Abstract
Angiogenic factors such as vascular endothelial growth factor (VEGF) are implicated in pulmonary hypertension (PH). However, the pathway of angiogenic factor-mediated pathologic angiogenesis in PH remains unclear. In this study, we evaluated the temporal expression of angiopoietin (Ang) 1, Ang2, and their receptor (Tie2) as well as VEGF, endothelial nitric oxide synthase (eNOS), inducible NOS (iNOS), and heme oxygenase 1 (HO1) in the monocrotaline-induced PH model. Histologic evaluation showed pathologic vascular remodeling in the arteries of lung sections 1 wk after monocrotaline treatment. Protein levels of Ang1, Ang2, eNOS, iNOS, HO1, and VEGF were increased 1 wk after monocrotaline treatment but Tie2 protein levels were decreased 2 wk afterward. These results suggest that Ang2 mediates vascular remodeling in PH by decreasing Tie2 expression. Therefore, the Ang–Tie2 system may play a role in the pathophysiology of PH.
Abbreviations: Ang, angiopoietin; eNOS, endothelial nitric oxide synthase; HO1, heme oxygenase 1; iNOS, inducible nitric oxide synthase; PH, pulmonary hypertension; VEGF, vascular endothelial growth factor
Pulmonary hypertension (PH) is a disease characterized by pathologic angiogenesis caused by diffuse smooth muscle cell hyperplasia and hypertrophy of the distal pulmonary vasculature, resulting in obliteration of small pulmonary arterioles.13 Vascular remodeling is governed by the interaction of several angiogenic factors on endothelial and smooth muscle cells. Vascular remodeling requires complex, multistep signaling pathways and a high degree of spatial and temporal coordination among endothelial and smooth muscle cells.29 However, the precise mechanisms of vascular remodeling at the cellular and molecular levels are not completely defined.
The angiopoietin (Ang) family and vascular endothelial growth factor (VEGF) are 2 types of vascular regulatory molecules that have been the subject of intense investigation in both physiologic and pathologic generation of blood vessels.2,38 Members of the Ang family have opposing effects on receptor activation, with Ang1 stimulating Tie2 and Ang2 antagonizing this stimulation.3,8,22 In particular, Ang1 plays an important role in the assembly of newly formed vasculature and in the maintenance of vascular integrity.7,14,36 In contrast, Ang2 antagonizes the activation of Tie2 by Ang1 and causes endothelial cell apoptosis and vascular regression.22 The functions of Ang2 appear to be more complex than those of Ang1, in that Ang2 binds to the Tie2 receptor, blocking Ang1–Tie2 signaling and acting as a vessel-destabilizing factor.26 However, prolonged exposure of endothelial cells to Ang2 activates Tie2 signaling.16 Thus, the precise roles of Ang2 during the development of PH are not well understood. Tie2 is a receptor tyrosine kinase that is expressed principally on vascular endothelium and that plays a role in integrity and survival of endothelial cells.27,30 Disrupting Tie2 function in mice results in embryonic lethality with defects in embryonic vasculature.11
Neither Ang1 nor Ang2 alone can trigger an angiogenic response, but both enhance angiogenesis or induce vascular remodeling, depending on the presence of VEGF or other angiogenic factors. Nitric oxide is produced by endothelial cells through the action of nitric oxide synthase (NOS). Northern blot analysis of hypoxic rat lungs showed significantly increased mRNA levels for both endothelial NOS (eNOS) and inducible NOS (iNOS).18 Increased NOS activity coincided with the beginning of the vascular remodeling process during chronic hypoxia.37 Hypoxia and nitric oxide stimulate VEGF production and induce HO1 expression in vascular tissue.10 In addition, several studies have shown that VEGF works in conjunction with other angiogenic factors to produce a stable and functional microvasculature.21,35
The purpose of the present study was to demonstrate the temporal changes of several angiogenic factors during the development of PH induced by treatment of rats with monocrotaline. This research was focused on the Ang–Tie2 system and other angiogenic factors and suggested that this system plays an important role in modulating vascular remodeling during PH.
Materials and Methods
Animals and drug treatment.
Adult male Sprague–Dawley rats weighing 250 to 300 g were purchased from Japan SLC (Hamamatsu, Japan). This study was performed in accordance with the Gyeongsang National University Institutional Guidelines for the Care and Use of Laboratory Animals (GLA-070215-R0003). To adapt the rats to their new surroundings, rats were housed in cages (n = 2 per cage) with littermates on 12:12-h light:dark cycle. Water and commercial rat chow (Nestle Purina PetCare Company, St Louis, MO) were available ad libitum. Rats were given a single injection of monocrotaline (60 mg/kg SC; Sigma, St Louis, MO) or an equivalent volume of its vehicle as a control.
