Summary
Morbidity and mortality associated with viral infections increase with age, although the underlying mechanisms are unclear. Here, we investigated whether aging alters inflammatory responses during systemic viral infection and whether such age-related alterations contribute to viral-induced death. We found that infection of aged mice with systemic herpes viruses led to rapid increases in serum IL-17, neutrophil activation, and mortality due to hepatocyte necrosis. In contrast, all young mice survived infection, displaying weaker IL-17 induction and neutrophil activation. During viral activation, natural killer T cells isolated from the livers of aged mice exhibited greater RORγT gene expression and IL-17 production than young cells. Importantly, IL-17 neutralization or neutrophil depletion during viral infection reduced liver damage and prevented death of aged mice. These results demonstrate that, during systemic viral infection, aging alters the host-pathogen interaction to overproduce IL-17, inducing liver injury and death.
Introduction
The elderly are at increased risk for microbial infection and malignancy (Linton and Dorschkind, 2004), indicating that aging impairs immunity. These individuals are less able to overcome viral infections and subsequently exhibit high morbidity and mortality after infection (Gorina et al., 2008), imposing a heavy burden on health care resources (Han et al., 1999; Kaplan and Angus, 2003; Kaplan et al., 2002). Several studies have demonstrated that aging adversely affects various components of immunity (Linton and Dorschkind, 2004; Miller, 1996). However, most studies have focused on adaptive T cell responses (Garcia and Miller, 2001; Haynes et al., 1999; Linton et al., 1996; Linton and Dorschkind, 2004; Thoman and Weigle, 1982), leaving it unclear as to how aging modifies other host defenses against infection.
Studies in humans and mice have shown that aging is associated with aberrant cytokine production and inflammation (Bruunsgard and Klarlund Pedersen, 2003; Rosenstiel et al., 2008). Aging is associated with elevated levels of several proinflammatory cytokines including IL-1, IL-6, and TNF-α. The elevation in IL-6 has been of particular interest, as this cytokine has recently been shown to be important for the differentiation of Th17 CD4+ T cells (Korn et al., 2007; Weaver et al., 2007). However, whether age-related aberrancies in inflammatory responses result from impaired host-pathogen interactions and whether these aberrant responses affect the outcome of systemic viral infections in older individuals is unclear. In this study, we compared inflammatory responses to systemic infection with either herpes simplex type 2 virus (HSV-2) or murine cytomegalovirus (MCMV) between young and aged mice. Our findings reveal that aberrant IL-17A responses to systemic viral infection contribute to the death of aged hosts via a neutrophil-dependent process. These findings suggest that therapies aimed at inactivating IL-17A may avert immune pathology in older individuals with systemic viral infections.
Results
Systemic viral infection induces proinflammatory cytokine overproduction and death in aged mice
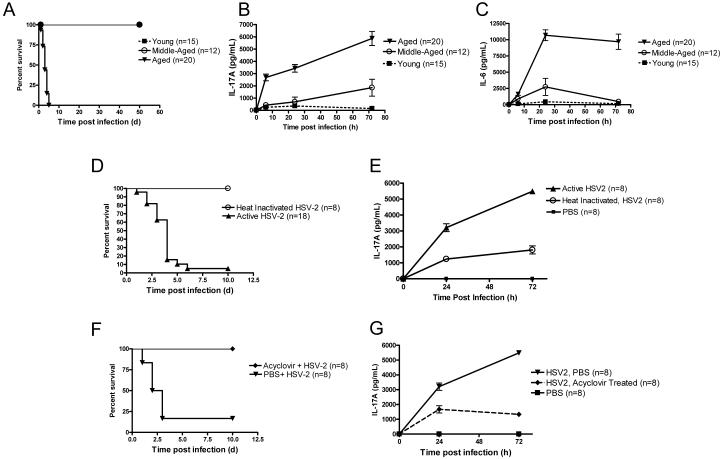
To begin to understand how aging modifies the inflammatory response to systemic viral infection, we infected young (2 – 4 months), middle-aged (8 – 10 months), and aged (18 – 20 months) C57BL/6 mice with HSV-2, a human viral pathogen that activates plasmacytoid dendritic cells (pDCs) (Lund et al., 2003). We found that all young and middle-aged mice survived infection, whereas all aged mice succumbed to it (Fig. 1 A). Mortality was associated with a significant elevation in systemic levels of IL-17A, IL-6 (Fig. 1 B - C), and TNF-α (Supplemental Fig. 1). The elevation in IL-17A levels and mortality in response to HSV-2 infection was not limited to one genetic background of mice, as similar responses were found in the BALB/c strain (Supplemental Fig. 2). We also found that infection of aged BALB/c mice with the alternate pathogen, MCMV, induced death as well as an elevation in IL-17A, IL-6, and TNF-α levels (Supplemental Fig. 3). On the other hand, young BALB/c mice survived infection and exhibited lower levels of these cytokines (Supplemental Fig. 3). Thus, in aged mice, systemic viral infection induces an aberrantly high IL-17A response, which is associated with mortality.
Figure 1. Aged mice succumb to systemic active HSV-2 infection and exhibit elevated serum inflammatory cytokine levels.
(A) Survival of aged (18 – 20 months), middle-aged (8 – 10 months), and young (2 – 4 months) C57BL/6 mice following HSV-2 infection. All aged mice died within 5 days of infection, whereas the other groups all survived up to 50 days after infection, at which time the experiment was terminated. p < 0.001, aged group vs. the other groups (Logrank test). (B and C) Serum levels of IL-17A (B) and IL-6 (C) following HSV-2 infection. Values represent the mean+/−SD of three independent experiments each performed in triplicate. p < 0.01, aged group vs. the other groups (ANOVA). (D and E) Survival (D) and IL-17A responses (E) in aged mice infected with active or heat-inactivated HSV-2. The difference in survival between the active and heat inactivated groups was significant (p < 0.001, Log rank). (F and G) Effect of acyclovir or PBS on survival (p = 0.005, Log rank) (F) and IL-17A responses (G) in aged mice infected with HSV-2. Values in E and G represent the mean+/−SD of two independent experiments each performed in triplicate.
Systemic HSV-2 infection elevates serum IL-17A levels in aged mice in a dose-dependent fashion
Next, the age-dependent augmentation of IL-17A responses to systemic viral infection was further characterized using dose-response experiments. We first detected differences in serum IL-17A levels between aged mice and their younger counterparts at a 1 × 105 PFU dose of HSV-2 (Supplemental Fig. 4 A). The difference in systemic IL-17A levels between young and aged mice grew larger with increasing doses of HSV-2 (Supplemental Fig. 4 A). In contrast to this age-dependent elevation in the systemic IL-17A response, systemic IFN-α responses to viral infection were lower in aged mice than in young mice (Supplemental Fig. 4 B). Moreover, measurement of viral plaques revealed that these age-related changes in IL-17A and IFN-α responses were accompanied by a reduced ability to clear HSV-2 (Supplemental Fig. 4 C). Finally, mortality was noted in aged mice infected with 1 × 107 PFU of HSV-2, but not in those infected with a 1 × 105 PFU (Supplemental Fig. 5). A comparison of aged mice infected with these two doses revealed that the higher infecting dose induced a greater increase in systemic IL-6, IL-17A and TNF-α levels (Supplemental Fig. 5).
