Abstract
Campylobacter jejuni is 1 of the most common enteric bacterial pathogens worldwide. The mechanisms of pathogenesis remain obscure, in part because of limitations of small animal models. Young ferrets develop diarrhea when fed C. jejuni, but their pathology and the immune response after infection have not been examined in detail. In the present study, we examined the pathogenesis of C. jejuni CG8421 and associated immune responses in ferrets. After oral infection with C. jejuni CG8421, 86.7% of the animals developed diarrhea and inflammatory responses that were similar to those seen in human infection. Pronounced histopathologic changes in the colonic mucosa of infected animals were observed during the acute phase (days 1 through 3) of infection. Electron micrographs of colonic epithelium revealed disruption of the villi and internalized bacteria that were not within membrane vacuoles. During the acute phase, C. jejuni was isolated from the livers of 7 of 9 (78%) animals, and bacteria were visualized immunohistochemically in the livers from 5 of the 7 animals (71%) from which C. jejuni was isolated. A vigorous systemic and mucosal immune response to Campylobacter antigens was elicited after infection of ferrets. The data presented contribute to the current knowledge of the pathogenicity of and immunologic response to C. jejuni CG8421 in ferrets and better understanding of this model.
Abbreviation: ASC, antibody-secreting cells
Campylobacter jejuni are gram-negative, spiral, microaerophilic, motile bacteria and 1 of the most common enteric bacterial pathogens globally.15,20 The annual incidence of C. jejuni diarrheal disease varies geographically, ranging from sporadic infections in young adults in developed countries28 to as frequent as 40,000 per 100,000 among children living in hyperendemic regions.15 In addition, C. jejuni is the primary cause of ‘traveler's diarrhea’ in various regions of the world.39,41,44 The clinical disease of C. jejuni infection has a wide spectrum of symptoms, varying from a mild, transient watery diarrhea to a bloody diarrhea with severe abdominal cramps and fever. C. jejuni infection also is associated with development of Guillain–Barré syndrome.51 Most strains of C. jejuni contain lipooligosaccharides that mimic human gangliosides structurally. Antibodies against these lipooligosaccharide structures lead to an autoimmune response, resulting in Guillain–Barré syndrome.52
Despite extensive study, little is understood about the mechanism by which C. jejuni causes diarrheal disease. Various Campylobacter small animal models have been reported, but these require surgical manipulation,13,46 administration of chemicals or antibiotics (or both),17,26,30 or inoculation by an unnatural route.5 None of these published models incorporate the natural route of infection. Although several immunodeficient mouse models have been reported,35,36,49 the applicability of these models for studying the pathogenesis and immune response of natural infection is limited. An adult mouse lung model has been described that may be useful for studying pathogenesis and inflammatory response and acquired immunity, but the route of infection is not the same as that in humans.1,5 Unlike other small animals, naïve ferrets (younger than 11 wk) intragastrically inoculated with C. jejuni developed mild to moderate diarrhea with mucus, fecal leukocytes or frank blood that lasted for as long as 3 d and remained colonized for as long as 8 d.9,10,18 Moreover, on rechallenge with the homologous bacterial strain, ferrets asymptomatically excreted C. jejuni in their stools.10 The observation of resistance to disease, but not to infection, is similar to findings from human studies, in which protection against disease developed before colonization resistance.11 Ferrets have been used to evaluate vaccines against C. jejuni14 and in molecular pathogenesis studies using strain 81-176 to confirm a role in virulence for capsular polysaccharide and flagellar glycans.3,21 However, we evaluated the histopathology and immune response after C. jejuni infection in ferrets in more detail in the present study.
C. jejuni CG8421 was isolated from a case of dysentery in Thailand. It has an lipooligosaccharide core that, as shown by chemical analyses, lacks all ganglioside mimicry.38 The absence of ganglioside mimicry is predicted to reduce the risk of Guillain–Barré syndrome. We selected strain CG8421 for the current studies as part of an evaluation of a potential strain for use in future human vaccine challenge studies. Here we report the pathogenesis, histopathology, inflammatory, and immune responses after oral infection of ferrets with C. jejuni strain CG8421.
Materials and Methods
Ferrets.