Tissue preparations.
Rats (n = 4 per group) were euthanized at 7, 14, and 21 d after monocrotaline injection; controls were euthanized at 7 d after vehicle injection. For Western analysis, rats were deeply anesthetized with ether and euthanized. The right lung was removed and stored at –80 °C until use. For histologic examination, rats were perfused through the right ventricle with a fixative solution containing 4% paraformaldehyde in 0.1 M PBS (pH 7.0). The left lung was removed after perfusion and fixed for 24 h at 4 °C. The tissue samples were processed for paraffin embedding, and 5-μm sections were cut.
Morphometric analysis.
Lung sections were stained by using Masson trichrome. Vessels with perceptible media within each cross-section were measured by a blinded observer under 40× magnification by using Image-Pro Plus (Media Cybernectics, Buckinghamshire, UK). All tangentially cut vessels were excluded. The medial wall thickness of the pulmonary arteries within each peripheral lung was analyzed as described previously.24 In brief, the external diameter and medial wall thickness were measured in 20 muscular arteries (ranging in external diameter from 31 to 50 and 51 to 100 μm) per lung section (4 sections per animal). For each artery, the medial wall thickness was calculated as follows: percentage wall thickness = [(medial thickness × 2)/external diameter] × 100%.
Immunohistochemical analysis.
After deparaffinization, section slides were treated sequentially with 1% H2O2 for 10 min and then rinsed thoroughly with PBS. Sections were blocked with 2% normal horse or goat serum in PBS at room temperature for 60 min to suppress nonspecific binding of IgG and then were incubated with primary antibodies of goat antiAng1 polyclonal IgG (1:500, Santa Cruz Biotech, Santa Cruz, CA), goat antiAng2 polyclonal IgG (1:500, Santa Cruz Biotech), and rabbit antiTie2 polyclonal IgG (1:500, Santa Cruz Biotech). After being washed with PBS, samples were incubated for 60 min at room temperature with biotin-conjugated secondary IgG (diluted 1:200, Vector Laboratories, Burlingame, CA) diluted in 2% normal blocking serum and then incubated for 60 min at room temperature with avidin–biotin–peroxidase complex (ABC Elite kit, Vector Laboratories). Samples were washed with PBS and then incubated for 3 min with diaminobenzidine tetrahydrochloride (DAB, Sigma) containing 0.05% hydrogen peroxidase to reveal the immunoreactivity of each antibody. The sections were visualized under light microscopy (model BX51, Olympus, Tokyo, Japan), and digital images were captured and documented.
Western blot analysis for Ang1, Ang2, Tie2, VEGF, eNOS, iNOS, and HO1.
Rat lungs were placed in plastic tubes with lysis buffer [10 mM HEPES–KOH (pH 7.9), 1.5 mM MgCl2, 10 mM KCl] containing leupeptin (0.5 μg/mL), aprotinin (1 μg/mL), 200 μM phenylmethylsulphonyl fluoride, and 500 μM dithiothreitol. Tissue samples were homogenized with a pestle and incubated for 10 min on ice, and lysates were collected by centrifugation at 14,240 × g for 20 min at 4 °C. The concentration of each lysate was determined using a bicinchoninic acid kit (Pierce Biotechnology, Rockford, IL) according to the manufacturer's protocol, with BSA as the standard.
Each lysate was diluted with sample buffer [0.15 M Tris–HCl (pH 6.8), 2% SDS, 10% glycerol, 5% 2-mercaptoethanol] and boiled for 5 min before Western blotting. Proteins from individual rats were combined in each experimental group. Equal amounts of protein (30 μg) were separated by SDS-PAGE and transferred to nitrocellulose membranes. The membranes were washed in Tris-buffered saline containing 0.5% Tween 20 (TBST) and incubated with the following primary antibodies diluted in TBST: goat anti-Ang1 (1:1000, Santa Cruz Biotech), goat anti-Ang2 (1:1000, Santa Cruz Biotech), rabbit anti-Tie2 (1:1000, Chemicon International, Temecula, CA), mouse anti-VEGF (1:200, Santa Cruz Biotech), mouse anti-eNOS (1:2500, BD Biosciences, San Jose, CA), rabbit anti-iNOS (1:5000, Chemicon International), and rabbit anti-HO1 (1:1000, Stressgen Bioreagents, Ann Arbor, MI). Control for protein loading was performed by reprobing membranes with antibodies against α tubulin (1:1000, Santa Cruz Biotech). Membranes were incubated with appropriate secondary antibodies (1:10,000), underwent chemiluminescence detection (NEN Life Science Products, Boston, MA), and exposed to X-ray film (X-OMAT, Kodak, Rochester, NY). The density (OD/mm2) of each band was measured with a scanner (UMAX, Techville, Dallas, TX) and was reported as mean ± SEM. The ratios of the density of each protein band to that of the α tubulin band were compared by using SigmaGel 1.0 (SPSS, Chicago, IL).
