Abstract
Population aging is underway globally, and dementias and neuropsychiatric disorders comprise a growing share of the disease burden. Using data from adults 50 years and older in Guatemala (n = 643), we assess to what extent measures of economic, social, intellectual, and biological individual capital are associated with and account for variation in cognitive functioning, as measured by the modified Mini-Mental Status Exam (M-MMSE). The M-MMSE is used widely in clinical and population-based research to assess for cognitive impairment and to screen for dementia. Measures of the above aspects of individual capital are positively associated with cognitive functioning, and with other demographic attributes, account for 29.6 per cent of the variance in cognitive functioning. Schooling accounts for the largest unique share (5.3 per cent) of the variance, followed by household standard of living (2.0 per cent), church attendance (1.3 per cent), and z-score for height (0.9 per cent). In a setting like Guatemala—with low schooling, widespread poverty, malnutrition, and infectious disease—early life investments that increase schooling and improve nutrition may be valuable investments to mitigate cognitive impairment in older adults and its contribution to the disease burden.
Keywords: aging, cognitive functioning, Guatemala, individual capital, life course
Research on aging and health around the world has focused on chronic physical conditions and disability; yet, dementias and neuropsychiatric disorders account for a growing share of the global disease burden (World Health Organization [WHO] 2007). In wealthier settings, aspects of economic, social, intellectual, and biological individual capital are important predictors of later-life cognitive functioning (e.g., Albert 1995; Abbott et al. 1998; Everson-Rose et al. 2003; Fujiwara and Kawachi 2008). In poorer settings, many older adults have accrued less economic, intellectual, and biological capital, often because of chronic exposure to poverty, infection, and malnutrition. Many of these older adults, however, may benefit from strong social and familial ties. Here, we assess the determinants of cognitive functioning—as measured by the modified Mini-Mental Status Exam (MMSE)—among adults 50 years and older in Guatemala. The results provide insights about the contributions of aspects of individual capital to cognitive aging in a poorer, malnourished population with little schooling but strong social ties.
Individual capital and cognitive aging
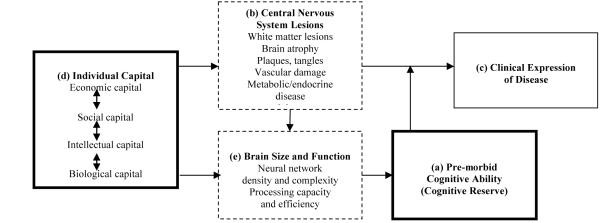
Research on cognitive aging reveals a strong association between increasing age and the onset of neuropathology and cognitive decline; yet, the onset and pace of age-related cognitive decline varies across age cohorts and settings (e.g., Zhang et al. 1990; Schaie 1994; Zhou et al. 2006). Gerontologists use the term reserve to explain variation in age-related levels and trajectories of cognitive decline (Stern et al. 1992). Reserve captures the idea that certain aspects of brain structure and function can delay the clinical effects of neuropathology (Richards and Deary 2005). In theory, two types of reserve have this effect. Structural reserve refers to the protective potential of certain anatomical features of the brain, such as its size, neural density, and synaptic connectivity. Functional reserve refers to the efficiency of neural networks and active compensation by other or more extensive networks following damage to the central nervous system. Our focus here is on the practical manifestations of both types of reserve-that is, observed cognitive functioning or performance.
Researchers have explored how various forms of individual capital-or a person’s accrued economic, social, intellectual, and biological resources-contribute to the nature and efficacy of both types of reserve. Economic capital refers to the ownership of financial and productive assets as well as consumer goods, which facilitate investment in various opportunities for mental and intellectual stimulation. Measures of economic capital in childhood and adulthood are positively associated with later-life cognitive functioning (Turrell et al. 2002; Everson-Rose et al. 2003;Wilson et al. 2005; Yount 2008). Social capital refers to those resources inherent in familial ties and community social organizations that offer opportunities for enhancing human development (Loury 1977; Coleman 1990). Social capital has value in that it facilitates access to other resources and creates well-being through a sense of connectedness (Terrion and Lagacé 2008). In research on aging, the strength of one’s informal ties and frequency of religious attendance are positively associated with cognitive functioning and mental health (e.g., Glei et al. 2005; Hill et al. 2006; Fujiwara and Kawachi 2008; Yount 2008). Two forms of individual human capital-intellectual and biological-contribute to reserve. Intellectual capital refers to a person’s knowledge or ability to know (Nahapiet and Goshal 1998); measures of intellectual and linguistic ability are associated with higher cognitive functioning and delayed onset of dementia (e.g., Snowdon et al. 1996; Schmand et al. 1997). Intellectual capital often is proxied by parental or own schooling attainment or occupational complexity. Such measures are positively associated with later-life cognitive functioning (Zhang et al. 1990; Albert 1995; Leibovici et al. 1996; Cagney and Lauderdale 2002; Richards and Sacker 2003; Andel et al. 2007; Anderson et al. 2007; Roe et al. 2007), but parental or own occupation may be more relevant in skilled labour markets (Yount 2008). Biological capital refers to a person’s stock of health and reflects accrued investments in health and exposures to disease. Poor investment in a child’s health and nutrition, for example, may lead to vitamin deficiencies or alterations in brain development (Albert 1995). Head size (e.g., Graves et al. 2001; Mortimer et al. 2003) and limb length or height (e.g., Abbott et al. 1998; Beeri et al. 2005; Kim et al. 2008) are inversely associated with dementia and cognitive decline. In theory, each aspect of individual capital may contribute separately to cognitive reserve and thereby delay the manifestation of cognitive impairment (Richards and Deary 2005; Bornstein et al. 2006).
Individual capital may also affect cognitive reserve indirectly (Figure 1). Schooling attainment, for example, may enhance one’s occupational trajectory, which reinforces mental stimulation throughout adulthood (e.g., Albert 1995; Schooler et al. 1999; Richards and Sacker 2003). Some occupational exposures also may cause central-nervous-system lesions, ultimately damaging brain size and function (Park et al. 2005). Finally, a poor childhood standard of living, low schooling attainment, or adverse occupational exposures may lead to behaviours (e.g., Blane et al. 1996; Lynch et al. 1997) and health conditions that are risk factors for cognitive impairment (e.g., Kuusisto et al. 1993; Breteler et al. 1994; Cerhan et al. 1998; Richards et al. 2003). While acknowledging such pathways, our aim here is to explore the adjusted associations of aspects of individual capital and cognitive functioning.
Figure 1.
Conceptual model for the determinants of cognitive functioning
Note. Adapted from Richards and Deary (2005). Boxes in bold depict central, measured components of this framework. Dashed boxes depict often unmeasured components of this framework.
