Abstract
The objective of this study was to determine whether a simple, noninvasive method involving administration of isoproterenol could be used to produce myocardial injury and cardiac dysfunction in the mouse heart with a low incidence of mortality. Adult Swiss–Webster mice were injected with isoproterenol (100 mg/kg SC) once daily for 5 d. Myocardial histology and left ventricular (LV) function were assessed 10 to 14 d after the last isoproterenol injection in 14 surviving isoproterenol-treated mice and 15 saline-treated control mice. Left ventricular systolic and diastolic pressures were evaluated in vitro by means of isovolumically contracting, perfused Langendorff preparations. Isoproterenol induced marked endocardial injury, associated with hypertrophy of surviving myocytes, and an increase in myocardial fibrosis (collagen types I and III according to picrosirius red microscopy). The hearts from isoproterenol-treated mice demonstrated decreased LV compliance, as evidenced by an upward shift in the diastolic pressure–volume relationship, with normal LV systolic function. Isoproterenol administration provides a simple, noninvasive means to induce endocardial injury and diastolic dysfunction without significant impairment of systolic function. This model has a low incidence of mortality and may be useful to assess the effects of gene or stem cell therapy on cardiac dysfunction without the potential confounding effects of invasive procedures.
Abbreviation: LV, left ventricular
The availability of genetically altered mice provides an opportunity to assess the structural and functional role of specific proteins that are involved in myocardial injury and potential therapeutic interventions. In general, techniques to induce myocardial injury and pathologic hypertrophy in the mouse have required surgical procedures such as ligation of the coronary artery,10,17,24 banding of the aorta,14,26 or implantation of an osmotic minipump to infuse β-adrenergic agonists,12,19,20,31 with associated risk of morbidity or mortality. Moreover, in vascular occlusion models, delivery of gene or stem cell therapy to damaged myocardium might require additional surgery because of the need for direct injection.13 For these reasons, a relatively noninvasive murine model that induces discrete myocardial injury in the presence of a patent coronary circulation, with a low incidence of mortality, would be of interest.
Previous investigations27-29 have demonstrated that a single subcutaneous injection of isoproterenol (85 mg/kg) in rats produced myocardial necrosis and fibrosis, while patent coronary vasculature was maintained. Others1,2,30 have shown that the extent of myocardial injury of the rat heart could be increased with repeated injections. A study of chronic isoproterenol infusion20 did not demonstrate significant fibrosis, necrosis, or hypertrophy in the mouse. However, repeated daily injection of isoproterenol (100 mg/kg SC) induces substantial LV fibrosis.7 A more recent study has demonstrated that histologic findings may be dependent on hemodynamic response to the mode of administration and on strain differences.11 The primary purpose of the present study was to define the functional consequences of the adverse structural changes induced in the mouse heart by multiple isoproterenol injections.
Materials and Methods
Animal model.
Thirty male Swiss–Webster mice (albino outbred strain, Tac:SW, Taconic, Germantown, NY; age, 8 mo; approximate weight, 40 g) were used to test the efficacy of this method. The relatively large size of male animals of this strain facilitated placement of the balloon in the left ventricle, which is a technically difficult procedure in a small heart. All animals received humane care in compliance with the Guide for the Care and Use of Laboratory Animals.15 The research and animal care protocols were approved by the institutional animal care and use committee. The mice were housed 2 per container and exposed to a 12:12-h light:dark cycle. Mice were maintained on an ad libitum diet (Rodent Laboratory Diet 5001, PM Nutrition International, St Louis, MO) with free access to water under conditions previously described.8 After acclimation of mice to the facility for 2 wk, isoproterenol (100 mg/kg) or isotonic saline was injected subcutaneously once daily for 5 consecutive days. Isoproterenol hydrochloride (Sigma Chemical, St Louis, MO) was dissolved in sterile saline.
Physiologic studies were carried out 10 to 14 d later, followed by histologic analysis of samples derived from the same animals. All mice were monitored with daily measurement of systolic arterial blood pressure while conscious by using an indirect tail-cuff technique. Of the 15 mice treated with isoproterenol, 1 died suddenly before the study, after 4 d of isoproterenol injections. Pathologic examination of the animal did not reveal any gross abnormalities; the possibility that death was related to a cardiac arrhythmia or acute isoproterenol-induced hemodynamic changes cannot be excluded.
Isolated heart preparation.
