screening program.
Design
Cost-effectiveness analysis with an empirically-calibrated model.
Data Sources
Published literature.
Target Population
U.S. women, ages 35–45.
Time Horizon
Lifetime.
Perspective
Societal.
Interventions
HPV vaccination added to screening strategies that differ by test (cytology, HPV DNA testing), frequency, and start age, versus screening alone.
Outcome Measures
Incremental cost-effectiveness ratios (2006 U.S. dollars per quality-adjusted life year (QALY) gained).
Results of Base-Case Analysis
In the context of annual or biennial screening, HPV vaccination of women ages 35–45 ranged from $116,950 to $272,350 per QALY using cytology with HPV DNA testing for triage of equivocal results, and from $193,690 to $381,590 per QALY using combination cytology and HPV DNA testing, depending on age and screening frequency.
Results of Sensitivity Analysis
Probabilistic sensitivity analysis revealed that the probability of HPV vaccination being cost-effective for women ages 35–45 was 0% when screening occurred annually or biennially, and <5% when screening occurred triennially, at thresholds considered good value for money.
Limitations
Uncertainty in the natural history of disease and vaccine efficacy in older women.
Conclusions
Given currently available information, the effectiveness of HPV vaccination of screened women over age 30 appears, on average, to be small. Compared with current screening that uses sensitive HPV DNA testing, HPV vaccination in this older population is associated with cost-effectiveness ratios that are less attractive than well-accepted interventions in the U.S.
Introduction
Organized screening is widely credited with reducing the incidence of cervical cancer, and today, women in the United States face an average lifetime risk of 0.7% (1). With the availability of human papillomavirus (HPV) DNA testing, clinical guidelines have been revised to provide several screening options including cytology screening at one- to three-year intervals with HPV DNA testing for triage of equivocal cytology results and HPV DNA testing in combination with cytology at two- to three-year intervals for women over the age of 30 (2–4). Previous analyses have reported that these strategies provide not only greater protection against cervical cancer than cytology-only strategies, but also good value for resources (i.e., are cost-effective) compared with other public health interventions that have been adopted (5, 6). As technologies continue to evolve, it is imperative to assess the comparative benefits, risks, and costs of all options in an objective analysis. This principle applies to newer screening tests, novel diagnostic algorithms for screen-positive women, and evolving technologies for primary prevention, such as the HPV vaccine.
In 2006, the U.S. Food and Drug Administration licensed a quadrivalent vaccine that protects against HPV types 16 and 18, two of the most common types that cause 70% of cervical cancer, as well as types 6 and 11, two types that cause over 90% of genital warts (7, 8). A bivalent vaccine, targeting only HPV types 16 and 18, is expected to be licensed soon in the U.S. Because of the high efficacy of the vaccines among females without prior exposure to these types (9–13), current guidelines for HPV vaccination in the U.S. have prioritized covering pre-adolescent girls prior to sexual debut (ages 11–12, and as early as age 9) (14, 15). The recommended upper age limit for a catch-up program, however, has been debated and ranges from age 18 (14) to 26 (15). In a recent cost-effectiveness analysis, we found that a policy of catch-up vaccination in females past age 21 generally does not provide as good value for money as vaccination of younger girls, even under favorable assumptions of the vaccine (16).
Women over age 30 have been the primary target for improved screening technology with HPV DNA testing but may soon be able to access the HPV vaccine. In comparison to adolescent girls, women over age 30 have a greater chance of previous HPV infection at some point in their lives, although there are no commercially available tests that can reliably distinguish those who have or have not been infected. As manufacturers of the vaccines seek approval for vaccinating women of older ages, there is mounting discussion about the magnitude of benefit and costs associated with vaccinating women up to age 45. If approved, current HPV vaccination guidelines will need to be reconsidered, and potentially revised, to provide scientifically-based guidance for this population.
The evaluation of cervical cancer prevention strategies presents particular challenges because of the long duration of cervical carcinogenesis, the uncertainty in a disease process that is largely unobservable, and the fact that interventions are applied at different time points along the disease spectrum. Further, clinical studies that compare screening strategies or assess vaccine efficacy mostly rely on surrogate endpoints, and the observation of these interventions on disease outcomes will be decades away. When this uncertainty is coupled with the inability to compare head-to-head all potential strategies, disease simulation models that synthesize the best available data and ensure consistency with epidemiological observations are valuable tools to estimate long-term outcomes of health interventions in a population. When used in a decision-analytic framework, model-based analyses can help assess the incremental benefits and cost-effectiveness of different interventions to inform policy decisions that are being made in the absence of complete information. In anticipation of potential vaccine approval for women over age 30, we used an empirically-calibrated model to conduct a comparative cost-effectiveness analysis of HPV vaccination of U.S. women up to the age of 45 in the context of available cervical cancer screening.
