Summary
Cell adhesion to the extracellular matrix (ECM) is mediated by the integrin family of transmembrane receptors. Integrins link ECM ligands to the cytoskeleton, providing strong attachment to enable cell-shape change and tissue integrity. This connection is made possible by an intracellular complex of proteins, which links to actin filaments and controls signalling cascades that regulate cytoskeletal rearrangements. We have identified stress-fibre-associated focal adhesions that change their composition during tissue morphogenesis. Early expression of αPS1βPS integrin decreases the levels of the actin-nucleating factors Enabled, Diaphanous and profilin, as well as downregulating the amount of F-actin incorporated into the stress fibres. As follicle cells mature in their developmental pathway and become squamous, the integrin in the focal adhesions changes from αPS1βPS to αPS2βPS. During the switch, stress fibres increase their length and change orientation, first changing by 90° and then reorienting back. The normal rapid reorientation requires new expression of αPS2βPS, which also permits recruitment of the adaptor protein tensin. Unexpectedly, it is the extracellular portion of the αPS2 subunit that provides the specificity for intracellular recruitment of tensin. Molecular variation of the integrin complex is thus a key component of developmentally programmed morphogenesis.
Keywords: Integrin, Stress fibres, Follicle cells
Introduction
Integrins mediate adhesion to the extracellular matrix (ECM) by connecting extracellular ligands to the cytoskeletal network, via an intracellular multiprotein complex: the integrin-cytoskeleton link (Hynes, 2002; Zaidel-Bar et al., 2007). The proteins that form or modulate this link are surprisingly numerous, suggesting that it can perform diverse functions. Insight into its range of activities came from the identification of adhesive structures whose composition varies. Even within a single mammalian cell cultured on an ECM substrate, distinct integrin adhesive structures are formed that differ in their morphology, position within the cell and molecular composition (Zamir et al., 1999; Zamir et al., 2000; Zamir and Geiger, 2001). Three categories of adhesions exist in fibroblasts cultured on two-dimensional (2D) matrices. Focal complexes are the earliest adhesions, forming at lamellipodia in migrating cells, and are not connected to stress fibres. Focal contacts are oval peripheral adhesions enriched with αVβ3 integrin, paxillin, vinculin and tyrosine-phosphorylated proteins, whereas fibrillar adhesions are central elongated structures containing α5β1 integrin, tensin and parvin (Zamir et al., 1999; Olski et al., 2001). Focal contacts attach to actin stress fibres and promote strong adhesion to rigid substrates (Geiger et al., 1995; Bershadsky et al., 1996), whereas fibrillar adhesions translocate centripetally from focal adhesions and promote fibrillogenesis on softer matrices, in a process called adhesion maturation (Katz et al., 2000; Zamir et al., 2000). Increasing the rigidity of the matrix blocks the formation of the fibrillar adhesions (Katz et al., 2000), and actomyosin contractility is required to form focal contacts and fibrillar adhesions, but only to maintain focal contacts (Zamir et al., 1999; Zamir et al., 2000). It was thus proposed that switching from focal contacts to fibrillar adhesions depends on local tension and matrix properties, allowing cells to adapt their adhesion to their environment (Zamir et al., 2000). However, the finding that culturing cells in a three-dimensional (3D) ECM resulted in a uniform adhesive structure (Cukierman et al., 2001) suggested that the diversity was artificially generated by culture on a 2D matrix. The biological significance of structural and molecular diversity of integrin adhesions is thus uncertain, and the relationship between molecular composition and functionality is still poorly understood. To address this issue, we searched for focal adhesions within tissues in the intact animal.
Adhesive structures similar to the focal contacts, i.e. associated with stress fibres, are rare within the intact organism, but examples include within mouse vascular endothelial cells, in goldfish fibroblasts, and in cells of the Drosophila pupal retina and follicular epithelium (Byers and Fujiwara, 1982; White et al., 1983; Cagan and Ready, 1989; Gutzeit, 1990). In the follicular epithelium, the apical surface of the cells contacts the oocyte, whereas the basal surface contacts the surrounding basement membrane (Gutzeit, 1990; Gutzeit, 1991). On the basal surface, actin filaments are bundled into stress fibres that terminate at protein aggregates containing integrins (Bateman et al., 2001). Additional components of these focal adhesions have been identified: the receptor tyrosine phosphatase Lar, α-actinin, Enabled and p21-activated kinase (PAK) (Bateman et al., 2001; Deng et al., 2003; Wahlstrom et al., 2006; Conder et al., 2007).
Here we show that molecular variation in the integrin adhesive structures occurs within the normal development of the follicular epithelium during Drosophila oocyte maturation. We show that these focal adhesions, which are linked to stress fibres, contain a full complement of integrin-associated proteins. As development proceeds we find that the integrin within these adhesions changes from the laminin-binding αPS1βPS to the RGD-binding αPS2βPS. Unique roles for each integrin have been identified, showing that the transition in integrin adhesive structures provides distinct activities during morphogenesis.
Results
A model of focal adhesions associated with stress fibres in a developing tissue
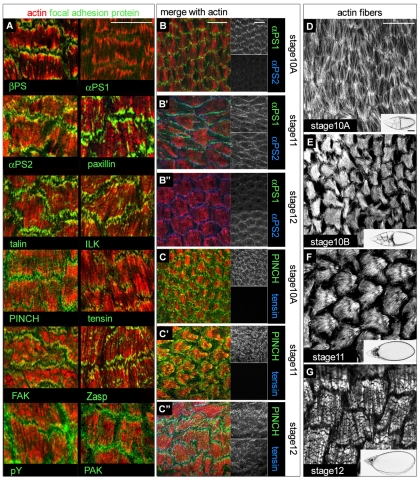
To confirm that the integrin adhesive structures associated with stress fibres in follicle cells are similar to those in vertebrate cells, we examined whether these structures also contain other integrin-associated proteins that we are able to visualize with existing reagents. We also examined whether the composition of the integrin structures changed as the oocyte matured and the surrounding follicular epithelial changed from columnar to squamous morphology. We found that paxillin, talin, integrin-linked-kinase (ILK), PINCH, tensin and Zasp were all present (Fig. 1A). The recently characterized NHL-domain protein Wech (Loer et al., 2008) was not found in the focal adhesions, although it colocalized with integrins in follicular stalk cells (data not shown), suggesting that Wech is not required at all sites of integrin function. In addition, phosphorylation pathways are active at these sites: focal adhesion kinase (FAK) was present (Fig. 1A) and in an active state, as indicated by phosphorylation of the autophosphorylation site Y397 (data not shown), as were proteins phosphorylated on tyrosine, and the serine/threonine kinase PAK (Fig. 1A) (see also Conder et al., 2007). Most proteins examined were present in focal adhesions at all developmental stages, with two exceptions: the integrin heterodimers changed, starting with the laminin-binding integrin αPS1βPS and making a transition to the RGD-binding αPS2βPS (Fig. 1B-B″), and tensin was only detectable at late stages (Fig. 1C-C″). These findings suggest that the function of the adhesive structure changes over time, because it undergoes a change in integrins and associated proteins.
Fig. 1.
Distribution of focal-adhesion proteins and compositional changes during egg-chamber morphogenesis. (A) Micrographs of the basal surface of stage 12 follicle cells showing the distribution of integrin subunits and associated proteins (green, as indicated) at actin stress-fibre (red) ends. (B-B″) Distribution of αPS1 (green), αPS2 (blue) and actin (red) at stages 10A, 11 and 12. Individual channels for αPS1 and αPS2 on the right of each panel show that αPS2 is not expressed at early stage 10A, overlaps with αPS1 at stage 10B-11 and is very strong at stage 12, whereas αPS1 becomes faint at this stage. (C-C″) Triple staining for PINCH (green), tensin (blue) and actin (red) shows that PINCH is present throughout oogenesis, whereas tensin is only detectable from stage 12. (D-G) Morphology of follicular stress fibres during morphogenesis from stage 10A to stage 12 with corresponding egg chamber shown in lower right-hand corner. Scale bars: 20 μm (A-G); 50 μm (D-G insets).
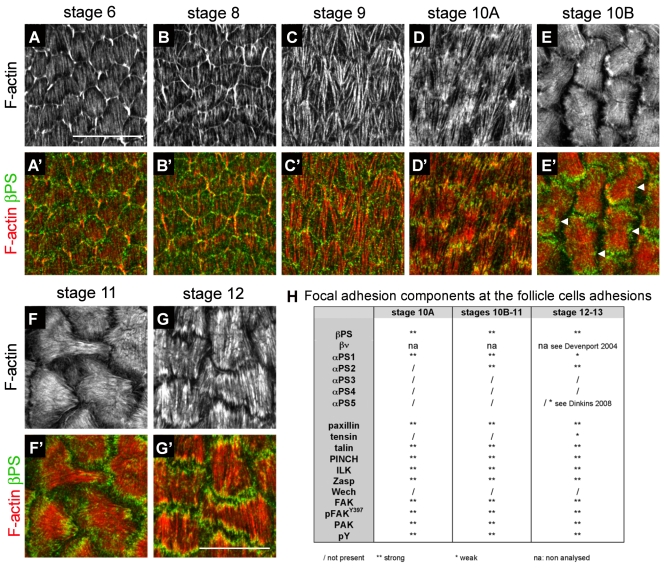
The molecular variation of these adhesive complexes raises the issue of why these changes occur and what mechanisms produce the changes. Possible functions for the changes were suggested by the developmental progression of stress-fibre and follicular-cell morphology. Follicular cells undergo a dramatic shape change from stages 10B to 12-13, flattening during oocyte growth (follicle-cell number remains constant). A particularly abrupt increase in oocyte volume occurs when the nurse-cell contents are squeezed into the oocyte (nurse-cell dumping), starting at stage 10B (Spradling, 1993). The F-actin stress fibres first become clearly organized midway through oogenesis, at stage 10A (Fig. 1D, Fig. 2A-G). At this stage, they are arranged perpendicular to the long axis of the egg chamber [the oocyte anterior-posterior (A-P) axis], encircling the oocyte. The stress fibres then change their orientation during nurse-cell dumping. They first become thicker and less well oriented at the start of dumping, stage 10B (Fig. 1E). By stage 11, they have acquired a fan-shaped organization oriented along the long axis, i.e. shifted 90° relative to their earlier orientation, with the fan base pointing posterior. They now cover a larger area, reflecting the flattening of each follicular cell (Fig. 1F). Finally, at stage 12 (dumping completed), the stress fibres have returned to their original orientation, become further thickened and have a striated appearance, with regular gaps in the phalloidin staining of F-actin, reminiscent of sarcomeric striations (Fig. 1G). Stress-fibre reorientation seems to occur by disassembly of existing fibres and synthesis of new ones, rather than the cell turning, because at intermediate stages a spot of actin and integrins forms at an internal point on the basal cell surface, in a position that suggests it will become the base of the fan (Fig. 2E′). In general, during all these changes integrin adhesive structures remain at actin fibre ends (Fig. 1B-B″, Fig. 2A′-G′).