An isolated isovolumically contracting blood-perfused mouse heart preparation was used as previously described.4 Briefly, mice were heparinized (1000 IU/kg IP), sedated with inhaled isoflurane (Abbott Laboratories, Abbott Park, IL), and then euthanized by decapitation. Hearts were removed quickly and placed in oxygenated Krebs–Henseleit solution containing heparin at 28 °C. The composition of the perfusate was: 118.5 mM NaCl; 4.69 mM KCl, 1.16 mM MgSO4, 1.18 mM KH2PO4, 22.0 mM glucose, 25.88 mM NaHCO3, and 2.2 mM CaCl2. The aorta was cannulated and perfused by using a 20-gauge cannula and the Langendorff technique with oxygenated perfusate. A reservoir was mounted above the heart to supply perfusate (nonrecirculating oxygenated Krebs–Henseleit solution with or without washed bovine red blood cells at a hematocrit of 20%) at constant perfusion pressure (approximately 80 mm Hg), with temperature maintained at 37 °C. Pressure in the aortic root was measured by a sidearm cannula connected to a pressure transducer. The perfusate was filtered (pore size, 5.0 µm) and bubbled with a gas mixture consisting of 95% O2 and 5% CO2, resulting in a pH of 7.4 and PO2 of approximately 600 mm Hg. Bovine red blood cells were suspended in Krebs–Henseleit solution to achieve a final hematocrit of 20%; 4 g% fatty-acid–free bovine albumin was added. The erythrocyte-enhanced solution was equilibrated with 20% O2, 3% CO2, 77% N2 to achieve a PO2 of 120 to 180 mm Hg and pH of 7.35 to 7.45, and was filtered to avoid microaggregation.
After initiation of coronary perfusion, a small custom latex fluid-filled balloon attached to a short length of PE50 polyethylene tubing was placed in the LV chamber via the left atrium. The tubing then was connected to a 1.4-French catheter-tip transducer (model SPR671, Millar Instruments, Houston, TX) to allow for measurement of LV end-diastolic pressure and for determination of LV pressure and dP/dt (rate of change of pressure).
Baseline pressure-volume determinations.
Baseline measurements (including a pressure–volume relation) were obtained when the preparation achieved a steady state after instrumentation (approximately 15 min). LV volume was increased by delivering 5-μL aliquots of saline to the balloon from a 50-μL glass syringe (Hamilton, Whittier, CA). The balloon volume was sufficiently large that a pressure of less than 2 mm Hg was developed by the balloon itself (out of the heart) when inflated to 50 μL, the maximum volume used in the present study. LV stiffness was calculated as described previously.22 The balloon volume was adjusted to achieve an LV end-diastolic pressure of 8 to 10 mm Hg and then held constant for physiologic measurements.
Physiologic interventions.
After baseline measurements with oxygenated perfusate with or without red cells, the influence of increasing stimulation rate was examined. Ventricular pacing was carried out at 4 Hz by using a right ventricular bipolar pacemaker wire driven by a stimulator (model SD9, Grass Technologies, West Warwick, RI) delivering 4-ms monophasic square-wave pulses. Stimulation threshold was determined by slowly increasing the stimulator voltage until consistent pacing occurred, and voltage was set to 10% above threshold (approximately 0.8 to 1 V). At completion of physiologic studies, approximately 50% of hearts were perfusion-fixed for histologic studies, and the remaining hearts were dissected and weighed.
Morphometric studies.
At the conclusion of the hemodynamic studies, 8 hearts in each group were perfusion-fixed with 10% neutral buffered formalin fixative and prepared for microscopy and morphologic analysis as previously described.5,6,9 Histologic cross-sections of the hearts were stained with Masson trichrome and picrosirius red, slides from 8 hearts were examined in each group, and morphologic analysis was performed twice in a blinded fashion.6 Measurements were derived from cross-sections at approximately half of the distance between the apex and base. Images were acquired by using a binocular microscope (Optiphot, Nikon, Melville, NY) with a camera and image processor (DKC5000, Sony, New York, NY). The connective tissue network and myocytes were quantified by using a semiautomated computer-based image analysis system and analysis software package (ImagePro Plus, Media Cybernetics, Silver Spring, MD). The software has the capability of identifying the area in an image corresponding to a specific spectral range. For each field of a given slide, a region of interest was identified, and the computer was used to discriminate all areas within that region meeting the specified spectral criteria. The fractional area occupied by interstitial space (representative of volume fraction, assuming a random 3-dimensional distribution) was determined from this analysis. The relative area occupied by connective tissue and myocytes was quantified in at least 15 fields from both right ventricular and LV endocardium and epicardium from each slide examined, and the results averaged. The cross-sectional area of myocytes from papillary muscles in which the nucleus was present was determined by image analysis. Polarized light microscopy was used for assessment connective tissue and collagen type in picrosirius-stained sections.5,6,23
Statistical analysis.