Methods
Model Overview
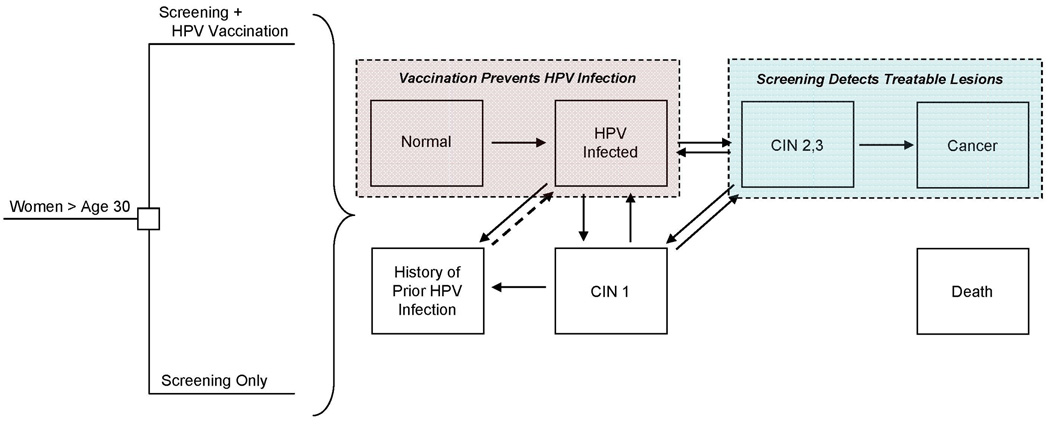
We used a previously-developed individual-based (“first-order”) Monte Carlo simulation model that simulates the natural history of HPV and cervical disease, as well as primary and secondary preventive interventions (16–18). The model is comprised of mutually-exclusive health states, among which individual women transition over time from entry in the model until death (Figure 1). The simulation begins with 9-year-old healthy girls, who at each month can acquire an HPV infection, categorized as HPV-16, HPV-18, other high-risk types, and low-risk types. Those with an HPV infection can develop precancerous lesions, categorized as cervical intraepithelial neoplasia, grade 1 (CIN 1) or grade 2–3 (CIN 2,3), and those with CIN 2,3 may develop invasive cancer. Females can clear their HPV infection or lesion; after first infection and clearance, women develop a degree of natural immunity to that same HPV type, after which future type-specific infections can be acquired at a reduced rate. Cancer states are stratified by stage (i.e., local, regional, distant) and detection status (i.e., undetected, symptom-detected, screen-detected). Death can occur from all-cause mortality from every health state and excess cancer-specific mortality from cancer states, depending on stage of cancer. The model can accommodate complex cervical cancer prevention strategies and tracks each individual woman’s history (e.g., vaccination, screening, treatment and past abnormalities) and resource use.
Figure 1. Schematic of Decision Tree and Cervical Cancer Natural History Model.
Women over age 30 (i.e., ages 35, 40, or 45) who participate in the U.S. screening program are faced with the decision to get vaccinated or continue with screening only. HPV vaccination (pink box) involves full adherence to the three-dose series at ages 35, 40, or 45 and provides complete, lifelong protection against incidence of vaccine-targeted HPV types among women without prior history of those types at vaccination. Screening (blue box) involves detection of precancerous lesions that can be treated before development of invasive cancer and may vary by test (i.e., cytology with HPV DNA testing as triage for equivocal results, with or without a switch to combined cytology and HPV DNA testing) and frequency (i.e., 1- to 5-year screening). At the start of the analysis, women may reside in any of the mutually-exclusive, collectively-exhaustive health states denoted by the boxes. Incidence and progression of HPV infection and cervical intraepithelial neoplasia, grade 1 (CIN 1) and grade 2,3 (CIN 2,3) depend on age and HPV type, categorized as type 16, type 18, other high-risk types, and low-risk types. Women who clear their infections or lesions are noted as having a history of prior HPV infection and face reduced risks of subsequent type-specific HPV infection due to natural immunity. Cancer states are stratified by stage (i.e., local, regional, distant) and detection status (i.e., undetected, symptom-detected, screen-detected). Death can occur from all-cause mortality from every health state and excess cancer-specific mortality from cancer states. Note, not all health states and transitions are shown.
Using data from epidemiological studies and cancer registries, we established initial input parameter values for the natural history model (i.e., without intervention). Using a likelihood-based approach, the model was then calibrated to fit to empirical data, including age-specific HPV prevalence, age-specific incidence of cervical cancer, and HPV type distribution among women with lesions and cancer, primarily from the U.S. (19–25). Data from clinical studies and controlled trials were synthesized to accurately reflect the performance of screening tests and HPV vaccine (4, 9–13, 26–28). Details of the model structure, parameterization, and calibration have been described in previous publications (16–18), and specific data used for the current analysis are available in the Supplemental Appendix.
Strategies
First, we evaluated the cost-effectiveness of vaccinating women of a particular age (e.g., 35 years) who had been participating in a specific screening strategy (e.g., biennial cytology). This analysis was intended to simulate a situation where women would continue with the same screening strategy before and after vaccination. We included screening strategies that have been recommended in clinical guidelines, including cytology with HPV DNA testing for triage of equivocal results (“cytology with HPV triage”) and combined cytology and HPV DNA testing after age 30, conducted annually and biennially (2–4). To account for women with less frequent or variable screening histories, we also included a scenario that reflects overall current screening practice. We restricted this analysis to only those who have ever been screened and assumed 53% are screened annually, 17% every two years, 11% every three years, and 15% every five years (29–31).
Second, we evaluated a broader array of technology options that confront women of a particular age. This analysis was intended to simulate a situation where a woman at a particular age (e.g., age 35) would consider all available screening options, with and without vaccination. We included currently recommended screening strategies (cytology with HPV triage, with or without a switch to combined cytology and HPV DNA testing at older ages), as well as promising strategies being evaluated in clinical studies (i.e., HPV DNA testing with cytology triage of HPV-positive results at older ages). For all strategies, we assumed women are screened using cytology with HPV triage prior to facing the full range of new options.
Vaccination involved the full three-dose series administered to women at the ages of 35, 40, or 45. In the base case analysis, we assumed 100% efficacy against HPV-16 and -18 over the lifetime among women without prior exposure to these specific types but explored the impact of lower efficacy and waning immunity in sensitivity analysis.
Analysis
Health benefits were expressed as reductions in lifetime risk of cervical cancer and gains in quality-adjusted life years (QALY), which reflect both morbidity (e.g., diminished quality of life due to cervical cancer) and mortality secondary to cervical cancer. Lifetime costs (in 2006 U.S. dollars) included direct medical costs associated with screening, diagnosis, and treatment (e.g., tests, procedures, hospitalizations) and with vaccination (e.g., three doses at $120 per dose, wastage, supplies and administration) (Table 1) (4, 5, 26–28, 32–40). Direct non-medical costs, such as patient time and transportation, were included for all strategies.
Table 1.