Fig. 2.
Time course of stress-fibre organization throughout oogenesis and summary of the distribution of all focal-adhesion molecules tested. (A-G′) Micrographs of basal actin stress fibres from stage 6 to stage 12, showing F-actin (A-G) and its merge with βPS (A′-G′). All images are oriented posterior to the right. Basal F-actin is present from the beginning of oogenesis (low staining in cell middles in A), but new filaments become visible from stage 6 (high staining originating from cell borders and pointing down). These new filaments extend at stage 8 (B) and, together with the early filaments, form long fibres covering the cells at stage 9 (C). These fibres are more apparent at stage 10A (D). Up to stage 10A, the fibres orient perpendicular to the long axis of the egg. At stage 10B, the fibres thicken (E) and change their orientation by 90° by stage 11 (F), adopting a fan shape. At stage 12, the original orientation is recovered and stress fibres are striated (G). βPS localizes at actin-fibre ends at all stages, and also at cell borders. (E′) Note that a central patch of βPS is also present, suggesting de novo adhesion (arrowheads). (H) Table summarizing all focal-adhesion components tested and their presence at stages 10A, 11 and 12. Scale bars: 20 μm.
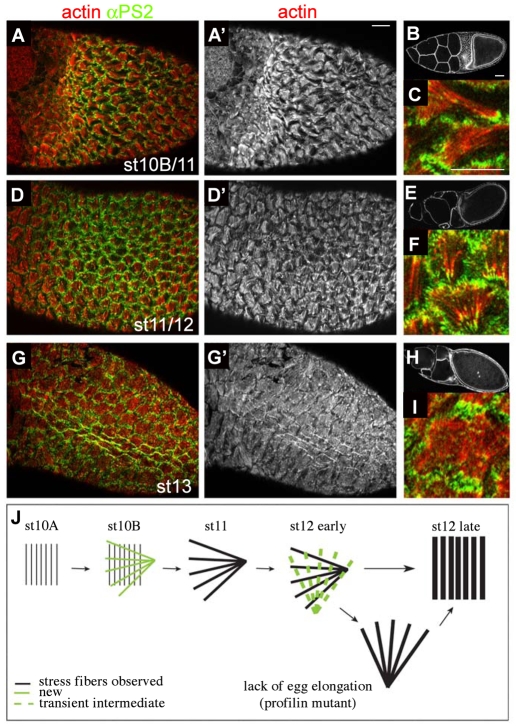
The observation that stress-fibre orientation changes as nurse cells dump their contents suggested that the reorientation and αPS2 expression could be triggered by oocyte enlargement and the resulting thinning of follicle cells. We therefore examined egg chambers that do not undergo dumping owing to a mutation in the gene encoding profilin that reduces its expression solely in the oocyte (Cooley et al., 1992). Despite the lack of dumping, αPS2 was still expressed, the stress fibres changed orientation (Fig. 3A-C) and then reoriented back (Fig. 3D-I). Thus, despite the temporal correlation, oocyte expansion is not required for changes in actin orientation nor αPS2 expression. However, some defective actin organization was observed, with the novel occurrence of fan-shaped stress fibres oriented perpendicular rather than parallel to the oocyte A-P axis (Fig. 3F). This suggests that the fan-shaped actin is an intermediate in the process of reorientation, and that the normally rapid conversion of a perpendicular fan shape to an array that fills the basal surface is delayed in the absence of oocyte elongation (see Fig. 3J).
Fig. 3.
Nurse-cell dumping does not trigger fibre reorientation or αPS2 expression. Stress-fibre orientation and αPS2 expression were examined in egg chambers of chic01230 females that are defective in nurse-cell dumping (see text). Stages are difficult to assess in these eggs; nonetheless, changes in actin morphology are clearly observed. (A-C) Stress fibres at stage 10B-11 (B) exhibited newly oriented fan shapes, and αPS2 was expressed (A,A′); (C) close-up of A. (D-F) When nurse cells start to degenerate at stages 11-12 (E), stress fibres were found oriented perpendicular to the long axis (D,D′) and mis-oriented fans were observed (F). (G-I) At stage 13, despite the lack of dumping, the oocyte is fully developed (H) and stress fibres were normally orientated; (I) close-up of G. (J) Schematic showing how reduction of profilin might prolong a normally brief intermediate. Scale bars: 20 μm (for all except B,E,H); 50 μm (B,E,H).
In summary, we have found a transition in the integrin heterodimer that occurs as stress fibres are remodelled. The changes in stress-fibre morphology could be the cause of the changes in adhesive junction, or reciprocally the adhesive-junction changes could change actin orientation. Furthermore, the stress fibres from stage 12 onwards, which terminate in adhesive contacts containing αPS2βPS and tensin, are much more substantial and striated than those found at the earlier stage that are in contact with αPS1βPS. To assess the importance of integrin adhesive structures in stress-fibre formation, we next examined the effects of removing all βPS-containing integrin heterodimers, thereby removing all integrin function, because the only other β-subunit, βν, is not functional in follicle cells (Devenport and Brown, 2004).
Integrins are required for stress-fibre attachment and control of F-actin levels
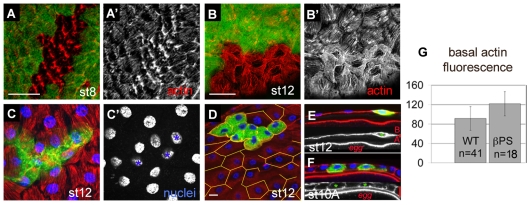
Because loss of integrins causes lethality, we used mitotic recombination to create cells lacking βPS, and examined stress-fibre distribution in clones of follicle cells derived from these mutant cells. As focal adhesions precede the formation of stress fibres in cells in culture (Couchman and Rees, 1979), we expected the integrin adhesive junctions to be required for the formation of stress fibres in follicle cells. We were therefore surprised to find that, in clones of cells lacking βPS, prominent bundles of F-actin were still present at the basal surface (Fig. 4A-B′). These bundles were not organized into the normal parallel arrangement and were displaced towards the periphery. The amount of F-actin at the basal surface of the mutant cells was greater than in adjacent wild-type cells, with quantification of the images revealing a 33% increase (Fig. 4G). Thus, integrins are not required to generate basal actin fibres, but instead are needed to reduce fibre number and organize them into parallel arrays. In addition to the change in stress fibres, lack of βPS-containing integrins resulted in changes to the shape of the follicle cells. The cells lost their hexagonal shape (Fig. 4D), and were unable to flatten fully, as revealed by the position of the nuclei in a lower focal plane (Fig. 4C,C′) and their shape in section (Fig. 4E). The morphology of the mutant cells within the columnar epithelium at earlier stages seemed normal (Fig. 4F) (Devenport and Brown, 2004). In some cases, integrin loss was accompanied by extensive filopodia-like actin spikes (Fig. 5A′).
Fig. 4.
Integrin adhesion is required for stress-fibre organization and the control of F-actin content at the basal surface. (A-F) Micrographs of clones lacking βPS, with clones marked either by the absence of GFP (A,B; no green) or the presence of GFP (C-F; green). (A′,B′) Show actin distribution; (A,B) show the merge of actin (red) and the position of the clone. (A,A′) Stage 6 egg chamber showing mutant cells forming thicker filaments than wild-type neighbours. (B,B′) Basal F-actin is stronger in cells lacking βPS at stage 12. (C,C′) In the absence of integrin, basal F-actin fails to assemble into stress fibres (C) and cells fail to flatten as indicated by nuclei at a lower focal plane (C′). (D) A mid-cell section shows the rounded shape of the cells lacking integrin (actin, red; nuclei, blue); the yellow lines show the shape of wild-type cells. (E,F) Transverse sections of follicular epithelia [actin, red; nuclei, blue; CD8-GFP, green (mutant cells); actin channel, grey). Red bars on the right indicate the thickness of the epithelia and apical (`A') and basal (`B') surfaces. The oocyte membrane is just above `egg'. Green asterisks on the actin channel indicate the position of mutant cell(s). Cells lacking integrin were rounded and failed to flatten at stage 12 (E), whereas stage 10A cells seemed normal (F). (G) Quantification of basal F-actin in cells lacking integrins (βPS) compared with wild type (WT) measured as integrated area (grey mean value multiplied by cell area). Wild-type value (92) divided by mutant value (122) is 0.75, which indicates a 33% increase in basal actin in the mutant cells. P=2.8×10–5 (Student's t-test two samples assuming equal variance). Scale bars: 20 μm. Scale bar shown in A is for A,A′; scale bar shown in B is for all other micrographs except D.