Physiologic and histologic parameters were compared by using the Student t test; a P level of less than 0.05 was used to determine statistical significance. Analysis was performed by using PROPHET Statistics (BBN Systems and Technologies, Cambridge, MA). Data are presented as mean ± SEM.
Results
Acute hemodynamic effects.
A single subcutaneous injection of isoproterenol (100 mg/kg) markedly decreased peak systolic blood pressure (mean ± SEM) of mice from 105 ± 6 to 55 ± 8 mm Hg within 10 min after administration. Blood pressure remained low for 15 to 20 min and gradually returned to baseline levels within 2 h.
Isoproterenol induced changes in left and right ventricular weight.
At 10 to 14 d after isoproterenol administration (100 mg/kg daily for 5 consecutive days) the LV and right ventricular weights and respective ventricle:body weight ratios were increased compared with those of age-matched controls injected with saline (P < 0.05; Table 1).
Table 1.
Effect of isoproterenol on LV and right ventricular weights and ventricle:body weight ratios
| Control (n = 7) | Isoproterenol (n = 6) | |
| Body weight (g) | 48.1 ± 2.0 | 46.7 ± 1.3 |
| LV (mg) | 166 ± 7 | 192 ± 9a |
| Right ventricle (mg) | 37 ± 2 | 47 ± 5a |
| LV:body weight ratio | 3.45 ± 0.15 | 4.11 ± 0.20a |
| Right ventricle:body weight ratio | 0.77 ± 0.05 | 1.00 ± 0.10a |
Data are presented as mean ± SEM.
Significant (P < 0.05) difference between values for isoproterenol-treated and saline-control samples.
LV function studies in vitro.
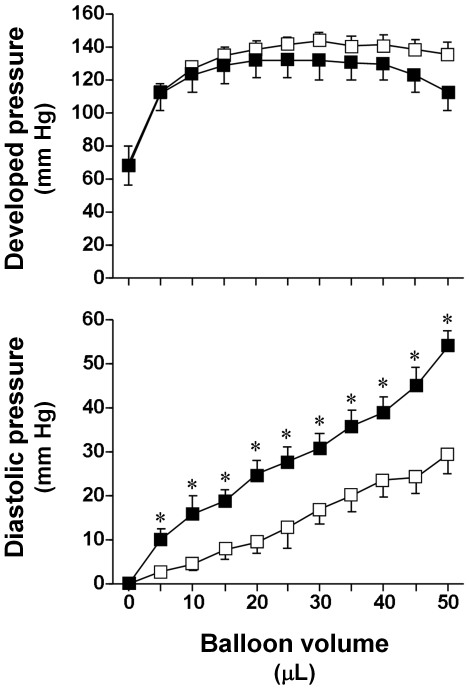
Figure 1 shows the LV pressure–volume relationships obtained from 8 isoproterenol-treated and 8 saline-treated control hearts. Compared with those from control mice, hearts from isoproterenol-treated mice demonstrated a significant (P < 0.05) upward shift of the entire LV diastolic pressure–volume relationship. Myocardial stiffness was significantly greater (P < 0.05) in isoproterenol-treated heart (41.2 ± 0.3 mm Hg) than control heart (26.7 ± 0.1 mm Hg). Developed pressure did not differ significantly between groups over a wide range of balloon volumes.
Figure 1.
Mean baseline developed pressure (top panel) and diastolic pressure (bottom panel) as a function of balloon volume for hearts from control mice (open squares) and isoproterenol-treated mice (solid squares). Data are presented as mean ± SEM. *, Significant (P < 0.05) difference between values for isoproterenol-treated and control mice.
Morphologic features and changes in response to isoproterenol administration.