Cervical Cancer Screening and Cost Parameters *
| Variable | Input values |
|---|---|
| Test characteristics (%) | |
| Cytology (26–28) † | |
| Sensitivity (CIN 1/CIN 2,3) | 70/80 |
| Specificity | 95 |
| HPV DNA test (4, 28) ‡ | |
| Sensitivity (CIN 1/CIN 2,3) | 83/93 |
| Specificity | 93 |
| Costs (2006 US dollars) § | |
| HPV vaccine (per dose) (32–35) ∥ | |
| Vaccine and wastage | 134 |
| Supplies and administration | 9 |
| Patient time and transport | 24 |
| Screening test (5, 36–39) | |
| Cytology | 32 |
| HPV DNA test (Hybrid Capture II) | 49 |
| Office visit | 27 |
| Patient time and transport | 26 |
| Diagnostic follow-up (5, 36–38) | |
| Colposcopy | 364 |
| Biopsy | 53 |
| Office visit | 61 |
| Patient time and transport | 51 |
| Treatment for CIN 2,3 (5) ¶ | 3,438 |
| Treatment for cervical cancer (5) ¶ | |
| Local invasive cancer | 26,123 |
| Regional invasive cancer | 27,958 |
| Distant invasive cancer | 44,780 |
CIN, cervical intraepithelial neoplasia, grade 1 (CIN 1) or grade 2,3 (CIN 2,3); HPV, human papillomavirus.
Sensitivity for detecting CIN 2,3 was calculated as the weighted average of values from two recent studies reporting conventional and liquid-based cytology sensitivities using an ASCUS+ threshold (27, 28).
HPV DNA testing is assumed to be 100% sensitive (specific) in detecting the presence (absence) of high-risk HPV types. When this assumption is made, the model generates an implied clinical sensitivity for detecting CIN 1 and CIN 2,3 of 83.0% and 93.0%, respectively, and specificity of 93.0%.
Costs were inflation-adjusted to constant 2006 U.S. dollars (USD) using the medical component of the Consumer Price Index (40).
Vaccination assumes three doses; cost per vaccinated individual is $500.
Treatment costs are inclusive of cost of procedures, office visit, and woman’s time.
We adopted a societal perspective and discounted costs and benefits by 3% annually (41). After eliminating strategies that were more costly and less effective (i.e., strongly dominated) or less costly and less cost-effective (i.e., weakly dominated) than an alternative strategy, incremental cost-effectiveness ratios were calculated as the additional cost divided by the additional health benefit associated with one strategy compared to the next-less-costly strategy.
Sensitivity analyses were conducted to explore how results were influenced by uncertainties, such as screening performance, vaccine efficacy and duration, and vaccine cost. A probabilistic sensitivity analysis was conducted using 50 good-fitting parameter sets.
Role of the Funding Source
This study was supported by the National Cancer Institute (R01 CA93435), and in part by the Centers for Disease Control and Prevention, and the American Cancer Society. The funding sources had no involvement in the design of the study; collection, analysis, or interpretation of the data; or preparation, review, or approval of the finished manuscript.
Results
HPV-16,-18 Vaccination of Screened Women, by Age and Specific Screening Algorithm
Adding HPV vaccination to screening resulted in gains in quality-adjusted life expectancy, although the incremental gains diminished with age. For example, for women who had been screened biennially using cytology with HPV DNA testing for triage of equivocal results, the QALY gains with vaccination were 0.0040 (35 hours) when vaccination occurred at age 35, and 0.0029 (26 hours) when vaccination occurred at age 45; these results correspond to additional reductions in lifetime cancer risk of 5.4% and 4.2%, respectively. Incremental benefits with vaccination were lower when screening was annual or when screening involved a switch to combined cytology and HPV DNA testing after age 30.
Among women undergoing annual or biennial screening using cytology with HPV triage, adding vaccination ranged from $116,950 to $272,350 per QALY gained compared to the corresponding strategies of screening without vaccination, depending on age and screening frequency (Figure 2, top). For those women who switched to combination cytology and HPV DNA testing after age 30, the incremental cost-effectiveness ratios were less attractive (i.e., higher), ranging from $193,690 to $381,590 per QALY (Figure 2, bottom). In the context of current U.S. screening patterns, in which women are screened with variable frequency, adding vaccination exceeded $125,000 per QALY irrespective of vaccination age or screening strategy. For all scenarios evaluated, the incremental cost-effectiveness of adding vaccination to screening was less attractive (i.e., had higher ratios) at older ages.
Figure 2. Cost-Effectiveness of HPV-16,-18 Vaccination of Screened Women and Specific Screening Algorithm.