Fig. 5.
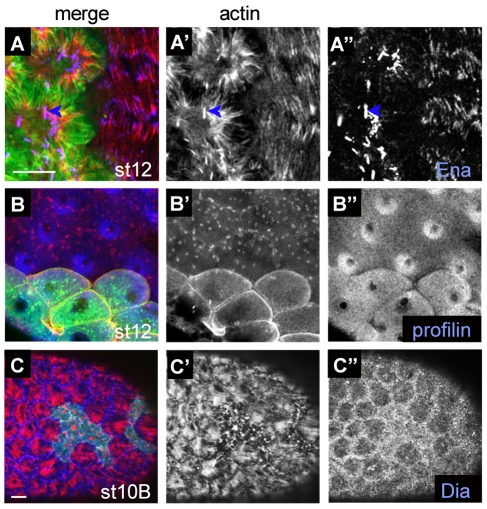
Integrins downregulate actin-nucleating factors. Micrographs show clones lacking βPS, marked by the presence of GFP (green) in stage 12 egg chambers, except for C-C′″, which is stage 10B. (A-A′″) In addition to not assembling F-actin into fibres, cells lacking integrins form F-actin aggregations associated with Enabled (blue arrow). (B-B″) Mid-cell section showing that cytoplasmic profilin is increased in cells lacking integrins (B″), and F-actin distribution reveals rounded cell shape (B′) [cytoplasmic profilin was increased throughout the cell (data not shown)]. (C-C″) Diaphanous levels were increased at the basal surface of cells lacking integrins. Scale bars: 20 μm.
We next explored possible mechanisms to explain the increase in F-actin in cells lacking integrins, by examining the distribution of proteins that stimulate actin-filament assembly: Enabled (Ena), profilin and Diaphanous (Dia) (Chakraborty et al., 1995; Reinhard et al., 1995; Butler et al., 2006). Ena was previously shown to localize at adhesion sites and genetic removal of Ena, profilin or Dia reduces basal F-actin in follicle cells (Baum and Perrimon, 2001) (data not shown). Furthermore, Ena overexpression produces aggregates of F-actin and Ena in both mammalian and follicle cells (Gertler et al., 1996; Baum and Perrimon, 2001), and similar aggregates were observed in cells lacking integrins (Fig. 5A-A″). Ena accumulated within these aggregates at levels higher than within focal adhesions in adjacent wild-type cells (Fig. 5A″), suggesting elevation of Ena levels. Cytoplasmic profilin was increased in mutant cells, so that the higher concentration of nuclear profilin seen in wild-type cells was no longer apparent (Fig. 5B-B″). This regulation occurs post-transcriptionally, because profilin mRNA levels were unchanged (data not shown). Basal surface levels of Dia were also increased, with this elevation clearer at earlier stages (Fig. 5C-C″). Similar to profilin, this occurred post-transcriptionally, because dia mRNA levels were unchanged (data not shown). Assay of monomeric actin levels by DNAse-I staining showed no difference between wild-type and mutant cells (data not shown), suggesting that the F-actin increase is not driven by increasing monomer concentration. Thus, integrin function is essential for regulating the amount of F-actin at the basal surface and its organization into ordered parallel arrays. This is correlated with an increase in levels of the three actin-polymerization factors Ena, Dia and profilin, suggesting that integrins normally downregulate the actin machinery to control the amount of F-actin incorporated into stress fibres.
αPS1βPS and αPS2βPS have distinct roles
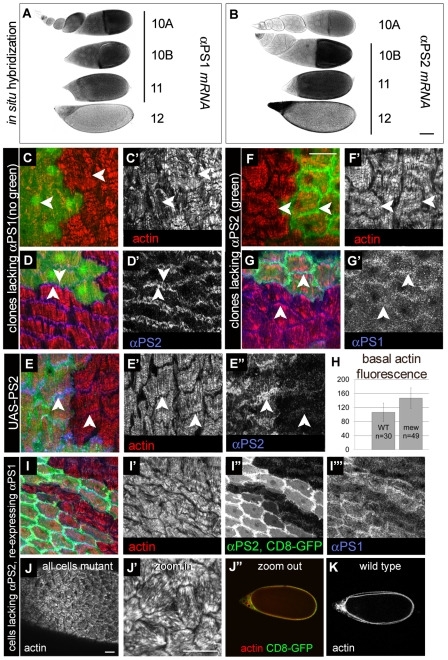
Having characterized the role of all βPS-containing integrins in the organization of follicle-cell stress fibres, we then examined the role of the two major integrin heterodimers expressed in follicle cells, αPS1βPS and αPS2βPS [expression of αPS3 or αPS4 mRNA was not detected in most follicular cells, whereas αPS5 mRNA was only detected at late stages 11 and 12 (Dinkins et al., 2008)]. We first examined the mechanism that results in the transition from one integrin to the other, and found that this occurs by mRNA regulation. The mRNA encoding αPS1 was first detected in follicle cells from stage 4, was retained through stage 11 and then decreased (Fig. 6A). The mRNA encoding αPS2 showed reciprocal expression, becoming first detectable at stage 10B and remaining from then onward (Fig. 6B). Expression of αPS2 mRNA at an earlier stage using the Gal4 system resulted in early production of αPS2βPS (data not shown), demonstrating the lack of a post-transcriptional mechanism to prevent early production of αPS2βPS. A similar switch of integrins in Caenorhabditis elegans is regulated by the transcription factor Pax6 (vab-3) (Meighan and Schwarzbauer, 2007). However, testing whether this is conserved was thwarted by possible redundancy between the four Drosophila Pax6 orthologues, all of which are expressed in follicle cells (supplementary material Fig. S1).
Fig. 6.
αPS1βPS and αPS2βPS have distinct functions for follicle-cell stress-fibre organization. (A,B) In situ hybridization on whole egg chambers showing in black the levels of mRNAs encoding αPS1 (A) and αPS2 (B) during oogenesis. (C-D′) Clones of cells lacking αPS1, marked by the absence of GFP. (C,D) Position of the mutant cells (not green) are shown together with the distribution of actin (red) and another protein if relevant (blue). (C,C′) Cells lacking αPS1 form fibres with brighter actin intensity than in wild-type cells; compare arrowheads. (D,D′) Loss of αPS1 elevates αPS2 levels; compare arrowheads. (E-E″) Overexpression of αPS2 using the UAS-GAL4 system increased αPS2 levels (E″) but did not change the actin fibres (E′). (F-G′) Clones of cells lacking αPS2, marked by the presence of GFP (MARCM clones of ifB4). (F,F′) Loss of αPS2 does not perturb stress fibres; compare arrowheads pointing to a wild-type and a mutant cell on F′. (G-G′) αPS1 level in focal adhesions was increased when αPS2 was removed; compare arrowheads in G′. (H) Quantification of basal F-actin in cells lacking αPS1 (mew) compared to wild type (WT) as shown in C and measured as integrated area (grey mean value multiplied by the cell area). Wild-type value (106) divided by mutant value (147) is 0.72, which indicates a rough increase of 38% in basal actin in the mutant cells, comparable to the increase observed in the total absence of integrins (see Fig. 2E). P=1.5.E-8 (Student's t-test two samples assuming equal variances). (I-I′″) Re-expression of αPS1 (blue, I′″) in cells lacking αPS2 (CD8-GFP, green, I″) and stained for actin (red, I′) and αPS2 (green also, I″) results in a delay in stress-fibre re-orientation at stage 12 (I′); most stress fibres in CD8-GFP-positive cells have failed to reorient compared to WT neighbours. (J-J″,L) Global (J) and magnified (J′) view of the basal surface of an egg chamber at early stage 12 where the whole follicular epithelium lacks αPS2 and re-expresses αPS1. In this extreme case, the stress fibres have random orientation yet the whole egg morphology is normal (J″) compared to a wild-type egg chamber (K). Scale bar in B: 50 μm (for A,B,J″,K); scale bar in F: 20 μm (for C-I′″); scale bars in J and J′: 20 μm.
Next, we removed each integrin heterodimer, by making clones of cells mutant for each α-subunit, and examined the consequences at late stages. In both, stress fibres were still organized, but cells that lacked αPS1βPS had elevated levels of basal F-actin. Quantification showed that αPS1 loss caused a similar level of F-actin increase as βPS loss (Fig. 6C,C′,H). Cells lacking αPS1 showed elevated levels of αPS2βPS (Fig. 6D,D′), most likely owing to the lack of competition between the α-subunits to form heterodimers with limited amounts of βPS. Thus, αPS2βPS is able to compensate for the loss of αPS1βPS for stress-fibre organization, but cannot control F-actin levels. We made sure that it was αPS1 loss rather than the resultant increase of αPS2βPS that was elevating F-actin levels, by overexpressing the αPS2 subunit with the Gal4 system. This successfully increased the levels of αPS2βPS, with no change in F-actin levels (Fig. 6E-E″). Absence of αPS1 also resulted in elevated levels of Ena, profilin and Dia, as observed for the absence of βPS (supplementary material Fig. S2) and this was not altered by αPS2 overexpression (data not shown). Conversely, cells lacking profilin still expressed αPS1, showing that the amount of basal F-actin does not influence integrin expression, but that actin fibres are required for normal distribution of focal adhesions (supplementary material Fig. S3). Thus, αPS1βPS is required to control F-actin levels, and αPS2βPS is not able to perform this function. Cells lacking αPS1 do not display cell-shape defects, and therefore F-actin increases are not a secondary consequence of cell-shape changes.
