Abstract
Several studies have indicated a strong association between asthma and aspiration of stomach contents. However, the complex association between these inflammatory processes has not been studied extensively in animal models. In the present study, we developed an animal model to evaluate the inflammatory cell, chemokine, and airway responses to asthma complicated by aspiration. The model was produced by sensitizing mice to cockroach allergens from house-dust extracts. Mice with asthma-like airway responses then were inoculated intratracheally with either an acidic solution or saline. Acid aspiration increased airway hyperresponsiveness in mice with asthma for at least 8 h. After 6 h, the combined injury caused an additive, not synergistic, increase in airway hyperresponsiveness and neutrophil recruitment to the airways. Although cysteinyl leukotrienes in bronchoalveolar lavage fluid were higher after acid aspiration, treatment with a receptor antagonist before aspiration did not diminish airway hyperresponsiveness. Vagal mechanisms reportedly mediate airway responses in acid aspiration; however, pretreatment with an anticholinergic agent did not reduce airway responses to acid. These results are consistent with an effective model of the acute effects of aspiration on the allergic lung. Further studies could examine how various forms of aspiration influence the severity of asthma.
Abbreviations: BAL, bronchoalveolar lavage; MIP, macrophage inflammatory protein; Penh, enhanced pause
Asthma is an escalating public health problem in children and adults.49 In patients with asthma, exaggerated immune responses to allergens produce lung inflammation and dysfunction. These responses lead to the characteristic airway hyperresponsiveness, obstructed airflow, and clinical symptoms associated with asthma.49 Although several conditions aggravate asthma, much recent attention has focused on the provocative association between asthma and aspiration of stomach acid. The prevalence of gastroesophageal reflux in some asthma patient populations is greater than 50% 21 and significantly exceeds the prevalence in nonasthmatic populations.20,47 This finding suggests an association between the 2 diseases and the possibility that gastroesophageal reflux promotes or aggravates symptoms that lead to the diagnosis asthma. In fact, several studies have shown a decrease in asthma symptoms after medical or surgical treatment of gastroesophageal reflux.4,11,18,19
Stomach acid may exacerbate asthma symptoms through 2 mechanisms. The first is a vagal reflex initiated in response to acid in the esophagus. Clinical studies in humans20,50 and experimental studies in animals34,48 have shown that acid in the esophagus promotes neurologic responses leading to bronchoconstriction and impaired airway function. Esophageal acid also may cause substance P- mediated neurogenic inflammation.16 The second mechanism is due to aspiration into the airways, which also has been documented to occur in asthma patients.25 The presence of acid in the trachea increases airway hyperresponsiveness in anesthetized animals through vagal mechanisms,48 particularly in the presence of preexisting lung inflammation.32 In addition to neurologic responses, aspiration of acid induces a pattern of pulmonary inflammation characterized by the release of proinflammatory cytokines and neutrophil recruitment.26,31 That inflammation may also increase airway responsiveness.6
Well-established models for both asthma6,10,14,27 and aspiration31,39 studies are available currently. However, the patterns of inflammation that occur after sequential insults are complex and may not be predicted by studies of the responses to individual insults.8 In addition, the mechanisms for exacerbation of airway hyperresponsiveness by aspiration in asthma have been limited to use of anesthetized animals. A model that allows recovery from the anesthesia after delivery of the aspirate permits the development and evaluation of pulmonary changes under more physiologic conditions. Therefore, the goals of this study were to: (1) describe acute exacerbation of asthma by acid aspiration in mice after recovery from anesthesia; (2) determine the effects of combined insults on airway hyperresponsiveness; and (3) profile the cellular and cytokine responses to the combined insults to assess potential mechanisms for the pulmonary responses to asthma complicated by aspiration.
Materials and Methods
Study design.
Asthma was induced according to an established protocol by using an extract containing cockroach allergens (Figure 1). After completion of the protocol, asthma-like responses were confirmed by measuring airway hyperresponsiveness by whole-body plethysmography and methacholine challenge. Groups of mice then received either saline or an acidic solution intratracheally either 24 or 48 h after the last allergen challenge (day 22 or 23, respectively; Figure 1). The effects of the intratracheal inoculation on pulmonary inflammation and airway hyperresponsiveness were assessed at various time points. Blood and bronchoalveolar lavage (BAL) fluid were obtained from some animals at 6 h after aspiration for analysis of cell counts and cytokine concentrations. In additional experiments, animals were pretreated with either montelukast or an anticholinergic agent, atropine, to examine their effects on asthma associated with aspiration.
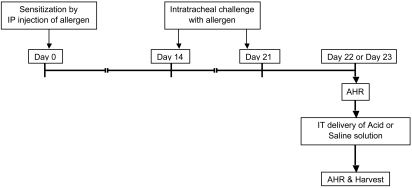
Figure 1.
Experimental design for induction of asthma followed by aspiration. Asthma was induced in mice through sensitization by intraperitoneal injection of an extract containing cockroach allergens (day 0) followed by intratracheal challenge (days 14 and 21) with the same allergen. Either 24 or 48 h (day 22 or 23) after the final challenge, airway responses were assessed by whole-body plethysmography to confirm asthma. Aspiration then was induced through intratracheal delivery of either saline or acid solution. Induction of airway hyperresponsiveness (AHR) was repeated, and mice were euthanized at various time points.
Animals.
Female BALB/c mice (18 to 20 g) were obtained from Harlan Sprague–Dawley (Indianapolis, IN). The mice were free of the following agents: minute virus of mice, mouse adenovirus types 1 and 2, cytomegalovirus, mouse hepatitis virus, mouse parvovirus, mouse polyoma virus, mouse rotavirus, mouse thymic virus murine norovirus, pneumonia virus of mice, respiratory enteric virus III, Sendai virus, Theiler murine encephalomyelitis virus, Ectromelia virus, Hantaan virus, K virus, lactic dehydrogenase elevating virus, lymphocytic choriomeningitis virus, cilia-associated respiratory bacillus, Clostridium piliforme, Corynebacterium kutscheri, Helicobacter spp., Mycoplasma pulmonis, Pasteurella pneumotropica, Salmonella spp., Streptobacillus moniliformis, Streptococcus pneumoniae, endo- and ectoparasites, dermatophytes, and Encephalitozoon cuniculi.
The mice were housed in a temperature-controlled room with a 12:12-h dark:light cycle and had ad libitum access to food and water. The University Committee on Use and Care of Animals approved all of the experiments.
Intratracheal inoculation.