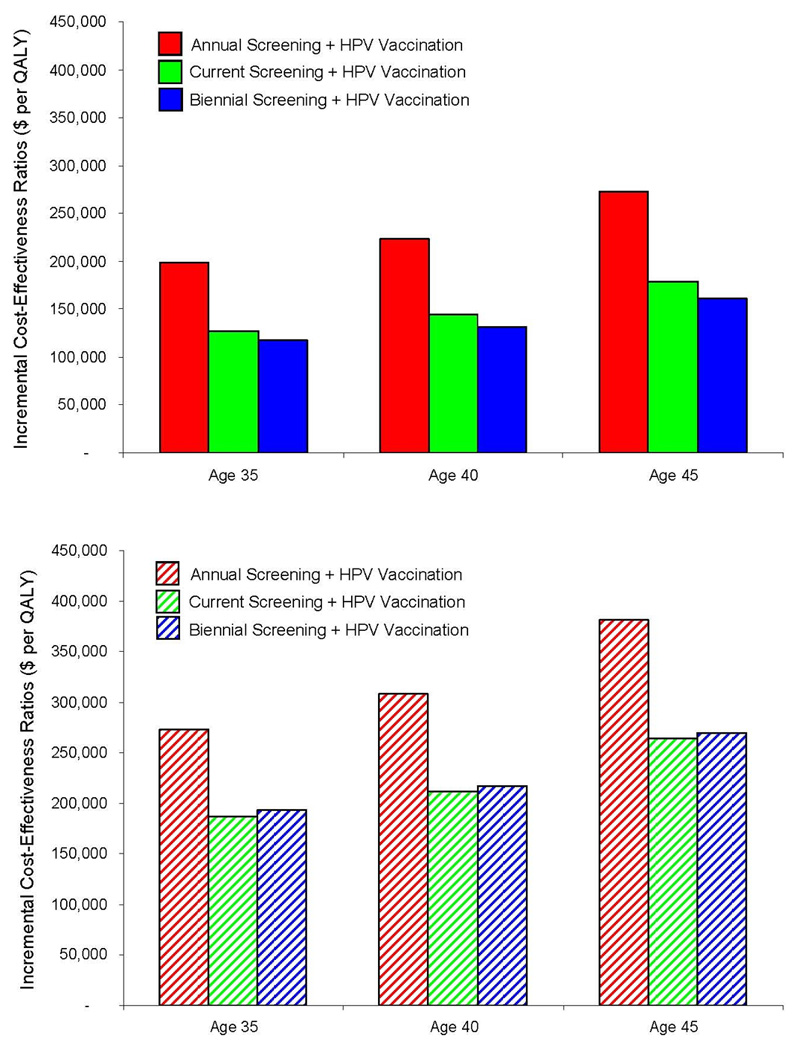
Bars indicate the incremental cost-effectiveness ratios of adding HPV-16,-18 vaccination to cervical cancer screening for women who are 35, 40, and 45 years of age, when screening involves cytology with HPV DNA testing for triage of equivocal results, with no switch (top) and with a switch to combined cytology and HPV DNA testing after age 30 (bottom). Red represents the strategy of HPV vaccination in the context of annual screening; green, current screening; blue, biennial screening. Current screening assumes that among women who have ever been screened, 53% are screened annually, 17% every two years, 11% every three years, and 15% every five years (29–31). Ratios for each vaccination strategy are calculated compared to the corresponding screening strategy without vaccination; for example, HPV vaccination in the context of biennial screening for 40-year-old women is compared with biennial screening without vaccination for 40-year-old women. All ratios are expressed as cost per quality-adjusted life year ($ per QALY). All costs are expressed in 2006 U.S. dollars.
HPV-16,-18 Vaccination and Screening Strategies, by Age and Screening Frequency
When we comparatively assessed the health and economic outcomes of a range of cervical cancer prevention options facing “today’s older woman” (i.e., different screening tests and frequencies, with and without vaccination), we found that most vaccination strategies were less efficient (i.e., strongly or weakly dominated) than strategies involving screening alone, or had cost-effectiveness ratios generally exceeding $100,000 per QALY (Table 2). For example, the cost-effectiveness of adding vaccination to annual screening for 35-year-old women ranged from nearly $200,000 per QALY (cytology with HPV triage over the lifetime) to over $400,000 per QALY (cytology with switch to combined testing). At 2- to 3-year screening intervals, vaccination strategies were either weakly dominated or exceeded $130,000 per QALY; ratios fell below $100,000 per QALY with less frequent screening. For 45-year-old women, these ratios were even less attractive. When HPV DNA testing with cytology triage for HPV-positive results was considered instead of combined cytology and HPV DNA testing for screening post-vaccination (not shown), the ratios associated with vaccination strategies were marginally more attractive, but the overall qualitative results were similar.
Table 2.
Cost-Effectiveness of HPV-16,-18 Vaccination and Screening Strategies, by Age and Screening Frequency *
| Strategy † | 1-year | 2-year | 3-year | 4-year | 5-year |
|---|---|---|---|---|---|
| For 35-year-old women | |||||
| Cytology with HPV triage + No vaccination |
--- | --- | --- | --- | --- |
| Cytology with HPV triage + Vaccination |
198,362 | not cost-effective | not cost-effective | not cost-effective | not cost-effective |
| Combined cytology and HPV testing + No vaccination |
not cost-effective | 99,315 | 51,319 | 35,996 | 28,366 |
| Combined cytology and HPV testing + Vaccination |
433,385 | 193,568 | 131,832 | 99,905 | 78,751 |
| For 45-year-old women | |||||
| Cytology with HPV triage + No vaccination |
--- | --- | --- | --- | --- |
| Cytology with HPV triage + Vaccination |
272,346 | not cost-effective | not cost-effective | dominated | dominated |
| Combined cytology and HPV testing + No vaccination |
not cost-effective | 102,703 | 53,631 | 38,851 | 27,517 |
| Combined cytology and HPV testing + Vaccination |
448,989 | 269,217 | 186,886 | 140,658 | 108,416 |
Values represent incremental cost-effectiveness ratios (additional cost divided by additional health benefit compared to the next less costly strategy) expressed as cost per quality-adjusted life year ($ per QALY). Strategies that are “dominated” are more costly and less effective than another strategy; strategies that are “not cost-effective” are less costly but less cost-effective than another strategy. All costs are expressed in 2006 U.S. dollars.
All screening tests include cytology with HPV DNA testing for triage of equivocal results up to the current age, with either continued cytology screening with HPV triage or switch to combined cytology and HPV DNA testing past current age. Strategies are listed in order of increasing lifetime costs.
Sensitivity Analyses
The general results were not influenced by plausible changes in screening test performance, screening and diagnostic follow-up costs, or the discount rate. Less favorable assumptions regarding vaccine properties, such as efficacy and duration of protection, resulted in higher (i.e., less attractive) cost-effectiveness ratios for the vaccination strategies. For example, when efficacy was reduced to 70% among those without prior exposure to vaccine-types, cost-effectiveness ratios associated with HPV vaccination increased by roughly 50% across all ages and screening scenarios, exceeding $400,000 per QALY for 45-year-old women. Similarly, cost-effectiveness ratios exceeded $400,000 per QALY and $200,000 per QALY for all ages when vaccine protection waned completely after 5 years and 10 years, respectively.