Few relevant studies of these direct relationships have been conducted in populations that experience endemic poverty, malnutrition, low schooling attainment, but strong social ties. Population aging is well underway in such settings and has outpaced historical trends in wealthier settings (United Nations 2007). As a result, dementias and neuropsychiatric disorders comprise a growing share of the global disease burden (WHO 2007). These epidemiologic shifts are outpacing investments to address them (e.g., Yount and Sibai 2009; Sinunu et al. 2008). So, aspects of individual capital may contribute differently to cognitive aging in such settings. In highly malnourished populations, for example, indicators of biological capital such as achieved height may account for a notable share of the variation in later-life cognitive functioning. Similarly, schooling may contribute less to later-life cognitive functioning in populations that have attained extremely little schooling. Our study thus explores the potential contributions of various facets of individual capital to cognitive functioning among older adults in Guatemala. The findings offer clues about suitable public investments to reduce the disease burden associated with cognitive impairment in such settings.
Setting
Participants in this study are from Guatemala City, the Department of El Progresso, and four Ladino (mixed Spanish-Mayan heritage), Spanish-speaking villages in El Progresso, about 100km northeast of Guatemala City. The Institute of Nutrition of Central America and Panama (INCAP) selected these four villages for a study in 1969-77 to assess the effects of protein and energy supplementation on the growth and development of preschool children (Habicht and Martorell 1992; Stein et al. 2008). The villages were chosen because of their comparable size, social isolation, low levels of schooling (30.0 per cent adult literacy), and high rates of malnutrition and gastrointestinal and respiratory infections. Since then, multiple follow-up studies have ensued, the last of which took place in 2005-07.
The rate of natural population growth—or births minus deaths per 1,000 population—in these villages historically has been high, 3.9 per cent in 1975-87 declining slightly to 3.2 per cent in 1996-2002. As a result, a high percentage of villagers are below age 15 years (48.0 per cent in 1967; 41.0 per cent in 2002). In these calendar years, a smaller but stable, percentage of villagers were older than age 50 years (12.0 per cent in 1967; 13.0 per cent in 2002) (Maluccio et al. 2005).
Village economies before the late 1960s were based on small-scale subsistence agriculture (Pivaral 1972). From the 1980s, a growing share of young adults left the villages for non-agricultural wage work or continued schooling (Maluccio et al. 2005). Seasonal migration for agricultural wage labour has been common in all villages, but the rates have varied with local demands for labour. Unskilled non-agricultural wage work in Guatemala City and along the Atlantic coastal highway in this region has become more accessible as roads and bus services have improved. Masonry among men and petty trade and piecework among women are common forms of self-employment in the villages (Pivaral 1972; Maluccio et al. 2005). Public primary schools have been available in all four villages since the 1960s; yet, their physical infrastructure at that time was poor. Only four grades were offered until the 1970s (Bergeron 1992), and teachers had limited training. In 1975, men aged 20-29 years had 2.0 grades of schooling and their female peers had 1.4 grades; by 2002, these figures were 5.8 and 5.0, respectively (Maluccio et al. 2005). Most often, married life for the current generation of older adults began on the groom’s family compound, where the living expenses were shared (Saenz de Tejada et al. 2005). Many couples eventually built homes on the same compound, so that they matured and aged surrounded by family. In general, sons are expected to provide financially for older parents, and daughters and daughters-in-law should provide the hands-on care (Saenz de Tejada et al. 2005). Rates of infectious disease and malnutrition have been high in the villages. In 1968, 73.0 per cent of children less than age six years were stunted (Stein et al. 2009), or at least two standard deviations below the median height-for-age in the WHO’s international reference population (de Onis et al. 2006). Although growth failure in early childhood (ages birth to two years) is negatively associated with educational achievements in adulthood (Li et al. 2004), the association of short stature in adulthood with later-life cognitive functioning is unknown.
Sample and data
For this study, we surveyed the biological parent(s), and their spouse if married, of participants in the original INCAP study who were studied in a prior survey (Grajeda et al. 2005). Of 756 identified and eligible participants of the present study, 85.2 per cent (n = 644) were interviewed. The analysis is based on 643 participants with complete data on cognitive functioning.
In interviews conducted in 2005-07, we collected data on marital history and goods brought to marriage, subjective well-being and objective and subjective cognitive functioning. By interview and physical exam, we obtained data on physical health, functioning, and nutrition. We also collected data on the economic and health-related shocks as well as crime victimization experienced by participants or their immediate families; transfers of money, goods, and services between the participants and their immediate kin; and wage, agricultural, and business labour and income.
The main outcome Yi is a 0-20 score for cognitive functioning, which is based on the sum of scores for items included in the Modified Mini-Mental State Exam (M-MMSE). The MMSE, or adaptations of it, have been widely used in clinical- and population-based research (e.g., Folstein et al. 1975; Escobar et al. 1986; Brayne and Calloway 1990; Farrag et al. 2002; Elhan et al. 2005; Yount 2008) to test for cognitive impairments and to screen for dementia. The instrument assesses orientation, registration, attention and calculation, recall, and language. The original MMSE is unsuitable for poor settings because literacy is required to complete the exam; yet, valid and reliable variants of the MMSE exist for illiterate populations (e.g., Kabir and Herlitz 2000). The M-MMSE in this study, which has been used in other studies of older adults in poor settings (Palloni, nd; Yount 2008), also does not require literacy, and assesses orientation, registration, attention and calculation, delayed recall, and language. The summative score is reasonably reliable (Cronbach’s α = 0.55, using standardized item scores).
The measure for economic capital Ei is a score derived from the first principal component of a principal components analysis of the respondent’s household’s ownership (coded 0 for no and 1 for yes) of 15 consumer durables and 7 housing amenities. The included items capture the economic success of these individuals and their spouses in accumulating assets over their lifetimes. Measures of social capital Si include the frequency of church attendance in the prior month and an indicator for whether the respondent lived alone five years before the interview. The respondent’s intellectual capital Ii is captured by the respondent’s mother’s and father’s grades of schooling and the respondent’s own grades of schooling and own grades of schooling squared, the latter two of which permit a non-linear association between own schooling and cognitive functioning (Cagney and Lauderdale 2002; Everson-Rose et al. 2003). Measures of biological capital Bi include the respondent’s z-score for height, with reference to Dutch adults aged 60 years and older in 2004 (a wealthier population not subject to chronic undernutrition in childhood; data available at http://dined.io.tudelft.nl/en,dined 2004,303), and indicators for reported doctor-diagnosed stroke and diabetes. Other control variables Xi include the respondent’s age in years, gender, and village of origin of respondents’ biological children who were part of the INCAP longitudinal study and the 2002-04 follow-up (Grajeda et al. 2005). Early marriage often is seen as limiting the options especially of women in poor settings (see Behrman et al. (2007) for a discussion), so we also include a control for the respondent’s age at first marriage. Other measures of individual capital, or its loss, in childhood and adulthood were considered (in childhood, the presence of a latrine in the household, work, residence with a grandparent, death or migration of a parent, and birth of a sibling; in adulthood, the respondent’s type of work at age 50, experience of economic hardship in the prior five years, religious affiliation, total number of living siblings by gender at the time of interview, total number of living and dead children including those of the spouse, death of a co resident in the prior five years, ever divorce or widowhood, disease suffered by him or her or his/her spouse in the prior five years, and blood pressure at the time of interview); however, analyses revealed that these variables lacked sufficient variability, were highly correlated with other covariates, or were not associated with cognitive functioning.