Statistics.
Values are expressed as mean ± SEM. Differences between groups (control and 1, 2, and 3 wk after monocrotaline treatment) were determined by 1-way ANOVA followed by post hoc analysis using the Bonferroni t test using SigmaPlot (10.0 version, Systat Software, Chicago, IL). A P value of less than 0.05 was considered statistically significant.
Results
Effect of monocrotaline on the vascular remodeling in rat lungs during PH.
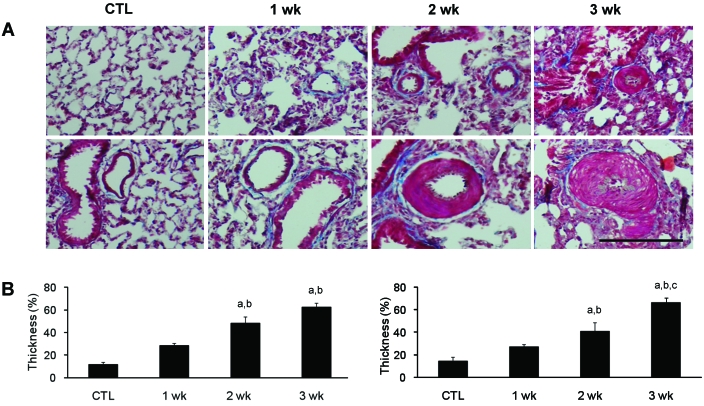
Pulmonary vessel walls in rats treated with monocrotaline were evaluated for hypertrophy by using tissue sections stained with Masson trichrome (Figure 1). Hypertrophy of the walls of pulmonary arteries was apparent 2 wk after monocrotaline treatment, as shown in a representative photomicrograph that illustrates hypertrophy and hyperplasia of small arteries (Figure 1 A). To demonstrate the hypertrophy and hyperplasia of small arteries in lung sections, we examined the thickness of the medial wall. The percentage wall thickness of pulmonary arteries between 31 to 50 μm and between 51 to 100 μm was increased 2 wk after monocrotaline injection, compared with controls (Figure 1 B).
Figure 1.
Effect of monocrotaline on the vascular remodeling in rats. (A) Representative photomicrographs of lung sections from control (CTL) or monocrotaline-injected rats at 1, 2, or 3 wk after treatment. Masson trichrome stain; bar, 100 μm. (B) Quantitative analyses of peripheral pulmonary arteries with an external diameter of 30 to 50 μm (left) or 51 to 100 μm (right). The percentage wall thickness was calculated as [(medial thickness × 2)/external diameter] × 100%. Data are shown as mean ± SEM a, Significantly (P < 0.05, ANOVA) different from CTL; b, significantly (P < 0.05, ANOVA) different from value at 1 wk; c, significantly (P < 0.05, ANOVA) different from value at 2 wk.
Effect of monocrotaline on Ang1, Ang2, and Tie2 in rat lungs during pulmonary hypertension.
