Abstract
Hepatocellular enzyme elevation is a known side effect of both bosentan and atorvastatin. However, a rise in liver enzyme level not characteristic of either agent individually may represent a reaction to their combination or an atypical reaction to bosentan alone.
The present case report describes a patient who had been taking atorvastatin for many years and was started on bosentan for chronic thromboembolic pulmonary hypertension. After 19 weeks of therapy, she developed severe liver enzyme elevation that necessitated the discontinuation of both bosentan and atorvastatin. Although the safety of reintroducing bosentan in such a case is unknown, it was reintroduced in this patient because of the severity of her disease, the demonstrated treatment benefit and the lack of alternative treatment options. On reintroduction of bosentan alone, she again demonstrated significant liver enzyme elevation – this time occurring after only two doses. The present case highlights that bosentan can cause more rapid and severe hepatocellular enzyme elevation than previously believed, thus necessitating more frequent monitoring.
Keywords: Adverse reaction, Atorvastatin, Bosentan, Hepatocellular enzymes, Pulmonary hypertension
Abstract
L’élévation des enzymes hépatocellulaires est un effet secondaire connu du bosentan et de l’atorvastatine. Toutefois, une élévation des taux d’enzymes hépatiques non caractéristique de ces agents pris individuellement pourrait représenter une réaction à leur administration concomitante ou une réaction atypique au bosentan seul.
Le présent rapport de cas décrit une patiente qui prenait de l’atorvastatine depuis de nombreuses années et qui a commencé un traitement par bosentan pour une hypertension pulmonaire thromboembolique chronique. Après 19 semaines de traitement, la patiente a développé une élévation marquée de ses enzymes hépatiques qui a nécessité l’arrêt du bosentan et de l’atorvastatine. Bien que l’innocuité de la reprise du traitement par bosentan dans un tel cas soit inconnue, l’agent a été redébuté chez cette patiente en raison de la gravité de sa maladie, des bienfaits thérapeutiques avérés du traitement et de l’absence de solutions de rechange au bosentan en monothérapie. À la reprise du traitement par bosentan, la patiente a de nouveau manifesté une augmentation marquée de ses enzymes hépatiques, cette fois, après deux doses seulement. Le présent cas rappelle que le bosentan peut provoquer une élévation des enzymes hépatocellulaires plus rapide et prononcée qu’on l’avait d’abord cru, d’où la nécessité d’une surveillance plus étroite.
Hepatocellular enzyme elevation is a known side effect of bosentan, a nonselective endothelin receptor antagonist used in the treatment of pulmonary arterial hypertension (PAH). An uncharacteristic rise in enzyme levels may represent an adverse drug interaction or, alternatively, bosentan alone may produce a more dramatic response than usually observed. In both cases, liver enzyme testing while using bosentan may need to be performed more frequently to ensure patient safety.
CASE PRESENTATION
A 71-year-old woman presented to the emergency department complaining of chest pain and shortness of breath. Clinical diagnoses of atrial fibrillation and congestive heart failure were made. A coronary angiogram ruled out significant coronary artery disease. An echocardiogram revealed an enlarged right ventricle (RV), with an RV systolic pressure of 65 mmHg. Initial pulmonary function tests showed a restrictive pattern with a normal diffusing capacity. Six-minute walk testing revealed moderately reduced performance at 270 m (59% of predicted). Right heart catheterization showed a mean pulmonary artery pressure of 43 mmHg, a pulmonary capillary wedge pressure of 21 mmHg and a calculated pulmonary vascular resistance of 4.3 Wood units, consistent with moderate to severe PAH. A computed axial tomographic (CT) scan of the chest was consistent with chronic thromboembolic disease. Other causes of pulmonary hypertension were excluded by history and extensive laboratory investigations. Based on this workup, a diagnosis of chronic thromboembolic pulmonary hypertension (CTEPH) was made.
Baseline liver enzyme testing revealed a very mild elevation of alanine transaminase (ALT). The patient had been receiving atorvastatin 80 mg daily for many years and refused to be considered for pulmonary thromboendarterectomy. Treatment for her CTEPH was initiated with bosentan at 62.5 mg twice daily, as well as warfarin, with an international normalized ratio (INR) target of 2.5 to 3.5. Liver enzymes were followed weekly and remained stable for four weeks, at which time bosentan was increased to 125 mg twice daily (the usual maintenance dose). The patient had a significant improvement in 6 min walk test exercise performance (from 270 m to 415 m [92% predicted]) after 16 weeks of treatment.