Pathogen-free (including Campylobacter and Helicobacter spp.) female domestic ferrets (Mustela putorius furo; age, 5.5 to 6 wk) were purchased from Triple F Farms (Sayre, PA). To ensure absence of Campylobacter infection, rectal swabs were obtained immediately on receipt of each animal from the vendor and plated on sheep blood agar plates containing cefoperazone, vancomycin, and amphotericin B (CVA agar, Remel, Lenexa, KS). Ferrets were housed singly in guinea pig polycarbonate cages (Lenderking Caging Products, Millersville, MD), with Alpha Pads (Quality Lab Products, Elkridge, MD) lining the bottom. Commercial ferret chow (Triple F Farms) and water were provided ad libitum. Animals were allowed to acclimate to housing conditions for 5 d before being used in experiments. All husbandry and experimental procedures were performed under Animal Biosafety Level 2 requirements in accordance with institutional policies regarding animal care and use and guidelines in the Guide for the Care and Use of Laboratory Animals24 and were approved by the institutional animal care and use committee.
Bacterial strain and growth conditions.
C. jejuni strain CG8421 (serotype HS23/36) was isolated from a United States military service member with acute dysentery in Khorat, Thailand, in May 1999. The subject presented to the clinic with acute diarrhea, dysentery, gross blood in stool, abdominal cramps, and fever. The strain was isolated by filtration of a stool suspension onto Brucella sheep blood agar, confirmed as C. jejuni, serotype HS23/36, and found to be susceptible to nalidixic acid, ciprofloxacin, and azithromycin with resistance to tetracycline. The genome sequence and lipooligosaccharide core structure of this strain have been determined.38 C. jejuni strain 81-176 (Penner serotype, HS23/36) was used as an infectivity reference strain; this strain initially was isolated from a child with watery diarrhea during a milkborne outbreak33 and then was used in a human challenge study.11 A recovered isolate from a subject with diarrhea11 was obtained, and a stock was kept frozen for animal studies.4
Frozen bacterial stock of each strain was inoculated onto Mueller–Hinton agar and incubated overnight at 37 °C under microaerophilic conditions. Biphasic cultures then were prepared by using Brucella broth over 5% sheep blood agar in Brucella agar.14,50 After additional overnight incubation at 37 °C, the broth portion was harvested and suspended in PBS. The bacterial concentration was adjusted spectrophotometrically to obtain approximately 2 × 109 CFU/mL. Precise bacterial counts of the inoculum were obtained by plating serial dilutions on blood agar plate (described later).
Study protocol and C. jejuni feeding.
Animals were randomized into 5 groups (to be euthanized on study days 1, 2, 3, 6, and 9) of 4 animals (3 to be infected, 1 PBS control). After withholding of food and water for 2 to 3 h, animals were anesthetized with ketamine (25 to 40 mg/kg IM) and xylazine (1 to 2 mg/kg; Phoenix Scientific, St Joseph, MO) and then were fed 10 mL of bacterial culture in NaHCO3 (5 mL of 2 × 109/mL bacteria + 5 mL 5% NaHCO3) via orogastric feeding tube. Five randomly assigned control animals received 5 mL PBS without bacteria and 5 mL 5% NaHCO3. One hour after inoculation with C. jejuni or PBS, 2.8 mL/kg of tincture of opium (Paregoric liquid, Henry Schein Animal Health, Reno, NV) was injected intraperitoneally; the detailed challenge procedure is described elsewhere.10,14 After infection, animals were observed twice daily for development of diarrhea (defined as loose stool with strings of nonteaseable mucus), presence of frank blood in the stool, signs of dehydration, and other infection-associated illness. To determine whether infection affected the rate of growth, body weights were measured before infection and daily for 9 d after infection.
Sample collection and processing.
While ferrets were under deep anesthesia [ketamine (25 to 40 mg/kg) and xylazine (1 to 2 mg/kg) mixed and delivered intramuscularly], cardiac blood (7 to 14 mL) was collected in a syringe and placed immediately in either tubes containing EDTA or serum separator tubes. A portion of the whole blood was used to determine complete blood cell count, and mononuclear cells were isolated from the remainder by density gradient, cryopreserved in DMSO-containing medium (Sigma Chemical, St Louis, MO) at a controlled rate, and stored in liquid nitrogen to be used later to determine antibody secreting cells.6,7 Serum was used to determine liver enzymes (alanine aminotransferase, alkaline phosphate, aspartate aminotransferase, total bilirubin), total protein, albumin, gammaglobulins, C-reactive protein, and Campylobacter antigen-specific serum IgA and IgG by ELISA. Once blood was obtained, the animals were euthanized with sodium pentobarbital (Euthasol, Henry Schein) through intracardiac injection, and tissues were collected for microbiology and histopathology.