Statistical methods
Mean and median scores for each item of the M-MMSE and for the 0-20 point score were compared, overall and across age groups (50-59, 60-69, and ≥ 70 years). Relative frequency distributions of the covariates were computed to assess their completeness and distributional properties. For any covariates with item non-response, the median or modal value based on the observed data was imputed, and indicators for item non-response were included. OLS regression was used to estimate the association of each covariate adjusting first for indicators of item non-response only, and then for these indicators and all other covariates, following equation (1):
| (1) |
Semi-partial correlations were estimated to assess the proportion of the total variance in the M-MMSE score that was uniquely accounted for by each covariate, accounting for all others.
Several sensitivity analyses were conducted to assess the robustness of the estimates and inferences. First, robust variance estimation was used to account for the possible clustering of responses separately within age cohort and village of origin. Second, multivariate models were estimated separately for men and women to account for possible variation in the estimates across these strata. Third, multivariate models were estimated for various transformations of the outcome. In none of these analyses were the inferences altered appreciably. Finally, several multivariate models were estimated using various transformations of the covariates, and their final measurement scales corroborate prior research and/or maximize the explained variance in cognitive functioning. Thus, the multivariate results that are presented are based on the pooled sample of older adults, the original M-MMSE score, and standard OLS estimation.
Results
On average, respondents’ parents have just over 0.5 grades of schooling, compared to 1.5 grades among the respondents themselves (Table 1). Respondents report having attended church 3.4 times in the prior month, and relatively few (5.6 per cent) report living alone five years prior. On average, the height of respondents is about 2.1 standard deviations below the Dutch reference population. About 10.0 per cent report doctor-diagnosed diabetes and 2.5 per cent report doctor-diagnosed stroke. The mean age of respondents is 64.2 years, more than one half (56.0 per cent) of respondents are women. The mean age at first marriage of respondents is 20.2 years.
Table 1.
Characteristics of the sample, adults 50 years and older (n =643), rural Guatemala, 2005-2007(1)
| Economic capital | |
| Household standard-of-living score (M±SD)(2) | 0.03±0.98 |
| Social capital | |
| Frequency of church attendance in prior month (M±SD) | 3.35±6.09 |
| Lived alone 5 years prior (per cent) | 5.6 |
| Intellectual capital | |
| Father’s grades of schooling (M±SD) | 0.57±1.25 |
| Mother’s grades of schoolng (M±SD) | 0.55±1.26 |
| Own grades of schooling (M±SD) | 1.53±1.91 |
| Biological capital | |
| Z-score for height(3) (M±SD) | -2.14±0.85 |
| Reported doctor-diagnosed diabetes (per cent) | 9.80 |
| Reported doctor-diagnosed stroke (per cent) | 2.49 |
| Other demographic characteristics | |
| Age in years (M±SD) | 64.23,8.53 |
| Gender (per cent women) | 55.99 |
| Age at first marriage (M±SD) | 20.17,4.14 |
| Village of origin (per cent)(4) | |
| Conacaste | 31.10 |
| Espiritu Santo | 19.91 |
| San Juan | 22.55 |
| Santo Domingo | 26.44 |
Except for the respondents’ father’s and mother’s schooling, all other characteristics refer to those of the respondent.
Mean plus or minus its standard deviation
With reference to Dutch adults 60 years and older in 2004.
Village of origin of those participants’ biological children who took part in the original INCAP longitudal study and the 2002-2004 followup (Grajeda et al. 2005).
Source. Author’s calculations of data collected as part of the Generational Transfers Study.
Respondents’ mean and median M-MMSE scores are 13.9 and 14.0, respectively, and the scores range from 4 to 20 (Table 2). As expected, the total M-MMSE score is lower at older ages (p-value for Cuzick’s non-parametric test for trend < 0.001), with a mean score of 14.8 among respondents aged 50-59 years and 12.8 among those aged 70 years and older. Mean scores for the subdomain of orientation are similar across age groups (3.8-3.9 on a 0-4 scale); however, respondents aged 70 years and older score worse than do those 50-59 years on the subdomains of registration and recall (e.g., 1.3 versus 2.1 on 0-3 score for delayed recall; p < 0.001), attention and calculation (1.7 versus 2.2 on 05 score for reverse recall of numbers, p < 0.01), and language (e.g., 2.2 versus 2.6 on 0-3 score for following a three-step command, p < 0.001).
Table 2.