Infection with active virus promotes higher IL-17A levels and mortality than infection with heat-inactivated virus
To determine whether systemic infection with active virus promotes death of aged mice, we infected aged mice with either active HSV-2 or heat-inactivated HSV-2. We found that, in aged mice, infection with 107 PFU of the active form of the virus was lethal and increased systemic IL-17A levels, whereas infection with the same dose of inactive form was not lethal and increased IL-17A to a lesser extent (Fig. 1 D and E). Moreover, administration of the antiviral agent, acyclovir, to aged mice just before HSV-2 infection and 24 h after infection reduced IL-17A production and prevented mortality (Fig. 1 F and G). Taken together, these results imply that the active form of the HSV-2 is superior to the inactive form in elevating IL-17A and inducing mortality in aged mice.
Aging augments neutrophil-attracting chemokine production, neutrophil activity, and liver neutrophil accumulation following HSV-2 infection
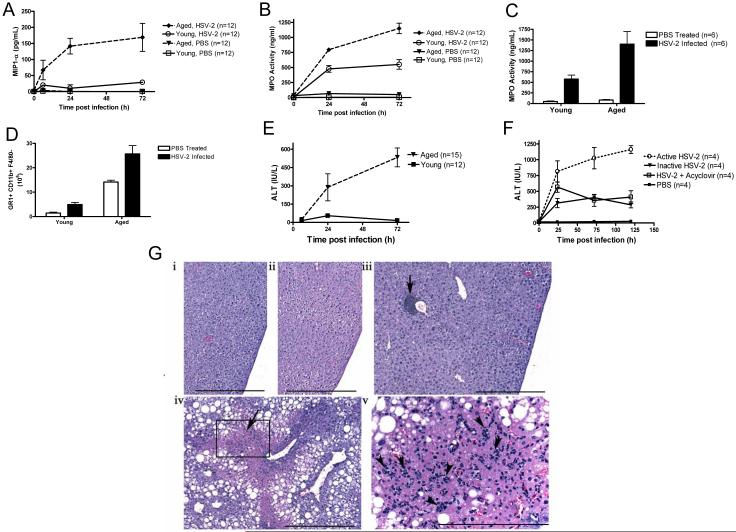
The finding that aging increases the IL-17A response prompted us to measure the production of macrophage inflammatory protein-1α (MIP-1α), a neutrophil- and macrophage-homing chemokine that is induced by IL-17A (Aggarwal and Gurney, 2002). We found that, following HSV-2 infection, serum levels of MIP-1α and myeloperoxidase (MPO, a marker of neutrophil activation) were higher in aged mice than in young mice (Fig. 2 A and B). MPO levels were also present at higher concentrations in the livers of aged infected mice than in those from young infected mice (Fig. 2 C). Accordingly, livers from aged infected mice contained a greater number of GR1+CD11b+ F4/80− cells than livers from young infected mice (Fig. 2 D).
Figure 2. Aged mice exhibit greater MIP1 production, neutrophil activity, liver neutrophil accumulation, and hepatocyte necrosis than young mice after HSV-2 infection.
(A) Effect of HSV-2 infection on serum MIP-1α levels in aged and young mice. p < 0.01, aged infected vs. young infected (ANOVA). (B) Serum neutrophil activity (MPO levels) in aged and young HSV-2-infected mice. Data are derived from three independent experiments. p = 0.007, aged infected vs. young infected (ANOVA). (C) Liver MPO levels in aged and young mice 12 h after HSV-2 infection. *p = 0.05 (t test). (D) Accumulation of liver GR1+CD11b+ F4/80− cells in aged and young HSV-2-infected mice at 12 h post infection. *p <0.01, (t test), n = 4 / group. (E) Serum ALT levels (measure of liver necrosis) in aged and young HSV-2-infected mice. p < 0.01, aged infected vs. young infected (ANOVA). (F) Serum ALT levels in aged HSV-2-infected mice treated with acyclovir or in aged mice infected with heat-inactivated HSV-2. Values in A, B and E represent the mean+/−SD of three independent experiments each performed in triplicate. Values in C and D and F represent the mean+/−SD of two independent experiments each performed in triplicate. (G) Histological images of livers from young and aged mice that were left uninfected [young (i) and aged (iii)] or infected for 24 h [young (ii) and aged (iv)]. Non-infected aged livers were similar to young infected and non-infected livers, except for normal age-related changes such as perivascular collections of lymphocytes and plasma cells (iii, arrow). Livers from infected aged mice exhibited marked multi-focal hepatocellular necrosis (iv, arrow), with multi-focal to diffuse hepatocellular fatty changes. Numerous polymorphonuclear cells were present in necrotic areas [arrowhead, v (inset of iv)]. Scale bars: i – iv = 500 μm; v = 200 μm. n = 3 – 6 mice per group.
HSV-2 infection induces hepatocyte necrosis in aged mice, but not in young mice
Neutrophils can induce acute hepatocyte injury and necrosis (Ramaiah and Jaeschke, 2007), which are accompanied by release of alanine aminotransferase (ALT). We found that, during the first 72 h after HSV-2 infection, aged mice had higher serum ALT levels than young mice (Fig. 2 E). Similar findings were noted in BALB/c mice infected with MCMV (Supplemental Fig. 6). Furthermore, a comparison of serum ALT levels in aged mice infected with either 105 or 107 PFU of HSV-2 demonstrated that the higher infecting dose was superior in inducing ALT release than the lower dose (Supplemental Fig. 7). Importantly, this age-related increase in serum ALT levels was partially prevented when mice were infected with heat-inactivated HSV-2 or given acyclovir just prior to viral infection (Fig. 2 F). Liver damage in aged mice was confirmed by histological analysis. As shown in Fig. 2 G, liver tissue from aged HSV-2-infected mice showed clear evidence of hepatocyte necrosis, while livers from young HSV-2-infected mice showed no histopathological changes. Histological examination of other organs and tissues from infected animals did not reveal any signs of age-specific necrosis (data not shown).