We did not detect any defects in cells lacking αPS2βPS (Fig. 6F,F′). The mutant cells showed elevated levels of αPS1βPS at late stages (Fig. 6G,G′), again suggesting that newly synthesized αPS2 competes with residual expression of αPS1 for limiting amounts of βPS subunit, and therefore that αPS2 expression contributes to αPS1βPS downregulation. Thus, within the limits of our analysis, residual αPS1βPS can substitute for αPS2βPS at late stage 12. Because stress fibres were better organized when αPS1βPS was removed, compared with all integrins removed, this demonstrates that αPS2βPS can organize fibres. We then tested whether the integrin switch per se had an important function for stress-fibre morphogenesis. To do so, we expressed αPS1 in clones of cells lacking αPS2 so that they maintained a high level of αPS1βPS and did not switch to αPS2βPS. These cells had not reoriented the stress fibres at early stage 12 (Fig. 6I-J′″), but recovered and completed reorientation by the end of stage 12 (data not shown). This shows that the switch from αPS1 to αPS2 is essential for the normal rapid return of the stress fibres to their circumferential orientation.
An additional function for αPS2 was suggested by the round egg phenotype caused by clones lacking αPS2 (Bateman et al., 2001) and in the eggs produced by females homozygous for a weak allele of the gene encoding αPS2, ifV2 (supplementary material Fig. S4). However, in our analysis of follicle-cell clones lacking αPS2 we found many examples in which all follicle cells lacked αPS2, yet the oocyte was normally elongated (data not shown). A possible explanation was that αPS2 also functions in the surrounding muscle layer (called the epithelial sheath), which contracts to help oocyte elongation. These muscles are unusual in that they do not fuse, and so clones of mutant cells can be produced (Hudson et al., 2008). We generated clones lacking αPS2 just in these muscle cells and found detachment of the actin from the ends of the muscles (supplementary material Fig. S4), similar to the muscle detachment in embryos (Brabant and Brower, 1993), and this was not rescued by αPS1 (supplementary material Fig. S4), but no round eggs were observed. It might therefore be that the round egg phenotype is caused by loss of αPS2 in both follicle cells and this muscle layer.
In order to define further the contribution of αPS2βPS integrin to stress-fibre organization, we examined a molecular phenotype specific to adhesive structures containing αPS2βPS: the recruitment of tensin.
Only αPS2βPS can recruit tensin in follicle cells
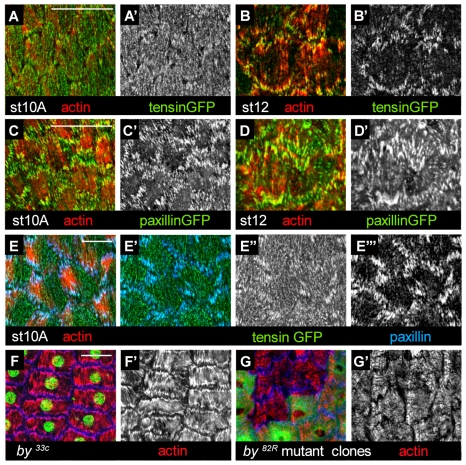
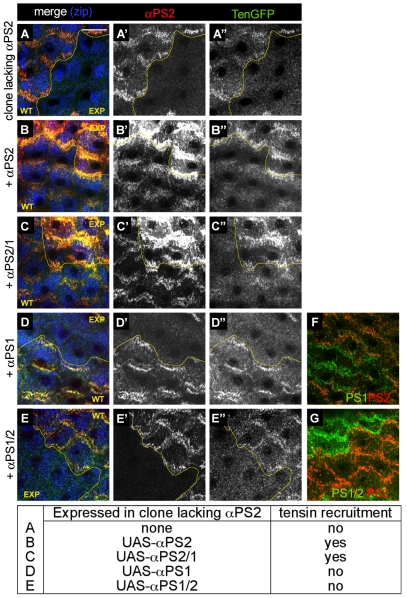
In follicle cells, tensin is only recruited after αPS2 is expressed (Fig. 1B-C″), suggesting that this might reflect a specific function for αPS2βPS. We first tested whether tensin could be associated with αPS1βPS-containing focal adhesions if we ectopically expressed tensin at early stages. The overexpressed tensin-GFP at early stage 10A was spotty at the basal surface of the cell and did not concentrate at αPS1βPS-containing focal adhesions (Fig. 7A,A′), whereas, at late stage 12 it was effectively recruited to those containing αPS2βPS (Fig. 7B,B′). In comparison, similarly expressed paxillin-GFP was effectively recruited at both stages (Fig. 7C-D′). Whereas most overexpressed tensin-GFP was mislocalized at stage 10A, some was recruited to focal adhesions, as shown by colocalization with paxillin (Fig. 7E-E′″). In clones of cells lacking αPS2 (which retain some αPS1βPS in focal adhesions) tensin-GFP expressed from its own promoter was no longer recruited (Fig. 8A-A″′). To rule out that it is not a threshold of integrin expression rather than αPS2βPS that is essential for tensin recruitment, we overexpressed αPS1 in cells lacking αPS2 but this was not able to recruit tensin (Fig. 8D-D″,F for αPS1 expression in cells lacking αPS2). Thus, tensin can only be recruited by αPS2βPS and not by αPS1βPS.
Fig. 7.
Tensin cannot be recruited at focal adhesions before stage 12. (A-D′) Clones of cells expressing Gal4 and consequently UAS constructs of tensin-GFP (A-B′) or paxillin-GFP (C-D′) at stages 10A and 12. Colour micrographs show the merge of actin (red) and the GFP-tagged protein (green), and the individual channel for the GFP protein is shown in grey. Tensin-GFP was localised at the ends of the stress fibres at stage 12 (B′) but not stage 10A (A′) compared to paxillin-GFP, which was localized at both stages (C′ and D′). (E-E′″) The co-localization experiment with paxillin antibody confirms that tensin-GFP is poorly recruited to focal adhesions at stage 10A compared to paxillin (E″ vs E′″). (F-F″) The lack of tensin is not deleterious for stress-fibre formation or organization, as shown in a stage 12 egg chamber where all cells lack tensin (G,G′; from a homozygous by33c female) or in clones of cells lacking tensin (by82R) (absence of GFP, no green). Egg chambers are stained for actin (red), βPS (blue) and nuclei (green) in A. Scale bars: 20 μm.
Fig. 8.
αPS2βPS is able to recruit tensin whereas αPS1βPS is not, and the specificity resides in the extracellular or transmembrane domain of αPS2. (A-G) Wild-type cells (WT) adjacent to experimentally perturbed clones of cells (EXP); a yellow line marks the interface. (A-A″) Cells lacking αPS2 (ifB4 mutant clones), revealed by absence of of αPS2 (red, A′) failed to recruit tensin-GFP (A″); the presence of stress fibres was shown by non-muscle myosin II (Zipper, Zip, blue in A). Re-expressing αPS2 (B-B″) or a chimera αPS2/1 with the cytoplasmic domains swapped (C-C″) in cells lacking αPS2 recruited tensin-GFP (note that αPS2 is `overexposed' in B′ and C′ because the level of re-expressed protein is elevated relative to wild type, and the level of αPS2 in the wild-type cells was used to set image levels). Conversely, αPS1 (D-D″) or the inverse chimera αPS1/2 (E-E″) failed to restore tensin-GFP recruitment. (F,G) Control images confirm the replacement of αPS2 with αPS1 or αPS1/2 (detected with the anti-αPS1 antibody) in similar clones to those in D and E. Scale bar: 20 μm.
The simplest mechanism for tensin recruitment is that it binds directly or indirectly to the αPS2 cytoplasmic domain. We tested this by replacing αPS2 with an αPS2/1 chimera having the cytoplasmic domain of αPS1 (Martin-Bermudo et al., 1997), or with αPS2 as control. In both cases, tensin was recruited and, furthermore, the amount of tensin was not limiting because more tensin-GFP was recruited by the increased levels of re-expressed αPS2 or αPS2/1 (Fig. 8B-C′″). By contrast, the reciprocal αPS1/2 chimera did not recruit tensin (Fig. 8E-G), demonstrating that only the extracellular (and/or transmembrane) domain of αPS2 can specify tensin recruitment. Adhesive structures in the imaginal disc containing primarily αPS1βPS contain high levels of tensin (data not shown), suggesting that αPS1βPS can recruit tensin in other cells.
Finally, we investigated the consequences of the lack of tensin in follicle cells. Tensin was found associated with major integrin adhesion sites in Drosophila, but the lack of tensin induced defects only in the wing, as flies completely lacking tensin are viable and fertile (Lee et al., 2003; Torgler et al., 2004). The follicle-cell stress fibres in animals lacking tensin seemed normal (Fig. 7F,F′). Owing to the variation in stress-fibre appearance, it is easier to see mutant phenotypes in clones, where mutant cells are compared with wild-type neighbours. However, even then we could not identify any defects caused by tensin loss (Fig. 7G,G′). Although this fails to explain the importance of recruiting tensin at late stages, it is fully consistent with the absence of a late phenotype in follicle cells lacking αPS2βPS, which do not recruit tensin. Thus, the recruitment of proteins such as tensin by αPS2βPS might provide robustness to follicle-cell behaviour that is not detected under normal culture conditions.
Discussion
We have shown that morphogenesis of the epithelial layer of follicle cells is accompanied by changes in the integrin-mediated focal adhesions on the basal surface. These changes, which are similar but not equivalent to those in vertebrate cells in culture, include exchanging one integrin for another, and new recruitment of tensin to mature integrin adhesions. Such switches in integrin heterodimers have been characterized in other developmental systems and are also regulated transcriptionally. Surprisingly, integrins organize actin filaments into well-organized arrays of parallel stress fibres but do not control their assembly into basally localized bundles. In fact, we found that integrins reduce basal F-actin levels, as well as reducing levels of proteins that contribute to new filament nucleation: Ena, Dia and profilin. This function is mediated by αPS1βPS integrin at early stages of oogenesis. Midway through the developmental process, αPS1βPS is replaced by αPS2βPS, which has the unique ability to recruit tensin in these cells. Finally, the changes in integrin adhesions and orientation of the actin filaments suggest novel functions for follicle-cell stress fibres.
