INTRODUCTION
Ninety percent of the estimated 2.2 million children infected with human immunodeficiency virus type 1 (HIV-1) worldwide live in sub-Saharan Africa (UNAIDS). This population of children has increased morbidity and mortality, the causes of which are largely infectious in nature and often vaccine preventable. HIV-1-infected children have greater rates of diarrhea, pneumonia, and measles than HIV-1-uninfected children1–3, and increased severity of disease, particularly measles4–6.
Many of the diseases affecting HIV-1-infected children are preventable with immunization, but HIV-1-infected children have decreased response rates to vaccines, including measles and tetanus4,7–9. Seroconversion rates of healthy, HIV-1 uninfected children for both measles and tetanus vaccine are greater than 90%10,11. In comparison, initial response rates to measles and tetanus vaccination among HIV-1-infected children are reported to range between 25%–88% and 60%–100%, respectively11–13. In addition to inadequate immune responses following vaccination, HIV-1-infected children may lose responses over time as they progress to AIDS. Measles and tetanus antibody responses post-vaccination have been shown to wane more rapidly among HIV-1-infected than uninfected children, with fewer than 50% maintaining protective levels 2 years after vaccine administration8,11,14.
In non-African cohorts, HAART and re-vaccination appear to be successful strategies for boosting immunity against measles and tetanus15–17, but current WHO guidelines do not include repeat pediatric immunization following HAART12,18. Lack of effective measles and tetanus immunization strategies among HIV-1-infected children may result in a growing disease-susceptible population which could have important public health implications19. Half of all measles-related deaths and the majority of the world’s tetanus burden are in sub-Saharan Africa, the region with the highest HIV/AIDS burden20. Measles deaths account for up to 45% of all vaccine preventable deaths in Africa, and measles directly or indirectly accounts for 20% of child mortality among children younger than 5 years in Kenya21.
In this study we determined immunity against measles and tetanus in a cohort of previously vaccinated HIV-1-infected Kenyan children before initiating HAART. We also assessed the impact of HAART on measles and tetanus IgG antibody responses before and after re-vaccination and defined correlates of immunity at baseline and during follow-up.
METHODS
Study subjects
Children enrolled into this study were participating in a longitudinal study of response to HAART among newly diagnosed HIV-1-infected children in Nairobi, Kenya22. Children and their guardians were referred from the pediatric wards at Kenyatta National Hospital (KNH) and the outpatient Comprehensive HIV Care Clinic from August 2004 through November 2005. Trained nurse counselors obtained written informed consent from guardians and written assent for participation from children older than 7 years. The study received ethical approval from the Institutional Review Boards of the University of Washington and the University of Nairobi. All children had positive HIV-1 enzyme-linked immunosorbent assays (ELISAs), were naïve to HAART at the time of enrollment, and had symptomatic HIV-1 disease (WHO stage III or IV, or CD4% <15%), as previously described22.
Clinical follow-up and specimen collection
At enrollment, childhood immunization history was obtained using immunization cards presented by the care provider or by verbal report from the care provider if a child’s card was not available. Blood was drawn for hematology, liver function, T-cell subsets, HIV-1 RNA viral load, measles and tetanus IgG titers. Children meeting eligibility criteria were then initiated on a HAART regimen according to the Kenyan national guidelines and monitored during monthly clinic visits. First line treatment included a non-nucleoside reverse transcriptase inhibitor (NNRTI), either nevirapine or efavirenz, and two of the following nucleoside reverse transcriptase inhibitors (NRTIs): stavudine, lamivudine, or zidovudine. After 6 and 9 months of HAART, the laboratory tests described above were repeated.
Re-vaccination against measles and/or tetanus was performed 8 months after HAART initiation for those children who were measles or tetanus antibody negative at 6 months and had immune reconstitution with a CD4 percent ≥15%. Two weeks and one month after re-vaccination additional blood was obtained to measure measles IgM and measles and tetanus IgG, respectively.
Laboratory assays
CD4 T cell subsets were measured with flow cytometry (FacsCount, Becton Dickenson, USA) and HIV-1 RNA viral load was quantified using the Gen-Probe viral load assay (San Francisco, USA), a transcription mediated amplification method sensitive for detection of HIV-1 subtypes A, C, and D23.