Results were modestly influenced by varying the cost of vaccination. When the cost per vaccinated woman was lowered to $250 (corresponding to a cost per dose of $70), adding vaccination to cytology with HPV triage for 35-year-old women decreased to $54,000 per QALY (biennial screening) and $92,000 per QALY (annual screening). When the cost per vaccinated woman was increased to $750, resembling a scenario in which the current costs are underestimated or a booster dose is required, the cost-effectiveness ratios ranged from $180,000 per QALY to $600,000 per QALY, depending on age, screening modality and frequency.
We conducted a probabilistic sensitivity analysis using 50 parameter sets with good fit to the epidemiological data, and estimated the probability that adding vaccination to screening is cost-effective according to lower- and upper-bound cost-effectiveness thresholds. For 35-year-old women, adding vaccination to annual or biennial screening with combined cytology and HPV testing resulted in cost-effectiveness ratios that exceeded $100,000 per QALY over all 50 simulations. In 96% and 72% of simulations involving 3-year and 4-year screening, respectively, adding vaccination was greater than $100,000 per QALY, and none of these scenarios resulted in ratios that were less than $50,000 per QALY across the 50 simulations. Across these same frequencies, for 45-year-old women, 100% of simulations resulted in ratios higher than $100,000 per QALY for vaccination and screening compared to screening alone.
Discussion
There is great promise in the availability of accurate HPV diagnostics, new screening strategies, and a preventive vaccine against HPV-16 and HPV-18 for cervical cancer prevention in the U.S. Model-based decision analyses of how to best use these new options alone or synergistically can provide insight for policy deliberations and professional guidelines, as well as aid in identifying research priorities. Although previous analyses have evaluated the HPV vaccine in the context of current guidelines for catch-up programs up to age 26 (16, 42, 43), to our knowledge, our study is the first to assess the vaccine’s routine use in an older population of U.S. women. Consistent with the general consensus that the value of HPV vaccination diminishes with increasing age of vaccination (16, 43–45), we found that HPV vaccination provides nominal benefits in the context of current screening recommendations and practice among women in their 30s and 40s. Considering the lifetime risk of cervical cancer in the U.S. is less than 1% (1), the absolute risk reductions provided by HPV vaccination in this age group are quite low. Likewise, the incremental cost-effectiveness ratios associated with adding vaccination to screening, given currently available information, exceeded $100,000 per QALY in most instances. These results were stable even when evaluating new, promising screening algorithms using HPV DNA testing with cytology triage, which is expected to have a higher positive predictive value than cytology testing alone post-vaccination (46).
There is no universal criterion that defines a threshold cost-effectiveness ratio, below which an intervention would be considered “good value for money.” Unlike some countries, the U.S. has not adopted an absolute cost-effectiveness threshold. Rather, informal heuristics have been cited, including the cost-effectiveness ratio associated with renal dialysis through the Medicare entitlement program (ranging from $55,000 to $108,500 per QALY (47–50)) to those associated with widely-adopted interventions, such as diabetes care, knee replacement, and mammography screening (generally below $100,000 - and often below $50,000 - per QALY (51–53)). Most recently, Shiroiwa et al. (54) measured thresholds in selected countries and reported $62,000 per QALY gained in the U.S. Based on these considerations, we feel that a range of $50,000 to $100,000 per QALY gained is a reasonable benchmark for cost-effectiveness in the U.S., although it is important to note that societal willingness to pay more than $100,000 per QALY may be based on other considerations. Using this threshold range, our results suggest that a policy of HPV vaccination in older women does not represent good value for resources expended, implying that more health can be gained by investing in alternative health interventions, such as screening previously unscreened women.
The vaccine’s impact in an older population is influenced in part by the level of prior exposure to the vaccine-targeted HPV types. Clinical trials have reported that the majority of female participants up to age 26 were not exposed to any vaccine-type at enrollment (55, 56), and therefore stand to benefit completely from the vaccine; however, because the trials excluded women with more than four sexual partners, it is unclear whether the trial population is representative of the general U.S. population with respect to exposure status and how these data extend to women up to 45 years of age. In order to explore the uncertainty in the natural history of disease, including prior exposure to HPV, we conducted a probabilistic analysis using 50 distinct parameter sets that fit well to the empirical data on HPV and cervical disease in the U.S. We found that the probability of HPV vaccination being cost-effective for screened women ages 35 to 45 was quite low at a threshold of $100,000 per QALY, even at extended screening intervals.
This analysis captures the average health and economic impact of the interventions over an entire population, intended primarily to inform the comparative effectiveness of health services, a priority recently highlighted in the American Recovery and Reinvestment Act of 2009 (57). Despite its policy focus, the analysis can also provide insights into clinical decision-making for women with particular screening histories who may benefit differentially from vaccination and screening. Specifically, our study provides estimates of the potential added value of vaccination compared not only to the counterfactual scenario (i.e., continuation of screening without vaccination), but also to new strategies that could involve revised screening algorithms and testing options (e.g., HPV DNA testing with cytology triage). Although, on average, we found that HPV vaccination does not provide good value among women of older age groups, there are undoubtedly individuals who could benefit from the vaccine. Because there is no conclusive test to identify an individual’s prior infection history, decision making for an individual will need to involve information about the person’s risk of HPV exposure (i.e., number of sexual partners) and particular screening history (i.e., compliance and frequency), as well as the woman’s preferences.
Limitations include uncertainty in the natural history of cervical disease, particularly in older women. As previously noted (58), whether an HPV infection detected at older ages is a newly-acquired infection or one that was acquired many years before and has re-emerged will influence the vaccine’s impact in older women, but is subject to much uncertainty and debate. Our probabilistic analysis attempts to explore plausible scenarios of natural history uncertainties, while maintaining a good fit to data that are available from empirical studies. Also, vaccine efficacy data using HPV infection and cervical disease endpoints are only available for five years and are only recently being presented for women in older age groups (59, 60). In our analysis, we optimistically assumed that women up to age 45 were fully compliant to the three-dose vaccine series, and that those without prior exposure to vaccine-types received complete lifelong protection from the vaccine. Given these optimistic assumptions, our results could be considered a “best-case scenario”; to the extent that efficacy is lower or of shorter duration, cost-effectiveness ratios for vaccination strategies may be even less attractive than presented in the current study.