Fecal samples were collected before infection and on days 1 through 4, and 6 and 9 after infection to determine excretion of C. jejuni in stool, presence of occult blood, lactoferrin, and the levels of C. jejuni antigen-specific IgA and IgG.
Due to difficulty in obtaining blood from 1 of the control animals (blood clotted in EDTA), that animal was removed from the study.
Necropsy and tissue collection.
Full necropsies were performed on all animals. To maintain consistency between animals, the entire intestine was measured and sampled obtained for histology from the greater curvature of the stomach; 1 cm distal to the pylorus, 20 cm distal to the pylorus and 80 cm distal to the pylorus for the small intestine; and 0.5 cm proximal to rectum, 2.5 cm proximal to rectum, and 5 cm proximal to rectum for large intestine. The tissues were placed in 10% buffered formalin, processed by standard methods, embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin and eosin. Numerical scores for superficial inflammation, mucosal changes, epithelial hyperplasia, and goblet cell loss were as follows: 0, none; 1, minimal; 2, mild; 3, moderate; 4, marked; and 5, severe. Peritonitis was scored as either 0 (absent) or 1 (present). Histopathologic scores for stomach, small intestine, and colon are presented as mean ± 1 SD during acute phase (days 1 through 3), recovery phase (days 6 and 9, and controls. Liver, kidney, lung, mesenteric lymph nodes, and any tissue that looked grossly abnormal were processed in addition to intestinal tissues. Immunohistochemistry was performed by using polyclonal antibodies prepared by immunizing rabbits with formalin-fixed C. jejuni CG8421 (Harlan, Indianapolis, IN). Sections of liver, spleen, and large and small intestine were used to determine the presence of C. jejuni by culture (described later).
Inflammatory responses.
Occult blood (Hemoccult, Beckman Coulter, Brea, CA) and lactoferrin (Leuko-Test, Inverness Medical Professional Diagnostics, Princeton, NJ) in stool and C- reactive protein (RapiTex, Dade Behring, Marburg, Germany) in serum were determined by using commercially available kits according to the manufacturer's instructions.
Campylobacter microbiology.
At various intervals after infection, blood, liver, spleen, large intestine, small intestine, and fecal material from 3 infected and 1 control animal were collected and individually processed to determine the presence of challenge organisms. Approximately 150 to 300 mg feces per animal was used to prepare a 1:4 (w/v) homogenous suspension in PBS. Portions of organs and tissues (300 to 500 g) were homogenized in cold PBS (1:5, w/v). Dilutions of each homogenate were plated in duplicate on CVA agar (Remel). After 48 h of incubation, C. jejuni colonies were counted. Data are expressed as mean CFU per gram of tissue or feces or per milliliter of blood.
Measurement of immune response.
Total protein was extracted from fecal samples collected from infected animals on days 4 (n = 6), 6 (n = 6), and 9 (n = 3) and from controls (n = 5), as described elsewhere.4 Serum samples were collected from control animals (n = 5) and from animals euthanized on days 6 and 9 (total, 6 animals). C. jejuni CG8421 glycine-extracted proteins (3 µg/mL) were used to detect antigen-specific fecal and serum IgA and IgG. Endpoint titers (highest dilution of the sample given a net OD405 of 0.12 for fecal samples, 0.15 for serum immunoglobulins) of individual samples were determined and log transformed (loge) and are presented as mean ± 1 SD.
Measurement of IgA antibody-secreting cells.