Measles IgG and IgM antibody status were determined using commercial ELISA kits (Calbiotech, Inc., Germany), as previously described24. All samples, as well as the standard, were diluted 1:20 in sample diluent and 3-fold serial dilutions were carried out in 96-well round bottomed plates following this initial dilution. Once samples were added to antigen coated plates contained in the kits, the manufacturer’s protocol was followed and the antibody index for each sample was calculated by multiplying the optical density value by the calibrator factor for the kit. The antibody index was interpreted as follows: negative <0.9; borderline 0.9 – 1.1; positive >1.1. When determining need for re-vaccination, both negative and borderline indices were considered negative.
Anti-tetanus antibody titers were measured with an in-house ELISA using tetanus antigen and an anti-tetanus IgG standard obtained from the World Health Organization (WHO). Briefly, 96-well flat bottom plates (Immulon 2HB, Thermo Scientific, USA) were coated with tetanus antigen at a concentration of 1 µg/mL and incubated overnight at 4°C. Serial dilutions of participant serum (initially diluted 1:50) or 2-fold dilutions of tetanus IgG standard were added to coated plates. Plates were incubated with horseradish peroxidase-conjugated antibody (Southern Biotech, USA) and after additional washing, TMB substrate was added. All plates were read on an Emax Precision microplate reader (Molecular Devices, USA) and data were acquired using Softmax software (Molecular Devices Corp., USA). A tetanus antibody concentration >0.01 IU/ml was considered protective and defined as a positive result.
Statistical analyses
Height and weight measurements were converted into z-scores using the Nutristat software program (EPI info-version 3.2, CDC Atlanta, USA). This software sets a z-score of 0 to correspond with the median score for height-for-age, weight-for-age, and weight-for-height ratios. A score of −1 or −2 represents 1 and 2 standard deviations below the median, respectively. HIV-1 RNA viral load values were log10 transformed. McNemar’s test was used for paired comparisons of antibody status at enrollment and after 6 months on HAART. Factors were compared between children with and without positive measles and tetanus antibody assays at baseline using independent t tests. To determine whether age, baseline HIV-1 viral load, CD4 percent, z-scores, or change in these were associated with conversion to antibody positivity after HAART, we restricted analyses to children who were initially antibody negative and those who developed antibody after 6 months of HAART were compared to those who remained antibody-negative using independent t tests. For all analyses, borderline measles results were classified as negative; to define sensitivity of the results, analyses were repeated classifying borderline measles as positive.
RESULTS
Cohort characteristics
Ninety HIV-1-infected children in the cohort had been vaccinated against both measles and tetanus during their first year of life, 24 (27%) of whom had vaccination cards to confirm immunization history. Median age at enrollment was 4.9 years (interquartile range [IQR] 2.6, 6.5) and 47 (52%) were female. Median weight-for-age z-score was −2.9 (IQR −4.8,−1.8) and median height-for-age z-score −2.3 (IQR −3.6,−1.4), indicating that more than half the children were >2 standard deviations below average for these measurements. Median CD4 percent was 6.3% (IQR 3.0, 10.6) and median plasma HIV-1 viral load was 6.0 log10 copies/ml (IQR 5.5–6.5). Sixty-two (69%) of enrolled children had been hospitalized during the preceding 6 months, 36 (40%) had been diagnosed with tuberculosis, 7 (8%) had history of meningitis, 2 (2%) had a history of malignancy, and 52 (58%) had history of pneumonia. Most (71%) of the 90 children completed 9 months of follow-up. Among those with incomplete follow-up, 11 (12%) died before 9 months and 15 (17%) were lost to follow-up. Age, gender, CD4%, HIV-1 viral load, and z-scores were not different for those with incomplete follow-up compared to those remaining in the study through 9 months.
Measles and tetanus IgG antibody at baseline
Thirty (33%) of the 90 children had positive measles IgG results at enrollment, 52 (58%) had negative measles antibody, and 8 (9%) had a borderline result. Seventy (78%) among the 90 children had a positive tetanus IgG at enrollment and 20 (22%) were negative.
Correlates of positive baseline antibody responses were determined for both measles and tetanus, and borderline measles results were considered negative in these analyses (Table 1). The child’s age and CD4 percentage at enrollment were not associated with having a positive measles IgG antibody test. There was a trend for infants with measles antibody at baseline to have a lower viral load (p=0.09). Median HIV-1 RNA viral load among children with IgG antibody against measles was 5.8 log10 copies/ml compared with a viral load of 6.0 log10 copies/ml among those without measles antibody. We found no significant associations between measles IgG results and clinical factors, such as weight-for-age z-score or height-for-age anthropometric z-score. The tetanus IgG result was not associated with age, laboratory, or clinical characteristics of children at baseline (Table 1). However, there was significant correlation between tetanus and measles IgG results (Pearson’s correlation coefficient=0.317; p=0.002).