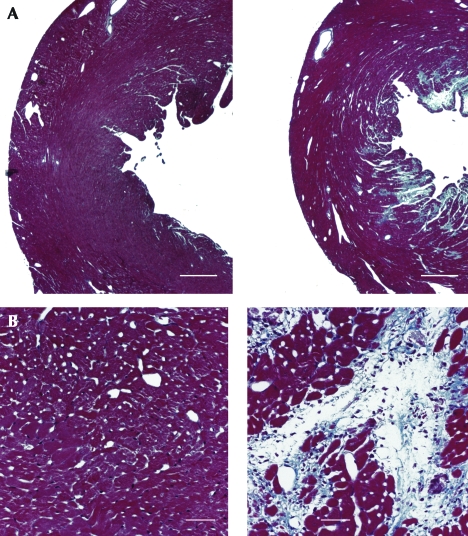
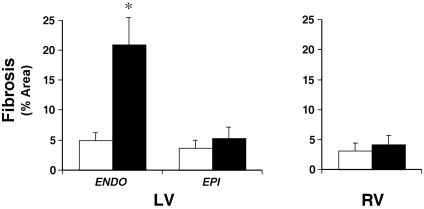
Treatment with isoproterenol (Figure 2 A, right panel) resulted in marked myocyte loss and increased fibrosis, primarily limited to the subendocardium of the LV free wall and septum, not extending to the epicardium. A high-power view of isoproterenol-treated LV endocardium (Figure 2 B, right panel) revealed a marked decrease in the proportional area occupied by viable myocardial cells and increased fibrosis which involved most of the subendocardium of the LV, not extending to the epicardium, compared with that in saline-treated control heart (left panel). Quantitative histologic analysis showed a marked increase in fibrosis in the endocardium of the LV, with no significant change in the epicardium of the LV or right ventricle (Figure 3, Table 2). With the picrosirius polarization technique, cross-sections of LV from isoproterenol-treated hearts demonstrated a marked increase in the collagen network of the myocardium. The red–yellow- (type I) and green- (type III) appearing collagen of the LV endocardium was significantly higher in isoproterenol-treated mice (5.91 ± 2.93 and 5.63 ± 3.37%, respectively) compared with that in control mice (2.13 ± 1.45 and 1.72 ± 0.84%, respectively; P < 0.01 for both comparisons). Isoproterenol also appeared to induce an increase in collagen density around small intramural coronary arteries.
Figure 2.
A) Low-power tissue cross-sections from control (left panel) and isoproterenol-treated (right panel) mice. The sections were obtained half way between the LV apex and base. Isoproterenol induced marked fibrosis of the LV endocardium. Masson trichrome stain; scale bar, 0.5 mm. B) Higher power sections of LV from control (left panel) and isoproterenol-treated (right panel) mouse. Masson trichrome stain; scale bar, 0.05 mm.
Figure 3.
Effect of isoproterenol administration (solid bars) compared with saline control (open bars) on fibrosis (expressed as % fractional area) in LV and right ventricular (RV) tissue cross-sections. Data are presented as mean ± SEM. ENDO, endocardium; EPI, epicardium. *, Significant (P < 0.05) difference between values for isoproterenol-treated and control mice.
Table 2.
Histologic analysis of LV and right ventricular cross-sections stained with Masson trichrome
| % of total area |
|||
| Fibrosis | Myocytes | ||
| LV endocardium | |||
| Control | 4.9 ± 1.3 | 82.4 ± 3.9 | |
| Isoproterenol | 20.9 ± 4.5a | 64.2 ± 3.8a | |
| LV epicardium | |||
| Control | 3.7 ± 1.3 | 85.4 ± 3.9 | |
| Isoproterenol | 5.3 ± 1.8 | 78.7 ± 3.6 | |
| Right ventricle | |||
| Control | 3.2 ± 1.2 | 86.7 ± 3.7 | |
| Isoproterenol | 4.2 ± 1.5 | 83.4 ± 4.0 | |
Data are presented as mean ± SEM (n = 8 per group).
Significant (P < 0.01) difference between values for isoproterenol-treated and saline-control samples.
Analysis of cross-sectional area of LV papillary muscles indicated a decrease in the area occupied by viable myocytes (Table 2). The size of individual surviving myocytes increased (P < 0.01) after isoproterenol treatment (cross-sectional area, 336 ± 40 μm2) compared with that of saline-treated control tissue (211 ± 20 μm2).
Discussion
The primary finding of this study is that myocardial injury and fibrosis induced by repetitive administration of isoproterenol in the mouse heart is associated with decreased LV compliance, with marked increase in LV filling pressure for any given volume, in association with hypertrophy of the surviving myocardium and no significant impairment of overall systolic function. These findings and model may be of interest with regard to the clinical syndrome of LV diastolic dysfunction and heart failure, in contrast to the myocardial infarction model, in which the primary impairment is related to systolic function.