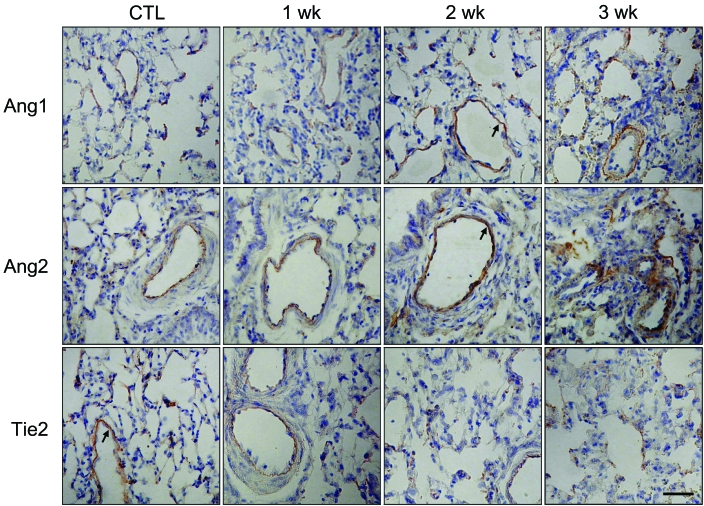
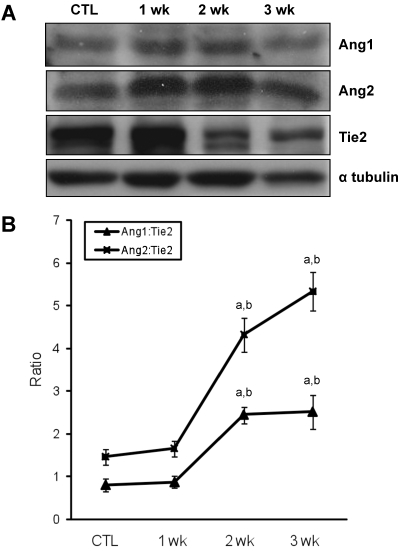
Immunohistochemistry was performed to determine which cell types increased expression of Ang1, Ang2, and Tie2 protein during the development of PH (Figure 2). Ang- and Tie2-immunostained endothelial cells were present in the pulmonary arteries of rats. Ang immunoreactivity was higher in the pulmonary arteries of MCT-treated rats than in those of the control rats 2 wk after monocrotaline injection. In contrast, a low level of Tie2 immunoreactivity was noted in endothelial cells of the pulmonary arteries of MCT-treated rats compared with those of the control rats 2 wk after monocrotaline injection. To confirm the immunoreactivity of each protein, we performed Western blot analysis of lung samples after monocrotaline injection (Figure 3 A). Ang1 and Ang2 protein levels were slightly increased over control levels 1 wk after monocrotaline injection. In contrast, Tie2 protein levels began to decrease 2 wk after monocrotaline injection. To assess the relative expression of Ang1 and Ang2 to Tie2 during monocrotaline-induced PH, we determined the ratios of Ang1 or Ang2:Tie2 expression intensities (Figure 3 B). Theoretically, steady-state ratios of Ang1:Ang2 or Ang2:Ang1 means that both Ang1 and Ang2 simultaneously increase during monocrotaline treatment. In particular, we noted a sharp and immediate increase (P < 0.05) in the Ang2:Tie2 ratio and a slight increase (P < 0.05) in the Ang1:Tie2 ratio between weeks 1 and 2 after monocrotaline treatment.
Figure 2.
Representative photomicrographs of Ang1, Ang2, and Tie2 immunostaining of lung sections after monocrotaline (60 mg/kg) treatment. Immunoreactivies of each protein are localized to the endothelium (arrows) of the pulmonary vessels of mice with monocrotaline-induced pulmonary hypertension. Bar, 50 μm.
Figure 3.
Changes of the expression of angiopoietins and Tie2 in rat lungs during the development of monocrotaline-induced PH. (A) Western blot analysis of Ang1, Ang2, and Tie2 in lungs from control and treated rats at 1, 2, and 3 wk after monocrotaline treatment. Ang1 and Ang2 expression is time-dependently increased in monocrotaline-induced PH. However, Tie2 expression is decreased in monocrotaline-induced PH. Immunoblots are representative of 4 pooled lungs from each group. (B) Ratio of Ang:Tie2 in rat lungs from control or treated rats at 1, 2, and 3 wk after monocrotaline treatment. This experiment was performed independently 3 times. Data are shown as mean ± SEM. a, Significantly (P < 0.05, ANOVA) different from CTL; b, significantly (P < 0.05, ANOVA) different from value at 1 wk; c, significantly (P < 0.05, ANOVA) different from value at 2 wk.
Effect of monocrotaline on other angiogenic factors in rat lungs during PH.
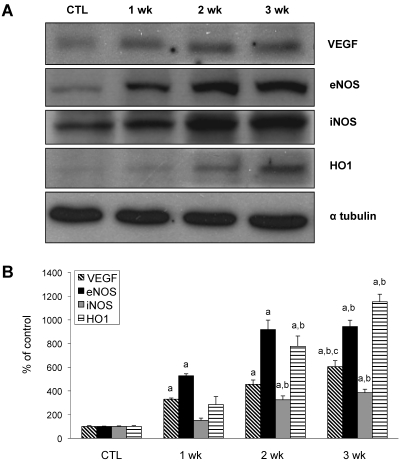
Western analysis was performed to determine the expression of the angiogenic factors VEGF, eNOS, iNOS, and HO1. Several of these proteins increased relative to controls as PH progressed (Figure 4). In particular, protein levels of VEGF and eNOS began to increase 1 wk after monocrotaline treatment.
Figure 4.