TABLE 1.
Correlates of Measles and Tetanus Antibody Seropositivity for 90 HIV-1-Infected Infants at Enrollment and After 6 Months on HAART
| Baseline Characteristics |
Measles Antibody Status | Tetanus Antibody Status | ||||
|---|---|---|---|---|---|---|
| Negative* | Positive | P | Negative | Positive | P | |
|
(n = 60) |
(n = 30) |
(n = 20) |
(n = 70) |
|||
| Age at enrollment (yrs) |
4.7 | 5.4 | 0.33 | 4.8 | 5.0 | 0.84 |
| CD4% | 9.3 | 8.4 | 0.70 | 8.0 | 9.3 | 0.65 |
| HIV-1 viral load (log10 copies/mL) |
6.0 | 5.8 | 0.09 | 6.1 | 5.9 | 0.35 |
| Weight for age z-score |
−3.8 | −3.6 | 0.91 | −3.0 | −3.9 | 0.50 |
| Height for age z-score |
−2.6 | −2.6 | 0.85 | −2.4 | −2.6 | 0.55 |
| After 6 Months of HAART†‡ | ||||||
| Baseline Characteristics |
Persistently Negative or Borderline† |
Seroconverted Antibody Positive† |
P | Persistently Negative‡ |
Seroconverted Antibody Positive‡ |
P |
|
(n = 26) |
(n = 17) |
(n = 10) |
(n = 3) |
|||
| Age (yrs) | 4.5 | 4.8 | 0.75 | 3.7 | 5.5 | 0.03 |
| CD4% | 9.9 | 10.4 | 0.91 | 11.6 | 4.7 | 0.3 |
| HIV-1 RNA viral load (log10 copies/mL) |
6.3 | 5.8 | 0.057 | 6.0 | 6.3 | 0.5 |
| Weight for age z-score |
−3.1 | −5.3 | 0.30 | −2.1 | −2.5 | 0.7 |
| Height for age z-score |
−2.9 | −2.4 | 0.35 | −1.9 | −3.0 | 0.25 |
| Follow-up Characteristics | ||||||
| CD4% at 6 mos |
14.2 | 20.7 | 0.087 | 14.2 | 17.2 | 0.51 |
| HIV-1 RNA viral load at 6 mos (log10 copies/mL) |
2.51 | 2.02 | 0.38 | 2.57 | 3.64 | 0.44 |
| Weight for age z-score at 6 mos |
−1.84 | −1.63 | 0.61 | −0.97 | −1.25 | 0.72 |
| Height for age z-score at 6 mos |
−2.45 | −2.20 | 0.58 | −1.65 | −2.18 | 0.64 |
| Change in Characteristics Between Baseline and 6 Months | ||||||
| CD4% | 4.76 | 10.33 | 0.22 | 1.27 | 10.9 | 0.084 |
| HIV-1 RNA viral load (log10 copies/mL) |
3.31 | 4.28 | 0.14 | 3.13 | 2.97 | 0.92 |
| Weight for age z-score |
1.03 | 3.87 | 0.21 | 0.10 | 1.36 | 0.90 |
| Height for age z-score |
0.45 | 0.06 | 0.086 | 0.32 | 0.26 | 0.51 |
Clinically borderline measles IgG results were included as negative results.
Among 60 children who were measles IgG negative at enrollment, 43 completed 6 mo of follow-up; 17 died or were lost to follow-up prior to study completion.
Among 20 children who were tetanus IgG negative at enrollment, 13 completed 6 mo of follow-up; 7 died or were lost to follow-up prior to study completion.
Measles and tetanus antibody status following six months of HAART
Sixty-two (69%) of the 90 children completed 6 months of HAART and had repeat measles and tetanus antibody results. Overall, 26 (42%) children were measles IgG positive at the 6-month visit and 37 (59%) were tetanus IgG positive. Median CD4 percentage increase was 9.5% and median 6-month CD4 percent was 15.8% (IQR 8.8, 20.0) at 6 months. The median HIV-1 viral load at follow-up was 2.3 log10 copies/ml, representing a decrease of 3.7 log10 copies/ml following 6 months of HAART.