Allergen challenges and acid aspiration were administered through the oropharyngeal route. Mice were anesthetized with 5% isoflurane delivered in a drop jar and then suspended vertically by the upper incisors on a board with a slight incline. The tongue was pulled gently from the mouth, and a pipette was used to deliver the fluid to the oropharynx. With the tongue held extended, the normal respiratory movements of the animal draw the fluid down the trachea and into the lung. In previous work, we have confirmed the diffuse dispersion of the fluid into the lower airways.40 Animals are fully ambulatory within 1 to 2 min after delivery of fluid.
Allergy protocol.
The allergy model we used was described previously.29,30,35 The allergens were extracted from house dust containing high levels of the cockroach antigens Blattella germanica 1 (Bla g1) and 2 (Bla g2) as confirmed by ELISA.29,30,35 Mice were sensitized (day 0) by intraperitoneal injection of 50 µL of the extract emulsified in 50 µL TiterMax Gold (CytRx Corporation, Norcross, GA). On days 14 and 21 after initial sensitization, the mice received challenge doses of extract (50 µL) intratracheally, as described earlier.
Aspiration protocol.
Mice were anesthetized with isoflurane for intratracheal administration of either saline or an acidic solution (volume, 35 µL) by the oropharyngeal route. The acid solution consisted of diluted saline (1:3, v/v) titrated to a pH of 1.15 with hydrochloric acid. Solutions of this pH were used in our previous studies of aspiration lung injury and, when delivered in aliquots of 80 µL, have produced consistent lung inflammation.41 The aspiration of acid or saline was performed either 24 or 48 h after the last allergen challenge.
Measurement of airway hyperreactivity.
Airway responses were evaluated after induction of asthma but before aspiration and were reassessed at various time points after aspiration. Airway reactivity was assessed by measuring responses to increasing doses of aerosolized acetyl β-methylcholine (Sigma, St Louis, MO) by using a 4-chamber, whole-body plethysmograph (Buxco, Troy, NY). Each chamber was equipped with a pneumotachograph (Halcyon™, Buxco) to measure flow and transmit this information to analysis software (Biosystem XA for Windows, Buxco). The software uses various algorithms to calculate several flow-derived parameters, including respiratory rate, lung volume, peak flow, and time intervals. The software reported data as ‘enhanced pause’ (Penh), an index of airway hyperreactivity17 that is derived from the equation:
Mice were placed individually into each of the 4 chambers and allowed to acclimate for 5 min. Then, a baseline recording period was initiated, with measurements and parameters recorded every second for 5 min. After baseline measurements, increasing concentrations of methacholine in PBS (0, 6.3, 12.5, 25.0, and 50.0 mg/mL) were nebulized through the inlet of the main chamber. Each dose was nebulized for 2 min and airway responses recorded for 5 min. The percentage increase in Penh over baseline Penh was calculated for each of the concentrations of methacholine, and these values were used to compare results among experimental groups.
Treatment with leukotriene receptor antagonist.
Previous studies have demonstrated that treatment with montelukast before induction of asthma reduces airway responsiveness and inflammation.10 However, the effect of leukotriene inhibition on the exacerbation of asthma by acid aspiration is unknown. Therefore, the asthma protocol was repeated in 2 groups of mice. Immediately after baseline recording of airway response, mice were anesthetized with isoflurane, and an 18-gauge oral gavage needle (Instech, Plymouth Meeting, PA) was passed into the stomach to administer either montelukast (10 mg/kg, Singular, Merck, Whitehouse Station, NJ) in PBS or an equal volume (100 μL) of PBS. This dosage of montelukast was effective in reducing airway hyperresponsiveness and inflammation in our model of asthma.27 At 1 h after montelukast treatment, the acidic solution was given intratracheally to both groups. All of the animals were euthanized after respiratory function testing 6 h later.
Treatment with anticholinergic agent.
Mice with airway hyperresponsiveness received subcutaneous injections of 0.05 mg/kg atropine sulfate (American Pharmaceutical Partners, Shaumburg, IL), a recommended preanesthetic dose for rodents;22 aspiration followed 30 min later. The atropine dose was repeated 2 h later. Control animals received injections of PBS, and all of the animals were euthanized 6 h after intratracheal inoculation.
Euthanasia and sample harvest.
Each mouse was anesthetized with intraperitoneal ketamine (87 mg/kg; Ketaset, Fort Dodge Laboratories, Fort Dodge, IA) and xylazine (13 mg/kg; Rompun, Bayer Corporation, Shawnee Mission, KS). Mice then underwent exsanguination through cardiocentesis with a heparin-washed syringe, followed by cervical dislocation. A ventral midline incision was made on the neck, with an opening in the trachea. A blunt, 26-gauge needle covered with polyethylene tubing was inserted in the trachea. A ligature was tied around the trachea to prevent leakage. The needle was connected to a stopcock fitted with two 3-mL syringes. Two separate 1-mL volumes of warm Hanks Balanced Salt Solution (without Ca2Cl, Mg2S04, or phenol red; Gibco, Grand Island, NJ) were injected into the trachea and recovered. After BAL, the thorax was opened, and the right ventricle was perfused with 2 mL saline. The lungs were removed and placed in buffered formalin.
Analysis of BAL samples.
The 1-mL samples were centrifuged (600 × g, 3 min), and the supernatant from the first sample retrieved from each mouse was stored at –20 °C for cytokine analysis. The cell pellets from both samples were pooled for total counts (model Z1, Beckman-Coulter, Miami, FL) after lysis of erythrocytes (Zap-Oglobin II, Beckman-Coulter, Fullerton, CA). Slides were loaded with 1 × 105 cells, centrifuged (700xg, 3 min), and stained with Diff-Quick (Baxter, Detroit, MI). Differentials (300 cells) were counted under light microscopy.
Histology.
The lung samples were fixed in 10% buffered formalin, embedded, sectioned, and stained with hematoxylin and eosin. Each section was evaluated under light microscopy by a blinded observer.
Cytokine measurements.
Cytokines in plasma (1:10 dilution) and lung lavage fluid (1:2 dilution) were measured by using ‘sandwich ELISAs.’ Matched pairs (biotinylated and nonbiotinylated) of antimurine antibodies against keratinocyte-derived chemokine, macrophage inflammatory protein (MIP)2α, and monocyte chemotactic protein 1with their recombinant proteins (R and D Systems, Minneapolis, MN) were used in methods previously described by this laboratory.42 Peroxidase-conjugated streptavidin (Jackson ImmunoResearch Laboratories, West Grove, PA) and the color reagent 3,3′,5,5′ tetramethyl benzidine TMB were used as the detection system. The reaction was stopped with 1.5 N sulfuric acid, and absorbance was read at 465 and 590 nm.