A single subcutaneous injection of isoproterenol (85 mg/kg) results in a rapid, marked increase in ‘infarct-like’ myocardial necrosis in rats.29 Subsequent studies have shown that the extent of myocardial injury in rat heart can be increased with repeated injections.1,2,30 Isoproterenol-induced myocardial necrosis and fibrosis in the rat characteristically involves the subendocardial area of the LV and papillary muscle.1,2,27-30 Chronic isoproterenol administration to mice reportedly did not induce marked structural changes.20 These findings suggest that apparent differences in the response to isoproterenol may be related to differences in the mode of administration or possibly species or strain differences; additional studies might be required to characterize the adverse effects of isoproterenol on structure and function in different murine strains.
The mechanism of myocardial injury might be related to a number of possible mechanisms, including hemodynamic and metabolic effects. In the present study, isoproterenol administration to mice induced a sudden and marked decrease in peak systolic blood pressure, suggesting that injury might be mediated by hypoperfusion. A previous study that used repeated isoproterenol injections in A/J and C57BL/6J mice demonstrated that the extent of myocardial injury on day 6 (24 h after the last isoproterenol injection) depended on hemodynamic response and on strain differences.11 In general, the histologic findings in the present study show a pattern of myocardial injury and fibrosis that appears similar to that produced by ischemia in rat18,27,30 and mouse8,10,17 heart, suggesting that isoproterenol may act by inducing myocardial hypoxia or ischemia. These findings of myocyte loss with associated myocyte hypertrophy and fibrosis appear to be similar to those reported to occur in transgenic mice with sustained endogenous sympathetic stimulation by overexpression of cardiac Gsα,16 suggesting that excessive β-adrenergic activation may induce similar structural abnormalities.
In the present study, isoproterenol did not significantly impair systolic function, despite evidence of myocyte loss in the endocardium (Table 2). This outcome may be related to the location and extent of injury or possibly to compensatory hypertrophy. Diastolic pressure after isoproterenol was increased at any given volume, consistent with decreased LV compliance, in association with increased myocardial stiffness.
The present study did not include protein determinations of collagen type, but morphometric analysis of the collagen profile by picrosirius microscopy suggested marked increases in collagen types I and III in the LV of isoproterenol-treated mouse heart relative to controls. Spectral analysis of myocardial sections stained with picrosirius red and viewed under polarized light has shown that type I collagen fibers appear primarily red–yellow, whereas type III collagen fibers appear green; the fractional area of collagen was positively correlated with type I and type III collagen mRNA and hydroxyproline content.23 However, additional evidence suggests that variables such as fiber size and section thickness may confound this determination,25 so that this method may not provide precise separation of collagen types. Nevertheless, these findings are consistent with an increased proportion of type I collagen fibers, a relatively nondistensible form that has been associated with increased myocardial stiffness and that has recently been shown to increase after high-dose isoproterenol infusion (that is, 15 mg/kg daily for 11 d) in C57BL/6 mice.31 In rats, expression of collagen types I and III mRNA increased within hours after isoproterenol administration;3 extracellular matrix transcripts are increased in the LV but not RV for as long as 7 d with continuous isoproterenol administration.21 Furthermore, repeated high-dose isoproterenol administration decreases LV compliance in rats.30 Taken together, these findings suggest that increased myocardial stiffness and decreased LV distensibility are related to isoproterenol-induced fibrosis.
To examine the possibility that limited oxygen availability influences diastolic compliance in this model, we studied isolated heart function by using perfusion media with and without red cell supplementation. Isoproterenol-treated hearts demonstrated elevated LV end-diastolic pressure at a given balloon volume with both perfusates. Systolic function under baseline conditions was comparable in both groups, suggesting that oxygen supply was not a limiting factor under the conditions studied.
In summary, we have shown that repeated isoproterenol administration affords a simple, noninvasive means for inducing myocardial injury and diastolic dysfunction in the mouse heart, with a low incidence of mortality and without the potentially confounding effects of invasive procedures. This model may be useful in assessing the therapeutic effects of pharmacologic agents and gene or stem cell therapy on LV diastolic dysfunction in a murine model of myocardial injury.