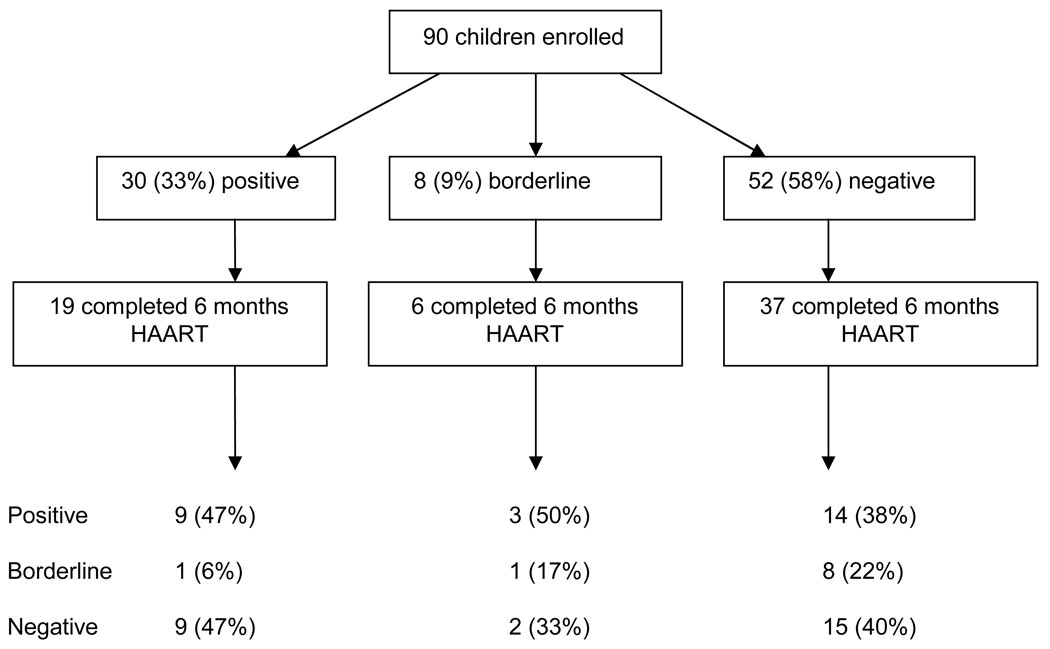
Of the 62 children, 37 (60%) were measles IgG negative and 6 (10%) had borderline results at baseline (Figure 1). Seventeen (40%) among these 43 (negative plus borderline) developed a positive IgG response post-HAART. However, we also observed a loss of antibody response among those initially positive. Ten (53%) of 19 children with baseline positive responses were antibody negative at 6 months (p=0.25). When comparing children who lost responses with those who remained measles IgG positive during follow-up, there was a significant difference in baseline measles antibody concentrations (7.1 versus 20.3 mg/ml; p=0.003). No consistent pattern characterized children with borderline responses followed for 6 months: 3 converted to a positive response, 1 remained borderline, and 2 reverted to negative.
Figure 1.
Enrollment and measles antibody status of study participants during follow-up
Among children who were measles IgG negative at baseline, there was a trend for lower baseline HIV-1 RNA viral loads to be associated with seroconversion to positive measles IgG antibody status after 6 months of HAART (p=0.057). At baseline, median viral load was 0.5 log10 copies/ml lower among children with positive responses after HAART than in those who remained measles IgG negative (Table 1). Children with responses tended to have higher CD4% after taking 6 months of HAART than those remaining antibody negative (20.7 versus 14.2; p=0.09). In addition, there was a trend for seroconverting children to have greater change in their height-for-age z-scores (p=0.09). Viral load at the 6-month follow-up visit was not associated with seroconversion (2.02 and 2.51 log10 copies/ml for seroconverters and non-converters, respectively; p=0.4)(Table 1). Seroreversion from a positive to negative response was not associated with any of these factors.