Leukotrienes in BAL fluid (1:2 dilution) were evaluated by using an enzyme immunoassay (Cysteinyl Leukotriene EIA Kit, Cayman Chemical, Ann Arbor, MI). Absorbance was read at 405 nm.
Statistics.
Summary statistics were expressed as mean ± SEM. Multiple groups were analyzed by ANOVA, and differences (P < 0.05) were compared post hoc by the Tukey multiple comparison method. When 2 groups were evaluated, a Student t test was performed. These analyses were performed using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego California USA).
Results
General observations.
Over the 3 wk between initial sensitization and the final intratracheal challenge of the allergy protocol, the mice were monitored for any abnormalities in weight, activity, or general condition. No abnormalities were noted. The animals showed no overt signs of respiratory disease. After completion of the allergy protocol, asthma-like disease was confirmed in all animals by methacholine challenge on the day of aspiration (either day 22 or 23). The responses in all mice were consistent with those reported previously in this model of asthma.28-30,35,36 Typically, increasing concentrations of methacholine (0 to 50 mg/mL) resulted in incremental increases in mean Penh. When expressed as a percentage increase over baseline reading, Penh reached values of 200% or more at high concentrations of methacholine. Aspiration of saline or acid solution (35 µL) through the oropharyngeal route appeared to increase the respiratory rate transiently. However, all of the mice remained active, and none demonstrated overt signs of distress (hunched posture, lethargy, isolation, and so on). Respiratory abnormalities were not noticeable 6 h later, at the completion of the experiment.
Effects of aspiration on airway hyperresponsiveness.
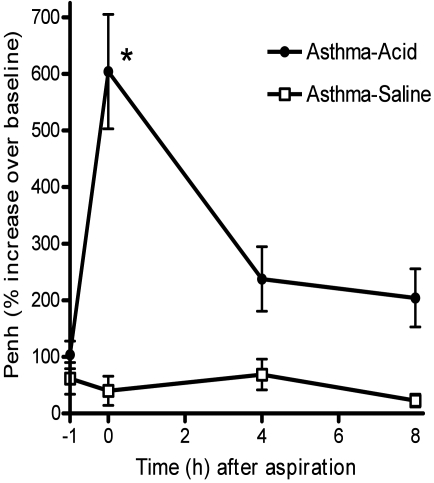
For an initial study of airway hyperresponsiveness, mice underwent the allergy protocol (n = 4 per group) followed by aspiration (saline or acid solution) at 48 h after the last allergen challenge. Airway responses to methacholine (12.5 mg/mL) were measured at 0, 4, and 8 h after aspiration (Figure 2). At 1 h before aspiration, both groups demonstrated asthma-like increases in airway hyperresponsiveness consistent with previously studies.29,30,35 Immediately after recovery from anesthesia, mice were evaluated for airway responses. These responses increased significantly (P < 0.05) in mice given the acidic solution intratracheally as compared with those given saline. Although the responses that occurred immediately after intratracheal acid decreased within 4 h, the effects of the acid were increased (P = 0.057) compared with those of saline for as long as 8 h after aspiration. Previous studies indicate that the inflammatory responses produced by aspiration tend to peak at 6 to 8 h.13,26,39 Based on these results, we used a 6-h time point after aspiration for subsequent investigation of both inflammation and airway responses.
Figure 2.
Duration of airway hyperresponsiveness after aspiration. For a pilot study, mice with asthma were given either saline (asthma–saline, n = 4) or an acid solution (asthma–acid, n = 4) intratracheally. At various time points after aspiration, respiratory function was measured by whole-body plethysmography. Penh, an indicator of airway responsiveness, was recorded for baseline readings followed by recording during methacholine (12.5 mg/mL) challenge. Responses were expressed as the percentage increase in Penh (relative to baseline) in response to methacholine challenge. Values are presented as mean ± SEM (n = 4 per group). Increased airway responses were readily apparent in the asthma–acid group throughout the 8 h of the study. *, P < 0.05 as compared to asthma-saline.
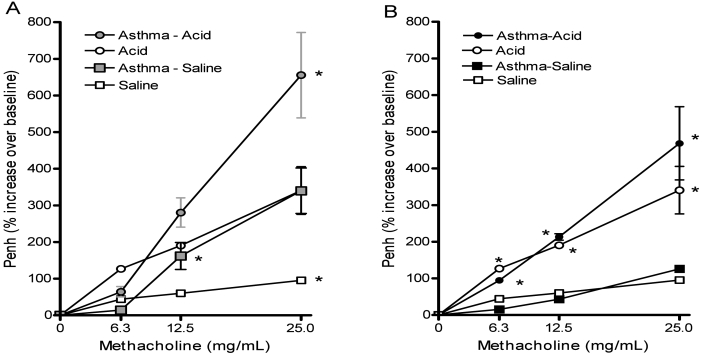
Airway responsiveness was assessed further through complete methacholine dose–response curves performed 6 h after aspiration (saline or acid) in animals with or without asthma. Aspiration was performed either 24 or 48 h after the last challenge of the allergy protocol. Compared with intratracheal saline, acid alone significantly (P < 0.05) increased airway responses to methacholine (Figure 3 A and B). When induction of allergy preceded acid aspiration, responses were further increased (P < 0.05). Mean airway hyperreponsiveness increased by 48% when the 2 insults were given 24 h apart and by 27% when 48 h apart. At both intervals, the combined effect of the insults appeared to be additive—the insults did not appear to have a synergistic effect on airway responses. Both intervals allowed comparison between groups with acid aspiration and their respective controls. However, the differences between the asthma–saline and saline groups were more evident when the insults were 24 h apart as compared with the 48-h interval. Although the initial intent was to use methacholine concentrations to 50 mg/mL, preliminary testing demonstrated escalating respiratory distress (reduced rate with increased effort) accompanied by respiratory secretions in animals from both the acid and asthma–acid groups at this highest dose. The distress was most noticeable at the 24-h interval, but also occurred in some mice at the 48-h interval. Although the mice recovered, this effect led to our decision to use the highest dose of methacholine only to confirm induction of asthma before aspiration. Testing was stopped if mice developed respiratory rates below 100 breaths per minute for more than 1 min or appeared to be in distress at any of the methacholine concentrations.
Figure 3.