Aging augments IL-17A production by NKT cells after HSV-2 infection
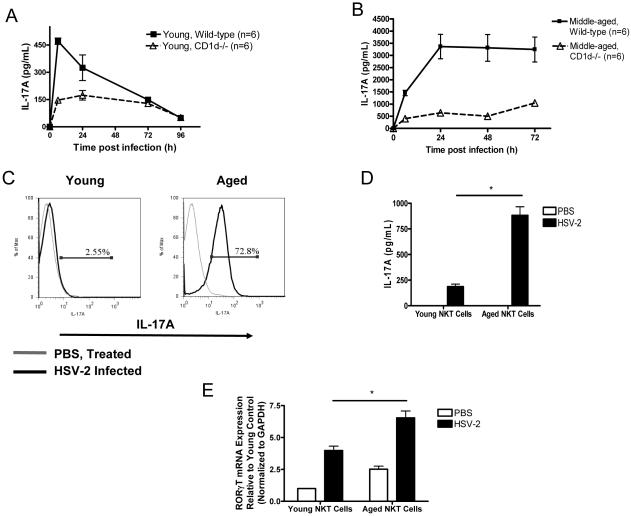
The rapid kinetics of IL-17A induction in aged HSV-2-infected mice indicate that IL-17A release primarily arises from the innate immune response. Recent studies have demonstrated that NKT cells produce IL-17A in response to synthetic NKT cell ligands (Michel et al., 2007; Rachitskaya et al., 2008). Furthermore, aging induces an accumulation of NKT cells (Faunce et al., 2005), which can mediate age-dependent liver injury (Inui et al., 2002; Kawabata et al., 2008). Hence, we infected 2- to 4-month-old WT and NKT cell-deficient (CD1d−/−) mice with HSV-2 and measured the serum IL-17A response. Six hours after infection, systemic IL-17A levels were lower in CD1d−/− mice than in WT mice (Fig. 3 A). We repeated this experiment with middle-aged mice (i.e., 9 months of age) and found that the difference in serum IL-17A levels between WT and CD1d−/− mice was even larger (Fig. 3 B). Similar results were noted in an alternate form of mutant mice that were deficient in NKT cells (Jα18−/−) (Supplemental Fig. 8).
Figure 3. Aging augments HSV-2-induced production of IL-17A by NKT cells.
(A and B) IL-17A serum levels in young (A) and middle-aged (9 months of age) (B) WT and NKT cell-deficient (CD1d−/−) mice infected with HSV-2. For both age groups, two independent experiments yielded similar results. p = 0.03, middle-aged WT vs. middle-aged CD1d−/− (ANOVA). (C) Intracellular IL-17A labeling in NKT cells (glycolipid-loaded CD1d tetramer+ TCRβ+ cells) gated within liver lymphocytes from aged and young mice receiving PBS or HSV-2. A representative experiment is shown from six independent experiments (n = 3 mice per group / experiment). (D) HSV-2-induced IL-17A production in cultured liver NKT lymphocytes isolated from aged and young mice. Data are derived from three independent experiments (n = 3 mice per group / experiment). *p < 0.01, young infected vs. aged infected cells (t test). (E) RORγT gene expression in liver NKT cells at rest and after HSV-2 infection (n = 3 mice per group). *p = 0.01, young infected vs. aged infected cells (t test). Values in A-E represent the mean+/−SD of two independent experiments each performed in triplicate.
Next, we analyzed IL-17A responses in liver NKT cells (PBS-57-loaded CD1d tetramer+, TCRβ+) from young and aged mice at 12 h post HSV-2 infection. The proportion of liver NKT cells that expressed IL-17A was greater in aged mice than in young mice (Fig. 3 C). Consistent with prior work (Inui et al., 2002), non-infected aged mice also manifested higher numbers of liver NKT cells than their younger counterparts, though these numbers did not increase after infection (Supplemental Fig. 9). To determine whether aging augments IL-17A responses on a per cell basis, we isolated liver NKT cells from non-infected aged and young mice. We then activated these cells ex vivo with HSV-2. We found that, during in vitro HSV-2 activation, aged NKT cells produced higher levels of IL-17A than young NKT cells (Fig. 3 D). Moreover, mRNA levels of RORγT, an IL-17-promoting transcription factor (Ivanov et al., 2006; Michel et al., 2007; Rachitskaya et al., 2008), were greater in aged NKT cells than in young NKT cells, both at rest and after HSV-2 infection (Fig. 3 E). Finally, NKT cells have been shown to produce IL-17A in an IL-6-independent manner, and experiments with middle-aged WT and IL-6-deficient mice revealed that HSV-2-induced production of IL-17A was IL-6 independent (Supplemental Fig. 10).
Impaired viral control and aged NKT cells synergize to induce liver injury during viral infection
Our results indicate that, in aged animals, liver injury and mortality during viral infection are associated with two factors: impaired containment of the virus (Supplemental Fig. 4C) and the presence of a cell with superior IL-17A-producing capabilities (i.e., NKT cell) (Fig. 3 C). Therefore, we investigated whether the presence of these two factors synergized to induce liver injury during viral infection in young mice. To impair viral control in young mice, we depleted these hosts of pDCs, which are critical to host defense to HSV-2 (Lund et al., 2006; Stout-Delgado et al., 2008). In young hosts, pDC depletion before viral infection using αPDCA-1 antibody led to a greater viral load in the liver than treatment with isotype control (Supplemental Fig. 11), confirming the critical role of pDCs in host defense to HSV-2.
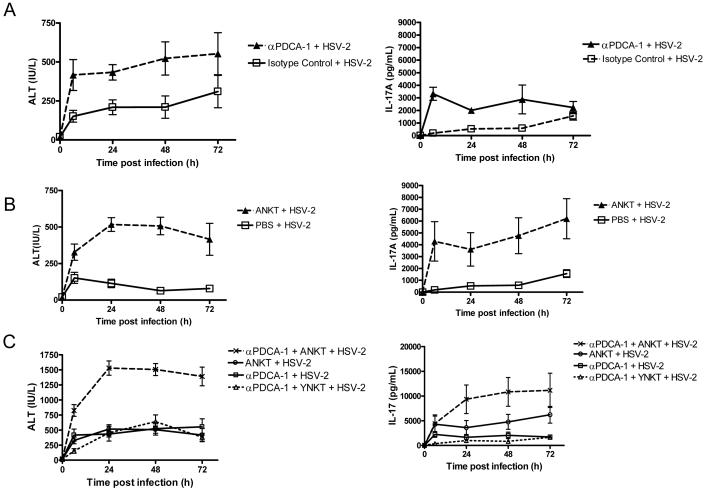
Next, we measured serum ALT and IL-17A levels in young mice that underwent either pDC depletion or adoptive transfer of aged NKT cells. Our results revealed that ALT and IL-17A levels were greater in mice undergoing pDC depletion than in control animals (mice receiving isotype control antibody) (Fig. 4 A). ALT and IL-17A levels were also greater in young infected hosts undergoing adoptive transfer of aged NKT cells than in those not receiving these cells (Fig. 4 B). However, elevating serum IL-17A levels in the absence of infection through administration of recombinant IL-17A did not induce ALT release or mortality, regardless of host age (Supplemental Fig. 12). In young infected hosts, a combination of pDC depletion and adoptive transfer of aged NKT cells increased ALT levels to a greater extent than a comparable degree of pDC depletion combined with adoptive transfer of young NKT cells (Fig 4 C). The pDC-depleted young mice that were adoptively transferred with aged, but not young, NKT cells exhibited signs of illness during viral infection (e.g., reduced movement, grooming and feeding); however, they eventually survived infection. Finally, we found that young infected hosts that underwent pDC depletion and received aged NKT cells exhibited higher ALT levels and IL-17A levels than young HSV-2 infected mice undergoing only pDC depletion or adoptive transfer of aged NKT cells (Fig. 4 C). Importantly, the effect of these combined manipulations on ALT and IL-17A production was greater than the sum of the effects resulting from either manipulation alone (Figure 4 C). These data indicate that impaired viral control and the presence aged NKT cells synergize to induce liver injury during HSV-2 infection.