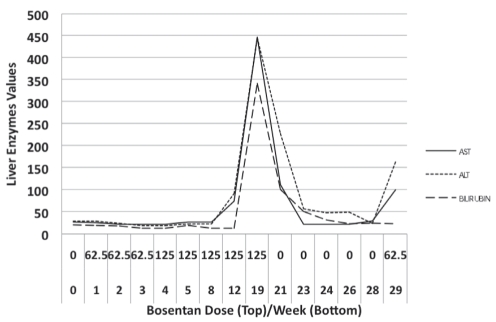
Liver enzymes were first noted to be elevated 12 weeks following treatment initiation to levels not requiring dose reduction or cessation of treatment (less than three times the upper limit of normal). Unfortunately, the patient did not return for follow-up until six weeks later when she presented with jaundice and a 10-day history of malaise and profuse vomiting. Laboratory investigations revealed a substantial elevation in her bilirubin and hepatocellular liver enzymes of approximately 15 times the upper limit of normal. Bosentan was immediately discontinued, as was atorvastatin because it was the only hepatotoxic drug identified as a possible cause of an adverse drug interaction with bosentan. During the following six weeks, the patient’s symptoms resolved and her liver enzymes returned to normal (Table 1 and Figure 1).
TABLE 1.
Liver enzyme trend in relation to bosentan dose
| Weeks from initiation of bosentan | Bosentan dose, mg (twice daily) |
Liver enzymes |
||
|---|---|---|---|---|
| Aspartate transaminase (U/L) | Alanine transaminase (U/L) | Bilirubin (μmol/L) | ||
| Baseline | 0 | 28 | 29 | 20 |
| 1 | 62.5 | 27 | 28 | 18 |
| 2 | 62.5 | 26 | 23 | 17 |
| 3 | 62.5 | 24 | 19 | 14 |
| 4 | 125 | 25 | 21 | 14 |
| 5 | 125 | 28 | 22 | 18 |
| 8 | 125 | 28 | 22 | 14 |
| 12 | 125 | 76 | 91 | 13 |
| 19 | 125 | 447 | 446 | 342 |
| 21 | 0 | 113 | 227 | 100 |
| 23 | 0 | 25 | 57 | 50 |
| 24 | 0 | 23 | 48 | 31 |
| 26 | 0 | 22 | 49 | 22 |
| 28 | 0 | 31 | 24 | 23 |
| 29 | 62.5 | 102 | 163 | 22 |
Figure 1).
Liver enzyme values over time after bosentan initiation. ALT Alanine transaminase (U/L); AST Aspartate transaminase (U/L); Bilirubin (μmol/L)
With the discontinuation of bosentan, the patient’s exercise performance on 6 min walk testing deteriorated significantly (from 415 m to 200 m [44% predicted]), and she was becoming increasingly dyspneic. Because the patient had demonstrated a significant treatment response to bosentan, and it was not clear whether the hepatocellular enzyme increase was due to bosentan alone or because of a drug interaction with atorvastatin, it was decided to reintroduce bosentan alone. After only two doses of 62.5 mg, her aspartate transaminase and ALT increased to four and six times the normal levels, respectively. Because of the extremely rapid rise in liver enzymes, bosentan was once again discontinued.
DISCUSSION
The patient had demonstrated tolerance to atorvastatin 80 mg daily for many years before the initiation of bosentan. She also tolerated both the initiation dose (62.5 mg twice daily) of bosentan for four weeks, and the maintenance dose (125 mg twice daily) for more than an additional three months. Aminotransferase elevations usually occur gradually at any time during the course of therapy (although 90% of events occur within the first four to six months of treatment), are dose-related, are asymptomatic and are generally reversible without sequelae both during continued treatment with bosentan or after discontinuation. The severity of enzyme elevation is usually approximately three times normal levels (incidence 11%), but in 6% to 8% of patients, more severe hepatic dysfunction occurs. Rare cases of hepatic cirrhosis and liver failure have also been reported (incidence less than 1%) (1,2). It is based on these data that the current recommendation for monthly liver enzyme testing was formed.
This patient demonstrated some features that did not fit the expected pattern. First, she had a sudden rise in her hepatocellular enzymes, particularly with the reintroduction of bosentan. Second, she experienced an elevation in bilirubin (at week 19) that is not usually associated with bosentan administration. Third, the elevation was far more dramatic (more than 15 times normal) than is usually seen. With such elevation, discontinuation of bosentan was mandatory. Because the liver enzyme elevation was uncharacteristic of an adverse effect from bosentan alone, it was hypothesized that the adverse effect could be the result of the combination of bosentan and atorvastatin. Hence, atorvastatin was also immediately discontinued.
In considering a potential drug interaction that has not previously been reported, a brief discussion of the pharmacology of the two drugs is warranted. Bosentan is a nonselective endothelin receptor antagonist that functions by blocking endothelin A and B receptors on vascular endothelium and smooth muscle, resulting in potent vasodilation. It is a major substrate and inducer of the cytochrome P450 3A4 (CYP3A4) component of the cytochrome P450 enzyme system (2,3). The exact mechanism by which bosentan causes liver enzyme elevation is not entirely understood, but studies have shown that it may relate to inhibition of the bile salt export pump (BSEP) in the canalicular membrane of hepatocytes. BSEP is the major bile salt excretory pump in mammalian livers. Inhibition of BSEP leads to accumulation of bile salts – some of which are hepatotoxic and may cause elevation of serum liver aminotransferases (3,4).