Effects of aspiration on airway hyperresponsiveness. Mice with asthma were inoculated intratracheally with either saline (asthma–saline) or an acid solution (asthma–acid). The allergy protocol was completed (A) 24 h or (B) 48 h before the aspiration insult. These groups were compared with mice without asthma after aspiration of identical solutions (acid or saline). At 6 h after aspiration, airway responses to methacholine challenges of increasing concentrations were assessed by whole-body plethysmography and expressed as the percentage increase in Penh over baseline. The combined insults induced an additive increase in airway responsiveness. Values are presented as mean ± SEM (n = 10 to 12 per group). *, P < 0.05 compared with the respective control group at the indicated concentration of methacholine.
Effects of acid aspiration on airway inflammation.
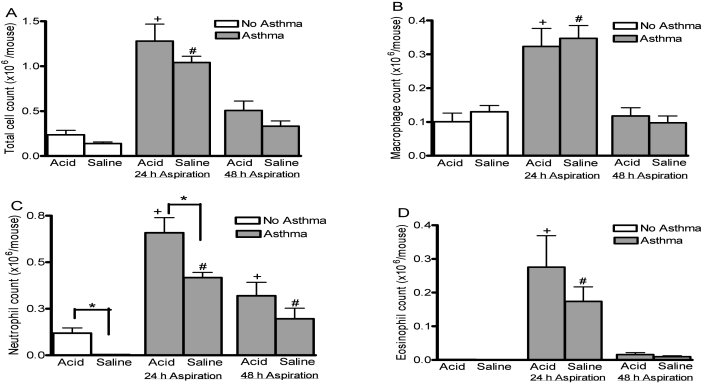
Mice given intratracheal acid alone did not show a significant change in total BAL fluid cell counts (Figure 4 A). but had a significant increase (P < 0.01) in neutrophil counts as compared to those given saline (Figure 4 C). In mice with preexisting asthma and saline innoculation, total cell counts (Figure 4 A) including macrophages, neutrophils and eosinophils (Figure 4 B through D) tended to be increased when compared to mice without asthma.. The asthma-associated increases in macrophages, neutrophils, and eosinophils were particularly evident (P < 0.001, P < 0.001, P < 0.01, respectively) when the intratracheal inoculation was given 24 h after the final allergen challenge. Intratracheal instillation of the acidic solution in animals with preexisting asthma resulted in significant increases (P < 0.05) of neutrophil counts as compared with saline when aspiration occurred at 24 h. A significant increase in the neutrophil counts did not occur when the acid insult was delivered 48 h after allergen challenge. Therefore, both insults to the lung (asthma and acid) appeared to influence the neutrophil counts, and the combined insults resulted in an additive increase in BAL neutrophils. However, by the asthma insult affected macrophage and eosinophil counts.
Figure 4.
Numbers of cells in BAL fluid after aspiration. Mice with asthma (gray bars) were given either saline or acid solution intratracheally at either 24 or 48 h after completion of the allergy protocol. These groups were compared with mice without asthma (white bars) after aspiration of identical solutions. At 6 h after aspiration, BAL fluid was retrieved for counting of (A) total cell number, (B) macrophages, (C) neutrophils, and (D) eosinophils. The combined insults caused a significant (P < 0.05) increase in neutrophil counts. Values are presented as mean ± SEM (n = 12 per group). *, P < 0.05; +, P < 0.05 as compared with all acid groups; #, P < 0.05 as compared with all saline groups.
The initial profiles of airway and inflammatory responses indicated that delivery of the asthma and acid insults at either a 24- or 48-h interval would create significant changes and could represent different aspects of asthma. However, we chose the model with 24 h between the last allergen challenge and aspiration insults for further study. This interval delivers the aspiration at a more active inflammatory phase of the asthma model.
Histology.
The asthmatic animals with aspiration of saline demonstrated lesions similar to those previously described in this model.28,30 A marked accumulation of inflammatory cells was evident in the peribronchial and perivascular spaces. The cell populations appeared to be primarily lymphocytes and eosinophils, with scattered neutrophils in these concentrated areas and occasional neutrophils in the alveolar walls. Animals in the asthma–acid groups yielded histological sections that were not distinguishable from those of the asthma–saline groups.
Chemokine concentrations.
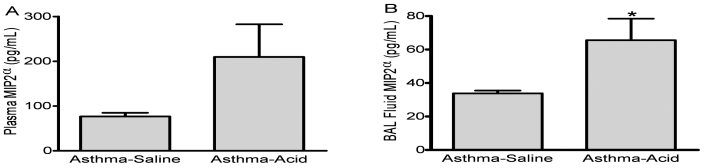
To profile potential mediators of the differences in airway inflammation, we used ELISA to measure concentrations of BAL fluid and plasma chemokines. The mean plasma level of MIP2α was greater in the asthma–acid group (209.7 ± 80.0 pg/mL) than in the asthma–saline group (76.8 ± 8.3 pg/mL), although the difference was not statistically significant (Figure 5 A). In the BAL fluid, MIP2α levels were significantly higher (P < 0.05) in the asthma–acid group compared with the asthma–saline group (Figure 5 B). Levels of keratinocyte-derived chemokine and monocyte chemotactic protein 1 for both the asthma–saline and asthma–acid groups were below the limit of detection in plasma and BAL fluid (28.0 and 50 pg/mL, respectively).
Figure 5.
Concentration of macrophage inhibitory protein 2α (MIP2α) after aspiration. The MIP2α levels in plasma (A) and BAL fluid (B) were measured by ELISA 6 h after asthmatic mice were inoculated intratracheally with either saline (asthma–saline) or an acid solution (asthma–acid). Aspiration of acid significantly (*, P < 0.05) increased the concentration of this chemokine. Values are presented as mean ± SEM (n = 10 to 12 per group).
Effect of leukotrienes after aspiration.
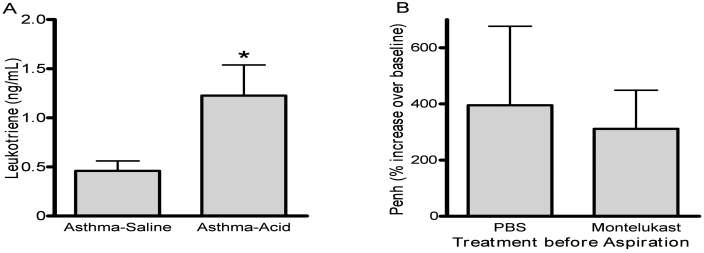
Levels of cysteinyl leukotrienes in BAL fluid were assayed and were increased (P < 0.05) in the asthma–acid versus the asthma–saline group (Figure 6 A). To further investigate whether the leukotrienes were responsible for increases in airway responsiveness, we pretreated mice with a leukotriene receptor antagonist (10 mg/kg montelukast) before aspiration of the acidic solution. Six hours after aspiration, airway responses to methacholine (0 to 25 mg/mL) did not differ from those of mice pretreated with PBS. We performed a follow-up experiment, in which mice with asthma (n = 4 per group) were given a doubled dose of leukotriene receptor (20 mg/kg) before aspiration. Antagonist pretreatment did not alter airway responses to 25 mg/mL of methacholine as compared with those of PBS controls (Figure 6 B).