Figure 4. Impaired viral control and aged NKT cells synergize to induce liver injury during HSV-2 infection.
Serum ALT and IL-17A levels were measured in young HSV-2 infected mice that were subjected to pDC depletion, adoptive transfer of NKT cells, or a combination of these procedures. (A) pDC depletion with α-PDCA-1 antibody [α-PDCA-1 antibody group vs. isotype control group, p = 0.01 for ALT and p < 0.05 for IL-17A (ANOVA)]. (B) Adoptive transfer of aged NKT (ANKT) cells [vs. PBS, p < 0.001 for ALT and p < 0.05 for IL-17A (ANOVA)]. (C) Combination of pDC depletion and adoptive transfer of NKT cells. Animals received ANKT cells or young NKT (YNKT) cells. ALT and IL-17A levels in mice undergoing a combination of pDC depletion and adoptive transfer of ANKT cells significantly differed from those in all other groups (p < 0.001, ANOVA). Values in A-C represent the mean+/−SD of two independent experiments each performed in triplicate. n = 4 mice per group / experiment. Young uninfected mice undergoing pDC depletion or adoptive transfer with NKT cells did not exhibit elevations in IL-17A and ALT (data not shown).
IL-17A inhibition prevents lethality of HSV-2 in aged mice
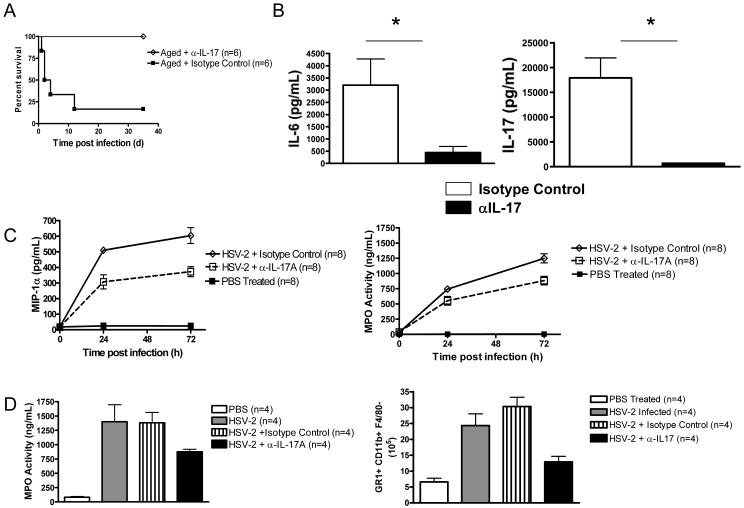
To understand the role of IL-17A in HSV-2-induced death of aged mice, we tested the effect of an IL-17A-neutralizing antibody on survival. Treatment of aged mice with the IL-17A-neutralizing antibody at the time of HSV-2 infection did not affect viral clearance from either the spleen or liver during the time course of study (Supplemental Fig. 13A). Similar findings were noted when serum viral load was measured by PCR (Supplemental Fig. 13B). Nevertheless, this treatment led to 100% survival in this group (6/6) (Fig. 5 A). On the other hand, administration of an isotype control antibody to aged mice led to the survival of only a single mouse (1/6 or 17%) (Fig. 5 A). A comparison of cytokine production between HSV-2-infected mice receiving IL-17A-neutralizing antibody and isotype control antibody revealed that IL-17A neutralization not only prevented death, but also significantly reduced serum IL-17A, IL-6, and TNF-α levels at 24 h after infection (Fig. 5 B and Supplemental Fig. 14). These changes were accompanied by a reduction in MIP-1α and MPO serum levels (Fig. 5 C). Liver MPO levels and neutrophil accumulation were also lower in the IL-17A neutralization group than in the isotype control group (Fig. 5 D). Importantly, these protective effects of IL-17A neutralization were associated with a reduction in ALT levels and hepatocyte necrosis (Fig. 6 A and B). In addition, the ability of anti-IL-17A treatment to prevent mortality was noted in aged BALB/c mice infected with MCMV (Supplemental Fig. 15 A and B). IL-17A neutralization was also capable of preventing death when administered at 24 h after HSV-2 infection (Supplemental Fig. 15 C and D). These data indicate that HSV-2-induced death in aged mice is IL-17A dependent.
Figure 5. IL-17A neutralization blocks HSV-2-induced death, MIP-1 production, and neutrophil activity in aged mice.
(A - D) Effect of anti-IL-17A antibody on survival of aged mice (A) (p =0.007, anti-IL-17A antibody vs. isotype control, Logrank test), serum IL-17A and IL-6 levels (B) (n = 6 mice per group; p < 0.01, anti-IL-17A antibody vs. isotype control, t test), serum MIP-1α levels and MPO activity (C) (p < 0.01, anti-IL-17A antibody vs. isotype control, ANOVA), as well as liver MPO production and GR1+CD11b+ F4/80− accumulation at 12 h post infection (D) (p < 0.01, anti-IL-17A antibody vs. isotype control, ANOVA). Values in B-D represent the mean+/−SD of two independent experiments each performed in triplicate.
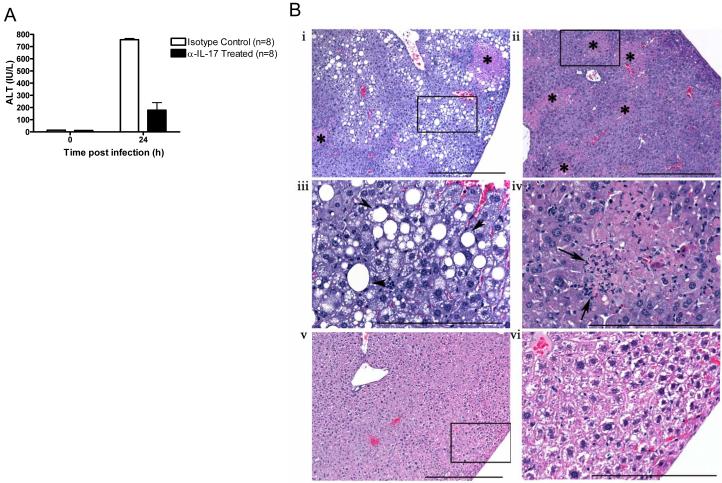
Figure 6. IL-17A neutralization inhibits HSV-2-induced hepatocyte necrosis in aged mice.