This analysis did not consider the effects of reduced HPV transmission attributable to vaccination of older women, resulting in herd immunity benefits. We also did not include the vaccine’s potential cross-protective effects against other high-risk HPV infections nor the benefits related to other HPV-associated conditions, such as other anogenital, oral, and oropharyngeal cancers; the natural histories of these conditions - and contribution of HPV-16,-18 - are far less certain, and vaccine efficacy data on these outcomes are limited. Previous cost-effectiveness studies that have included some or all of these factors have suggested that their inclusion does not offset the diminished efficacy among women of older ages (16, 61).
It is important to note that we did not incorporate the risks of adverse events or diminished quality of life from vaccination (e.g., side effects) or screening (e.g., overtreatment). Even though small risks of minor adverse events from either intervention will likely be outweighed by the overall average benefits at the population level, as data become available, studies should incorporate all risks and costs associated with a vaccination program. We also assumed that a woman’s screening interval would not change post-vaccination; since a woman’s particular history of vaccine-type HPV exposure - and therefore her level of vaccine protection - is unknowable with certainty in clinical practice, we assumed that extending a woman’s screening interval without more information would be unjustifiable. Finally, this analysis is not relevant for women who have never been screened, who may comprise up to 5% of screen-eligible women in the U.S. (29–31). Both vaccination and screening will likely have beneficial effects in this population, but the magnitude of benefit from either approach will depend on important factors, such as prior exposure to vaccine-targeted HPV types and compliance with the three necessary doses and screening visits.
Using information that is available now, our results indicate that HPV vaccination of older women participating in the U.S. screening program provides much lower benefits than vaccination of pre-adolescent girls and does not provide good health value for the resources invested, compared with well-accepted health interventions in the U.S. It will be important to revisit this analysis as more information becomes available regarding the natural history of HPV and vaccine impact in older women.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the contributions of the cervical cancer prevention team at the Center for Health Decision Science (Harvard School of Public Health).
Grant Support
The authors are supported by grants from the National Cancer Institute (R01 CA93435), the Centers for Disease Control and Prevention, and the American Cancer Society, and are also supported by the Bill and Melinda Gates Foundation (30505) for related work in developing countries.
Footnotes
Publisher's Disclaimer: This is the prepublication, author-produced version of a manuscript accepted for publication in Annals of Internal Medicine. This version does not include post-acceptance editing and formatting. The American College of Physicians, the publisher of Annals of Internal Medicine, is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to www.annals.org or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record.
References
- 1.National Cancer Institute. [Last accessed July 1, 2009];Surveillance, Epidemiology, End Results (SEER) Cancer Statistics Review. 1975–2001 http://seer.cancer.gov/csr/1975_2001/
- 2.Saslow D, Runowicz CD, Solomon D, Moscicki AB, Smith RA, Eyre HJ, et al. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin. 2002;52(6):342–362. doi: 10.3322/canjclin.52.6.342. [DOI] [PubMed] [Google Scholar]
- 3.American College of Obstetricians and Gynecologists. ACOG practice bulletin. Cervical cytology screening. Number 45, August 2003. Int J Gynaecol Obstet. 2003;83(2):237–247. doi: 10.1016/s0020-7292(03)00412-0. [DOI] [PubMed] [Google Scholar]
- 4.Wright TC, Jr, Schiffman M, Solomon D, Cox JT, Garcia F, Goldie S, et al. Interim guidance for the use of human papillomavirus DNA testing as an adjunct to cervical cytology for screening. Obstet Gynecol. 2004;103(2):304–309. doi: 10.1097/01.AOG.0000109426.82624.f8. [DOI] [PubMed] [Google Scholar]
- 5.Goldie SJ, Kim JJ, Wright TC. Cost-effectiveness of human papillomavirus DNA testing for cervical cancer screening in women aged 30 years or more. Obstet Gynecol. 2004;103(4):619–631. doi: 10.1097/01.AOG.0000120143.50098.c7. [DOI] [PubMed] [Google Scholar]
- 6.Goldhaber-Fiebert JD, Stout NK, Salomon JA, Kuntz KM, Goldie SJ. Cost-effectiveness of cervical cancer screening with human papillomavirus (HPV) DNA testing and HPV-16,18 vaccination. J Natl Cancer Inst. 2008;100(5):308–320. doi: 10.1093/jnci/djn019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006;24 Suppl 3:S11–S25. doi: 10.1016/j.vaccine.2006.05.111. [DOI] [PubMed] [Google Scholar]
- 8.Lacey CJ, Lowndes CM, Shah KV. Chapter 4: Burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine. 2006;24 Suppl 3:S35–S41. doi: 10.1016/j.vaccine.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 9.Future II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 10.Ault KA. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369(9576):1861–1868. doi: 10.1016/S0140-6736(07)60852-6. [DOI] [PubMed] [Google Scholar]
- 11.Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 12.Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369(9580):2161–2170. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 13.Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006;367(9518):1247–1255. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 14.Saslow D, Castle PE, Cox JT, Davey DD, Einstein MH, Ferris DG, et al. American Cancer Society Guideline for human papillomavirus (HPV) vaccine use to prevent cervical cancer and its precursors. CA Cancer J Clin. 2007;57(1):7–28. doi: 10.3322/canjclin.57.1.7. [DOI] [PubMed] [Google Scholar]