Acknowledgment
This work was supported by Medical Research Funds from the Department of Veterans Affairs (REAP and VA Merit review grants).
References
- 1.Balazs T. 1972. Cardiotoxicity of isoproterenol in experimental animals. Influence of stress, obesity, and repeated dosing. Recent Adv Stud Cardiac Struct Metab 1:770–778 [PubMed] [Google Scholar]
- 2.Benjamin IJ, Jalil JE, Tan LB, Cho K, Weber KT, Clark WA. 1989. Isoproterenol-induced myocardial fibrosis in relation to myocyte necrosis. Circ Res 65:657–670 [DOI] [PubMed] [Google Scholar]
- 3.Boluyt MO, Long X, Eschenhagen T, Mende U, Schmitz W, Crow MT, Lakatta EG. 1995. Isoproterenol infusion induces alterations in expression of hypertrophy-associated genes in rat heart. Am J Physiol 269:H638–H647 [DOI] [PubMed] [Google Scholar]
- 4.Brooks WW, Apstein CS. 1996. Effect of treppe on isovolumic function in the isolated blood-perfused mouse heart. J Mol Cell Cardiol 28:1817–1822 [DOI] [PubMed] [Google Scholar]
- 5.Brooks WW, Bing OH, Robinson KG, Slawsky MT, Chaletsky DM, Conrad CH. 1997. Effect of angiotensin-converting enzyme inhibition on myocardial fibrosis and function in hypertrophied and failing myocardium from the spontaneously hypertensive rat. Circulation 96:4002–4010 [DOI] [PubMed] [Google Scholar]
- 6.Brooks WW, Conrad CH. 2000. Myocardial fibrosis in transforming growth factor β1 heterozygous mice. J Mol Cell Cardiol 32:187–195 [DOI] [PubMed] [Google Scholar]
- 7.Brooks WW, Conrad CH, Hayes JA, Apstein CS. 1996. Effect of isoproterenol-induced fibrosis on left ventricular function in the isolated blood-perfused mouse heart. J Am Coll Cardiol 27:86 [Google Scholar]
- 8.Brooks WW, Garibaldi BA, Conrad CH. 1998. Myocardial injury in the mouse induced by transthoracic cauterization. Lab Anim Sci 48:374–378 [PubMed] [Google Scholar]
- 9.Conrad CH, Brooks WW, Hayes JA, Sen S, Robinson KG, Bing OH. 1995. Myocardial fibrosis and stiffness with hypertrophy and heart failure in the spontaneously hypertensive rat. Circulation 91:161–170 [DOI] [PubMed] [Google Scholar]
- 10.Eberli FR, Sam F, Ngoy S, Apstein CS, Colucci WS. 1998. Left-ventricular structural and functional remodeling in the mouse after myocardial infarction: assessment with the isovolumetrically contracting Langendorff heart. J Mol Cell Cardiol 30:1443–1447 [DOI] [PubMed] [Google Scholar]
- 11.Faulx MD, Ernsberger P, Vatner D, Hoffman RD, Lewis W, Strachan R, Hoit BD. 2005. Strain-dependent β-adrenergic receptor function influences myocardial responses to isoproterenol stimulation in mice. Am J Physiol Heart Circ Physiol 289:H30–H36 [DOI] [PubMed] [Google Scholar]
- 12.Gaspard GJ, Pasumarthi KB. 2008. Quantification of cardiac fibrosis by colour-subtractive computer-assisted image analysis. Clin Exp Pharmacol Physiol 35:679–686 [DOI] [PubMed] [Google Scholar]
- 13.Guzman RJ, Lemarchand P, Crystal RG, Epstein SE, Finkel T. 1993. Efficient gene transfer into myocardium by direct injection of adenovirus vectors. Circ Res 73:1202–1207 [DOI] [PubMed] [Google Scholar]
- 14.Hamawaki M, Coffman TM, Lashus A, Koide M, Zile MR, Oliverio MI, DeFreyte G, Cooper G, 4th, Carabello BA. 1998. Pressure-overload hypertrophy is unabated in mice devoid of AT1A receptors. Am J Physiol 274:H868–H873 [DOI] [PubMed] [Google Scholar]
- 15.Institute of Laboratory Animal Resources 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academy Press [Google Scholar]