(A) Effect of anti-IL-17A antibody on HSV-2 induced increases in systemic ALT levels. Values represent the mean+/−SD of two independent experiments each performed in triplicate. (B) Effect of anti-IL-17A antibody on HSV-2-induced histopathological changes in the aged liver at 48 h after infection (n = 3 mice per group). Isotype antibody treatment (i – iv) was associated with random multifocal necrosis (i, ii *), scattered polymorphonuclear cells [arrows, iv (inset of ii)], and marked multifocal to diffuse fatty changes (steatosis) in some hepatic lobes [iii; arrowheads, v (inset of i)]. Treatment with anti-IL-17A prior to infection [v, vi (inset of v)] prevented the induction of histopathological changes. Scale bars: i, ii, v = 500 μm; iii, iv, and vi = 200 μm.
Neutrophil depletion prevents HSV-2-induced death in aged mice
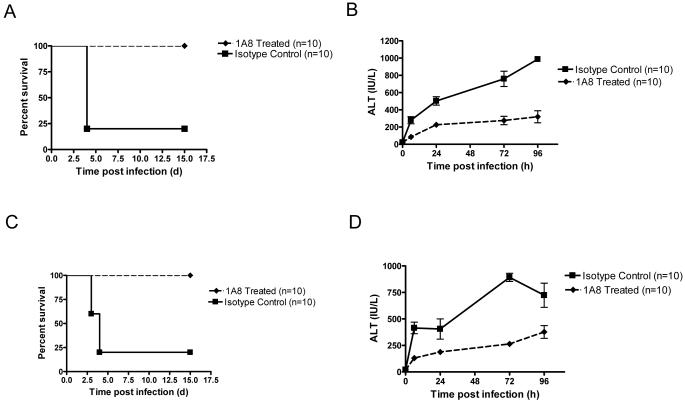
The above results suggest that neutrophils are the cellular mediators of HSV-2-induced liver damage and death in aged mice. To further investigate this possibility, we depleted aged mice of neutrophils with a specific neutrophil depleting mAb (Daley et al., 2008) and then infected these animals with HSV-2. All neutrophil depleted mice (8/8) survived HSV-2 infection, while none of the isotype control mice survived (0/8) (Fig. 7 A). In addition, ALT levels were lower in neutrophil-depleted mice than in isotype control animals (Fig. 7 B). Similar results were noted in aged BALB/c mice infected with MCMV (Fig. 7 C and D). These data indicate that neutrophils are the cellular mediators of herpes virus-induced death in aged mice.
Figure 7. Depletion of 1A8+ cells prevents HSV-2 or MCMV-induced death in aged mice.
(A and B) Effect of depletion of 1A8+ cells on survival (A) (p < 0.001, Logrank test) and serum ALT levels (B) (1A8+ vs. isotype, p < 0.001, ANOVA) in aged C57BL/6 mice infected with HSV-2. Non-infected mice did not exhibit an increase in serum ALT (data not shown). (C and D) Effect of depletion of 1A8+ cells on survival (C) (p < 0.001, Logrank test) and serum ALT levels (D) (1A8+ vs. isotype, p < 0.001, ANOVA) in aged BALB/c mice infected with MCMV. Non-infected mice did not exhibit an increase in serum ALT (data not shown). Values in B and D represent the mean+/−SD of two independent experiments each performed in triplicate.
Discussion
Aging is known to augment inflammatory responses, but whether these alterations contribute to death following viral infection is unknown. In the current study, we found that, following either HSV-2 or MCMV infection, aged mice produced higher serum levels of several inflammatory cytokines, in particular IL-17A. Importantly, neutralizing IL-17A in aged mice during HSV-2 or MCMV infection reduced liver injury and increased survival (Fig. 5, and 6). These findings are consistent with prior work showing that IL-17 signaling induces liver injury in non-aging models (Nagata et al., 2008). IL-17A neutralization also reduced serum levels of other inflammatory cytokines including IL-6 and TNF-α, indicating that elevated IL-17A levels control the production of other cytokines in this experimental system. This is consistent with prior work demonstrating that IL-17A can induce the production of IL-6 by a variety of cells (Fossiez et al., 1996). Our findings demonstrate that aging induces an aberrant overproduction of IL-17A during systemic viral infection and that this IL-17A overproduction can lead to death secondary to liver damage.
In the aged mice studied here, neutrophil-mediated liver necrosis was responsible for HSV-2-induced death. Prior work in non-aging models has demonstrated that neutrophils are key mediators of liver cell injury (Ramaiah and Jaeschke, 2007). We found that 24 h after infection, aged virally infected mice, but not their young infected counterparts, exhibited severe hepatocyte necrosis that was associated with neutrophil activation (Fig. 2). Moreover, both liver injury and mortality were blocked by neutrophil depletion (Fig. 7). Both outcomes were driven by IL-17A, as seen by the ability of IL-17A neutralization to reduce levels of MIP-1α (a neutrophil-attracting chemokine) and decrease neutrophil enzyme activity (Fig. 5). These data indicate that neutrophils are a key cellular mediator of IL-17A-dependent death in aged mice with systemic viral infections.
Our study provides evidence that NKT cells are important for the rapid production of IL-17A during HSV-2 infection, because NKT-deficient mice exhibited a much weaker IL-17A response than WT mice (Fig. 3). These results indicate that NKT cells are a major source of IL-17A in our experimental systems but do not exclude the possibility that other cell types with known IL-17-producing capabilities, such as γδ T cells and CD4+ T cells (O’Brien et al., 2009; Stockinger and Veldhoen, 2007), contribute to the age-induced IL-17 response in our study. Recent studies have demonstrated that NKT cells produce IL-17A when activated by α-galactosylceramide or LPS instillation (Michel et al., 2007; Rachitskaya et al., 2008). However, to our knowledge, the ability of NKT cells to produce IL-17A during a viral infection has not been previously described. Our work suggests that IL-17-producing NKT cells may have pathological consequences during viral infection. Prior work indicates that NKT cells are important for host defenses against infection with HSV and other viruses (Grubor-Bauk et al., 2008; Grubor-Bauk et al., 2003). Furthermore, studies with MCMV have indicated that NKT cells are activated via the presence of an accessory cell to maximally induce IFN-γ production during infection (Tyznik et al., 2008; Wesley et al., 2008). Our results indicate that NKT cells can respond directly to in vitro stimulation by HSV-2 to produce IL-17A, although our study does not exclude the possibility that an accessory cell is involved in vivo. In the future, it will be important to identify the viral components (e.g., viral glycolipids) that stimulate NKT cells to produce IL-17A.
We found that aging increased expression of RORγT, a transcription factor that may control IL-17A production by NKT cells (Michel et al., 2007; Rachitskaya et al., 2008). Why aging led to an upregulation of the RORγT gene in NKT cells is unclear. One possible avenue for future investigation is exploration of the role of oxidative stress, as many studies have shown that age-induced phenotypes correlate with increasing levels of oxidative stress [reviewed in (Bokov et al., 2004)].