Follicle-cell adhesions vs focal adhesions in cultured cells: similarities and differences
The changes observed in the follicular epithelium have both similarities and differences with the changes in focal adhesions that occur within vertebrate cells cultured on a 2D ECM. In both cases, distinct adhesive structures arise through a process of maturation. In vertebrate cells, the first structures that form are focal complexes, some of which mature into focal contacts, which in turn can mature into fibrillar adhesions (Zamir et al., 1999). In both vertebrate cells and the follicular epithelium, the adaptor protein tensin is enriched in the most mature structures: fibrillar adhesions and post-stage-11 follicle cells, respectively. Also, in both systems the integrin that forms the connection to the ECM changes. In vertebrate cells this is a change between two fibronectin receptors, αVβ3 and α5β1, whereas in the follicular epithelium the change in integrin implies a change in the ECM ligand from laminin to an RGD-containing ligand (Bunch and Brower, 1992; Gotwals et al., 1994). The main difference is that proteins that are lost in vertebrate cells from the adhesive structures as they mature, such as paxillin and proteins phosphorylated on tyrosine, remain present in late follicle-cell adhesions, coexisting with tensin. Furthermore, both the different integrin structures coexist in vertebrate cells, whereas all adhesions change simultaneously in the follicular epithelium. Finally, the time frame differs, with the transition from a focal complex to a fibrillar adhesion taking approximately 1 hour (Zamir et al., 2000), whereas follicle-cell maturation takes 10.5 hours (Spradling, 1993).
Our findings demonstrate that the composition of adhesive structures does change in normal cells within an intact tissue, and therefore such changes are not just a phenomenon reserved for cells in culture. This indicates that 3D culture does not in all cases represent intact tissues more accurately than 2D culture. The differences between 2D and 3D culture might still highlight an important distinction between integrin adhesion in cells surrounded by ECM compared with those that contact the ECM with part of their cell surface, as epithelial layers do when contacting the basement membrane. It could be that focal-adhesion transitions are restricted to cells in contact with a flat ECM, but to confirm this will require examining integrin adhesions in cells surrounded by an ECM in their native environment.
Integrin switching
Our findings establish a new example of integrin switching during a developmental programme. It is the first example of such a transition in fly tissues, but similar transitions have been observed during the differentiation of mammalian myoblasts and adipocytes, and in C. elegans distal tip cells (reviewed in Meighan and Schwarzbauer, 2008). In all these examples, the developmental switch involves a laminin-binding integrin and an RGD-binding integrin, but the direction of the switch differs. In mammalian cells undergoing differentiation, RGD-binding α5β1 is present in the starting non-differentiated cells and promotes proliferation, and is replaced by laminin-binding α6β1, which promotes differentiation. By contrast, in invertebrates, cells start with laminin-binding αina-1βpat-3/αPS1βPS and switch to αpat-2βpat-3/αPS2βPS. So far, in all cases the switch occurs by reciprocal transcriptional regulation of the two α-subunits. In C. elegans, the single transcription factor vab-3 (Pax6) represses expression of ina-1, while initiating the expression of pat-2 (Meighan and Schwarzbauer, 2007). Early expression of αina-1βpat-3 mediates distal-tip cell migration and its downregulation arrests migration to specify gonad length. Expression of αpat-2βpat-3 turns the gonad, creating organ shape. Our finding that preventing the integrin switch alters the timing of stress-fibre reorientation, rather than stress-fibres structure, suggests that the switch provides robustness to a complicated orchestration of morphogenetic changes, recruitment of intracellular components and development of the egg chamber.
Regulation of the actin cytoskeleton by integrins
Removal of all βPS integrins or just αPS1βPS from follicle cells results in elevation of F-actin and the actin-nucleating proteins Ena, Dia and profilin. With no integrins, the basal actin fibres are completely disorganized, whereas at late stages the actin fibres are well organized in the absence of αPS1βPS. This shows that regulation of actin and actin-nucleating protein levels is independent from organizing stress fibres into parallel arrays.
In the follicular epithelium, cells lacking Ena, profilin or Dia have reduced basal actin (Baum and Perrimon, 2001) (our unpublished results), consistent with a role in stimulating the formation of the stress fibres. In mammalian and follicle cells, Ena localizes at integrin sites and overexpressed Ena causes the formation of actin aggregates (Gertler et al., 1996; Baum and Perrimon, 2001), which we also observed in cells lacking integrins. A link between integrins and the actin machinery was revealed by de novo actin polymerization at integrin adhesions via recruitment of actin-polymerization factors Arp2/3, Ena, profilin and Dia (Chakraborty et al., 1995; Reinhard et al., 1995; Butler et al., 2006). Moroever, profilin co-precipitates with paxillin, indicating that they are in a complex (Mayhew et al., 2006). Consistent with Dia function regulating basal F-actin in follicle cells, stress-fibre formation in cultured cells is mainly mediated by Dia1 and ROCK (Watanabe et al., 1999), and removing ROCK from follicle cells reduces the thickness of stress fibres (our unpublished data). The enhancement of Dia1-induced actin-polymerization by external pulling force (reviewed in Bershadsky et al., 2006) is consistent with the transient increase in Dia protein levels at stage 10B, when stress fibres thicken and the cells flatten, which seems to be regulated by increasing dia mRNA levels from stage 10A (our unpublished results).
Altogether, these data support the view that an increase in actin-nucleating factors could account for the increase in F-actin in follicle cells lacking integrins. Why integrins downregulate the actin machinery while being required for the maintenance of a complex cytoskeletal structure is unknown, but it might be that organizing fibres into parallel arrays works more efficiently if actin polymerization is partially suppressed.
Specific function of αPS2 and tensin recruitment
The specificity of function between αPS1 and αPS2 was previously shown to be dependent on their extracellular domains (Martin-Bermudo et al., 1997). We had interpreted this to show that the important property that individual α-subunits bring to the heterodimer is specific interaction with extracellular ligands. In addition, we have now found that extracellular specificity also changes intracellular events. This raises the key issue of how the extracellular (and/or transmembrane) domain could specify the recruitment of an intracellular protein.
We can envision three possible explanations: (1) the different ECM interaction alters the ability of the integrin to resist force. Actomyosin-driven unfolding of proteins bound to the integrin might expose new interaction sites (e.g. del Rio et al., 2009). The specific integrin-ECM interaction could differ in ligand-binding strength or matrix rigidity, affecting the ability to resist force, and thus the level of new site exposure. Supporting this, increasing ECM rigidity strengthened the integrin-cytoskeleton link in fibroblasts (Choquet et al., 1997). Thus, the interaction of αPS2βPS with its ligand might be able to resist more force than αPS1βPS, thus exposing tensin recruitment sites. The proposed role of tensin in α5β1 translocation from focal contacts to fibrillar adhesions and fibronectin fibril formation (Pankov et al., 2000) suggests that tensin recruitment feeds back to increase matrix rigidity. (2) The different ECM interactions affect the density of integrin clusters, which affects tensin recruitment. The density of αPS2βPS clusters might be higher than αPS1βPS clusters, providing higher avidity for a low-affinity interaction with tensin. (3) Recruitment or activation of another transmembrane protein in the same membrane, which in turn recruits tensin through its intracellular domain. For example, specific integrin heterodimers bind tetraspanins, which in turn bind protein kinase C, bringing it into proximity with integrins (Zhang et al., 2001). In another example, a combination of signals from α5β1 and the proteoglycan syndecan-4, which both bind extracellular fibronectin, leads to the activation of p190RhoGAP and the suppression of Rho (Bass et al., 2008). Thus, switching the integrin extracellular binding might bring the integrin adjacent to other receptors that synergize to recruit tensin.
Function of the stress fibres for egg morphogenesis and the role of the integrin switch
The orientation of the stress fibres encircling the oocyte suggested that they act as a `contractile corset' to elongate the egg (Gutzeit, 1991), similar to a series of rubber bands exerting pressure so that oocyte growth is not uniform. Identification of mutations that disrupted fibre polarity and caused round eggs supported this idea (Gutzeit et al., 1991; Bateman et al., 2001). However, disruption of actin-fibre polarity could be a cause or consequence of round eggs. Our finding that stress-fibre orientation changes (see also Wahlstrom et al., 2006) shows that stress fibres cannot continuously exert a circumferential contractile force. Therefore, rather than exerting a contractile force, stress fibres might become oriented in response to forces exerted on follicle cells. We tested whether the rapid increase in oocyte volume during nurse-cell dumping triggered changes in stress-fibre orientation, but found that blocking dumping did not disrupt orientation changes. This suggests either that other forces trigger these changes or that the changes are part of a developmentally timed programme of follicular development that runs independently of morphological changes.