Atorvastatin, an antilipemic agent, is a 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitor that functions by inhibiting the rate-limiting enzyme in cholesterol synthesis – HMG-CoA reductase. There is a compensatory increase in low-density lipoprotein receptor expression on hepatocytes that effectively decreases plasma low-density lipoprotein cholesterol levels. Statin drugs, as a class, also have many other beneficial effects that are beyond the scope of the present paper. Atorvastatin is a major substrate of the CYP3A4 component of the cytochrome P450 enzyme system (5,6).
The interaction between bosentan and simvastatin was studied by Dingemanse et al (7). Simvastatin, similar to atorvastatin, is an HMG-CoA reductase inhibitor that is metabolized via the CYP3A4 pathway. Bosentan, as an inducer of CYP3A4, lowers the levels of CYP3A4 substrates such as atorvastatin. Therefore, the combination of these two drugs should not lead to a toxic effect that can be attributed to either drug and, in fact, strengthens the hypothesis that this patient’s liver enzyme elevation was due to bosentan alone.
Before deciding to reintroduce bosentan, consideration of all future medical treatment options was necessary. The safety of reintroduction of bosentan after such a significant adverse event is unknown. Three other treatment options were considered: sildenafil, sitaxsentan and ambrisentan. Sildenafil, a phosphodiesterase inhibitor, functions by causing vasodilation in the pulmonary vasculature. However, this drug was already tried in the patient and was not tolerated due to symptomatic side effects that included dizziness and severe headaches. Sitaxsentan, a selective endothelin receptor antagonist, may be used when bosentan treatment failure or intolerance is proven; however, it potentiates the effect of warfarin and it can be difficult to maintain a safe international normalized ratio in patients taking both drugs who require frequent adjustments of their warfarin dose. Because studies on the use of sitaxsentan in the setting of CTEPH are not available, and the drug was not available to us in the present setting, it was not used. Ambrisentan is another selective endothelin receptor antagonist that is not available but is expected to be in the near future. Early data (8) show a safer liver enzyme abnormality profile than bosentan or sitaxsentan, with only a 1% to 3% incidence of liver enzyme elevation.
With these limited treatment options, and considering the severity of the patient’s disease, the poor prognosis and the demonstrated significant treatment benefit, the decision was made to reintroduce bosentan under close monitoring. Unfortunately, the observed immediate and substantial liver enzyme elevation precluded this patient from receiving any endothelin receptor antagonists in the future.
The present case report demonstrates that bosentan can cause more severe and more rapid increases in hepatocellular enzymes than previously believed. This necessitates a change in the recommendation for liver enzyme testing to more frequent monitoring.
REFERENCES
- 1.Segal ES, Valette C, Oster L, et al. Risk management strategies in the postmarketing period. Drug Safety. 2005;28:971–80. doi: 10.2165/00002018-200528110-00001. [DOI] [PubMed] [Google Scholar]
- 2.Lexi-Comp Online Bosentan drug monograph. (Version current at May 1, 2008).
- 3.Dingemanse J, van Giersbergen PL. Clinical pharmacology of bosentan, a dual endothelin receptor antagonist. Clin Pharmacokinet. 2004;43:1089–115. doi: 10.2165/00003088-200443150-00003. [DOI] [PubMed] [Google Scholar]
- 4.Fattinger K, Funk C, Pantze M, et al. The endothelin antagonist bosentan inhibits the canalicular bile sale export pump: A potential mechanism for hepatic adverse reactions. Clin Pharmacol Thera. 2001;69:223–31. doi: 10.1067/mcp.2001.114667. [DOI] [PubMed] [Google Scholar]
- 5.Lexi-Comp Online Atorvastatin drug monograph. <http://online.lexi.com/crlsql/servlet/crlonline>. (Version current at May 1, 2008).
- 6.Stancu C, Sima A. Statins: Mechanism of action and effects. J Cell Mol Med. 2001;5:378–8. doi: 10.1111/j.1582-4934.2001.tb00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dingemanse J, Schaarschmidt D, van Giersbergen PL. Investigation of the mutual pharmacokinetic interactions between bosentan, a dual endothelin receptor antagonist, and simvastatin. Clin Pharmacokinet. 2003;42:293–301. doi: 10.2165/00003088-200342030-00004. [DOI] [PubMed] [Google Scholar]
- 8.Galiè N, Olschewski H, Oudiz RJ, et al. Ambrisentan for the treatment of pulmonary arterial hypertension: Results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. 2008;117:3010–19. doi: 10.1161/CIRCULATIONAHA.107.742510. [DOI] [PubMed] [Google Scholar]