Our work provides evidence that two factors (i.e., impaired viral control and aged NKT cells) cooperate in inducing liver injury during viral infection in our experimental models. A recent study demonstrated that aging impairs pDC function and in this way, disrupts the IFN-α response, inhibiting HSV-2 or MCMV clearance in aged mice (Stout-Delgado et al., 2008). In young infected mice, impeding viral clearance via pDC depletion and adoptively transferring aged, but not young, NKT cells induced greater ALT release than either manipulation alone and imparted a similar phenotype to that seen in aged mice (Fig. 4). Although these young mice became ill, they eventually survived viral infection, suggesting that young hosts contain unidentified protective factors or that aged hosts contain unidentified disease-promoting factors that contribute to mortality during viral infection. A recent study found that IL-17A inhibits NK function, reducing clearance of vaccinia virus (Kawakami et al., 2009). This study may explain the synergy between impaired viral control and elevated IL-17A levels in our model, as it is possible that the augmented IL-17A response alters other components of the aging immune system, further exacerbating liver injury. Nevertheless, our results indicate that, in aged hosts, viral infection induces liver injury through at least two factors: impaired containment of the virus and aged NKT cells, which exhibit an exaggerated IL-17A response.
In future studies, it will be important to determine whether local or organ-specific infection in aged hosts induces a phenotype similar to that described here in response to systemic viral infections. This may depend on whether NKT cells accumulate with age at local sites of infection. In addition, clinical studies should be conducted to determine whether older people exhibit increased levels of IL-17 during viral infection. A recent study showed that Th17 cells enhance viral persistence in a murine chronic viral infection model (Hou et al., 2009). If Th17 cells are found to be elevated in aged humans with viral infections, then perhaps age-dependent increases in IL-17 responses may not only contribute to immune pathology during systemic viral infections, but also promote chronic viral infections. In any case, our data clearly show that aging alters the host-virus interaction by inducing liver damage and mortality through a novel pathway involving IL-17A. Thus, anti-IL-17A therapies may hold promise for preventing immune pathology and increasing survival in older individuals with systemic viral infections.
Experimental Procedures
Mice
Aged (18 – 20 months), middle-aged (8 – 10 months), and young (2 – 4 months) C57BL/6 or BALB/c mice were purchased from the NIA rodent facility. C57BL/6 CD1d−/− mice and BALB/c Jα18−/− were generously provided by Dr. Erol Fikrig and Dr. McMahon-Pratt, respectively (both of Yale University, New Haven, CT) and were aged in our colony. The institutional animal care and use committee at Yale University approved the use of animals in this study. Animals with any evidence of skin lesions, weight loss, or lymphadenopathy were excluded from the study.
In vivo infection
HSV-2 virus was generously provided by Dr. Akiko Iwasaki (Yale University) and grown in Vero cells as previously described (Lund et al., 2003). Gradient-purified virus was administered via tail vein injection at a dose of 1 × 107 PFU or at other doses as indicated. HSV-2 viremia was measured via a plaque assay as previously described (Gadjeva et al., 2004). Briefly, diluted serum or supernatants obtained from homogenized spleen and liver samples were plated on a confluent layer of Vero cells, seeded at 1 × 106 cells / well in 10% FCS DMEM. The cells were incubated overnight at 37°C and then plaques were counted by visual inspection following a Giemsa stain. Results are expressed as units/ml of serum or units/liver. Viral load was also assessed by real time PCR (see below). HSV-2 was heat inactivated at 56°C for 1 h. The MCMV Smith strain was generously provided by Anthony Van Den Pol (Yale University). Mice received 1 × 103 PFU of MCMV via i.v. injection as described elsewhere (Delale et al., 2005).
In vivo reagents
IL-17A neutralizing antibody (rat anti-mouse mAb, clone M210, IgG2a) was generously provided by Amgen (Seattle, WA) (Cong-Qiu Chu et al., 2007). It was administered i.p., at a dose of 150 μg/mouse at 24 h prior to viral infection or at other times indicated. Anti-1A8 mAb and isotype control were obtained from BioXcell (Lebanon, NH). Both were used at a concentration of 0.5 mg/mouse and administered i.p., 72 h prior to infection. Acyclovir (Sigma-Aldrich) or PBS (control) was administered i.p. at a dose of 0.6 mg/mouse. These agents were administered at the time of HSV-2 infection and 24 h after infection. For pDC depletion, mice received 0.5 mg of anti-PDCA-1 monoclonal antibody (Miltenyi Biotec, CA) or isotype control (BioXcell) i.p., 24 h before infection, as previously reported (Stout-Delgado et al., 2008). Recombinant IL-17A (eBiosciences, San Diego, CA) was administered i.p., at a dose of 250pg / gm body weight.
Histology
A routine selection of tissues from all organ systems was subjected to histopathologic examination. The tissues were processed, embedded in paraffin, sliced into 5 μm-thick sections, and stained with hematoxylin and eosin using routine methods. Samples were evaluated by investigators blinded to the experimental group. Digital light microscopic images were acquired using a Zeiss Axioskop microscope, an AxoCam MRC Camera, and AxioVision 4.4 imaging software (Carl Zeiss Microimaging, Inc., Thornwood, NY). The resulting images were optimized using Adobe Photoshop 8.0 (San Jose, CA).
Cell sorting, flow cytometry, and NKT culture
Liver lymphocytes were isolated as previously described (Crisp, 2003). These cells were stained with fluorescently labeled PBS-57 glycolipid-loaded or unloaded CD1d tetramers (NIAID Tetramer Facility, Atlanta, GA) and FITC-tagged rat anti-mouse TCRβ+ mAb (eBiosciences, San Diego, CA). The cells were subjected to intracellular cytokine staining with PE-tagged rat anti-mouse IL-17 mAb (eBiosciences), and NKT cells were identified by gating on the PBS-57-loaded CD1d tetramer+ TCRβ+ population, as previously described (Liu et al., 2006). Liver NKT cells (i.e., PBS-57-loaded CD1d tetramer+ TCRβ+) were also sorted by FACs for further in vitro studies. For cytokine staining, NKT cells were harvested 12 h after HSV-2 infection and then cultured in the presence of cell permeability agents and golgi stop (eBiosciences). For in vitro studies, 1 × 105 FACs-purified NKT cells were plated in RPMI supplemented with 10% FBS (200 μL and treated with 50 PFU heat-inactivated HSV-2 for 18 h at 37°C. Cells were then processed for real time PCR and cell supernatants for ELISA.
Adoptive transfer of NKT cells
Liver NKT cells were purified as described above and 1 × 105 cells were adoptively transferred via i.v. tail vein injection at 72 h prior to infection.