The dynamic changes in stress-fibre orientation and morphology suggest that stress fibres have a different function than exerting force on the oocyte. Therefore, we propose an alternative primary function for these stress fibres, namely to strengthen integrin-mediated adhesion. As we examined the progression of integrin adhesion and stress fibre morphology in the different stages of oogenesis, what we found striking is the dramatic expansion of the basal surface of the follicle cells, and the coordinated increase in the size of the integrin adhesions and stress fibres. This suggests that much stronger adhesion is required as cells flatten and retain tight apposition to the basement membrane. As we have shown, the proper flattening of follicle cells requires integrin adhesion. A novel mechanism of integrin insertion has been hypothesized to contribute to the rapid increase in follicle-cell basal surface area (Schotman et al., 2008). When one combines the need for strong yet dynamic adhesion to the basement membrane with the importance of mechanical force for the strengthening of integrin adhesions, then a role for the stress fibres in providing such contractile force to expand integrin adhesions becomes apparent. This fits with data from vertebrate cells in culture demonstrating that focal-contact size is proportional to applied force (reviewed in Bershadsky et al., 2003), and our own demonstration that the number of focal adhesions was reduced when stress fibres are absent due to loss of profilin. Thus, adoption of a cell shape that is far away from spherical (or cuboidal within and epithelium) requires strong adhesion and, by forming internal contractile structures, the strength of the adhesive site becomes sufficiently strong. In the case of follicle cells, such adhesion is required for a squamous cell shape, whereas, in the pseudostratified epithelium of the imaginal discs, integrin adhesion is required to maintain the elongated columnar shape (Dominguez-Gimenez et al., 2007). In the case of the imaginal disc cells, strong integrin adhesion is achieved without stress fibres, perhaps because, in elongated cells, the forces driving a return to cuboidal shape will be exerted perpendicular to the basement membrane rather than in the same plane. To date, all mutations that give the round egg phenotype are either transmembrane proteins or cytoplasmic proteins tightly associated with them (Gutzeit, 1991; Bateman et al., 2001; Frydman and Spradling, 2001; Deng et al., 2003; Conder et al., 2007; Mirouse et al., 2009), whereas mutations that disrupt the cytoskeleton alone do not cause round eggs (Wahlstrom et al., 2006). Adding our newly characterized function for αPS2 in the attachment of muscles of the epithelial sheath, this suggests that generating an elongated egg requires the tripartite interaction between the follicular epithelium, the basement membrane and surrounding muscle layer, perhaps directing the expansion of the basement membrane non-uniformly.
The switch of integrin heterodimers suggests that the role of integrins changes during the morphogenetic programme of follicular-cell development. Our ability to manipulate integrin expression has allowed the identification of specific roles for each integrin and transient defects when the integrin switch is perturbed. However, we have yet to find an essential function for the integrin switching. We speculate that, at early stages, the ability of αPS1βPS to repress actin-fibre production is important and the switch to αPS2βPS relieves this repression so that more substantial actin fibres form later, which strengthen integrin adhesions as the cells flatten. If this is true, there must be a redundant mechanism that inhibits the αPS1-specific pathway to downregulate actin-fibre formation at late stages, otherwise we would expect to see a reduction in filamentous actin when αPS2βPS was replaced with αPS1βPS.
In conclusion, follicle-cell stress fibres provide a useful model system for the study of developmentally programmed morphogenetic changes. The transcriptional regulation of the integrin α-subunits contributes to the temporal control of actin-cytoskeletal changes and the change in the composition of the focal adhesions, as revealed by the ability to recruit tensin. Important challenges for the future are to identify the mechanisms of stress-fibre reorientation and the molecular pathway between integrin adhesion and repression of the actin machinery.
Materials and Methods
Fly genetics
Clones lacking βPS were induced by crossing a null allele, mysXG43 FRT101/FM6 (Bunch et al., 1992), to Ubi-GFP FRT101; hsFLP38 (negatively marked clones) or mysXG43 FRT19A/FM6 to tub::Gal80, hsFlp FRT19A; UAS-CD8-GFP; Tub-GAL4/TM6b (MARCM stock for positive marking) (Lee and Luo, 2001). Clones lacking αPS1 were induced with a null allele, mewM6FRT18A/FM6 (Brower et al., 1995), crossed to Ubi-GFP FRT18A; hsFlp38. Lack of tensin phenotype was studied using a null allele, by33c (Torgler et al., 2004), and for clones the allele by3R-B FRT82B/TM6B (Prout et al., 1997) was crossed to hsFlp; Ubi-GFP FRT82B. Clones lacking αPS2 were induced by crossing a null allele, if B4 FRT19A/FM7c (Brown, 1994), to the MARCM stock. The ifB4 mutant clones re-expressing αPS2, αPS1, αPS2/1 and αPS1/2 were obtained by crossing ifB4 FRT19A; UAS-αPSx to the MARCM stock. To visualize tensin-GFP (Torgler et al., 2004) in ifB4 mutant clones, ifB4 FRT19A/FM6; tensin-GFP was crossed to FRT19A; hsFlp38. FlpOUT (overexpressing) clones were induced by crossing UAS-paxillin-GFP (Victoria Williams, Christos G. Zervas and N.H.B., unpublished) and UAS-tensin-GFP (Torgler et al., 2004) to hsFlp; arm::FRTstopf+FRTGal4 (N.H.B., unpublished). A stock of w f36a was used as wild type. To generate cells lacking profilin and Dia, we used chicP5025 FRT40A (Baum and Perrimon, 2001) and dia5 FRT40A (Wang and Riechmann, 2007), respectively. Follicle-cell clones were induced during larval stages L1 and L2 by two 1- to 2-hour heat-shocks at 37°C in a water bath. Females were dissected 2-3 days after eclosion. Clones removing αPS2 in epithelial sheath muscles were generated in ifB4FRT19A/FRT19A; Vg-Gal4 UAS-Flp/+; Mef-GAL4/+c females.
Immunostainings
Antibody stainings were carried out on female ovaries dissected in PBS, fixed 10 minutes in 4% formaldehyde, permeabilized, blocked for 1 hour in PBS 0.3% Triton X-100, 0.5% BSA, and stained overnight at 4°C for both primary and secondary antibodies. The following antibodies were used (HB stands for The Developmental Studies Hybridoma Bank): anti-βPS [HB CF.6G11 mouse monoclonal antibody (MmAb); 1:100] (Brower et al., 1984), anti-αPS1 (HB DK.1A4 MmAb; 1:10 (Brower et al., 1984), anti-αPS2 (5D6 rat mAb; 1:10) (Bogaert et al., 1987), anti-αPS3 [rabbit polyclonal antibody (RpAb); 1:100] (Grotewiel et al., 1998), anti-αPS4 (RpAb; 1:200) (Krzemien et al., 2007), anti-talin (E16B; MmAb; 1:50) (Brown et al., 2002), anti-PINCH (RpAb; 1:500) (Clark et al., 2003), anti-PY (4G10 MmAb; 1:500; Upstate #05-321), anti-pFAKY397 (RpAb; 1:500; Biosource), anti-FAK (RpAb; 1:500) (Palmer et al., 1999), anti-PAK (RpAb; 1:1000) (Harden et al., 1996), anti-Zipper (RpAb; 1:500) (Jordan and Karess, 1997), anti-profilin (HB ch1J MmAb; 1:50) (Verheyen and Cooley, 1994), anti-Ena (HB 5G2 MmAb; 1:10) (Bashaw et al., 2000), anti-Dia (RpAb; 1:100) (Afshar et al., 2000), anti-GFP (RpAb; 1:500; Abcam Ab290), anti-GFP (MmAb; 1:500; Roche), anti-paxillin (RpAb; 1:250) (Chen et al., 2005), anti-Wech (RpAb; 1:20) (Loer et al., 2008), anti-Eyeless (RpAb; 1:200) (Adachi et al., 2003), anti-Twin-of-eyeless (RpAb; 1:500) (Jacobsson et al., 2009). To visualize tensin and ILK we used GFP-tagged genomic rescue constructs (Zervas et al., 2001; Torgler et al., 2004) and Zasp, a GFP gene trap (G00189) (Jani and Schock, 2007).
Secondary antibodies were Alexa-Fluor-488-conjugated anti-rabbit and anti-mouse from Molecular Probes, and Cy5-conjugated anti-rabbit, anti-mouse and anti-rat from Jackson Laboratories, all used at 1:200. F-actin was stained using rhodamine-phalloidin (#R-415, Molecular Probes, 1:500). The DNAse-I staining was performed using a DNAseI–Alexa-Fluor-488 conjugate (0.3 μM, Molecular Probes). Samples were mounted in Vectashield H-1000 or Vectashield H-1200 with DAPI (Vector Laboratories). Confocal images from an Olympus Fluoview FV1000 were assembled with Adobe Photoshop CS3.
In situ hybridization on ovaries
In situ hybridization was performed as in Palacios and St Johnston (Palacios and St Johnston, 2002) with antisense DIG probes against fragments of mew and if mRNA, and pax6 orthologue mRNAs were transcribed from DGRC cDNAs: GH01157 (ey), GH14454 (toy), AT09010 (eg), GH22493 (toe). Ovaries were mounted in 1:1 PBS/glycerol and imaged using a LEICA DMR microscope and an Optronics digital camera.
FISH was performed to detect profilin and dia mRNA using antisense DIG probes transcribed from DGRC cDNAs LD15581 and LD14246, respectively. Hybridized ovaries were incubated for 2 hours with an anti-GFP to detect mysXG43 clones and an anti-DIG-HRP (Roche, 1:2000) followed by amplification with Cy5 tyramide reagent (Perkin Elmer).
Basal actin quantifications
Quantifications of basal actin in the absence of βPS and αPS1 were done with ImageJ on very basal single sections of the epithelium, measuring the integrated area of actin fluorescence (mean grey value × area).
Supplementary Material
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/122/23/4363/DC1
We thank our colleagues for kind gifts of antibodies and fly stocks, Bloomington Stock Center and Boris Egger for fly stocks, the Iowa Developmental Hybridoma Bank for antibodies, and the DGRC for ESTs. We also thank John Overton for technical assistance and members of the Brown laboratory for helpful discussions. This work was supported by BBSRC grant BB/D013011 and Wellcome Trust grant 069943. Deposited in PMC for release after 6 months.