Point estimates for the Modified Mini-Mental Status Exam (M-MMSE) score and its component items, adults 50 years and older (n =643), rural Guatemala, 2005-2007
| Median | Mean | p | SD | Min | Max | |
|---|---|---|---|---|---|---|
| M-MMSE (0–20) | *** | |||||
| Total | 14 | 13.90 | 3.19 | 4 | 20 | |
| 50–59 years | 15 | 14.76 | 3.01 | 4 | 20 | |
| 60–69 years | 14 | 13.88 | 3.30 | 4 | 20 | |
| ≥70 years | 12 | 12.83 | 2.95 | 4 | 19 | |
| Orientation | ||||||
| Know residence and day of week (0–4) | ||||||
| Total | 4 | 3.83 | 0.48 | 1 | 4 | |
| 50–59 years | 4 | 3.86 | 0.42 | 2 | 4 | |
| 60–69 years | 4 | 3.80 | 0.57 | 1 | 4 | |
| ≥70 years | 4 | 3.82 | 0.43 | 2 | 4 | |
| Registration and recall | ||||||
| Immediate recall of three words (0–3) | *** | |||||
| Total | 3 | 2.89 | 0.41 | 0 | 3 | |
| 50–59 years | 3 | 2.97 | 0.17 | 2 | 3 | |
| 60–69 years | 3 | 2.88 | 0.42 | 0 | 3 | |
| ≥70 years | 3 | 2.79 | 0.57 | 0 | 3 | |
| Delayed recall of three words (0–3) | ** | |||||
| Total | 2 | 1.72 | 1.15 | 0 | 3 | |
| 50–59 years | 2 | 2.08 | 1.06 | 0 | 3 | |
| 60–69 years | 2 | 1.71 | 1.16 | 0 | 3 | |
| ≥70 years | 1 | 1.27 | 1.11 | 0 | 3 | |
| Attention and calculation | ||||||
| Reverse recall of numbers (0–5) | ** | |||||
| Total | 1 | 1.96 | 1.89 | 0 | 5 | |
| 50–59 years | 1 | 2.15 | 1.93 | 0 | 5 | |
| 60–69 years | 1 | 1.99 | 1.88 | 0 | 5 | |
| ≥70 years | 1 | 1.66 | 1.81 | 0 | 5 | |
| Language | ||||||
| Following a three-step command (0–3) | *** | |||||
| Total | 3 | 2.39 | 0.77 | 0 | 3 | |
| 50–59 years | 3 | 2.55 | 0.65 | 0 | 3 | |
| 60–69 years | 3 | 2.38 | 0.77 | 0 | 3 | |
| ≥70 years | 2 | 2.22 | 0.87 | 0 | 3 | |
| Copying a drawing (proportion correct) | *** | |||||
| Total | 1 | 0.83 | 0.37 | 0 | 1 | |
| 50–59 years | 1 | 0.89 | 0.32 | 0 | 1 | |
| 60–69 years | 1 | 0.83 | 0.38 | 0 | 1 | |
| ≥70 years | 1 | 0.77 | 0.42 | 0 | 1 | |
| Repeating a local proverb (proportion correct) | ** | |||||
| Total | 0 | 0.23 | 0.42 | 0 | 1 | |
| 50–59 years | 0 | 0.28 | 0.45 | 0 | 1 | |
| 60–69 years | 0 | 0.24 | 0.43 | 0 | 1 | |
| ≥70 years | 0 | 0.17 | 0.38 | 0 | 1 | |
p < .10
p < .05
p < .01
p < .001
for Cuzick’s non-parametric test for trend.
Source. As for Table 1.
Almost all covariates are, as expected, significantly and positively associated with the M-MMSE score, with more individual capital associated with higher M-MMSE scores (Table 3). One exception is reported doctor-diagnosed stroke, which is rare in this sample and not associated with the M-MMSE score. Two other exceptions are living alone five years prior and reported doctor-diagnosed diabetes, signifying less social and biological capital, respectively, which are significantly positively associated with the M-MMSE score.
Table 3.
Simple and multivariate ordinary least squares regressions of the M-MMSE score on measures of individual capital and other demographic attributes, adults 50 years and older (n = 643), rural Guatemala, 2005-2007
| Simple OLS regressions | Multivariate OLS regression | |||||||
|---|---|---|---|---|---|---|---|---|
| β(1) | (se) | p | R 2 | β(2) | (se) | p | sR 2(3) | |
| Intercept | 14.436 | *** | ||||||
| Economic capital | ||||||||
| Household standard-of-living score | 1.099 | (0.121) | *** | 0.120 | 0.522 | (0.125) | *** | 0.020 |
| Social capital | ||||||||
| Frequency of church attendance | 0.053 | (0.020) | ** | 0.049 | 0.036 | (0.018) | † | 0.013 |
| Lived alone 5 years prior | 1.575 | (0.544) | ** | 0.013 | 0.748 | (0.488) | 0.003 | |
| Intellectual capital | ||||||||
| Father’s grades of schooling | 0.328 | (0.102) | *** | 0.017 | 0.028 | (0.104) | 0.002 | |
| Mother’s grades of schooling | 0.230 | (0.101) | * | 0.008 | -0.045 | (0.104) | 0.000 | |
| Own grades of schooling | 0.817 | (0.101) | *** | 0.130 | 0.654 | (0.124) | *** | 0.053 |
| Own grades of schooling squared | -0.037 | (0.101) | * | -0.041 | (0.017) | * | ||
| Biological capital | ||||||||
| Z-score for height | 0.789 | (0.145) | *** | 0.054 | 0.277 | (0.138) | * | 0.009 |
| Reported doctor-diagnosed diabetes | 1.057 | (0.423) | * | 0.012 | 0.931 | (0.378) | * | 0.007 |
| Reported doctor-diagnosed stroke | -0.518 | (0.809) | 0.003 | -0.596 | (0.706) | 0.001 | ||
| Other demographic attributes | ||||||||
| Age in years | -0.935 | (0.014) | *** | 0.063 | -0.069 | (0.014) | *** | 0.027 |
| Female gender | -0.709 | (0.252) | ** | 0.012 | -0.759 | (0.261) | ** | 0.010 |
| Age at first marriage | 0.482 | (0.183) | ** | 0.037 | 0.232 | (0.171) | 0.004 | |
| Age at first marriage squared | -0.009 | (0.004) | * | -0.004 | (0.004) | |||
| Village of origin | ||||||||
| Conacaste | -0.744 | (0.332) | * | 0.012 | -0.406 | (0.293) | 0.007 | |
| Espiritu Santo | -0.708 | (0.372) | † | -0.512 | (0.332) | |||
| San Juan | -0.080 | (0.359) | 0.189 | (0.318) | ||||
| R 2 (full model) | 0.296 | |||||||
Coefficients are adjusted only for the imputation of missing values.
Coefficients are adjusted for the imputation of missing values and all other variables.
Proportion of total variance in the M-MMSE score uniquely accounted for by X (controlling for other covariates).
p < .10
p < .05
p < .01
p < .001
Source. As for Table 1.
In the multivariate model (Table 3), associations with the M-MMSE score remain significant for the measure of economic capital (household standard of living score), two measures of intellectual capital (own grades of schooling and own schooling squared), and two measures of biological capital (z-score for height and reported doctor-diagnosed diabetes). Also, one measure of social capital—the frequency of church attendance in the prior month—remains marginally significantly associated with this score. The M-MMSE score does not vary significantly with indicators for whether the respondent was living alone five years prior, the schooling of the respondents’ parents, and the respondents’ age at first marriage.
For all of these measures, except reported doctor-diagnosed diabetes, associations with the M-MMSE score are in the expected directions. A unit increase in the score for household standard of living, for example, is associated with an increase of 0.52 points (0.16SD) in the M-MMSE score. An increase in the respondent’s grades of schooling is associated with a diminishing increment in the M-MMSE score (as indicated by the negative coefficient for grades of schooling squared). An increase of one visit per month to church is marginally associated (p < 0.10) with a small (0.04) increment in the M-MMSE score. A unit increase in the z-score for height is associated with a 0.23-point increase in the M-MMSE score, and reporting doctor-diagnosed diabetes is associated unexpectedly with a 0.93-point higher M-MMSE score. Even after adjustment for all measures of individual capital, a one-year increase in age is associated with a small (0.07), albeit significant, decrease in the M-MMSE score, and women on average have a 0.76-point lower M-MMSE score than do men.