RNA and DNA purification
RNA was extracted using the Qiagen RNAeasy mini kit (Qiagen, Valencia, CA) and quantified by A260/280 absorbance readings. Superscript III reverse transcriptase (Invitrogen) was used, according to the manufacturer’s instructions, to create complementary DNA from mRNA. DNA was extracted from 10-μL of serum samples using the Qiagen DNeasy blood and tissue kit (Qiagen, Valencia, CA), eluted into 50-μL of sterile, double-distilled water, and quantified by A260/280 absorbance readings.
Real time PCR
Real time PCR for RORγT was performed using TaqMan gene expression probe / primer sets (Applied Biosystems, Foster City, CA). Reaction mixtures had a final volume of 25 μL and contained complementary DNA from 20 ng of reverse-transcribed total RNA, 1X TaqMan Gene expression primer / probe mix, and 1X TaqMan Gene Expression Master Mix. Real time PCR for HSV-2 DNA was performed using SYBR Green universal PCR master mix (Applied Biosystems). The subsequent RT-PCR was performed in a final volume of 25 μl containing 20 ng of DNA, 150 nM forward and reverse primers (Kaoru et al., 2009), and SYBR Green universal PCR master mix. Known concentrations of HSV-2 DNA were included in all PCR reactions to assess sensitivity. PCR was performed in 96-well plates using the MJ Research detection system (MJ Research Inc, MA). All reactions were performed in triplicate. An amplification curve analysis was performed and dilution curve standards were generated to identify primer sets and conditions yielding specific products with 100% amplification efficiency. Relative mRNA levels were calculated using the comparative cycle threshold method (User Bulletin No. 2, Applied Biosystems). GAPDH and cyclophilin mRNA levels served as an invariant controls.
ELISA
Culture supernatants or sera were analyzed for IL-6, IL-17A, IFN-α, TNF-α, and MIP-1α using ELISA kits from BD Biosciences (San Diego, CA), R&D Systems (Minneapolis, MN), or PBL (Piscataway, NJ) according to the manufacturer’s instructions. Neutrophil activity was determined by measuring MPO levels, which were also analyzed using a commercially available ELISA kit (Hycult Biotechnology, Uden, The Netherlands) according to the manufacturer’s protocol.
ALT assay
Serum ALT levels were measured using the Infinity ALT Assay Kit (Fisher Scientific) according to the supplied instructions.
Statistical analysis
Survival analysis was performed using the Logrank method. Comparison of means was performed using a two-tailed t test or repeated measures analysis of variance. All data were analyzed using GraphPad prism software (San Diego, CA). Values of p less than 0.05 were considered significant.
Supplementary Material
Acknowledgments
This work was supported by NIH grant RO1 AG028082 to D. R. G. H. W. S.-D. is supported by NIH grant T32AG019134 and A.S. is supported by NIH grant 5T32DK 007276-30.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicting financial interests.
References
- Aggarwal S, Gurney AL. IL-17: prototype member of an emerging cytokine family. J Leukoc Biol. 2002;71:1–8. [PubMed] [Google Scholar]
- Bokov A, Chaudhuri A, Richardson A. The role of oxidative damage and stress in aging. Mech Ageing Dev. 2004;125:811. doi: 10.1016/j.mad.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Bruunsgard H, Klarlund Pedersen B. Age-related inflammatory cytokines and disease. Immunol Allergy Clin North Am. 2003;23:15–39. doi: 10.1016/s0889-8561(02)00056-5. [DOI] [PubMed] [Google Scholar]
- Chu Cong-Qiu, Swart D, Alcorn D, Tocker J, Elkon KB. Interferon-gamma regulates susceptibility to collagen-induced arthritis through suppression of interleukin-17. Arthritis & Rheumatism. 2007;56:1145–1151. doi: 10.1002/art.22453. [DOI] [PubMed] [Google Scholar]
- Crisp N. Isolation of Mouse Intrahepatic Lymphocytes. Curr Prot in Immunol. 2003:3.21. doi: 10.1002/0471142735.im0321s22. [DOI] [PubMed] [Google Scholar]
- Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- Delale T, Paquin A, Asselin-Paturel C, Dalod M, Brizard G, Bates EEM, Kastner P, Chan S, Akira S, Vicari A, et al. MyD88-Dependent and -Independent Murine Cytomegalovirus Sensing for IFN-{alpha} Release and Initiation of Immune Responses In Vivo. J Immunol. 2005;175:6723–6732. doi: 10.4049/jimmunol.175.10.6723. [DOI] [PubMed] [Google Scholar]
- Faunce DE, Palmer JL, Paskowicz KK, Witte PL, Kovacs EJ. CD1d-Restricted NKT Cells Contribute to the Age-Associated Decline of T Cell Immunity. J Immunol. 2005;175:3102–3109. doi: 10.4049/jimmunol.175.5.3102. [DOI] [PubMed] [Google Scholar]
- Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadjeva M, Paludan SR, Thiel S, Slavov V, Ruseva M, Eriksson K, Lowhagen GB, Shi L, Takahashi K, Ezekowitz A, Jensenius JC. Mannan-binding lectin modulates the response to HSV-2 infection. Clin Exp Immun. 2004;138:304–311. doi: 10.1111/j.1365-2249.2004.02616.x. I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia GG, Miller RA. Single-Cell Analyses Reveal Two Defects in Peptide-Specific Activation of Naive T Cells from Aged Mice. J Immunol. 2001;166:3151–3157. doi: 10.4049/jimmunol.166.5.3151. [DOI] [PubMed] [Google Scholar]
- Gorina Y, Kelly T, Lubitz J, Hines Z. Trends in Influenza and Pneumonia Among Older Persons in the United States. Aging Trends. 2008;8:1–11. [PubMed] [Google Scholar]
- Grubor-Bauk B, Arthur JL, Mayrhofer G. Importance of NKT cells in resistance to herpes simplex virus, fate of virus infected neurons and level of latency in mice. J. Virol. 2008;82:11073–11083. doi: 10.1128/JVI.00205-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubor-Bauk B, Simmons A, Mayrhofer G, Speck PG. Impaired Clearance of Herpes Simplex Virus Type 1 From Mice Lacking CD1d or NKT Cells Expressing the Semivariant V{alpha}14-J{alpha}281 TCR. J Immunol. 2003;170:1430–1434. doi: 10.4049/jimmunol.170.3.1430. [DOI] [PubMed] [Google Scholar]
- Han L, Alexander JP, Anderson LJ. Respiratory syncytial virus pneumonia among the elderly: an assessment of disease burden. J. Infect. Dis. 1999;179:25–30. doi: 10.1086/314567. [DOI] [PubMed] [Google Scholar]