References
- Adachi, Y., Hauck, B., Clements, J., Kawauchi, H., Kurusu, M., Totani, Y., Kang, Y. Y., Eggert, T., Walldorf, U., Furukubo-Tokunaga, K. et al. (2003). Conserved cis-regulatory modules mediate complex neural expression patterns of the eyeless gene in the Drosophila brain. Mech. Dev. 120, 1113-1126. [DOI] [PubMed] [Google Scholar]
- Afshar, K., Stuart, B. and Wasserman, S. A. (2000). Functional analysis of the Drosophila diaphanous FH protein in early embryonic development. Development 127, 1887-1897. [DOI] [PubMed] [Google Scholar]
- Bashaw, G. J., Kidd, T., Murray, D., Pawson, T. and Goodman, C. S. (2000). Repulsive axon guidance: Abelson and Enabled play opposing roles downstream of the roundabout receptor. Cell 101, 703-715. [DOI] [PubMed] [Google Scholar]
- Bass, M. D., Morgan, M. R., Roach, K. A., Settleman, J., Goryachev, A. B. and Humphries, M. J. (2008). p190RhoGAP is the convergence point of adhesion signals from alpha 5 beta 1 integrin and syndecan-4. J. Cell Biol. 181, 1013-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman, J., Reddy, R. S., Saito, H. and Van Vactor, D. (2001). The receptor tyrosine phosphatase Dlar and integrins organize actin filaments in the Drosophila follicular epithelium. Curr. Biol. 11, 1317-1327. [DOI] [PubMed] [Google Scholar]
- Baum, B. and Perrimon, N. (2001). Spatial control of the actin cytoskeleton in Drosophila epithelial cells. Nat. Cell. Biol. 3, 883-890. [DOI] [PubMed] [Google Scholar]
- Bershadsky, A., Chausovsky, A., Becker, E., Lyubimova, A. and Geiger, B. (1996). Involvement of microtubules in the control of adhesion-dependent signal transduction. Curr. Biol. 6, 1279-1289. [DOI] [PubMed] [Google Scholar]
- Bershadsky, A. D., Balaban, N. Q. and Geiger, B. (2003). Adhesion-dependent cell mechanosensitivity. Annu. Rev. Cell Dev. Biol. 19, 677-695. [DOI] [PubMed] [Google Scholar]
- Bershadsky, A. D., Ballestrem, C., Carramusa, L., Zilberman, Y., Gilquin, B., Khochbin, S., Alexandrova, A. Y., Verkhovsky, A. B., Shemesh, T. and Kozlov, M. M. (2006). Assembly and mechanosensory function of focal adhesions: experiments and models. Eur. J. Cell Biol. 85, 165-173. [DOI] [PubMed] [Google Scholar]
- Bogaert, T., Brown, N. and Wilcox, M. (1987). The Drosophila PS2 antigen is an invertebrate integrin that, like the fibronectin receptor, becomes localized to muscle attachments. Cell 51, 929-940. [DOI] [PubMed] [Google Scholar]
- Brabant, M. C. and Brower, D. L. (1993). PS2 integrin requirements in Drosophila embryo and wing morphogenesis. Dev. Biol. 157, 49-59. [DOI] [PubMed] [Google Scholar]
- Brower, D. L., Wilcox, M., Piovant, M., Smith, R. J. and Reger, L. A. (1984). Related cell-surface antigens expressed with positional specificity in Drosophila imaginal discs. Proc. Natl. Acad. Sci. USA 81, 7485-7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower, D. L., Brabant, M. C. and Bunch, T. A. (1995). Role of the PS integrins in Drosophila development. Immunol. Cell Biol. 73, 558-564. [DOI] [PubMed] [Google Scholar]
- Brown, N. H. (1994). Null mutations in the alpha PS2 and beta PS integrin subunit genes have distinct phenotypes. Development 120, 1221-1231. [DOI] [PubMed] [Google Scholar]
- Brown, N. H., Gregory, S. L., Rickoll, W. L., Fessler, L. I., Prout, M., White, R. A. and Fristrom, J. W. (2002). Talin is essential for integrin function in Drosophila. Dev. Cell 3, 569-579. [DOI] [PubMed] [Google Scholar]
- Bunch, T. A. and Brower, D. L. (1992). Drosophila PS2 integrin mediates RGD-dependent cell-matrix interactions. Development 116, 239-247. [DOI] [PubMed] [Google Scholar]
- Bunch, T. A., Salatino, R., Engelsgjerd, M. C., Mukai, L., West, R. F. and Brower, D. L. (1992). Characterization of mutant alleles of myospheroid, the gene encoding the beta subunit of the Drosophila PS integrins. Genetics 132, 519-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler, B., Gao, C., Mersich, A. T. and Blystone, S. D. (2006). Purified integrin adhesion complexes exhibit actin-polymerization activity. Curr. Biol. 16, 242-251. [DOI] [PubMed] [Google Scholar]
- Byers, H. R. and Fujiwara, K. (1982). Stress fibers in cells in situ: immunofluorescence visualization with antiactin, antimyosin, and anti-alpha-actinin. J. Cell Biol. 93, 804-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagan, R. L. and Ready, D. F. (1989). The emergence of order in the Drosophila pupal retina. Dev. Biol. 136, 346-362. [DOI] [PubMed] [Google Scholar]
- Chakraborty, T., Ebel, F., Domann, E., Niebuhr, K., Gerstel, B., Pistor, S., Temm-Grove, C. J., Jockusch, B. M., Reinhard, M., Walter, U. et al. (1995). A focal adhesion factor directly linking intracellularly motile Listeria monocytogenes and Listeria ivanovii to the actin-based cytoskeleton of mammalian cells. EMBO J. 14, 1314-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, G. C., Turano, B., Ruest, P. J., Hagel, M., Settleman, J. and Thomas, S. M. (2005). Regulation of Rho and Rac signaling to the actin cytoskeleton by paxillin during Drosophila development. Mol. Cell. Biol. 25, 979-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choquet, D., Felsenfeld, D. P. and Sheetz, M. P. (1997). Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell 88, 39-48. [DOI] [PubMed] [Google Scholar]
- Clark, K. A., McGrail, M. and Beckerle, M. C. (2003). Analysis of PINCH function in Drosophila demonstrates its requirement in integrin-dependent cellular processes. Development 130, 2611-2621. [DOI] [PubMed] [Google Scholar]
- Conder, R., Yu, H., Zahedi, B. and Harden, N. (2007). The serine/threonine kinase dPak is required for polarized assembly of F-actin bundles and apical-basal polarity in the Drosophila follicular epithelium. Dev. Biol. 305, 470-482. [DOI] [PubMed] [Google Scholar]
- Cooley, L., Verheyen, E. and Ayers, K. (1992). chickadee encodes a profilin required for intercellular cytoplasm transport during Drosophila oogenesis. Cell 69, 173-184. [DOI] [PubMed] [Google Scholar]
- Couchman, J. R. and Rees, D. A. (1979). The behaviour of fibroblasts migrating from chick heart explants: changes in adhesion, locomotion and growth, and in the distribution of actomyosin and fibronectin. J. Cell Sci. 39, 149-165. [DOI] [PubMed] [Google Scholar]
- Cukierman, E., Pankov, R., Stevens, D. R. and Yamada, K. M. (2001). Taking cell-matrix adhesions to the third dimension. Science 294, 1708-1712. [DOI] [PubMed] [Google Scholar]
- del Rio, A., Perez-Jimenez, R., Liu, R., Roca-Cusachs, P., Fernandez, J. M. and Sheetz, M. P. (2009). Stretching single talin rod molecules activates vinculin binding. Science 323, 638-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, W. M., Schneider, M., Frock, R., Castillejo-Lopez, C., Gaman, E. A., Baumgartner, S. and Ruohola-Baker, H. (2003). Dystroglycan is required for polarizing the epithelial cells and the oocyte in Drosophila. Development 130, 173-184. [DOI] [PubMed] [Google Scholar]
- Devenport, D. and Brown, N. H. (2004). Morphogenesis in the absence of integrins: mutation of both Drosophila beta subunits prevents midgut migration. Development 131, 5405-5415. [DOI] [PubMed] [Google Scholar]
- Dinkins, M. B., Fratto, V. M. and Lemosy, E. K. (2008). Integrin alpha chains exhibit distinct temporal and spatial localization patterns in epithelial cells of the Drosophila ovary. Dev. Dyn. 237, 3927-3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Gimenez, P., Brown, N. H. and Martin-Bermudo, M. D. (2007). Integrin-ECM interactions regulate the changes in cell shape driving the morphogenesis of the Drosophila wing epithelium. J. Cell Sci. 120, 1061-1071. [DOI] [PubMed] [Google Scholar]
- Frydman, H. M. and Spradling, A. C. (2001). The receptor-like tyrosine phosphatase lar is required for epithelial planar polarity and for axis determination within Drosophila ovarian follicles. Development 128, 3209-3220. [DOI] [PubMed] [Google Scholar]
- Geiger, B., Yehuda-Levenberg, S. and Bershadsky, A. D. (1995). Molecular interactions in the submembrane plaque of cell-cell and cell-matrix adhesions. Acta. Anat. (Basel) 154, 46-62. [DOI] [PubMed] [Google Scholar]
- Gertler, F. B., Niebuhr, K., Reinhard, M., Wehland, J. and Soriano, P. (1996). Mena, a relative of VASP and Drosophila Enabled, is implicated in the control of microfilament dynamics. Cell 87, 227-239. [DOI] [PubMed] [Google Scholar]
- Gotwals, P. J., Paine-Saunders, S. E., Stark, K. A. and Hynes, R. O. (1994). Drosophila integrins and their ligands. Curr. Opin. Cell Biol. 6, 734-739. [DOI] [PubMed] [Google Scholar]
- Grotewiel, M. S., Beck, C. D., Wu, K. H., Zhu, X. R. and Davis, R. L. (1998). Integrin-mediated short-term memory in Drosophila. Nature 391, 455-460. [DOI] [PubMed] [Google Scholar]