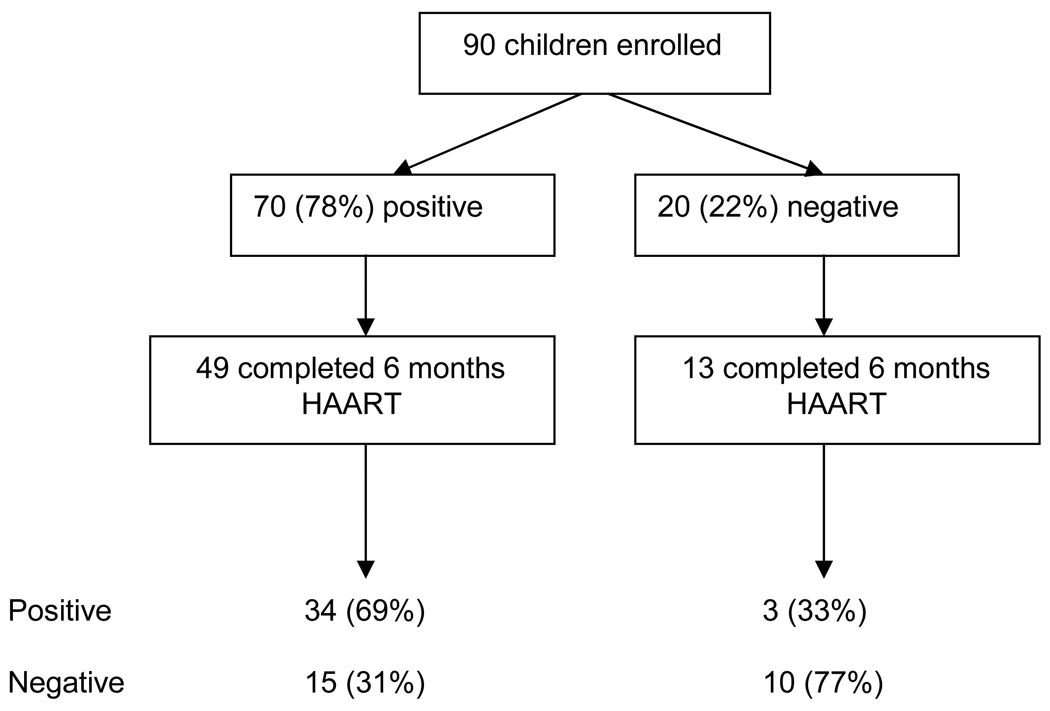
Tetanus antibody status at enrollment and during follow-up is diagrammed in Figure 2. Among 62 children followed for 6 months on HAART, 13 (21%) were antibody negative at baseline (Figure 2). Ten (77%) of these children remained tetanus antibody negative and 3 (13%) became tetanus antibody positive. For the 49 children with positive baseline responses, 34 (69%) remained antibody positive and 15 (31%) reverted to a negative antibody status, resulting in a significant difference in status (p=0.008). Median age for children converting to antibody positivity was 5.5 years compared to 3.7 years for those remaining antibody negative (p=0.03). There was a trend for the change in CD4 percent from enrollment to month 6 to be greater for children who converted to tetanus positive status (10.9 versus 1.3% change for seroconverters and non-converters, respectively; p=0.08). HIV-1 viral load and growth z-scores were no different among those who did and did not seroconvert. The 15 children who reverted from positive to negative tetanus IgG tended to be younger (4.1 versus 5.6 years at enrollment; p=0.098). This was not the case for 10 children reverting from positive to negative measles IgG and there was no association between tetanus and measles reversion in this cohort. Tetanus IgG concentration was also no different for children reverting compared to children remaining antibody positive (0.54 versus 0.43 IU/ml; p=0.30)
Figure 2.
Enrollment and tetanus antibody status of study participants during follow-up
Post-HAART response to measles and tetanus immunization
Eighteen children were re-immunized against measles and 24 were re-immunized against tetanus. Among those not immunized against measles were 17 (47%) who did not meet criteria for measles immunization because their CD4 percent remained <15% and 3 children who were lost to follow-up between 6 and 9 months. Median CD4 percent for children re-vaccinated against measles was 20% (IQR 17.1, 25.4) and for children re-vaccinated against tetanus it was 16% (IQR 9.7, 20.3). Overall, positive responses were observed in 14 (78%) of 18 children re-immunized against measles and 18 (75%) children re-immunized against tetanus. Eleven of the 18 had measles IgM assays performed on specimens obtained 2 weeks after re-vaccination and none was positive. There were no adverse reactions to re-vaccination.