Changes of other angiogenic factors in rat lungs during the development of monocrotaline-induced PH. (A) Western blot analysis was used to assess VEGF, eNOS, iNOS, and HO1 expression in rat lungs from control and treated rats at 1, 2, and 3 wk after monocrotaline treatment. Immunoblots are representative of 4 pooled lungs from each group. (B) Quantitative expression of each protein is time-dependently increased in monocrotaline-induced rat lungs. This experiment was performed independently 3 times. Data are shown as mean ± SEM. a, Significantly (P < 0.05, ANOVA) different from CTL; b, significantly (P < 0.05, ANOVA) different from value at 1 wk.
Discussion
Angiogenesis plays an important role in the physiologic and pathologic mechanism of PH. Many studies have examined the roles of various proteins involved in angiogenesis, including VEGF and the angiopoietin family. The temporal and quantitative balance of angiogenic factor expression is critical for the regulation of physiologic as well as PH-induced vascular remodeling. Currently, no studies have reported the temporal expression pattern of several angiogenic factors, including the angiopoietin family, in rat lungs during PH development. The present study confirms that expression of Ang1, Ang2 increased and Tie2 expression decreased after monocrotaline treatment. Furthermore, our study demonstrates that the levels of VEGF, eNOS, iNOS, and HO1 steadily increased during PH development. These results suggest that, in addition to other angiogenic factors, the Ang–Tie2 system may be an important modulator of the pathologic vascular process.
Ang1 induces pulmonary hypertensive vascular pathology and clinical development of PH leading to right ventricular failure and death.6 In PH patients undergoing embolectomy, Ang1 expression was increased in the lung parenchyma proportional to the increase in pulmonary vascular resistance and medial wall hypertrophy.33 These findings confirmed that increased Ang1 production is a compensatory response to vascular damage in PH. Ang1 protects against the development of PH in the rat monocrotaline model.40 Endothelial apoptosis could lead to decreased vessel numbers in the pulmonary bed, causing increased resistance, and Ang1 may protect against such endothelial loss. In contrast, high steady-state levels of Ang1 induced by viral injection reportedly led to development of advanced PH, resulting in vascular obstruction.32 Ang1 has potent effects on adult vessels, including promoting vessel survival, inhibiting vascular leakage, and suppressing inflammatory gene expression.4 Evidence for increased vessel survival in response to Ang1 in vivo has been shown in radiation-exposed mice.5 These contradictory findings of elevated Ang1 in PH and the protective effects of this ligand require further clarification. In the current study, Ang1 expression began to increase steadily in rat lungs 1 wk after monocrotaline treatment, strongly supporting the role of Ang1 as a compensatory response for stabilizing vessels rather than an initiating factor.
Ang2 is an antagonist of Ang1 and competes for Tie2 binding, inducing loosening of the interactions between endothelial and perivascular support cells and facilitating access to angiogenic inducers.20 A recent study demonstrated that increases in Ang2 in sepsis patients correlates with impaired oxygenation.25 The report suggested that Ang2 contributes to endothelial barrier disruption in sepsis-associated lung injury, a condition that involves mature vasculature. Ang2 is expressed only in the endothelium of adult tissue undergoing vascular remodeling and in highly vascularized tumors.22,34 Consistent with its action as inhibitor of the Ang1–Tie2 system, Ang2 overexpression results in vascular defects similar to those observed in Ang1 or Tie2 knockouts.22 This evidence suggests that Ang2 acts not only as an antagonist for Tie2 receptors expressed in vitro and in vivo on endothelial cells but also as an agonist for ectopically expressed Tie2 receptors on nonendothelial cells. Up until now, there was little evidence that Ang2 elevation contributes to vascular remodeling in PH. The present study shows that like Ang1, Ang2 protein levels gradually increased beginning 1 wk after monocrotaline injection. These results suggest that both Ang1 and Ang2 are increased in lung with monocrotaline-induced PH as endothelial response to vascular remodeling. Based on the patterns of expression (Figure 3 B), the more dominant Ang2-mediated inhibition of Tie2 activation serves to destabilize vessels, making them responsive to other angiogenic factors. Subsequently, the less dominant Ang1-mediated activation of Tie2 stimulates remodeling and stabilization of the neovasculature. Taken together, our evidence suggests that Ang2 is no less important than Ang1 in vascular remodeling and maturation.