- Gutzeit, H. O. (1990). The microfilament pattern in the somatic follicle cells of mid-vitellogenic ovarian follicles of Drosophila. Eur. J. Cell Biol. 53, 349-356. [PubMed] [Google Scholar]
- Gutzeit, H. O. (1991). Organization and in vitro activity of microfilament bundles associated with the basement membrane of Drosophila follicles. Acta Histochem. Suppl. 41, 201-210. [PubMed]
- Gutzeit, H. O., Eberhardt, W. and Gratwohl, E. (1991). Laminin and basement membrane-associated microfilaments in wild-type and mutant Drosophila ovarian follicles. J. Cell Sci. 100 (Pt 4), 781-788. [DOI] [PubMed] [Google Scholar]
- Harden, N., Lee, J., Loh, H. Y., Ong, Y. M., Tan, I., Leung, T., Manser, E. and Lim, L. (1996). A Drosophila homolog of the Rac- and Cdc42-activated serine/threonine kinase PAK is a potential focal adhesion and focal complex protein that colocalizes with dynamic actin structures. Mol. Cell. Biol. 16, 1896-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson, A. M., Petrella, L. N., Tanaka, A. J. and Cooley, L. (2008). Mononuclear muscle cells in Drosophila ovaries revealed by GFP protein traps. Dev. Biol. 314, 329-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes, R. O. (2002). Integrins: bidirectional, allosteric signaling machines. Cell 110, 673-687. [DOI] [PubMed] [Google Scholar]
- Jacobsson, L., Kronhamn, J. and Rasmuson-Lestander, A. (2009). The Drosophila Pax6 paralogs have different functions in head development but can partially substitute for each other. Mol. Genet. Genomics 282, 217-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jani, K. and Schock, F. (2007). Zasp is required for the assembly of functional integrin adhesion sites. J. Cell Biol. 179, 1583-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan, P. and Karess, R. (1997). Myosin light chain-activating phosphorylation sites are required for oogenesis in Drosophila. J. Cell Biol. 139, 1805-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz, B. Z., Zamir, E., Bershadsky, A., Kam, Z., Yamada, K. M. and Geiger, B. (2000). Physical state of the extracellular matrix regulates the structure and molecular composition of cell-matrix adhesions. Mol. Biol. Cell 11, 1047-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzemien, J., Dubois, L., Makki, R., Meister, M., Vincent, A. and Crozatier, M. (2007). Control of blood cell homeostasis in Drosophila larvae by the posterior signalling centre. Nature 446, 325-428. [DOI] [PubMed] [Google Scholar]
- Lee, S. B., Cho, K. S., Kim, E. and Chung, J. (2003). blistery encodes Drosophila tensin protein and interacts with integrin and the JNK signaling pathway during wing development. Development 130, 4001-4010. [DOI] [PubMed] [Google Scholar]
- Lee, T. and Luo, L. (2001). Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 24, 251-254. [DOI] [PubMed] [Google Scholar]
- Loer, B., Bauer, R., Bornheim, R., Grell, J., Kremmer, E., Kolanus, W. and Hoch, M. (2008). The NHL-domain protein Wech is crucial for the integrin-cytoskeleton link. Nat. Cell Biol. 10, 422-428. [DOI] [PubMed] [Google Scholar]
- Martin-Bermudo, M. D., Dunin-Borkowski, O. M. and Brown, N. H. (1997). Specificity of PS integrin function during embryogenesis resides in the alpha subunit extracellular domain. EMBO J. 16, 4184-4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew, M. W., Webb, D. J., Kovalenko, M., Whitmore, L., Fox, J. W. and Horwitz, A. F. (2006). Identification of protein networks associated with the PAK1-betaPIX-GIT1-paxillin signaling complex by mass spectrometry. J. Proteome Res. 5, 2417-2423. [DOI] [PubMed] [Google Scholar]
- Meighan, C. M. and Schwarzbauer, J. E. (2007). Control of C. elegans hermaphrodite gonad size and shape by vab-3/Pax6-mediated regulation of integrin receptors. Genes Dev. 21, 1615-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meighan, C. M. and Schwarzbauer, J. E. (2008). Temporal and spatial regulation of integrins during development. Curr. Opin. Cell Biol. 20, 520-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirouse, V., Christoforou, C. P., Fritsch, C., St Johnston, D. and Ray, R. P. (2009). Dystroglycan and perlecan provide a basal cue required for epithelial polarity during energetic stress. Dev. Cell 16, 83-92. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Olski, T. M., Noegel, A. A. and Korenbaum, E. (2001). Parvin, a 42 kDa focal adhesion protein, related to the alpha-actinin superfamily. J. Cell Sci. 114, 525-538. [DOI] [PubMed] [Google Scholar]
- Palacios, I. M. and St Johnston, D. (2002). Kinesin light chain-independent function of the Kinesin heavy chain in cytoplasmic streaming and posterior localisation in the Drosophila oocyte. Development 129, 5473-5485. [DOI] [PubMed] [Google Scholar]
- Palmer, R. H., Fessler, L. I., Edeen, P. T., Madigan, S. J., McKeown, M. and Hunter, T. (1999). DFak56 is a novel Drosophila melanogaster focal adhesion kinase. J. Biol. Chem. 274, 35621-35659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankov, R., Cukierman, E., Katz, B. Z., Matsumoto, K., Lin, D. C., Lin, S., Hahn, C. and Yamada, K. M. (2000). Integrin dynamics and matrix assembly: tensin-dependent translocation of alpha(5)beta(1) integrins promotes early fibronectin fibrillogenesis. J. Cell Biol. 148, 1075-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prout, M., Damania, Z., Soong, J., Fristrom, D. and Fristrom, J. W. (1997). Autosomal mutations affecting adhesion between wing surfaces in Drosophila melanogaster. Genetics 146, 275-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard, M., Giehl, K., Abel, K., Haffner, C., Jarchau, T., Hoppe, V., Jockusch, B. M. and Walter, U. (1995). The proline-rich focal adhesion and microfilament protein VASP is a ligand for profilins. EMBO J. 14, 1583-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotman, H., Karhinen, L. and Rabouille, C. (2008). dGRASP-mediated noncanonical integrin secretion is required for Drosophila epithelial remodeling. Dev. Cell 14, 171-182. [DOI] [PubMed] [Google Scholar]
- Spradling, A. (1993). Developmental genetics of oogenesis. In The Development of Drosophila melanogaster (ed. B. Martinez-Arias). New York: Cold Spring Harbor Laboratory Press.
- Torgler, C. N., Narasimha, M., Knox, A. L., Zervas, C. G., Vernon, M. C. and Brown, N. H. (2004). Tensin stabilizes integrin adhesive contacts in Drosophila. Dev. Cell 6, 357-369. [DOI] [PubMed] [Google Scholar]
- Verheyen, E. M. and Cooley, L. (1994). Profilin mutations disrupt multiple actin-dependent processes during Drosophila development. Development 120, 717-728. [DOI] [PubMed] [Google Scholar]
- Wahlstrom, G., Norokorpi, H. L. and Heino, T. I. (2006). Drosophila alpha-actinin in ovarian follicle cells is regulated by EGFR and Dpp signalling and required for cytoskeletal remodelling. Mech. Dev. 123, 801-818. [DOI] [PubMed] [Google Scholar]
- Wang, Y. and Riechmann, V. (2007). The role of the actomyosin cytoskeleton in coordination of tissue growth during Drosophila oogenesis. Curr. Biol. 17, 1349-1355. [DOI] [PubMed] [Google Scholar]
- Watanabe, N., Kato, T., Fujita, A., Ishizaki, T. and Narumiya, S. (1999). Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat. Cell Biol. 1, 136-143. [DOI] [PubMed] [Google Scholar]
- White, G. E., Gimbrone, M. A., Jr and Fujiwara, K. (1983). Factors influencing the expression of stress fibers in vascular endothelial cells in situ. J. Cell Biol. 97, 416-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel-Bar, R., Itzkovitz, S., Ma'ayan, A., Iyengar, R. and Geiger, B. (2007). Functional atlas of the integrin adhesome. Nat. Cell Biol. 9, 858-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir, E. and Geiger, B. (2001). Molecular complexity and dynamics of cell-matrix adhesions. J. Cell Sci. 114, 3583-3590. [DOI] [PubMed] [Google Scholar]
- Zamir, E., Katz, B. Z., Aota, S., Yamada, K. M., Geiger, B. and Kam, Z. (1999). Molecular diversity of cell-matrix adhesions. J. Cell Sci. 112, 1655-1669. [DOI] [PubMed] [Google Scholar]
- Zamir, E., Katz, M., Posen, Y., Erez, N., Yamada, K. M., Katz, B. Z., Lin, S., Lin, D. C., Bershadsky, A., Kam, Z. et al. (2000). Dynamics and segregation of cell-matrix adhesions in cultured fibroblasts. Nat. Cell Biol. 2, 191-196. [DOI] [PubMed] [Google Scholar]
- Zervas, C. G., Gregory, S. L. and Brown, N. H. (2001). Drosophila integrin-linked kinase is required at sites of integrin adhesion to link the cytoskeleton to the plasma membrane. J. Cell Biol. 152, 1007-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. A., Bontrager, A. L. and Hemler, M. E. (2001). Transmembrane-4 superfamily proteins associate with activated protein kinase C (PKC) and link PKC to specific beta(1) integrins. J. Biol. Chem. 276, 25005-25013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.