Cardiac blood mononuclear cells collected from controls (n = 4) and animals euthanized on days 6 and 9 (n = 6) were used to determine Campylobacter-specific IgA antibody-secreting cells (ASC) by ELISpot using a modification of a procedure published previously.5,7 After thawing and washing, viability of mononuclear cells was determined using Guava ViaCount Reagent (Guava Technologies, Hayward, CA). Viable cells (average viability 92.8 ± 1.9%) were adjusted to 5 × 106/mL in medium containing 10% FCS, 2 mM L-glutamine, and 50 μg/mL gentamicin (complete medium). Multiscreen immunoplates (catalog no. S2EM004M99, Millipore, Bedford, MA) were coated with C. jejuni glycine-extracted antigens, and control wells were coated with bovine serum albumin (10 µg/mL). Plates were blocked with 5% FCS in RPMI; then 100 μL complete medium containing 5 × 105 mononuclear cells was added in duplicate. Plates were incubated overnight at 37 °C in a 5% CO2 environment. After addition of 0.025 μg alkaline-phosphate–conjugated goat antiferret IgA (Rockland Immunochemical, Gilbertsville, PA), plates were incubated for an additional 2 h at 37 °C. Spots were developed by using nitroblue tetrazolium, 5-bromo-4-chloro-3-indolyl phosphate substrate (SigmaFast, Sigma Chemicals, St Louis, MO). After an additional 15 min of incubation, plates were rinsed extensively with water, air-dried, and counted by using a spot analyzer (Cellular Technology, Shaker Heights, OH). Data are expressed as the number (loge) of ASC per 106 mononuclear cells.
Transmission electron microscopy.
Samples for transmission electron microscopy were obtained distal to the samples from the small intestine and proximal to those from the large intestine. The samples were fixed in 4% formaldehyde–1% glutaraldehyde, washed twice in phosphate buffer (Dako, Carpentaria, CA), postfixed in 1% osmium tetroxide, dehydrated in graded ethanol solutions and propylene oxide, and embedded in EMbed 812 (Electron Microscopy Sciences, Hatfield, PA). Thin (80 to 100 nm) sections were stained in lead citrate and uranyl acetate and examined by electron microscopy (model LEO912, Carl Zeiss SMT, Thornwood NY).
Statistical analyses.
Animals were assigned randomly by using the PROC PLAN procedure (SAS version 8.1, SAS Institute, Cary, NC) to either acute (day 1 to 3, n = 9), recovery (day 4 to 9, n = 6), or control (n = 5) groups. Analysis of variance was used to compare results of complete blood counts and serum chemistry analyses between groups. Pairwise comparisons were used to detect differences between the acute and recovery groups. Repeated measures ANOVA was used to analyze body weights. Data were compared by ANOVA with Tukey–Kramer correction to adjust for multiple comparisons. ANOVA was used to analyze fecal IgA and IgG data, and t tests were used to analyze IgA–ASC, serum IgA, and serum IgG results. The significance (alpha) level was set at 0.05. All analyses were done by using SAS (SAS Institute).
Results
C. jejuni infection outcome.
After oral challenge with 1010 CFU C. jejuni 81-176 or C. jejuni GC8421, all 15 (100%) ferrets were infected, by fecal culture, whereas 7 of 8 (87.6%) strain 81-176 recipients and 5 of 7 (71.4%) strain CG8421 recipients developed diarrhea. The preliminary results showed similar infectivity for the 2 strains. In the subsequent study, oral challenge with 1010 CFU C. jejuni GC8421, all 15 (100%) ferrets were infected, and 13 of 15 (86.7%) developed diarrhea. In contrast, neither strain was isolated from animals that received PBS only, and a change in their fecal consistency was not noted. Of the 13 animals infected with CG8421 that developed diarrhea, the first diarrheal stool was within 12 h for 3 animals (23%), 24 h for 9 animals (69%), and 60 h for 1 animal (8%). Duration of diarrhea varied between 1 and 3 d, but the precise diarrheal day for all animals could not be determined because animals were selected randomly for euthanasia for the first 3-d of the experiment. These findings are similar to those reported for other strains of C. jejuni.3,10,14,50
Lactoferrin and gross and occult blood in feces were measured as markers for inflammation. One day after challenge, 80% of all infected animals (92% of animals that developed diarrhea) showed gross blood in their stool; this value gradually declined to 22% by day 3, but no gross blood was present on or beyond day 4 (Table 1). Compared with occult blood, lactoferrin was more sensitive in detecting inflammation (Table 1). C-reactive protein was not detected in blood at any time during the study period. No difference in growth rates between infected and control animals were observed.
Table 1.
Inflammatory response to C. jejuni infection
| Fecal inflammatory marker [no. positive/no. tested (%)] |
||||
| Group | Day | Gross blood | Occult blood | Lactoferrin |
| C. jejuni sCG8421 | 1 | 12/15 (80) | 8/15 (53) | 10/15 (67) |
| 2 | 5/12 (42) | 5/12 (42) | 6/12 (50) | |
| 3 | 2/9 (22) | 3/9 (33) | 5/9 (56) | |
| 6 or 9 | 0/6 (0) | 0/6 (0) | 0/6 (0) | |
| Control | Any day | 0/5 (0) | 0/5 (0) | 0/5 (0) |
Stool samples collected on days 6 (n = 6) or day 9 (n = 3) were negative for all markers. C-reactive protein was not detected in any serum sample during the study.