The expression of Ang1, Ang2, and Tie2 mRNA in rat lung with monocrotaline-induced PH has been assessed previously.40 In that study, Ang1 and Ang2 mRNA levels remained unchanged, even in the presence of severe PH. In contrast, both Tie2 mRNA and protein were reduced in the monocrotaline-treated lungs compared with control lungs. Tie2 receptor expression was markedly decreased in rats treated with monocrotaline.40 However, another study documented that Tie2 expression was increased in lungs and cultured pulmonary endothelial cells from patients with idiopathic PH compared with control subjects.9 This evidence suggests that the Ang1–Tie2 pathway was abnormal in idiopathic PH and contributed to hyperplasia of smooth muscle cell through excessive release of growth factors by endothelial cells of pulmonary vessels. The results of the present study correspond with those of the first-cited work,40 except for the absence of changes in lung Ang1 and Ang2 expression. Our results show that Ang1 and Ang2 were increased and Tie2 expression was decreased in monocrotaline-treated rats compared with controls. Our results suggest that the strongly increased expression of Ang2 protein during PH augments angiogenesis by inhibiting Tie2, thereby destabilizing the vasculature and increasing the response to angiogenesis initiators, such as VEGF.
VEGF is a protein secreted by endothelial cells, and the only known targets of VEGF are endothelial cells themselves, perhaps to initiate proliferation. In animal models of PH, VEGF and receptor expression were higher during PH than in controls.23 VEGF may also take part in the development of the vascular remodeling in PH.23 Serum VEGF levels were elevated markedly in patients with primary and secondary PH compared with normal controls, potentially indicating increased VEGF at sites of lung injury.12 Our results also show that VEGF was increased in rat lungs during PH development. The role of Ang2 in vascular remodeling was suggested initially by observations that VEGF and Ang2 showed coincidental expression patterns during angiogenic sprouting and that Ang2 expression was induced in the absence of VEGF during vessel regression.39 A recent report indicates that transgenic cardiac overexpression of both VEGF and Ang2 leads to a synergistic induction of angiogenesis.35 In the presence of VEGF, Ang2 destabilizes the preexisting vasculature and consequently makes it more responsive to angiogenic stimuli.17
HO1 activity regulates both NO-mediated VEGF expression and VEGF-mediated NO production by eNOS, depending upon the amount of NO produced.19 These reciprocal relations between NO and VEGF may contribute to angiogenesis regulation in normal tissues. Dysfunction caused by endothelial injury may play a key role in the initiation of PH and typically occurs in human cases of PH.1 In the present study, our results show that the eNOS was increased significantly during the PH development. A similar increase in pulmonary arterial eNOS levels and associated augmentation of endothelium-derived nitric-oxide–dependent responsiveness of the arterial vasculature occurred in a monocrotaline model of PH.28
HO1 is activated by a variety of stress-inducing factors that overlap with those that activate iNOS: heat shock, peroxide, cytokines, endotoxin, hypoxia, and shear stress.31 In a rat model of prehepatic portal hypertension, pulmonary artery pressure was elevated, and iNOS and HO1 protein expression were increased in the pulmonary endothelium and bronchial epithelium, respectively.31 Treatment with large amounts of monocrotaline produced prominent inflammatory changes in the lung, PH, and right ventricle hypertrophy concurrent with HO1 induction.15 Mice treated with an HO inhibitor exhibited more severe pathologic changes than untreated animals.15 Our current study shows that iNOS and HO1 proteins were increased during PH development. This result suggests that HO1 induction represents an intrinsic defense system during monocrotaline-induced PH.
In conclusion, our data show that expression of both Ang1 and Ang2, as well as other angiogenic factors, increased during PH but that Tie2 expression decreased. Alterations in the levels of angiogenic factors may have an important role in PH vascular remodeling and may be relevant to the pathologic and structural changes in monocrotaline-induced PH.
Acknowledgment
This work was supported by the MRC program of MOST/KOSEF (grant R13-2005-012-01001-0).