Figure 6.
Leukotriene concentrations in BAL fluid after aspiration. (A) Levels of cysteinyl-leukotrienes were measured in BAL fluid harvested 6 h after asthmatic mice were inoculated intratracheally with either saline (asthma–saline) or an acid solution (asthma–acid). Aspiration of acid significantly (*, P < 0.05) increased leukotriene concentrations. (B) Asthmatic animals were treated with a leukotriene receptor antagonist (montelukast, 20 mg/kg) or a control (PBS) before aspiration of an acid solution. After aspiration, airway responses at baseline and after 25 mg/mL methacholine were measured by whole-body plethysmography. Pretreatment did not significantly affect airway responses. Values are expressed as mean ± SEM (n = 10 to 12 per group).
Anticholinergic treatment.
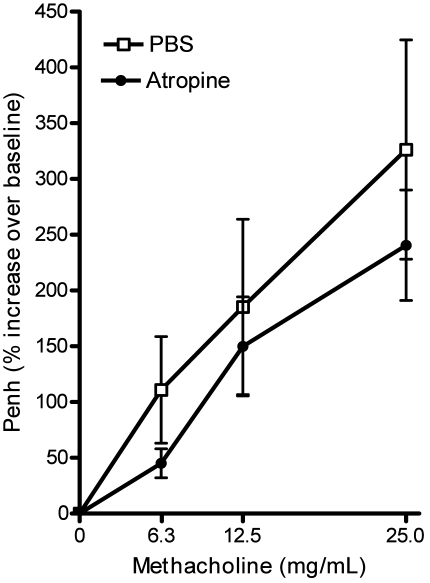
Because vagal nerve responses are implicated in airway responses to acid aspiration,20,34,48,50 we treated a group of mice with the anticholinergic agent atropine. Although the mean values for percentage increase in Penh of the atropine-treated mice were lower than those in the PBS-treated mice, there were no statistically significant differences between the groups at any methocholine dose (Figure 7).
Figure 7.
Airway hyperresponsiveness after anticholinergic treatment. Mice with confirmed airway hyperresponsiveness were treated with PBS or atropine before microaspiration of an acidic solution. Pretreatment with the anticholinergic agent atropine did not alter airway responses significantly. Values are expressed as mean ± SEM (n = 8 to 12 per group).
Discussion
The relationship of aspiration with exacerbation of asthma is of considerable interests, yet few models are available to study the effects of the combined lung insults. Studies of anesthetized dogs23 and guinea pigs7,16,44 have demonstrated increased bronchoconstriction and plasma extravasation into airways in response to either esophageal or tracheal deposition of acid. In addition, in anesthetized guinea pigs, bronchiolar hyperresponsiveness is exacerbated by preexisting lung inflammation induced by sensitization to ovalbumin.32 The use of anesthetized animals allows direct stimulation or transection of the vagal nerve, establishing a neurologic mechanism for the acute airway responses to aspiration.7,16,44 In a chronic model of gastroesophageal reflux, ovalbumin-sensitized mice were anesthetized and given multiple, 10-µL volumes of acid by endotracheal tube over 8 wk.2 This model demonstrated the tendency toward a Th2 cytokine profile and significant lung pathology in response to chronic aspiration.2 However, lung pathology did not differ between sensitized and unsensitized animals after exposure to acid.2 Because the mice did not have overt lung pathology throughout the chronic course,2 the effects of preexisting inflammation on acute responses to aspiration could not be examined.
The model presented here differs importantly from other models of asthma complicated by aspiration and may offer advantages. Unlike earlier studies on normal lung or ovalbumin allergy,32 the current study examined the effects of acid aspiration with asthma induced by clinically relevant (that is, cockroach) allergens. Our previous work30 has shown that the allergy protocol we used here produces lung pathology including peribronchiolar inflammatory infiltrates and airway hyperresponsiveness. Furthermore, in contrast to studies in guinea pigs, our mice recovered from anesthesia, thereby allowing pulmonary changes to develop under more physiologic conditions with regard to blood pressure and temperature regulation. In addition, the oropharyngeal route of administration eliminates the need for intubation or tracheal cut-down procedures. Our previous work40 demonstrated good distribution of solutions to the lower airways by using this method. Therefore, the procedures described yield a relatively noninvasive model to study the effects of aspiration on lungs affected by preexisting inflammation associated with asthma.
Using this model, we characterized the effects of the combined lung insults on airway responses and lung inflammation. Our studies revealed that the effects of the combined injury on both the airway and inflammatory parameters were additive rather than synergistic. These responses were more robust if aspiration occurred within 24 h, rather than 48 h, of allergen challenge, consistent with greater inflammation associated with the asthma insult at the 24-h time point. In effect, these pronounced inflammatory responses were primarily a background consequence of the initial, allergy insult. The combined injury significantly increased concentrations of the CXC chemokine macrophage inhibitory protein 2α and cysteinyl leukotrienes in the BAL fluids, suggesting that these compounds play a role in mediating the increase in the inflammatory responses produced by aspiration.
The airway hyperresponsiveness after acid aspiration may be related to vagal nerve stimulation. Studies in anesthetized animals have shown that acid in the esophagus can trigger neurogenic inflammation16 and bronchoconstriction, 48 yet acid in the larynx or trachea elicits a greater response.24,32,48 In response to microaspiration, tracheal irritant receptors are believed to initiate reflex bronchoconstriction,5 a response that is heightened by previous sensitization to allergens and pulmonary inflammation.32 Because vagotomy can abolish bronchoconstriction in response to tracheal acid, the effect appears to occur through a vagal afferent pathway.48 In addition, exposure to allergens heightens vagal nerve responses to electrical stimuli.7,44 The current study shows that the bronchoconstriction seen in awake, asthmatic animals after aspiration of acid is negligibly attenuated by routinely recommended doses of atropine. However, our findings do not completely rule out a vagal nerve mechanism. First, the atropine may not have provided a complete blockade of cholinergic pathways. Second, a study performed in anesthetized guinea pigs demonstrated that atropine-pretreatment did not completely abolish the increase in pulmonary insufflation pressures when nonadrenergic, noncholinergic vagal nerves were stimulated.44 Our preliminary findings lend further support that these noncholinergic pathways are important to acid-induced airway hyperresponsiveness in the awake animal. However, further studies are necessary before definitive conclusions are possible.