- Haynes L, Linton P-J, Eaton SM, Tonkonogy SL, Swain SL. Interleukin 2, but Not Other Common {gamma} Chain binding Cytokines, Can Reverse the Defect in Generation of CD4 Effector T Cells from Naive T Cells of Aged Mice. J. Exp. Med. 1999;190:1013–1024. doi: 10.1084/jem.190.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou W, Kang HS, Kim BS. Th17 cells enhance viral persistence and inhibit T cell cytotoxicity in a model of chronic virus infection. J. Exp. Med. 2009;206:313–328. doi: 10.1084/jem.20082030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui T, Nakagawa R, Ohkura S, Habu Y, Koike Y, Motoki K, Kuranaga N, Fukasawa M, Shinomiya N, Seki S. Age-Associated Augmentation of the Synthetic Ligand-Mediated Function of Mouse NK1.1 Ag+ T Cells: Their Cytokine Production and Hepatotoxicity In Vivo and In Vitro. J Immunol. 2002;169:6127–6132. doi: 10.4049/jimmunol.169.11.6127. [DOI] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The Orphan Nuclear Receptor ROR[gamma]t Directs the Differentiation Program of Proinflammatory IL-17+ T Helper Cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Kaplan V, Angus DC. Community-acquired pneumonia in the elderly. Critical Care Clinics. 2003;19:729–748. doi: 10.1016/s0749-0704(03)00057-5. [DOI] [PubMed] [Google Scholar]
- Kaplan V, Angus DC, Griffin MF, Clermont G, Scott Watson R, Linde-Zwirble WT. Hospitalized community-acquired pneumonia in the elderly: age- and sex-related patterns of care and outcome in the United States. Am J Resp & Critic Care Med. 2002;165:766–772. doi: 10.1164/ajrccm.165.6.2103038. [DOI] [PubMed] [Google Scholar]
- Kaoru W, Sachiko M, Yoshinori I, Jun-ichi K, Yohei Y, Tsuneo M, Yukihiro N, Hiroshi K. Multiplex real-time PCR for the simultaneous detection of herpes simplex virus, human herpesvirus 6, and human herpesvirus 7. Microbiol & Immunol. 2009;53:22–29. doi: 10.1111/j.1348-0421.2008.00090.x. [DOI] [PubMed] [Google Scholar]
- Kawabata T, Kinoshita M, Inatsu A, Habu Y, Nakashima H, Shinomiya N, Seki S. Functional alterations of liver innate immunity of mice with aging in response to CpG-oligodeoxynucleotide. Hepatology. 2008;5:1586–1597. doi: 10.1002/hep.22489. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Tomimori Y, Yumoto K, Hasegawa S, Ando T, Tagaya Y, Crotty S, Kawakami T. Inhibition of NK cell activity by IL-17 allows vaccinia virus to induce severe skin lesions in a mouse model of eczema vaccinatum. J. Exp. Med. 2009;206:1219–1225. doi: 10.1084/jem.20082835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Oukka M, Kuchroo V, Bettelli E. Th17 cells: Effector T cells with inflammatory properties. Semin Immunol. 2007;19:362–371. doi: 10.1016/j.smim.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton P, Haynes L, Klinman N, Swain S. Antigen-independent changes in naive CD4 T cells with aging. J. Exp. Med. 1996;184:1891–1900. doi: 10.1084/jem.184.5.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton P-J, Dorschkind K. Age related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- Liu Y, Goff RD, Zhou D, Mattner J, Sullivan BA, Khurana A, Cantu Iii C, Ravkov EV, Ibegbu CC, Altman JD, et al. A modified [alpha]-galactosyl ceramide for staining and stimulating natural killer T cells. J Immunol Meth. 2006;312:34–39. doi: 10.1016/j.jim.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like Receptor 9-mediated Recognition of Herpes Simplex Virus-2 by Plasmacytoid Dendritic Cells. J. Exp. Med. 2003;198:513–520. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund JM, Linehan MM, Iijima N, Iwasaki A. Cutting Edge: Plasmacytoid Dendritic Cells Provide Innate Immune Protection against Mucosal Viral Infection In Situ. J Immunol. 2006;177:7510–7514. doi: 10.4049/jimmunol.177.11.7510. [DOI] [PubMed] [Google Scholar]
- Michel M-L, Keller AC, Paget C, Fujio M, Trottein F, Savage PB, Wong C-H, Schneider E, Dy M, Leite-de-Moraes MC. Identification of an IL-17-producing NK1.1neg iNKT cell population involved in airway neutrophilia. J. Exp. Med. 2007;204:995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA. The aging immune system: primer and prospectus. Science. 1996;273:70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- Nagata T, McKinley L, Peschon JJ, Alcorn JF, Aujla SJ, Kolls JK. Requirement of IL-17RA in Con A Induced Hepatitis and Negative Regulation of IL-17 Production in Mouse T Cells. J Immunol. 2008;181:7473–7479. doi: 10.4049/jimmunol.181.11.7473. [DOI] [PubMed] [Google Scholar]
- Rachitskaya AV, Hansen AM, Horai R, Li Z, Villasmil R, Luger D, Nussenblatt RB, Caspi RR. Cutting Edge: NKT Cells Constitutively Express IL-23 Receptor and ROR{gamma}t and Rapidly Produce IL-17 upon Receptor Ligation in an IL-6-Independent Fashion. J Immunol. 2008;180:5167–5171. doi: 10.4049/jimmunol.180.8.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaiah SK, Jaeschke H. Role of Neutrophils in the Pathogenesis of Acute Inflammatory Liver Injury. Toxicol Pathol. 2007;35:757–766. doi: 10.1080/01926230701584163. [DOI] [PubMed] [Google Scholar]
- O’Brien RL, Roark CL, Born W. IL-17-producing gammadelta T cells. European J Immunol. 2009;39:662–666. doi: 10.1002/eji.200839120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstiel P, Derer S, Till A, Hasler R, Eberstein H, Bewig B, Nikolaus S, Nebel A, Schreiber S. Systematic expression profiling of innate immune genes defines a complex pattern of immunosenescence in peripheral and intestinal leukocytes. Genes Immun. 2008;9:103–114. doi: 10.1038/sj.gene.6364454. [DOI] [PubMed] [Google Scholar]
- Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Stout-Delgado HW, Yang X, Walker WE, Tesar BM, Goldstein DR. Aging Impairs IFN Regulatory Factor 7 Up-Regulation in Plasmacytoid Dendritic Cells during TLR9 Activation. J Immunol. 2008;181:6747–6756. doi: 10.4049/jimmunol.181.10.6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoman ML, Weigle WO. Cell-mediated immunity in aged mice: an underlying lesion in IL 2 synthesis. J Immunol. 1982;128:2358–2361. [PubMed] [Google Scholar]
- Tyznik AJ, Tupin E, Nagarajan NA, Her MJ, Benedict CA, Kronenberg M. Cutting Edge: The Mechanism of Invariant NKT Cell Responses to Viral Danger Signals. J Immunol. 2008;181:4452–4456. doi: 10.4049/jimmunol.181.7.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Ann Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- Wesley JD, Tessmer MS, Chaukos D, Brossay L. NK cell-like behavior of Valpha14i NK T cells during MCMV infection. PLoS Pathog. 2008;4:e1000106. doi: 10.1371/journal.ppat.1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.