- 16.Iwase M, Bishop SP, Uechi M, Vatner DE, Shannon RP, Kudej RK, Wight DC, Wagner TE, Ishikawa Y, Homcy CJ, Vatner SF. 1996. Adverse effects of chronic endogenous sympathetic drive induced by cardiac GSα overexpression. Circ Res 78:517–524 [DOI] [PubMed] [Google Scholar]
- 17.Johns TN, Olson BJ. 1954. Experimental myocardial infarction. I. A method of coronary occlusion in small animals. Ann Surg 140:675–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalkman EA, van Suylen RJ, van Dijk JP, Saxena PR, Schoemaker RG. 1995. Chronic aspirin treatment affects collagen deposition in non-infarcted myocardium during remodeling after coronary artery ligation in the rat. J Mol Cell Cardiol 27:2483–2494 [DOI] [PubMed] [Google Scholar]
- 19.Krishnamurthy P, Subramanian V, Singh M, Singh K. 2007. β1 integrins modulate β-adrenergic receptor-stimulated cardiac myocyte apoptosis and myocardial remodeling. Hypertension 49:865–872 [DOI] [PubMed] [Google Scholar]
- 20.Kudej RK, Iwase M, Uechi M, Vatner DE, Oka N, Ishikawa Y, Shannon RP, Bishop SP, Vatner SF. 1997. Effects of chronic β-adrenergic receptor stimulation in mice. J Mol Cell Cardiol 29:2735–2746 [DOI] [PubMed] [Google Scholar]
- 21.Masson S, Arosio B, Luvara G, Gagliano N, Fiordaliso F, Santambrogio D, Vergani C, Latini R, Annoni G. 1998. Remodelling of cardiac extracellular matrix during β-adrenergic stimulation: upregulation of SPARC in the myocardium of adult rats. J Mol Cell Cardiol 30:1505–1514 [DOI] [PubMed] [Google Scholar]
- 22.Mirsky I, Parmley WW. 1973. Assessment of passive elastic stiffness for isolated heart muscle and the intact heart. Circ Res 33:233–243 [DOI] [PubMed] [Google Scholar]
- 23.Nicoletti A, Heudes D, Hinglais N, Appay MD, Philippe M, Sassy-Prigent C, Bariety J, Michel JB. 1995. Left ventricular fibrosis in renovascular hypertensive rats. Effect of losartan and spironolactone. Hypertension 26:101–111 [DOI] [PubMed] [Google Scholar]
- 24.Patten RD, Aronovitz MJ, Deras-Mejia L, Pandian NG, Hanak GG, Smith JJ, Mendelsohn ME, Konstam MA. 1998. Ventricular remodeling in a mouse model of myocardial infarction. Am J Physiol 274:H1812–H1820 [DOI] [PubMed] [Google Scholar]
- 25.Rich L, Whittaker P. 2005. Collagen and picrosirius red staining: a polarized light assessment of fibrillar hue and spatial distribution. Braz J Morphol Sci 22:97–104 [Google Scholar]
- 26.Rockman HA, Wachhorst SP, Mao L, Ross J., Jr 1994. AngII receptor blockade prevents ventricular hypertrophy and ANF gene expression with pressure overload in mice. Am J Physiol 266:H2468–H2475 [DOI] [PubMed] [Google Scholar]
- 27.Rona G. 1985. Catecholamine cardiotoxicity. J Mol Cell Cardiol 17:291–306 [DOI] [PubMed] [Google Scholar]
- 28.Rona G, Boutet M, Huttner I, Peters H. 1973. Pathogenesis of isoproterenol-induced myocardial alterations: functional and morphological correlates. Recent Adv Stud Cardiac Struct Metab 3:507–525 [PubMed] [Google Scholar]
- 29.Rona G, Chappel CI, Balazs T, Gaudry R. 1959. An infarct-like myocardial lesion and other toxic manifestations produced by isoproterenol in the rat. Am Med Assoc Arch Pathol 67:443–455 [PubMed] [Google Scholar]
- 30.Teerlink JR, Pfeffer JM, Pfeffer MA. 1994. Progressive ventricular remodeling in response to diffuse isoproterenol-induced myocardial necrosis in rats. Circ Res 75:105–113 [DOI] [PubMed] [Google Scholar]
- 31.Zhang GX, Ohmori K, Nagai Y, Fujisawa Y, Nishiyama A, Abe Y, Kimura S. 2007. Role of AT1 receptor in isoproterenol-induced cardiac hypertrophy and oxidative stress in mice. J Mol Cell Cardiol 42:804–811 [DOI] [PubMed] [Google Scholar]