The full multivariate model accounts for 29.6 per cent of the total variance in the M-MMSE score (Table 3). Own grades of schooling and grades of schooling squared together account for the largest unique percentage of the total variance in the M-MMSE score (5.3 per cent). Age accounts for the next largest percentage (2.7 per cent), followed by household standard of living (2.0), frequency of church attendance (1.3 per cent), gender (1.0 per cent), z-score for height (0.9 per cent), and reported doctor-diagnosed diabetes (0.7 per cent). Other measures of individual capital each uniquely account for ≤ 0.3 per cent of the total variance in the M-MMSE score.
To give some idea of the possible importance of two components of individual capital for cognitive functioning in old age, we used the full model in Table 3 to predict M-MMSE scores for four hypothetical groups of older adults: (1) those who are short in stature (height 2 SD below the reference) and received no schooling, (2) those who are short but completed primary schooling (6 grades), (3) those who are tall (height equal to the median height in the reference) but received no schooling, and (4) those who are tall and completed primary school. Assuming that all other attributes in these groups reflect the mean or mode in this sample (Table 1), those who are tall and completed primary school would have an M-MMSE score three points higher than those who are short and not schooled, an increase, relative to the mean score (13.6) in this population of approximately 22.0 per cent. Although schooling accounts for the largest direct proportion of this increase, poor nutrition as a preschooler is associated with the M-MMSE score both through its causal impact on adult height and through its causal impact on schooling (Behrman et al., 2008).
Discussion
This analysis has explored the predictors of cognitive functioning among older adults from four Guatemalan villages, and has assessed the extent to which measures of economic, social, intellectual, and biological capital account for variation in cognitive functioning. The analysis draws on a rich set of data on adults aged 50 years or older in Guatemala, where levels of schooling have been low and levels of poverty, disease, and malnutrition have been high, but where social and familial ties tend to be strong. A lack of research on aging in such settings raises questions about which form of individual capital confers the greatest benefit in terms of cognitive functioning.
Older adults in this part of Guatemala have very little schooling, but have more schooling than their parents, on average. They also tend to be short, which is suggestive of chronic exposure to malnutrition and disease, especially in early childhood. The prevalence of chronic diseases, such as diabetes, appears to be low in this cohort, perhaps partly because of poor access to health care and under-diagnosis, resulting in under-reporting and increased mortality. These older adults have intensive contact with religious organizations, and rarely live apart from other family members. Thus, they appear to be well integrated into their home communities.
Consistent with the conceptual framework described at the beginning of the paper, measures of economic, social, intellectual, and biological capital all are significantly, independently associated with cognitive functioning in this sample. These measures, along with other demographic attributes, account for 29.6 per cent of the variance in cognitive functioning, which parallels similar studies in the U.S. and abroad (e.g., Ofstedal et al. 1999). Of the measures of individual capital under consideration, schooling accounts for the largest unique share (5.3 per cent) of the total variance in cognitive functioning. While consistent with the findings from wealthier populations noted in the introduction, it is nonetheless striking that even in a setting characterized by poverty, low schooling, endemic malnutrition, and infectious disease, but strong social ties, the intellectual stimulation associated with formal schooling seems to be a major determinant of later-life cognitive functioning.
The weak or unexpected relationships between other measures of individual capital and cognitive functioning merit remark. First, the lack of a direct adjusted association between the respondents’ parents’ schooling and the respondents’ cognitive functioning could be attributable to universally low levels of schooling among the parents. Alternatively, parental schooling may operate only indirectly through other measures of individual capital. Second, the lack of a direct adjusted association between living alone and cognitive functioning could result from the infrequency (5.6 per cent) of solitary living in this sample or from the intensive exchanges that occur among non-co resident family members. Research is needed on the nature and intensity of such exchanges and their association with cognitive functioning in older adults. Third, the lack of any direct association between reported stroke and cognitive functioning is surprising, but the negative direction of this association is as expected. So, the event may have been too rare or was underreported, precluding estimation of a significant association in this sample. Finally, the unexpected, positive association between reported doctor-diagnosed diabetes and cognitive functioning may be suggestive of reverse causality: those with higher cognitive functioning may have better access to care and better recollections of diagnosed diabetes. Because the inclusion or exclusion of reported doctor-diagnosed diabetes does not notably alter other model coefficients, and because of the documented contributions of impaired glucose tolerance and Type II diabetes to cognitive aging (Messier 2005), this variable is retained in the final models that are presented.
Certain limitations of the study are notable. First, most covariates in this analysis, as in other studies of older adults, are based on retrospective reports by study participants. Such reports may suffer from various threats to validity, such as recall bias of more distant behaviours and events (e.g., Wolk et al. 1997). In the present study, some self-reported measures, such as doctor-diagnosed high blood pressure, can be validated with objective measures. In the case of blood pressure, the estimate of chance-corrected agreement (kappa) is ~0.33, but does not vary monotonically with the M-MMSE score. If this pattern is similar for all reported measures of health, then estimates of association with cognitive functioning may tend toward the null.
Second, selection bias is a potential threat to generalisability, of which variability in the association of cognitive functioning and survival is one example. If this association differs across subgroups that vary in their levels of individual capital, then observed gaps in cognitive functioning may not reflect the true gaps among older adults in this region. Elsewhere, dementia and mortality are more strongly associated among men than women (e.g., Perls et al. 1993), in which case any observed female deficits in cognitive functioning in this sample may exaggerate the true gap. Efforts to adjust for selective survival in studies of cognitive functioning, however, yield small differences in model estimates (Yount 2008). For this reason, our analyses do not adjust for selective survival.
Taken together, the findings from this analysis suggest that investments in multiple forms of individual capital may enhance cognitive functioning among older adults in Guatemala. Given the real constraints on public resources for investment, however, early life investments that increase schooling and improve nutrition may offer substantial benefits.
Acknowledgements
We gratefully acknowledge the assistance of Ms. Meng Wang for aspects of the data analysis for this research and Dr. Paul Melgar, Field Director for this study. This research was supported by grant HD-045627 from the National Institutes of Health. Funding for earlier waves of data collection on which this study builds has been provided by the U.S. National Institutes of Health (NIH), the Nestle Foundation, and the Thrasher Research Foundation and funding for data analysis provided by NIH, the U.S. National Science Foundation, and the American Heart Association. The authors are indebted to the many investigators and support staff, who over 40 years, have developed and sustained this unique cohort. Finally, the investigators thank the cohort members for their continued co operation.