DISCUSSION
In this prospective study, only one-third of antiretroviral-naïve HIV-1-infected Kenyan children with a history of prior measles vaccination had protective titers of measles antibody, a rate comparable to that reported in some studies and lower in others4,12,13. One explanation for low rates of measles seropositivity despite prior vaccination is that children in this cohort had advanced HIV disease, with a median CD4 percent of 6.3% and median HIV-1 viral load of 1,000,000 copies/ml. All children were infected via mother-to-child HIV-1 transmission and had progressed to clinical AIDS before enrollment into the study. Recruitment into this study involved identification of clinically ill children, thus our results reflect typical pediatric HIV clinic attendees who may be sicker than infants identified via prevention of mother-to-child transmission of HIV-1 programs.
Lack of measles antibody for children with advanced HIV disease may be because of failed induction after initial vaccination or of loss of measles-specific antibody responses over time with progressive HIV disease. Other studies would suggest that it is a combination of these two factors11. We did not observe measles-specific IgM responses 2 weeks following re-vaccination, suggesting children had previously been exposed to measles. In addition, approximately 40% of children who were measles antibody negative at enrollment converted to measles antibody positive status after immune reconstitution, which would be consistent with prior immunity. However, 60% of children did not convert after HAART and a large proportion (53%) of children who were initially measles IgG positive reverted to antibody negative status during the 6-month follow-up period. For tetanus, change in status from positive to negative was statistically significant, with 15 (31%) of 49 losing tetanus antibody on HAART. Other studies have also found defective humoral immunity among children on HAART and possible explanations include slow functional recovery of B-cell memory and a shorter life-span of plasma cells in HIV-infected children25. Regardless of the underlying mechanism, HAART alone was not sufficient to restore immune responses in this study and re-vaccination was necessary for a significant number of children.
There may be significant public health implications of these findings. As a result of the decreased ability of HIV-1-infected children to generate and maintain protective vaccine-induced immunity, a growing number of vulnerable children may compromise the World Health Organization’s Expanded Program on Immunizations. In past years, increased vaccine failure among HIV-1-infected children would have been offset by high mortality in this population. However, with the increased accessibility of HAART around the world, and in sub-Saharan Africa in particular, HIV-1 infected children will have extended life expectancies, and could potentially have a greater impact on measles epidemics. This makes it even more important to assess the impact of HAART on immunity against routine childhood immunizations in order to develop appropriate immunization strategies to optimize vaccination.
In contrast to measles immune responses which were present in a minority of children at baseline, the majority (>75%) had positive tetanus IgG antibody titers at enrollment. This is consistent with data showing that cellular immune compromise adversely impacts measles but not tetanus vaccine responses26. While the humoral response is of paramount importance for vaccine-induced protection against both measles and tetanus, cellular immunity is critically important for mounting a measles vaccine response26, as demonstrated by studies among immunosuppressed individuals vaccinated against measles showing compromised and more transient responses9. The fact that tetanus vaccine is first administered during the first 2 months of life whereas measles vaccine is administered at 9 months of age may also contribute to the differential responses. More children are infected with HIV-1 by 9 months, with many experiencing severe immune dysfunction that may not have been present earlier.
All children demonstrated immune reconstitution following 6 months of HAART, with increased CD4 percentage, decreased viral loads, and improved z-score measurements. However, no clinical or immunologic markers were found to be significantly associated with either baseline or 6-month vaccine immune status, or with change in measles antibody status. There was a trend for children with lower baseline HIV-1 viral load to be more likely to be measles antibody positive at baseline and to seroconvert to measles antibody positive status after 6 months of HAART. Children converting from negative to positive for measles antibody had a baseline HIV-1 viral load that was approximately one-half log10 lower than children who remained antibody negative. It would therefore be difficult to identify children receiving HAART who would be more or less likely to require repeat immunization in the absence of antibody testing. Programs would need to re-vaccinate all children with immune reconstitution meeting CD4% criteria or measure measles antibody concentrations and only re-immunize those with low negative or borderline titers, as was done in this study, estimating that approximately 75% of these children would seroconvert to measles and tetanus antibody positive status following re-vaccination. Empiric re-vaccination may be more cost-effective and provide longer term benefits to programs. Notably, WHO policies do not include recommendations for either approach. This study provides further evidence for the need for such recommendations.