Course of infection.
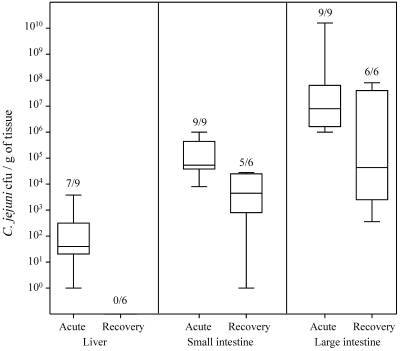
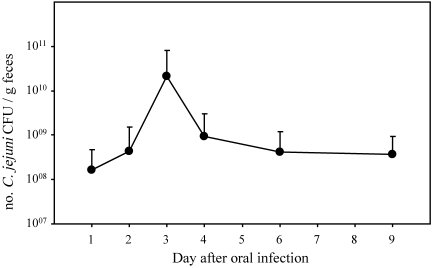
After oral infection of ferrets with 1010 CFU C. jejuni, 3 patterns emerged. First, initial colonization during the acute phase and then reduction in bacterial load during the recovery phase occurred in both the small and large intestine (Figure 1). C. jejuni was isolated from all animals, although the bacterial counts per gram of large intestine were approximately 2 logs lower (P < 0.05) during recovery phase than acute phase. The reduction in bacterial counts in the small intestine did not differ between acute and recovery periods. The second pattern that emerged involved the secretion of C. jejuni in feces. The number of C. jejuni shed in feces gradually increased, peaked at day 3, and then declined by 1 log and persisted essentially at unchanged levels through the course of the study (Figure 2). The third pattern of infection occurred in the liver. C. jejuni was isolated from livers of 7 of 9 animals during the acute phase of infection, but no bacteria were isolated during the recovery phase (Figure 1). C. jejuni was not isolated from the blood of any animal or from 14 of 15 spleens (data not shown).
Figure 1.
Isolation of C. jejuni from tissues. Acute-phase samples were collected during days 1 through 3, and recovery-phase samples were collected on days 6 and 9. Numbers over each box indicate the number of ferrets positive / number tested. In liver and large intestine, levels of colonization were significantly (P < 0.05) different during acute and recovery phases, whereas no difference was observed for the small intestine (P > 0.05). Compared with the large intestine, small intestine yielded significantly (P > 0.05) lower levels of C. jejuni.
Figure 2.
Fecal excretion of C. jejuni after oral infection. All ferrets challenged with C. jejuni excreted bacteria in their stools until the day of euthanize. None of the 5 controls were positive for C. jejuni excretion at any given time (17 fecal samples from controls were evaluated during the study). The magnitude of colonization throughout the study did not differ significantly (P > 0.05).
Clinical pathology.
Results of complete blood counts and serum chemistries are summarized in Table 2. White blood cell counts during the acute phase of infection were significantly lower (P < 0.05) than those of controls, whereas neutrophils in infected animals during both phases of infection were significantly (P < 0.05) lower than those of control ferrets. Compared with control animals, total serum protein in infected animals was increased (P < 0.05) during the recovery phase.
Table 2.
Clinical pathology
| Experimental group (mean ± 1 SD) |
|||
| Infected with C. jejuni |
|||
| Parameter | Control | Acute phase | Recovery phase |
| WBC (x106 /mL) | 10.1 ± 4.8 | 5.4 ± 1.9a | 5.7 ± 1.4a |
| Neutrophils (x106 /mL) | 4.8 ± 2.8 | 2.1 ± 1.1a | 2.1 ± 0.9a |
| Total protein (mg /mL) | 44.5 ± 3.7 | 44.2 ± 3.6 | 50.0 ± 2.7 |
| Albumin (mg /mL) | 23.3 ± 2.2 | 22.0 ± 1.4 | 26.2 ± 2.1 |
| Globulin (mg /mL) | 21.3 ± 9.0 | 22.1 ± 2.7 | 23.8 ± 4.0 |
No changes in other parameters (RBC and liver enzymes, as listed in Material and Methods) occurred.