References
- Abbott, Robert D, White Lon R., Ross G. Webster, Petrovitch Helen, Masaki Kamal H., Snowdon David A., Curb J. David. Height as a marker of childhood development and late-life cognitive function: The Honolulu-Asia aging study. Pediatrics. 1998;102:602–609. doi: 10.1542/peds.102.3.602. [DOI] [PubMed] [Google Scholar]
- Albert MS. How does education affect cognitive function? Annals of Epidemiology. 1995;5:76–78. doi: 10.1016/1047-2797(94)00044-t. [DOI] [PubMed] [Google Scholar]
- Alderman, Harold, Behrman Jere R., Ross David R., Sabot Richard. Decomposing the gender gap in cognitive skills in a poor rural economy. The Journal of Human Resources. 1996;31(1):229–254. [Google Scholar]
- Andel, Ross, Karehold Ingleman, Parker Marti G., Thorslund Mats, Gatz Margaret. Complexity of primary lifetime occupation and cognition in advanced old age. Journal of Aging and Health. 2007;19(3):397–415. doi: 10.1177/0898264307300171. [DOI] [PubMed] [Google Scholar]
- Anderson, Tracy M, Perminder S. Sachdev, Brodaty Henry, Trollor Julian N., Andrews Gavin. Effects of sociodemographic and health variables on Mini-Mental State Exam Scores in older Australians. American Journal of Psychiatry. 2007;15(6):467–476. doi: 10.1097/JGP.0b013e3180547053. [DOI] [PubMed] [Google Scholar]
- Beeri, Schnaider Michal, Davidson Michael, Silverman Jeremy M., Noy Shlomo, Schmeidler James, Goldbourt Uri. Relationship between body height and dementia. American Journal of Geriatric Psychiatry. 2005;13(2):116–123. doi: 10.1176/appi.ajgp.13.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrman, Jere R, Murphy Alexis, Quisumbing Agnes, Ramakrishnan Usha, Yount Kathyrn M. New evidence from Guatemala, background paper to the 2007 World Development Report WPS4023. 2007. What is the real impact of schooling on age of first union and age of first parenting? [Google Scholar]
- Behrman, Jere R, Hoddinott John F., Maluccio John A., Soler-Hampejsek Erica, Behrman Emily, Martorell Reynaldo, Ramirez-Zea Manuel, Stein Aryeh. Impacts of pre-school, schooling and post-school experiences in Guatemala, PSC Working Paper Series PSC 06-03. 2008. What determines adult cognitive skills? [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron G. Social and economic development in four Ladino communities of Eastern Guatemala: A comparative description. Food and Nutrition Bulletin. 1992;14(3):221–236. [Google Scholar]
- Blane, David, Hart Carole L., Smith George D., Gillis Charles R., Hole David J., Hawthorne Victor M. Association of cardiovascular disease risk factors with socioeconomic position during childhood and during adulthood. British Medical Journal. 1996;313:1434–1438. doi: 10.1136/bmj.313.7070.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayne, Carol, Calloway Paul. The Association of education and socioeconomic status with the Mini-Mental State Examination and the clinical diagnosis of dementia in elderly people. Age and Ageing. 1990;19:91–96. doi: 10.1093/ageing/19.2.91. [DOI] [PubMed] [Google Scholar]
- Bornstein, Amy R, Copenhaver Cathleen I., Mortimer James A. Early-life risk factors for Alzheimer disease. Alzheimer Disease and Associated Disorders. 2006;20(1):63–72. doi: 10.1097/01.wad.0000201854.62116.d7. [DOI] [PubMed] [Google Scholar]
- Breteler, Monique MB, Claus JJ, Grobbee DE, Hofman A. Cardiovascular disease and distribution of cognitive function in elderly people: The Rotterdam study. British Medical Journal. 1994;308:1604–1608. doi: 10.1136/bmj.308.6944.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagney, Kathleen A, Lauderdale Diane .S. Education, wealth, and cognitive function in later life. The Journals of Gerontology: Psychological Sciences. 2002;57B(2):163–172. doi: 10.1093/geronb/57.2.p163. [DOI] [PubMed] [Google Scholar]
- Cerhan, James R, Folsom Aaron R., Mortimer James A., Shahar Eval, Knopman David S., McGovern Paul G., Hays Melissa A., Crum Larry D., Heiss Gerardo. Correlates of cognitive function in middle-aged adults. Atherosclerosis risk in communities (ARIC) study investigators. Gerontology. 1998;44:95–105. doi: 10.1159/000021991. [DOI] [PubMed] [Google Scholar]
- Coleman, James . Foundations of Social Theory. Harvard University Press; Cambridge: 1990. [Google Scholar]
- de Onis Mercedes, Garza Cutberto, Onyango Adelheid W., Martorell Reynaldo., editors. The WHO child growth standards. Acta Paediatrica Supplementum. 2006;450:1–101. [Google Scholar]
- Elhan, Atilla H, Kutlay Sehim, Kucukdeveci Ayse A., Cotuk Cotuk, Ozturk Gulsah, Tesio Luigi, Tennant Alan. Psychometric properties of the Mini-Mental State Examination in patients with acquired brain injury in Turkey. Journal of Rehabilitative Medicine. 2005;37(5):306–311. doi: 10.1080/16501970510037573. [DOI] [PubMed] [Google Scholar]
- Escobar, Javier, Burnam Audrey, Karno Marvin, Forsythe Alan, Landsverk John, Golding Jacqueline M. Use of the Mini-Mental State Examination (MMSE) in a community population of mixed ethnicity. Journal of Nervous and Mental Disorders. 1986;174:607–613. doi: 10.1097/00005053-198610000-00005. [DOI] [PubMed] [Google Scholar]
- Everson-Rose, Susan A, Mendes de Leon Carlos F., Bienias Julia L., Wilson Robert S., Evans Denis A. Early life conditions and cognitive functioning in later life. American Journal of Epidemiology. 2003;158(11):1083–1089. doi: 10.1093/aje/kwg263. [DOI] [PubMed] [Google Scholar]
- Farrag, Abdul-kader F, Khedr Eman M., Abdel-Aleem Hany, Rageh Tarek A. Effect of surgical menopause on cognitive functions. Dementia and Geriatric Cognitive Disorder. 2002;13(3):193–198. doi: 10.1159/000048652. [DOI] [PubMed] [Google Scholar]
- Folstein, Marshal F, Folstein SE, McHugh Paul R. Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fujiwara, Takeo, Kawachi Ichiro. Social capital and health: A study of adult twins in the U.S. American Journal of Preventive Medicine. 2008;35(2):139–144. doi: 10.1016/j.amepre.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Glei, Dana A, Landau David A., Goldman Noreen, Chuang Yi L., Rodríguez German, Weinstein Maxine. Participating in social activities helps to preserve cognitive function: An analysis of a longitudinal, population-based study of the elderly. International Journal of Epidemiology. 2005;34(4):864–871. doi: 10.1093/ije/dyi049. [DOI] [PubMed] [Google Scholar]