In conclusion, these data suggest that the WHO recommended vaccination schedules for HIV-1 infected children in developing countries may be inadequate. We found no significant increase in measles or tetanus responses post-HAART, and observed waning responses for 15 children who were initially tetanus antibody positive and later antibody negative and 10 children who were initially measles IgG positive. However, response to re-vaccination post-HAART was excellent. Additional boosters after immune reconstitution in regions where HAART is available appear to be necessary to achieve adequate immune responses. With increased access and use of HAART in pediatric populations, re-vaccination may have great benefit at the individual and community level for measles, tetanus and potentially other vaccine-preventable disease.
ACKNOWLEDGMENTS
Research was funded by the NIH Fogarty International Center D43 TW000007, RO1 TW007632, Puget Sound Partners for Global Health Research and Technology Project Award #26145, the UW CFAR Humoral Immunity Core, and NIH P30 AI27757. D. Wamalwa was a scholar in the AIDS International Training and Research Program supported by the NIH Fogarty International Center grant D43 TW00007. C. Farquhar was supported by NICHD K23 HD41879 and S. Selig was a fellow in the NIH Fogarty Clinical Research Scholars Program. G. John-Stewart was an Elizabeth Glaser Pediatric AIDS Foundation (EGPAF) Scientist and D. Mbori-Ngacha had an EGPAF Leadership Award. Antiretroviral drugs were provided by the US Presidents Emergency Program for AIDS Relief (PEPFAR).
Footnotes
The authors have no associations that pose a conflict of interest.
REFERENCES
- 1.Obimbo EM, Mbori-Ngacha DA, Ochieng JO, et al. Predictors of early mortality in a cohort of human immunodeficiency virus type 1-infected african children. Pediatr Infect Dis J. 2004;23(6):536–543. doi: 10.1097/01.inf.0000129692.42964.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taha TE, Graham SM, Kumwenda NI, et al. Morbidity among human immunodeficiency virus-1-infected and -uninfected African children. Pediatrics. 2000;106(6):E77. doi: 10.1542/peds.106.6.e77. [DOI] [PubMed] [Google Scholar]
- 3.Zijenah L, Mbizvo MT, Kasule J, et al. Mortality in the first 2 years among infants born to human immunodeficiency virus-infected women in Harare, Zimbabwe. J Infect Dis. 1998;178(1):109–113. doi: 10.1086/515604. [DOI] [PubMed] [Google Scholar]
- 4.Krasinski K, Borkowsky W. Measles and measles immunity in children infected with human immunodeficiency virus. JAMA. 1989;261(17):2512–2516. [PubMed] [Google Scholar]
- 5.Sension MG, Quinn TC, Markowitz LE, et al. Measles in hospitalized African children with human immunodeficiency virus. Am J Dis Child. 1988;142(12):1271–1272. doi: 10.1001/archpedi.1988.02150120025021. [DOI] [PubMed] [Google Scholar]
- 6.Mbori-Ngacha D, Nduati R, John G, et al. Morbidity and mortality in breastfed and formula-fed infants of HIV-1-infected women: A randomized clinical trial. JAMA. 2001;286(19):2413–2420. doi: 10.1001/jama.286.19.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernstein CN, Rawsthorne P, Blanchard JF. Population-based case-control study of measles, mumps, and rubella and inflammatory bowel disease. Inflamm Bowel Dis. 2007;13(6):759–762. doi: 10.1002/ibd.20089. [DOI] [PubMed] [Google Scholar]
- 8.Brena AE, Cooper ER, Cabral HJ, Pelton SI. Antibody response to measles and rubella vaccine by children with HIV infection. J Acquir Immune Defic Syndr. 1993;6(10):1125–1129. [PubMed] [Google Scholar]
- 9.Rudy BJ, Rutstein RM, Pinto-Martin J. Responses to measles immunization in children infected with human immunodeficiency virus. J Pediatr. 1994;125(1):72–74. doi: 10.1016/s0022-3476(94)70125-3. [DOI] [PubMed] [Google Scholar]
- 10.Krugman S, Muriel G, Fontana VJ. Combined live measles, mumps, rubella vaccine. Immunological response. Am J Dis Child. 1971;121(5):380–381. doi: 10.1001/archpedi.1971.02100160050003. [DOI] [PubMed] [Google Scholar]