References
- 1.Archer S, Rich S. 2000. Primary pulmonary hypertension: a vascular biology and translational research ‘work in progress’. Circulation 102:2781–2791 [DOI] [PubMed] [Google Scholar]
- 2.Asahara T, Chen D, Takahashi T, Fujikawa K, Kearney M, Magner M, Yancopoulos GD, Isner JM. 1998. Tie2 receptor ligands, angiopoietin 1 and angiopoietin 2, modulate VEGF-induced postnatal neovascularization. Circ Res 83:233–240 [DOI] [PubMed] [Google Scholar]
- 3.Bouïs D, Kusumanto Y, Meijer C, Mulder NH, Hospers GA. 2006. A review on pro- and antiangiogenic factors as targets of clinical intervention. Pharmacol Res 53:89–103 [DOI] [PubMed] [Google Scholar]
- 4.Brindle NP, Saharinen P, Alitalo K. 2006. Signaling and functions of angiopoietin 1 in vascular protection. Circ Res 98:1014–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho CH, Kammerer RA, Lee HJ, Steinmetz MO, Ryu YS, Lee SH, Yasunaga K, Kim KT, Kim I, Choi HH, Kim W, Kim SH, Park SK, Lee GM, Koh GY. 2004. COMP–Ang1: a designed angiopoietin 1 variant with nonleaky angiogenic activity. Proc Natl Acad Sci USA 101:5547–5552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu D, Sullivan CC, Du L, Cho AJ, Kido M, Wolf PL, Weitzman MD, Jamieson SW, Thistlethwaite PA. 2004. A new animal model for pulmonary hypertension based on the overexpression of a single gene, angiopoietin 1. Ann Thorac Surg 77:449–456 [DOI] [PubMed] [Google Scholar]
- 7.Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD. 1996. Isolation of angiopoietin 1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell 87:1161–1169 [DOI] [PubMed] [Google Scholar]
- 8.Davis S, Yancopoulos GD. 1999. The angiopoietins: yin and yang in angiogenesis. Curr Top Microbiol Immunol 237:173–185 [DOI] [PubMed] [Google Scholar]
- 9.Dewachter L, Adnot S, Fadel E, Humbert M, Maitre B, Barlier-Mur AM, Simonneau G, Hamon M, Naeije R, Eddahibi S. 2006. Angiopoietin–Tie2 pathway influences smooth muscle hyperplasia in idiopathic pulmonary hypertension. Am J Respir Crit Care Med 174:1025–1033 [DOI] [PubMed] [Google Scholar]
- 10.Dulak J, Jozkowicz A, Foresti R, Kasza A, Frick M, Huk I, Green CJ, Pachinger O, Weidinger F, Motterlini R. 2002. Heme oxygenase activity modulates vascular endothelial growth factor synthesis in vascular smooth muscle cells. Antioxid Redox Signal 4:229–240 [DOI] [PubMed] [Google Scholar]
- 11.Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, Auerbach A, Breitman ML. 1994. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev 8:1897–1909 [DOI] [PubMed] [Google Scholar]
- 12.Eddahibi S, Humbert M, Sediame S, Chouaid C, Partovian C, Maitre B, Teiger E, Rideau D, Simonneau G, Sitbon O, Adnot S. 2000. Imbalance between platelet vascular endothelial growth factor and platelet-derived growth factor in pulmonary hypertension. Effect of prostacyclin therapy. Am J Respir Crit Care Med 162:1493–1499 [DOI] [PubMed] [Google Scholar]
- 13.Gaine SP, Rubin LJ. 1998. Primary pulmonary hypertension. Lancet 352:719–725 [DOI] [PubMed] [Google Scholar]
- 14.Gale NW, Yancopoulos GD. 1999. Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development. Genes Dev 13:1055–1066 [DOI] [PubMed] [Google Scholar]
- 15.Goto J, Ishikawa K, Kawamura K, Watanabe Y, Matumoto H, Sugawara D, Maruyama Y. 2002. Heme oxygenase 1 reduces murine monocrotaline-induced pulmonary inflammatory responses and resultant right ventricular overload. Antioxid Redox Signal 4:563–568 [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto T, Pittet JF. 2006. Angiopoietin-2: modulator of vascular permeability in acute lung injury? PLoS Med 3:e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holash J, Wiegand SJ, Yancopoulos GD. 1999. New model of tumor angiogenesis: dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene 18:5356–5362 [DOI] [PubMed] [Google Scholar]
- 18.Igari H, Tatsumi K, Sugito K, Kasahara Y, Saito M, Tani T, Kimura H, Kuriyama T. 1998. Role of EDRF in pulmonary circulation during sustained hypoxia. J Cardiovasc Pharmacol 31:299–305 [DOI] [PubMed] [Google Scholar]
- 19.Kimura H, Esumi H. 2003. Reciprocal regulation between nitric oxide and vascular endothelial growth factor in angiogenesis. Acta Biochim Pol 50:49–59 [PubMed] [Google Scholar]