We further investigated the potential contribution of cysteinyl leukotrienes in the aspiration component of the combined injury. The role of cysteinyl leukotrienes, particularly LTC4 and LTD4, in asthma is well established.10 These compounds mediate many of the clinical signs of asthma including bronchoconstriction, airway hyperresponsiveness, vascular permeability, mucus secretion, and proliferation of smooth muscle. In fact, LTC4 and LTD4 are 100 times more potent in producing bronchoconstriction than is histamine, an important mediator of asthma.43 Studies in guinea pigs have shown that LTD4 mediates bronchoconstriction and plasma exudation to some extent through the stimulation of C fibers and release of tachykinins.23 In a murine model of asthma, inhibition of cysteinyl leukotrienes by treatment with the receptor antagonist montelukast before antigen challenge reduced airway eosinophilia and decreased airway hyperresponsiveness.10 In addition, cysteinyl leukotriene antagonists inhibit bronchoconstriction induced by direct vagal nerve stimulation.9 Because vagal nerve stimulation has also been associated with bronchoconstriction after aspiration, we attempted to inhibit the effects of aspiration by using montelukast. Although the dosage we used is effective in a murine asthma model,10,27 it did not block the effects of acid in our study. These findings contrast with the conclusion that cysteinyl leukotriene antagonists inhibit the bronchoconstriction induced by direct vagal nerve stimulation.9 However, the earlier evidence was derived from a guinea pig model, and the findings may be species-specific given the lack of similar findings in humans.43 Our preliminary results in mice suggest that, although cysteinyl leukotrienes have a known role in asthma and are increased significantly after acid aspiration, they do not have a key mechanistic role in the airway hyperresponsiveness after combined injury.
As demonstrated, the current model of asthma and aspiration has produced results that warrant further investigation. However, additional factors must be considered when extrapolating the results of aspiration studies in mice to the effects of aspiration in humans. The amount of inflammation and lung injury caused by acid aspiration is dependent on several factors including the pH, volume, composition, and frequency of the aspirate.26 We chose a solution with an acidic pH to induce lung injury in our model; however, many patients with diagnosed gastroesophageal reflux may aspirate fluid with a relatively high pH due to treatment with agents to reduce the acidity of stomach contents. In addition, stomach contents may contain particulate material that produces a more pronounced pathology involving different mediators. With regard to volume, experimental animal models generally require a larger volume of aspirate to cause lung injury, as compared with humans. We performed preliminary studies using 50 µL of the acidic solution (approximately 2.5 mL/kg), a volume known to readily distribute throughout the lung after oropharyngeal inoculation.40 This volume resulted in extreme airway hyperresponsiveness that prompted the use of a smaller volume, 35 µL, which made methacholine challenge and respiratory function testing a possibility. Although still considered a large volume in relation to body size when compared with that aspirated by patients with gastroesophageal reflux, this volume (1.75 mL/kg) allowed the study of quantifiable airway inflammation with minimal distress to the animals..2 Using an even smaller and therefore more clinically representative volume of fluid may be possible. A murine model of chronic aspiration was developed by repeated administration of 10-µL volumes of gastric acid by gavage. Compared with our current model, the cited model used a level clinically relevant allergen, ovalbumin, to induce asthma and did not produce chronic lung inflammation. Recently, a wide range of mouse models of chronic allergic asthma have been developed and reviewed.14,38,46 Such models could provide the background for studies of the effect of chronic aspiration on preexisting lung inflammation. Therefore, detailed studies of asthma and aspiration may require several iterations of the model described here depending on the goals of the investigations.
Another consideration of the current study is the use of Penh, a unitless parameter derived mathematically from the respiratory waveform produced by whole-body plethysmography. Specifically, the expiratory phase of respiration is the source of several parameters used to calculate Penh as a function of peak flow rates and expiratory time. Although not a direct measure of resistance, Penh correlates with airway resistance17 and indicates that changes in resistance have occurred. Because of the availability of Penh, whole-body plethysmography allows noninvasive and repeated evaluation of airway hyperresponsiveness in unrestrained, alert mice. However, using Penh to evaluate airway resistance is controversial.12,15,33,37 A key concern in this controversy is that Penh is influenced not only by changes in pulmonary mechanics but also breathing patterns.1,3,45 In addition, whole-body plethysmography might be modified by factors that are not directly related to bronchoconstriction, such as movement, humidity, and temperature as well as upper airway resistance.15 Some studies have concluded that the relationship of Penh to airway resistance is most accurate under uniform conditions of temperature and humidity.37 Despite these concerns, many investigators identify a correlative relationship between Penh and airway resistance.12 We have used Penh to indicate changes in airway responsiveness in several asthma studies.28-30,35,36 In the current studies, we conducted plethysmography on equal numbers of mice from each treatment group on each day of testing. In addition, our use of a multichamber plethysmograph allowed simultaneous testing of subjects from each group. Although these additional efforts may help reduce artifacts related to environmental conditions, investigators still reluctant to use Penh as an indicator of airway resistance would require an invasive method for airway evaluation. Because the allergy protocol we present here induced reliable and repeatable changes in airway responses, the initial assessment of airway responses after induction of allergy but before aspiration of acid is not mandatory. This situation might circumvent the need for repeated assessment of pulmonary function in some studies.
In conclusion, the prevalence of asthma with concurrent aspiration is high, but the pathophysiology of the combined conditions is not well understood. We developed a murine model of asthma exacerbated by acid aspiration. The model essentially combines 2 well-established, relatively noninvasive protocols that lead to lung inflammation in unanesthetized animals. Our preliminary studies using this model suggest that the combination of asthma and aspiration stimulates additive effects on lung inflammation and airway constriction. Although aspiration may be mediated by the vagal nerve, our results suggest that neither a leukotriene receptor antagonist nor an anticholinergic agent would be of much value in treatment of acute bronchoconstriction. Further studies are necessary to fully understand the complex mechanisms that mediate combined asthma and aspiration.
Acknowledgment
This work was supported in part by the National Institutes of Health (grant P01 GM067189).