- 11.Moss WJ, Scott S, Mugala N, et al. Immunogenicity of standard-titer measles vaccine in HIV-1-infected and uninfected Zambian children: an observational study. J Infect Dis. 2007;196(3):347–355. doi: 10.1086/519169. [DOI] [PubMed] [Google Scholar]
- 12.Moss WJ, Clements CJ, Halsey NA. Immunization of children at risk of infection with human immunodeficiency virus. Bull World Health Organ. 2003;81(1):61–70. [PMC free article] [PubMed] [Google Scholar]
- 13.Palumbo P, Hoyt L, Demasio K, Oleske J, Connor E. Population-based study of measles and measles immunization in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 1992;11(12):1008–1014. doi: 10.1097/00006454-199211120-00004. [DOI] [PubMed] [Google Scholar]
- 14.Ching N, Deville JG, Nielsen KA, et al. Cellular and humoral immune responses to a tetanus toxoid booster in perinatally HIV-1-infected children and adolescents receiving highly active antiretroviral therapy (HAART) Eur J Pediatr. 2007;166(1):51–56. doi: 10.1007/s00431-006-0184-2. [DOI] [PubMed] [Google Scholar]
- 15.Aurpibul L, Puthanakit T, Sirisanthana T, Sirisanthana V. Response to measles, mumps, and rubella revaccination in HIV-infected children with immune recovery after highly active antiretroviral therapy. Clin Infect Dis. 2007;45(5):637–642. doi: 10.1086/520651. [DOI] [PubMed] [Google Scholar]
- 16.Berkelhamer S, Borock E, Elsen C, Englund J, Johnson D. Effect of highly active antiretroviral therapy on the serological response to additional measles vaccinations in human immunodeficiency virus-infected children. Clin Infect Dis. 2001;32(7):1090–1094. doi: 10.1086/319591. [DOI] [PubMed] [Google Scholar]
- 17.Melvin AJ, Mohan KM. Response to immunization with measles, tetanus, and Haemophilus influenzae type b vaccines in children who have human immunodeficiency virus type 1 infection and are treated with highly active antiretroviral therapy. Pediatrics. 2003;111(6 Pt 1):e641–e644. doi: 10.1542/peds.111.6.e641. [DOI] [PubMed] [Google Scholar]
- 18.WHO. Weekly Epidemiologic Record: Measles Vaccines. 2004;14:132–142. [Google Scholar]
- 19.Moss WJ, Cutts F, Griffin DE. Implications of the human immunodeficiency virus epidemic for control and eradication of measles. Clin Infect Dis. 1999;29(1):106–112. doi: 10.1086/520136. [DOI] [PubMed] [Google Scholar]
- 20.UNAIDS. AIDS Epidemic Update: December 2007. Geneva: 2007. http://data.unaids.org/pub/EPISlides/2007/2007epiupdateen.pdf. [Google Scholar]
- 21.Siringi S. Largest ever measles vaccination programme launched in Africa. Lancet. 2002;359(9324):2175. doi: 10.1016/S0140-6736(02)09193-6. [DOI] [PubMed] [Google Scholar]
- 22.Wamalwa DC, Farquhar C, Obimbo EM, et al. Early response to highly active antiretroviral therapy in HIV-1-infected Kenyan children. J Acquir Immune Defic Syndr. 2007;45(3):311–317. doi: 10.1097/QAI.0b013e318042d613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emery S, Bodrug S, Richardson BA, et al. Evaluation of performance of the Gen-Probe human immunodeficiency virus type 1 viral load assay using primary subtype A, C, and D isolates from Kenya. J Clin Microbiol. 2000;38(7):2688–2695. doi: 10.1128/jcm.38.7.2688-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farquhar C, Nduati R, Haigwood N, et al. High maternal HIV-1 viral load during pregnancy is associated with reduced placental transfer of measles IgG antibody. J Acquir Immune Defic Syndr. 2005;40(4):494–497. doi: 10.1097/01.qai.0000168179.68781.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bekker V, Scherpbier H, Pajkrt D, Jurriaans S, Zaaijer H, Kuijpers TW. Persistent humoral immune defect in highly active antiretroviral therapy-treated children with HIV-1 infection: loss of specific antibodies against attenuated vaccine strains and natural viral infection. Pediatrics. 2006;118(2):e315–e322. doi: 10.1542/peds.2005-2616. [DOI] [PubMed] [Google Scholar]
- 26.Plotkin S. Immunologic correlates of protection induced by vaccination. Pediatr Infect Dis J. 2001;20(1):63–75. doi: 10.1097/00006454-200101000-00013. [DOI] [PubMed] [Google Scholar]