- 15.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56(RR2):1–24. [PubMed] [Google Scholar]
- 16.Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med. 2008;359(8):821–832. doi: 10.1056/NEJMsa0707052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JJ, Kuntz KM, Stout NK, Mahmud S, Villa LL, Franco EL, et al. Multiparameter calibration of a natural history model of cervical cancer. Am J Epidemiol. 2007;166(2):137–150. doi: 10.1093/aje/kwm086. [DOI] [PubMed] [Google Scholar]
- 18.Goldhaber-Fiebert JD, Stout NK, Ortendahl J, Kuntz KM, Goldie SJ, Salomon JA. Modeling human papillomavirus and cervical cancer in the United States for analyses of screening and vaccination. Popul Health Metr. 2007;5:11. doi: 10.1186/1478-7954-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunne EF, Unger ER, Sternberg M, McQuillan G, Swan DC, Patel SS, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297(8):813–819. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- 20.Clifford G, Franceschi S, Diaz M, Munoz N, Villa LL. Chapter 3: HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine. 2006;24 Suppl 3:S26–S34. doi: 10.1016/j.vaccine.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 21.Clifford GM, Gallus S, Herrero R, Munoz N, Snijders PJ, Vaccarella S, et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: a pooled analysis. Lancet. 2005;366(9490):991–998. doi: 10.1016/S0140-6736(05)67069-9. [DOI] [PubMed] [Google Scholar]
- 22.Clifford GM, Rana RK, Franceschi S, Smith JS, Gough G, Pimenta JM. Human papillomavirus genotype distribution in low-grade cervical lesions: comparison by geographic region and with cervical cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(5):1157–1164. doi: 10.1158/1055-9965.EPI-04-0812. [DOI] [PubMed] [Google Scholar]
- 23.Clifford GM, Smith JS, Aguado T, Franceschi S. Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: a meta-analysis. Br J Cancer. 2003;89(1):101–105. doi: 10.1038/sj.bjc.6601024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clifford GM, Smith JS, Plummer M, Munoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer. 2003;88(1):63–73. doi: 10.1038/sj.bjc.6600688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parkin DM, Whelan SL, Ferlay J, Storm H. Cancer incidence in five continents, vol. I–VIII. IARC CancerBase No. 7. Lyon: 2005. [Google Scholar]
- 26.Nanda K, McCrory DC, Myers ER, Bastian LA, Hasselblad V, Hickey JD, et al. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann Intern Med. 2000;132(10):810–819. doi: 10.7326/0003-4819-132-10-200005160-00009. [DOI] [PubMed] [Google Scholar]
- 27.Arbyn M, Bergeron C, Klinkhamer P, Martin-Hirsch P, Siebers AG, Bulten J. Liquid compared with conventional cervical cytology: a systematic review and meta-analysis. Obstet Gynecol. 2008;111(1):167–177. doi: 10.1097/01.AOG.0000296488.85807.b3. [DOI] [PubMed] [Google Scholar]
- 28.Mayrand MH, Duarte-Franco E, Rodrigues I, Walter SD, Hanley J, Ferenczy A, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357(16):1579–1588. doi: 10.1056/NEJMoa071430. [DOI] [PubMed] [Google Scholar]
- 29.Eltoum IA, Roberson J. Impact of HPV testing, HPV vaccine development, and changing screening frequency on national Pap test volume: projections from the National Health Interview Survey (NHIS) Cancer. 2007;111(1):34–40. doi: 10.1002/cncr.22487. [DOI] [PubMed] [Google Scholar]
- 30.Soni A. Use of the Pap test as a cancer screening tool among women age 18–64, U.S. noninstitutionalized population. [Last accessed July 1, 2009];Agency for Healthcare Research and Quality (AHRQ) Statistical Brief #173. 2005 http://www.meps.ahrq.gov/mepsweb/data_files/publications/st173/stat173.pdf.
- 31.Insinga RP, Glass AG, Rush BB. Pap screening in a U.S. health plan. Cancer Epidemiol Biomarkers Prev. 2004;13(3):355–360. [PubMed] [Google Scholar]
- 32.U.S. Centers for Disease Control and Prevention. [Last accessed July 1, 2009];HPV Vaccine Information for Young Women. http://www.cdc.gov/std/hpv/STDFact-HPV-vaccine-young-women.htm.
- 33.Wallace LA, Young D, Brown A, Cameron JC, Ahmed S, Duff R, et al. Costs of running a universal adolescent hepatitis B vaccination programme. Vaccine. 2005;23(48–49):5624–5631. doi: 10.1016/j.vaccine.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 34.Iskedjian M, Walker JH, Hemels ME. Economic evaluation of an extended acellular pertussis vaccine programme for adolescents in Ontario, Canada. Vaccine. 2004;22(31–32):4215–4227. doi: 10.1016/j.vaccine.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 35.U.S. Centers for Disease Control and Prevention. HIV counseling and testing system, version 4.0. Atlanta: National Center for HIV, STD, and TB Prevention; 1999. [Google Scholar]
- 36.Kulasingam SL, Kim JJ, Lawrence WF, Mandelblatt JS, Myers ER, Schiffman M, et al. Cost-effectiveness analysis based on the atypical squamous cells of undetermined significance/low-grade squamous intraepithelial lesion Triage Study (ALTS) J Natl Cancer Inst. 2006;98(2):92–100. doi: 10.1093/jnci/djj009. [DOI] [PubMed] [Google Scholar]
- 37.Cantor SB, Levy LB, Cardenas-Turanzas M, Basen-Engquist K, Le T, Beck JR, et al. Collecting direct non-health care and time cost data: application to screening and diagnosis of cervical cancer. Med Decis Making. 2006;26(3):265–272. doi: 10.1177/027298906288679. [DOI] [PubMed] [Google Scholar]
- 38.Shireman TI, Tsevat J, Goldie SJ. Time costs associated with cervical cancer screening. Int J Technol Assess Health Care. 2001;17(1):146–152. doi: 10.1017/s0266462301104137. [DOI] [PubMed] [Google Scholar]
- 39.Centers for Medicare and Medicaid Services. [Last accessed July 1, 2009];Clinical Lab Fee Schedule. 2006 http://www.cms.hhs.gov/ClinicalLabFeeSched/02_clinlab.asp#TopOfPage.
- 40.U.S. Bureau of Labor Statistics. [Last accessed July 1, 2009];Consumer Price Index, Medical Care Component. http://www.bls.gov/cpi/#data.