References
- 1.Adler A, Cieslewicz G, Irvin CG. 2004. Unrestrained plethysmography is an unreliable measure of airway responsiveness in BALB/c and C57BL/6 mice. J Appl Physiol 97:286–292 [DOI] [PubMed] [Google Scholar]
- 2.Barbas AS, Downing TE, Balsara KR, Tan HE, Rubinstein GJ, Holzknecht ZE, Collins BH, Parker W, Davis RD, Lin SS. 2008. Chronic aspiration shifts the immune response from Th1 to Th2 in a murine model of asthma. Eur J Clin Invest 38:596–602 [DOI] [PubMed] [Google Scholar]
- 3.Bates J, Irvin C, Brusasco V, Drazen J, Fredberg J, Loring S, Eidelman D, Ludwig M, Macklem P, Martin J, Milic-Emili J, Hantos Z, Hyatt R, Lai-Fook S, Leff A, Solway J, Lutchen K, Suki B, Mitzner W, Pare P, Pride N, Sly P. 2004. The use and misuse of Penh in animal models of lung disease. Am J Respir Cell Mol Biol 31:373–374 [DOI] [PubMed] [Google Scholar]
- 4.Bowrey DJ, Peters JH, DeMeester TR. 2000. Gastroesophageal reflux disease in asthma: effects of medical and surgical antireflux therapy on asthma control. Ann Surg 231:161–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coleridge HM, Coleridge JC. 1994. Pulmonary reflexes: neural mechanisms of pulmonary defense. Annu Rev Physiol 56:69–91 [DOI] [PubMed] [Google Scholar]
- 6.Colombo JL, Hallberg TK. 2000. Airway reactivity following repeated milk aspiration in rabbits. Pediatr Pulmonol 29:113–119 [DOI] [PubMed] [Google Scholar]
- 7.Costello RW, Fryer AD, Belmonte KE, Jacoby DB. 1998. Effects of tachykinin NK1 receptor antagonists on vagal hyperreactivity and neuronal M2 muscarinic receptor function in antigen-challenged guinea pigs. Br J Pharmacol 124:267–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deitch EA. 1992. Multiple organ failure. Pathophysiology and potential future therapy. Ann Surg 216:117–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellis JL, Undem BJ. 1991. Role of peptidoleukotrienes in capsaicin-sensitive sensory fibre-mediated responses in guinea-pig airways. J Physiol 436:469–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eum SY, Maghni K, Hamid Q, Campbell H, Eidelman DH, Martin JG. 2003. Involvement of the cysteinyl-leukotrienes in allergen-induced airway eosinophilia and hyperresponsiveness in the mouse. Am J Respir Cell Mol Biol 28:25–32 [DOI] [PubMed] [Google Scholar]
- 11.Field SK, Sutherland LR. 1998. Does medical antireflux therapy improve asthma in asthmatics with gastroesophageal reflux? A critical review of the literature. Chest 114:275–283 [DOI] [PubMed] [Google Scholar]
- 12.Finkelman FD. 2008. Use of unrestrained, single-chamber barometric plethysmography to evaluate sensitivity to cholinergic stimulation in mouse models of allergic airway disease. J Allergy Clin Immunol 121:334–335 [DOI] [PubMed] [Google Scholar]
- 13.Folkesson HG, Matthay MA, Hebert CA, Broaddus VC. 1995. Acid aspiration-induced lung injury in rabbits is mediated by interleukin-8-dependent mechanisms. J Clin Invest 96:107–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuchs B, Braun A. 2008. Improved mouse models of allergy and allergic asthma—chances beyond ovalbumin. Curr Drug Targets 9:495–502 [DOI] [PubMed] [Google Scholar]
- 15.Glaab T, Taube C, Braun A, Mitzner W. 2007. Invasive and noninvasive methods for studying pulmonary function in mice. Respir Res 8:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamamoto J, Kohrogi H, Kawano O, Iwagoe H, Fujii K, Hirata N, Ando M. 1997. Esophageal stimulation by hydrochloric acid causes neurogenic inflammation in the airways in guinea pigs. J Appl Physiol 82:738–745 [DOI] [PubMed] [Google Scholar]
- 17.Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG, Gelfand EW. 1997. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med 156:766–775 [DOI] [PubMed] [Google Scholar]
- 18.Harding SM. 2005. Gastroesophageal reflux: a potential asthma trigger. Immunol Allergy Clin North Am 25:131–148 [DOI] [PubMed] [Google Scholar]
- 19.Harding SM, Richter JE, Guzzo MR, Schan CA, Alexander RW, Bradley LA. 1996. Asthma and gastroesophageal reflux: acid-suppressive therapy improves asthma outcome. Am J Med 100:395–405 [DOI] [PubMed] [Google Scholar]
- 20.Harding SM, Schan CA, Guzzo MR, Alexander RW, Bradley LA, Richter JE. 1995. Gastroesophageal reflux-induced bronchoconstriction: is microaspiration a factor? Chest 108:1220–1227 [DOI] [PubMed] [Google Scholar]
- 21.Havemann BD, Henderson CA, El-Serag HB. 2007. The association between gastro-oesophageal reflux disease and asthma: a systematic review. Gut 56:1654–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hawk C, Leary S. 1999. Formulary for laboratory animals. Ames (IA): Iowa State University Press [Google Scholar]
- 23.Ishikawa J, Ichinose M, Miura M, Kageyama N, Yamauchi H, Tomaki M, Sasaki Y, Shirato K. 1996. Involvement of endogenous tachykinins in LTD4-induced airway responses. Eur Respir J 9:486–492 [DOI] [PubMed] [Google Scholar]
- 24.Ishikawa T, Sekizawa SI, Sant'Ambrogio FB, Sant'Ambrogio G. 1999. Larynx versus esophagus as reflexogenic sites for acid-induced bronchoconstriction in dogs. J Appl Physiol 86:1226–1230 [DOI] [PubMed] [Google Scholar]
- 25.Jack CI, Calverley PM, Donnelly RJ, Tran J, Russell G, Hind CR, Evans CC. 1995. Simultaneous tracheal and oesophageal pH measurements in asthmatic patients with gastro-oesophageal reflux. Thorax 50:201–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kennedy TP, Johnson KJ, Kunkel RG, Ward PA, Knight PR, Finch JS. 1989. Acute acid aspiration lung injury in the rat: biphasic pathogenesis. Anesth Analg 69:87–92 [PubMed] [Google Scholar]