- 20.Laurén J, Gunji Y, Alitalo K. 1998. Is angiopoietin 2 necessary for the initiation of tumor angiogenesis? Am J Pathol 153:1333–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lobov IB, Brooks PC, Lang RA. 2002. Angiopoietin 2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci USA 99:11205–11210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. 1997. Angiopoietin 2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277:55–60 [DOI] [PubMed] [Google Scholar]
- 23.Mata-Greenwood E, Meyrick B, Soifer SJ, Fineman JR, Black SM. 2003. Expression of VEGF and its receptors Flt1 and Flk1/KDR is altered in lambs with increased pulmonary blood flow and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 285:L222–L231 [DOI] [PubMed] [Google Scholar]
- 24.Ono S, Voelkel NF. 1991. PAF antagonists inhibit monocrotaline-induced lung injury and pulmonary hypertension. J Appl Physiol 71:2483–2492 [DOI] [PubMed] [Google Scholar]
- 25.Parikh SM, Mammoto T, Schultz A, Yuan HT, Christiani D, Karumanchi SA, Sukhatme VP. 2006. Excess circulating angiopoietin 2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med 3:e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peters KG, Kontos CD, Lin PC, Wong AL, Rao P, Huang L, Dewhirst MW, Sankar S. 2004. Functional significance of Tie2 signaling in the adult vasculature. Recent Prog Horm Res 59:51–71 [DOI] [PubMed] [Google Scholar]
- 27.Puri MC, Rossant J, Alitalo K, Bernstein A, Partanen J. 1995. The receptor tyrosine kinase TIE is required for integrity and survival of vascular endothelial cells. EMBO J 14:5884–5891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Resta TC, Gonzales RJ, Dail WG, Sanders TC, Walker BR. 1997. Selective upregulation of arterial endothelial nitric oxide synthase in pulmonary hypertension. Am J Physiol 272:H806–H813 [DOI] [PubMed] [Google Scholar]
- 29.Risau W. 1997. Mechanisms of angiogenesis. Nature 386:671–674 [DOI] [PubMed] [Google Scholar]
- 30.Schnürch H, Risau W. 1993. Expression of tie 2, a member of a novel family of receptor tyrosine kinases, in the endothelial cell lineage. Development 119:957–968 [DOI] [PubMed] [Google Scholar]
- 31.Schroeder RA, Ewing CA, Sitzmann JV, Kuo PC. 2000. Pulmonary expression of iNOS and HO1 protein is upregulated in a rat model of prehepatic portal hypertension. Dig Dis Sci 45:2405–2410 [DOI] [PubMed] [Google Scholar]
- 32.Sullivan CC, Du L, Chu D, Cho AJ, Kido M, Wolf PL, Jamieson SW, Thistlethwaite PA. 2003. Induction of pulmonary hypertension by an angiopoietin 1–TIE2–serotonin pathway. Proc Natl Acad Sci USA 100:12331–12336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thistlethwaite PA, Lee SH, Du LL, Wolf PL, Sullivan C, Pradhan S, Deutsch R, Jamieson SW. 2001. Human angiopoietin gene expression is a marker for severity of pulmonary hypertension in patients undergoing pulmonary thromboendarterectomy. J Thorac Cardiovasc Surg 122:65–73 [DOI] [PubMed] [Google Scholar]
- 34.Vajkoczy P, Farhadi M, Gaumann A, Heidenreich R, Erber R, Wunder A, Tonn JC, Menger MD, Breier G. 2002. Microtumor growth initiates angiogenic sprouting with simultaneous expression of VEGF, VEGF receptor 2, and angiopoietin 2. J Clin Invest 109:777–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Visconti RP, Richardson CD, Sato TN. 2002. Orchestration of angiogenesis and arteriovenous contribution by angiopoietins and vascular endothelial growth factor (VEGF). Proc Natl Acad Sci USA 99:8219–8224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong AL, Haroon ZA, Werner S, Dewhirst MW, Greenberg CS, Peters KG. 1997. Tie2 expression and phosphorylation in angiogenic and quiescent adult tissues. Circ Res 81:567–574 [DOI] [PubMed] [Google Scholar]
- 37.Xue C, Rengasamy A, Le Cras TD, Koberna PA, Dailey GC, Johns RA. 1994. Distribution of NOS in normoxic vs hypoxic rat lung: upregulation of NOS by chronic hypoxia. Am J Physiol 267:L667–L678 [DOI] [PubMed] [Google Scholar]
- 38.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. 2000. Vascular-specific growth factors and blood vessel formation. Nature 407:242–248 [DOI] [PubMed] [Google Scholar]
- 39.Zagzag D, Hooper A, Friedlander DR, Chan W, Holash J, Wiegand SJ, Yancopoulos GD, Grumet M. 1999. In situ expression of angiopoietins in astrocytomas identifies angiopoietin 2 as an early marker of tumor angiogenesis. Exp Neurol 159:391–400 [DOI] [PubMed] [Google Scholar]
- 40.Zhao YD, Campbell AI, Robb M, Ng D, Stewart DJ. 2003. Protective role of angiopoietin 1 in experimental pulmonary hypertension. Circ Res 92:984–991 [DOI] [PubMed] [Google Scholar]