- Grajeda, Ruben, Behrman Jere R., Flores Rafael, Maluccio John A., Martorell Reynaldo, Stein Aryeh D. The human capital study 2002-2004: Tracking, data collection, coverage, and attrition. Food and Nutrition Bulletin. 2005;26(2Supp 1):S15–S24. doi: 10.1177/15648265050262S103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves, Borenstein A, Mortimer James A., Bowen JD, McCormick Wayne C., McCurry Susan M., Schellenberg Gerald D., Larson EB. Head circumference and incident Alzheimer’s disease: modification by apolipoprotein. Neurology. 2001;57:1453–1460. doi: 10.1212/wnl.57.8.1453. [DOI] [PubMed] [Google Scholar]
- Habicht, Jean-Pierre, Martorell Reynaldo. Objectives, design and implementation of the INCAP longitudinal study. Food Nutrition Bulletin. 1992;14:176–190. [Google Scholar]
- Hill, Terence D, Burdette Amy M., Angel Jacqueline L., Angel Ronald J. Religious attendance and cognitive functioning among older Mexican Americans. The Journals of Gerontology: Psychological Sciences. 2006;61B(1):3–9. doi: 10.1093/geronb/61.1.p3. [DOI] [PubMed] [Google Scholar]
- Kabir, Zarina N, Herlitz Agneta. The Bangla adaptation of Mini-Mental State Examination (BAMSE): An instrument to assess cognitive function in illiterate and literate individuals. International Journal of Geriatric Psychiatry. 2000;15:441–450. doi: 10.1002/(sici)1099-1166(200005)15:5<441::aid-gps142>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Kim, Jae-Min, Stewart Robert, Il-Seon Shin, Sung-Wan Kim, Su-Jin Yang, Jin-Sang Yoon. Associations between head circumference, leg length and dementia in a Korean population. International Journal of Geriatric Psychiatry. 2008;23:41–48. doi: 10.1002/gps.1833. [DOI] [PubMed] [Google Scholar]
- Kuusisto, Koivisto J,K, Mykkanen L, Helkala EL, Vanhanen M, Hanninen T, Pyorala K, Riekkinen P, Laakso M. Essential hypertension and cognitive function: The role of hyperinsulinemia. Hypertension. 1993;22:771–779. doi: 10.1161/01.hyp.22.5.771. [DOI] [PubMed] [Google Scholar]
- Leibovici, Didier, Ritchie Karen, Ledésert Bernard, Touchon Jacques. Does education level determine the course of cognitive decline? Age and Ageing. 1996;25:392–397. doi: 10.1093/ageing/25.5.392. [DOI] [PubMed] [Google Scholar]
- Li, Haojie, DiGirolamo Ann M., Barnhart Huiman X., Stein Aryeh D., Martorell Reynaldo. Relative importance of birth size and postnatal growth for women’s educational achievement. Early Human Development. 2004;76:1–16. doi: 10.1016/j.earlhumdev.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Loury, Glenn C. A dynamic theory of racial income differences. In: Wallace PA, LaMonde AM, editors. Women, Minorities and Employment Discrimination. Lexington Books; Lexington, MA: 1977. pp. 153–186. [Google Scholar]
- Lynch, George JW, Kaplan A, Salonen Jukka T. Why do poor people behave poorly? Variation in adult health behaviours and psychosocial characteristics by stages of the socioeconomic lifecourse. Social Science and Medicine. 1997;44:809–819. doi: 10.1016/s0277-9536(96)00191-8. [DOI] [PubMed] [Google Scholar]
- Maluccio, John A, Melgar Paúl, Méndez Humberto, Murphy Alexis, Yount Kathryn M. Social and economic development and change in four Guatemalan villages: Demographics, schooling, occupation and assets. Food and Nutrition Bulletin. 2005;26(2 supplement 1):S25–S45. doi: 10.1177/15648265050262S104. [DOI] [PubMed] [Google Scholar]
- Messier C. Impact of impaired glucose tolerance and type 2 diabetes on cognitive aging. Neurobiology of Aging. 2005;26(1):26–30. doi: 10.1016/j.neurobiolaging.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Mortimer, James A, Snowdon David A., Markesbery William R. Head circumference, education, and risk of dementia: findings from the nun study. Journal of Clinical and Experimental Neuropsychology. 2003;25:625–633. doi: 10.1076/jcen.25.5.671.14584. [DOI] [PubMed] [Google Scholar]
- Nahapiet, Janine, Ghoshal Sumantra. Social capital, intellectual capital, and the organizational advantage. Academy of Management Review. 1998;23(2):42–66. [Google Scholar]
- Ofstedal, Mary B, Zimmer ZS, Lin Hsien S. A comparison of correlates of cognitive functioning in older persons in Taiwan and the United States. Journals of Gerontology. 1999;54B(5):S291–S301. doi: 10.1093/geronb/54b.5.s291. [DOI] [PubMed] [Google Scholar]
- Palloni, Alberto . Protocol of the multicenter study: health, well-being, and aging in Latin American and the Caribbean. Pan American Health Organization; Washington, D.C.: n.d. [Google Scholar]
- Park, Robert M, Schulte Paul A., Bowman Joseph D., Walker James T., Bondy Stephen C., Yost Michael G., Touchstone Jennifer A., Dosemeci Mustafa. Potential occupational risks for neurodegenerative diseases. American Journal of Industrial Medicine. 2005;48(1):63–77. doi: 10.1002/ajim.20178. [DOI] [PubMed] [Google Scholar]
- Perls TT, Morris JN, Ooi WL, Lipsitz LA. The relationship between age, gender, and cognitive performance in the very old: The effect of selective survival. Journal of the American Geriatric Society. 1993;41(11):1193–1201. doi: 10.1111/j.1532-5415.1993.tb07302.x. [DOI] [PubMed] [Google Scholar]
- Pivaral VM. Características económicas y socioculturales de cuatro aldeas ladinas de Guatemala. Ministerio de Educación Pública, Instituto Indigenista Nacional; Guatemala: 1972. Socio-economic characteristics of four Ladino villages of Guatemala. Guatemala: Ministry of Education, National Institute for Indigenous. [Google Scholar]
- Richards, Marcus, Deary Ian J. A life course approach to cognitive reserve: A model for cognitive aging and development. Annals of Neurology. 2005;58:617–622. doi: 10.1002/ana.20637. [DOI] [PubMed] [Google Scholar]