- 27.Kim J, McKinley L, Bolgos GL, Siddiqui J, Remick DG. 2004. Montelukast treatment reduced pulmonary inflammation and airway hyperresponsiveness in a murine model of asthma induced by house dust containing high levels of cockroach allergens. Am J Respir Crit Care Med 169S:A803 [Google Scholar]
- 28.Kim J, McKinley L, Natarajan S, Bolgos GL, Siddiqui J, Copeland S, Remick DG. 2006. Antitumor necrosis factor α antibody treatment reduces pulmonary inflammation and methacholine hyper-responsiveness in a murine asthma model induced by house dust. Clin Exp Allergy 36:122–132 [DOI] [PubMed] [Google Scholar]
- 29.Kim J, McKinley L, Siddiqui J, Bolgos GL, Remick DG. 2004. Prevention and reversal of pulmonary inflammation and airway hyperresponsiveness by dexamethasone treatment in a murine model of asthma induced by house dust. Am J Physiol Lung Cell Mol Physiol 287:L503–L509 [DOI] [PubMed] [Google Scholar]
- 30.Kim J, Merry AC, Nemzek JA, Bolgos GL, Siddiqui J, Remick DG. 2001. Eotaxin represents the principal eosinophil chemoattractant in a novel murine asthma model induced by house dust containing cockroach allergens. J Immunol 167:2808–2815 [DOI] [PubMed] [Google Scholar]
- 31.Knight PR, Druskovich G, Tait AR, Johnson KJ. 1992. The role of neutrophils, oxidants, and proteases in the pathogenesis of acid pulmonary injury. Anesthesiology 77:772–778 [DOI] [PubMed] [Google Scholar]
- 32.Lopes FD, Alvarenga GS, Quiles R, Dorna MB, Vieira JE, Dolhnikoff M, Martins MA. 2002. Pulmonary responses to tracheal or esophageal acidification in guinea pigs with airway inflammation. J Appl Physiol 93:842–847 [DOI] [PubMed] [Google Scholar]
- 33.Lundblad LK, Irvin CG, Adler A, Bates JH. 2002. A reevaluation of the validity of unrestrained plethysmography in mice. J Appl Physiol 93:1198–1207 [DOI] [PubMed] [Google Scholar]
- 34.Mansfield LE, Hameister HH, Spaulding HS, Smith NJ, Glab N. 1981. The role of the vagus nerve in airway narrowing caused by intraesophageal hydrochloric acid provocation and esophageal distention. Ann Allergy 47:431–434 [PubMed] [Google Scholar]
- 35.McKinley L, Kim J, Bolgos GL, Siddiqui J, Remick DG. 2004. Reproducibility of a novel model of murine asthma-like pulmonary inflammation. Clin Exp Immunol 136:224–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKinley L, Kim J, Bolgos GL, Siddiqui J, Remick DG. 2006. Allergens induce enhanced bronchoconstriction and leukotriene production in C5 deficient mice. Respir Res 7:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitzner W, Tankersley C. 2003. Interpreting Penh in mice. J Appl Physiol 94:828–831 [DOI] [PubMed] [Google Scholar]
- 38.Munitz A, Bachelet I, Levi-Schaffer F. 2006. Reversal of airway inflammation and remodeling in asthma by a bispecific antibody fragment linking CCR3 to CD300a. J Allergy Clin Immunol 118:1082–1089 [DOI] [PubMed] [Google Scholar]
- 39.Nemzek JA, Call DR, Ebong SJ, Newcomb DE, Bolgos GL, Remick DG. 2000. Immunopathology of a 2-hit murine model of acid aspiration lung injury. Am J Physiol Lung Cell Mol Physiol 278:L512–L520 [DOI] [PubMed] [Google Scholar]
- 40.Nemzek JA, Ebong SJ, Kim J, Bolgos GL, Remick DG. 2002. Keratinocyte growth factor pretreatment is associated with decreased macrophage inflammatory protein 2α concentrations and reduced neutrophil recruitment in acid aspiration lung injury. Shock 18:501–506 [DOI] [PubMed] [Google Scholar]
- 41.Nemzek JA, Fry C, Abatan O. 2008. Low-dose carbon monoxide treatment attenuates early pulmonary neutrophil recruitment after acid aspiration. Am J Physiol Lung Cell Mol Physiol 294:L644–L653 [DOI] [PubMed] [Google Scholar]
- 42.Nemzek JA, Siddiqui J, Remick DG. 2001. Development and optimization of cytokine ELISAs using commercial antibody pairs. J Immunol Methods 255:149–157 [DOI] [PubMed] [Google Scholar]
- 43.Nicosia S, Capra V, Rovati GE. 2001. Leukotrienes as mediators of asthma. Pulm Pharmacol Ther 14:3–19 [DOI] [PubMed] [Google Scholar]
- 44.Perretti F, Ballati L, Evangelista S, Argentino-Storino A, Manzini S. 1995. Hyperresponsiveness to nonadrenergic, noncholinergic vagal stimulation following multiple-antigen challenge in guinea pigs. Pulm Pharmacol 8:21–30 [DOI] [PubMed] [Google Scholar]
- 45.Petak F, Habre W, Donati YR, Hantos Z, Barazzone-Argiroffo C. 2001. Hyperoxia-induced changes in mouse lung mechanics: forced oscillations vs. barometric plethysmography. J Appl Physiol 90:2221–2230 [DOI] [PubMed] [Google Scholar]
- 46.Siegle JS, Hansbro N, Herbert C, Yang M, Foster PS, Kumar RK. 2006. Airway hyperreactivity in exacerbation of chronic asthma is independent of eosinophilic inflammation. Am J Respir Cell Mol Biol 35:565–570 [DOI] [PubMed] [Google Scholar]
- 47.Sontag SJ, O'Connell S, Khandelwal S, Miller T, Nemchausky B, Schnell TG, Serlovsky R. 1990. Most asthmatics have gastroesophageal reflux with or without bronchodilator therapy. Gastroenterology 99:613–620 [DOI] [PubMed] [Google Scholar]
- 48.Tuchman DN, Boyle JT, Pack AI, Scwartz J, Kokonos M, Spitzer AR, Cohen S. 1984. Comparison of airway responses following tracheal or esophageal acidification in the cat. Gastroenterology 87:872–881 [PubMed] [Google Scholar]
- 49.Wills-Karp M. 1999. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol 17:255–281 [DOI] [PubMed] [Google Scholar]
- 50.Wu DN, Tanifuji Y, Kobayashi H, Yamauchi K, Kato C, Suzuki K, Inoue H. 2000. Effects of esophageal acid perfusion on airway hyperresponsiveness in patients with bronchial asthma. Chest 118:1553–1556 [DOI] [PubMed] [Google Scholar]