- 41.Gold MR, Siegel JE, Russel LB, Weinstein MC, editors. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 42.Elbasha EH, Dasbach EJ, Insinga RP. Model for assessing human papillomavirus vaccination strategies. Emerg Infect Dis. 2007;13(1):28–41. doi: 10.3201/eid1301.060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taira AV, Neukermans CP, Sanders GD. Evaluating human papillomavirus vaccination programs. Emerg Infect Dis. 2004;10(11):1915–1923. doi: 10.3201/eid1011.040222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.French KM, Barnabas RV, Lehtinen M, Kontula O, Pukkala E, Dillner J, et al. Strategies for the introduction of human papillomavirus vaccination: modelling the optimum age- and sex-specific pattern of vaccination in Finland. Br J Cancer. 2007;96(3):514–518. doi: 10.1038/sj.bjc.6603575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skinner SR, Garland SM, Stanley MA, Pitts M, Quinn MA. Human papillomavirus vaccination for the prevention of cervical neoplasia: is it appropriate to vaccinate women older than 26? Med J Aust. 2008;188(4):238–242. doi: 10.5694/j.1326-5377.2008.tb01593.x. [DOI] [PubMed] [Google Scholar]
- 46.Franco EL, Cuzick J, Hildesheim A, de Sanjose S. Chapter 20: Issues in planning cervical cancer screening in the era of HPV vaccination. Vaccine. 2006;24 Suppl 3:S171–S177. doi: 10.1016/j.vaccine.2006.05.061. [DOI] [PubMed] [Google Scholar]
- 47.Wong JB, Mulrow C, Sox HC. Health policy and cost-effectiveness analysis: yes we can. Yes we must. Ann Intern Med. 2009;150(4):274–275. doi: 10.7326/0003-4819-150-4-200902170-00010. [DOI] [PubMed] [Google Scholar]
- 48.King JT, Jr, Tsevat J, Lave JR, Roberts MS. Willingness to pay for a quality-adjusted life year: implications for societal health care resource allocation. Med Decis Making. 2005;25(6):667–677. doi: 10.1177/0272989X05282640. [DOI] [PubMed] [Google Scholar]
- 49.Eichler HG, Kong SX, Gerth WC, Mavros P, Jonsson B. Use of cost-effectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge? Value Health. 2004;7(5):518–528. doi: 10.1111/j.1524-4733.2004.75003.x. [DOI] [PubMed] [Google Scholar]
- 50.Winkelmayer WC, Weinstein MC, Mittleman MA, Glynn RJ, Pliskin JS. Health economic evaluations: the special case of end-stage renal disease treatment. Med Decis Making. 2002;22(5):417–430. doi: 10.1177/027298902236927. [DOI] [PubMed] [Google Scholar]
- 51.Huang ES, Zhang Q, Brown SE, Drum ML, Meltzer DO, Chin MH. The cost-effectiveness of improving diabetes care in U.S. federally qualified community health centers. Health Serv Res. 2007;42(6 Pt 1):2174–2193. doi: 10.1111/j.1475-6773.2007.00734.x. discussion 2294-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Losina E, Walensky RP, Kessler CL, Emrani PS, Reichmann WM, Wright EA, et al. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169(12):1113–1121. doi: 10.1001/archinternmed.2009.136. discussion 1121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stout NK, Rosenberg MA, Trentham-Dietz A, Smith MA, Robinson SM, Fryback DG. Retrospective cost-effectiveness analysis of screening mammography. J Natl Cancer Inst. 2006;98(11):774–782. doi: 10.1093/jnci/djj210. [DOI] [PubMed] [Google Scholar]
- 54.Shiroiwa T, Sung YK, Fukuda T, Lang HC, Bae SC, Tsutani K. International survey on willingness-to-pay (WTP) for one additional QALY gained: what is the threshold of cost effectiveness? Health Econ. 2009 doi: 10.1002/hec.1481. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 55.Barr E, Gause CK, Bautista OM, Railkar RA, Lupinacci LC, Insinga RP, et al. Impact of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, 18) L1 virus-like particle vaccine in a sexually active population of North American women. Am J Obstet Gynecol. 2008;198(3):261, e1–e11. doi: 10.1016/j.ajog.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 56.Paavonen J. Baseline demographic characteristics of subjects enrolled in international quadrivalent HPV (types 6/11/16/18) vaccine clinical trials. Curr Med Res Opin. 2008;24(6):1623–1634. doi: 10.1185/03007990802068151. [DOI] [PubMed] [Google Scholar]
- 57.Steinbrook R. Health care and the American Recovery and Reinvestment Act. N Engl J Med. 2009;360(11):1057–1060. doi: 10.1056/NEJMp0900665. [DOI] [PubMed] [Google Scholar]
- 58.Goldie SJ, Kohli M, Grima D, Weinstein MC, Wright TC, Bosch FX, et al. Projected clinical benefits and cost-effectiveness of a human papillomavirus 16/18 vaccine. J Natl Cancer Inst. 2004;96(8):604–615. doi: 10.1093/jnci/djh104. [DOI] [PubMed] [Google Scholar]
- 59.Schwartz TF, Spaczynski M, Schneider A, Wysocki J, Galaj A, Perona P, et al. Immunogenicity and tolerability of an HPV-16/18 AS04-adjuvanted prophylactic cervical cancer vaccine in women aged 15–55 years. Vaccine. 2009;27:581–587. doi: 10.1016/j.vaccine.2008.10.088. [DOI] [PubMed] [Google Scholar]
- 60.Munoz N, Manalastas R, Jr, Pitisuttithum P, Tresukosol D, Monsonego J, Ault K, et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24–45 years: a randomised, double-blind trial. Lancet. 2009;373(9679):1949–1957. doi: 10.1016/S0140-6736(09)60691-7. [DOI] [PubMed] [Google Scholar]
- 61.Jit M, Choi YH, Edmunds WJ. Economic evaluation of human papillomavirus vaccination in the United Kingdom. BMJ. 2008;337:a769. doi: 10.1136/bmj.a769. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.