- Richards, Marcus, Jarvis Martin J., Thompson Neil, Wadsworth Michael E. J. Cigarette smoking and cognitive decline in midlife: Evidence from a prospective birth cohort study. American Journal of Public Health. 2003;93(6):994–948. doi: 10.2105/ajph.93.6.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards, Marcus, Sacker Amanda. Lifetime antecedents of cognitive reserve. Journal of Clinical and Experimental Neuropsychology. 2003;25(5):614–624. doi: 10.1076/jcen.25.5.614.14581. [DOI] [PubMed] [Google Scholar]
- Roe, Catherine M, Xiong Chengjie, Phillip Miller J, Morris John C. Education and Alzheimer disease without dementia: support for the cognitive reserve hypothesis. Neurology. 2007;68:223–228. doi: 10.1212/01.wnl.0000251303.50459.8a. [DOI] [PubMed] [Google Scholar]
- Saenz de Tejada, Sandra, María Mazariegos Luisa, Asturias de Barrios Linda. Intergenerational exchanges in four Guatemalan communities, Final Report. Estudio 1360 and International Food Policy Research Institute; Washington, D.C.: 2005. [Google Scholar]
- Schaie KW. The course of adult intellectual development. American Psychologist. 1994;49(4):304–313. doi: 10.1037//0003-066x.49.4.304. [DOI] [PubMed] [Google Scholar]
- Schmand B, Smit JH, Geerlings MI, Lindboom J. The effects of intelligence and education on the development of dementia: A test of the brain reserve hypothesis. Psychological Medicine. 1997;27:1337–1344. doi: 10.1017/s0033291797005461. [DOI] [PubMed] [Google Scholar]
- Schooler, Carmi, Mulatu Mesfin S., Oates Gary. The continuing effects of substantively complex work on the intellectual functioning of older workers. Psychology and Aging. 1999;14(3):483–506. doi: 10.1037//0882-7974.14.3.483. [DOI] [PubMed] [Google Scholar]
- Sinunu, Michelle A, Yount Kathryn M., Abdel-Wahab El Afifi Nadia. Formal and informal care of frail older adults in Cairo, Egypt. Journal of Cross-Cultural Gerontology. 2008;24(1):63–76. doi: 10.1007/s10823-008-9074-6. [DOI] [PubMed] [Google Scholar]
- Snowdon, David A, Kemper Susan J., Mortimer James A., Greiner Lydia H., Wekstein David R., Markesbery William R. Linguistic ability in early life and cognitive function and Alzheimer’s disease in later life, findings from the nun study. Journal of the American Medical Association. 1996;275:528–532. [PubMed] [Google Scholar]
- Stein, Aryeh D, Wang Meng, DiGirolamo Ann, Grajeda Ruben, Ramakrishnan Usha, Ramirez-Zea Manuel, Yount Kathryn M., Martorell Reynaldo. Nutritional supplementation in early childhood, schooling, and intellectual functioning in adulthood: a prospective study in Guatemala. Archives of Pediatrics and Adolescent Medicine. 2008;162(7):612–618. doi: 10.1001/archpedi.162.7.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein, Aryeh D, Wang Meng, DiGirolamo Ann, Hoddinott John, Martorell Reynaldo, Ramirez-Zea Manuel, Yount Kathryn M. Trends in height for age and body mass index for age among Guatemalan children between 1967 and 2007. American Journal of Nutrition. 2008;58:636–640. doi: 10.3945/jn.108.098343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein Aryeh D., Wang Meng, DiGirolamo Ann, Hoddinott John, Martorell Reynaldo, Ramirez-Zea Manuel, Yount Kathryn M. Height for age increased while body mass index for age remained stable between 1968 and 2007 among Guatemalan children. The Journal of Nutrition. 2009;139:365–369. doi: 10.3945/jn.108.098343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Alexander GE, Prohovnik Isak, Mayeux R. Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer’s disease. Annals of Neurology. 1992;32:371–375. doi: 10.1002/ana.410320311. [DOI] [PubMed] [Google Scholar]
- Terrion, Jenepher L, Lagacé Martine. Communication as precursor and consequence of subjective social capital in older people: A new perspective on the communication predicament model. Social Theory and Health. 2008;6(3):239–249. [Google Scholar]
- Turrell, Gavin, Lynch John W., Kaplan George A., Everson Susan A., Helkala Eeva L., Kauhanen Jukka, Salonen JT. Socioeconomic position across the lifecourse and cognitive function in late middle age. Journals of Gerontology. 2002;57B(1):S43–S51. doi: 10.1093/geronb/57.1.s43. [DOI] [PubMed] [Google Scholar]
- United Nations. Population Division. Department of Economic and Social Affairs World Population Prospects: The 2006 Revision and World Urbanization Prospects: The 2005 Revision. Retrieved August 20, 2007 from http://esa.un.org/unpp.
- Wilson, S R, Scherr Paul A., Bienias Julia L., Mendes de Leon Carlos F., Everson-Rose Susan A., D, Bennett A, Evans DA. Socioeconomic characteristics in the community in childhood and cognition in old age. Experimental Aging Research. 2005;31:393–407. doi: 10.1080/03610730500206683. [DOI] [PubMed] [Google Scholar]
- Wolk A, Bergström R, Hansson LE, Nyrén O. Reliability of retrospective information on diet 20 years ago and consistency of independent measurements of remote adolescent diet. Nutrition and Cancer. 1997;29(3):234–241. doi: 10.1080/01635589709514630. [DOI] [PubMed] [Google Scholar]
- World Health Organization [WHO] Revised Global Burden of Disease (GBD) 2002 Estimates. 2007 Retrieved August 20, 2007 from http://www.who.int/healthinfo/bodgbd2002revised/en/index.html.
- Yount, Kathryn M. Gender, resources across the life course, and cognitive functioning in Ismailia, Egypt. Demography. 2008;45(5):907–926. doi: 10.1353/dem.0.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yount, Kathryn M, Sibai Abla. The demography of aging in Arab countries. In: Uhlenberg Peter., editor. International Handbook of Population Aging. Springer; Dordrecht: 2009. pp. 277–315. [Google Scholar]
- Zhang, Mingyuan Y, Katzman Robert, Salmon David, Jin Hua, Cai Guojun J., Wang Zhengyu Y., Qu Guangya.Y., Grant Igor, Yu Elena, Levy Paul, Klauber MelvilleR., Liu William T. The prevalence of dementia and Alzheimer’s disease in Shanghai, China: impact of age, gender, and education. Annals of Neurology. 1990;27(4):428–437. doi: 10.1002/ana.410270412. [DOI] [PubMed] [Google Scholar]
- Zhou, Dong F, Wu Cheng S., Qi Hai, Fan Jun H., Sun Xiu D., Como Peter, Qiao You L., Zhang Lin, Kieburtz Karl. Prevalence of dementia in rural China: impact of age, gender, and education. Acta Neurologica Scandinavica. 2006;114:273–280. doi: 10.1111/j.1600-0404.2006.00641.x. [DOI] [PubMed] [Google Scholar]