Mean was significantly (P < 0.05) different from that of controls; no significant changes between infection phases were noted.
Histopathology.
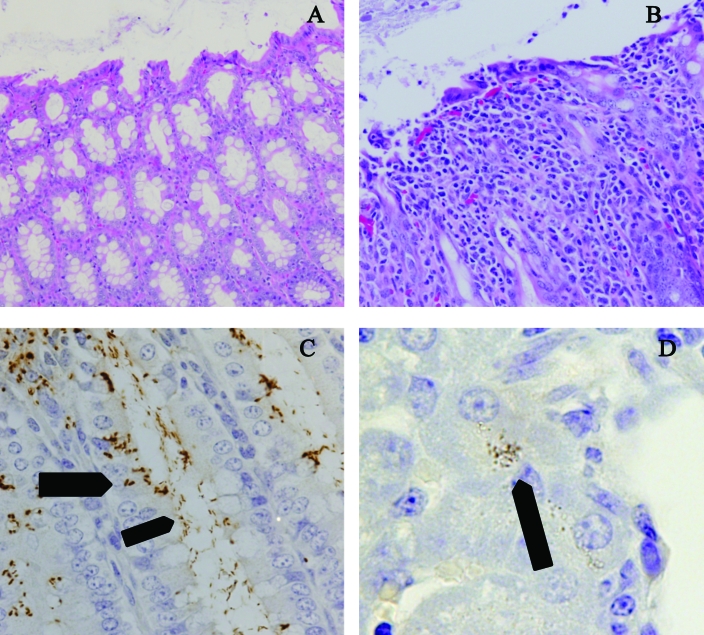
Histopathologic changes in the small intestine of infected and control animals during various phases of infection were fewer and less intense than those in the colon (score: acute phase, 1.1 ± 0.7; recovery phase, 1.1 ± 0.8; controls, 0.5 ± 0.5; P < 0.05 for all comparisons). Changes in the colon appeared more representative of pathology related to infection with C. jejuni. Colonic lesions were more severe earlier in the course of the experiment: pronounced changes in the colonic mucosa were present primarily on the first and second days of infection (score: acute phase, 2.3 ± 1.0; recovery phase, 0.9 ± 0.8; controls, 0.5 ± 0.7). There were areas of epithelial cell loss, and the remaining epithelial cells often were attenuated and diffuse, presumably in response to a loss of enterocytes (Figure 3 B compared with normal shown in Figure 3 A). Increases in the mitotic rate of enterocytes were noted in both the crypts and superficial surface tissues. Cells in the affected sections were less mature, and as a result, few goblet cells were present. Initially the primary inflammatory cells in these areas were neutrophils, but during recovery, mucosal changes were much less prominent. In most cases, epithelial coverage was nearly normal by day 6. The initial infiltration of neutrophils into the mucosa was followed by macrophages. Peritoneal histopathologic evaluation revealed no significant changes in infected or control animals at anytime during the study. No histologic changes were seen in livers, kidneys, lungs, or mesenteric lymph nodes.
Figure 3.
Histopathology and immunohistochemistry of C. jejuni. (A) Colon of a control ferret, showing abundance of goblet cells, the columnar epithelium, and the distance between colonic crypts. Hematoxylin and eosin; magnification, ×20. (B) Colon of an infected ferret on day 3, showing the lack of goblet cells, attenuation and loss of epithelium, and separation of the colonic crypts by numerous inflammatory cells. Hematoxylin and eosin; magnification, ×20. (C) Colon of an infected ferret on day 3, showing presence of Campylobacter along the luminal surface(narrow arrow) as well as within or between enterocytes (wide arrow) by using immunohistochemistry for antiCampylobacter antibodies and hematoxylin counterstain. Magnification, ×40. (D) Liver of an infected ferret on day 1, showing Campylobacter within hepatocytes (arrow) by using immunohistochemistry for antiCampylobacter antibodies and hematoxylin counterstain (oil immersion). Magnification, ×100.
Immunohistochemistry.
C. jejuni were found throughout the lumen of the small and large intestines and were present both intra- and extracellularly in the large intestine (Figure 3 C – wide and thin arrows). Bacteria reactive to antiC. jejuni antibody were apparent in liver from 5 of 7 (71%) infected animals (Figure 3 D).
Transmission electron microscopy.
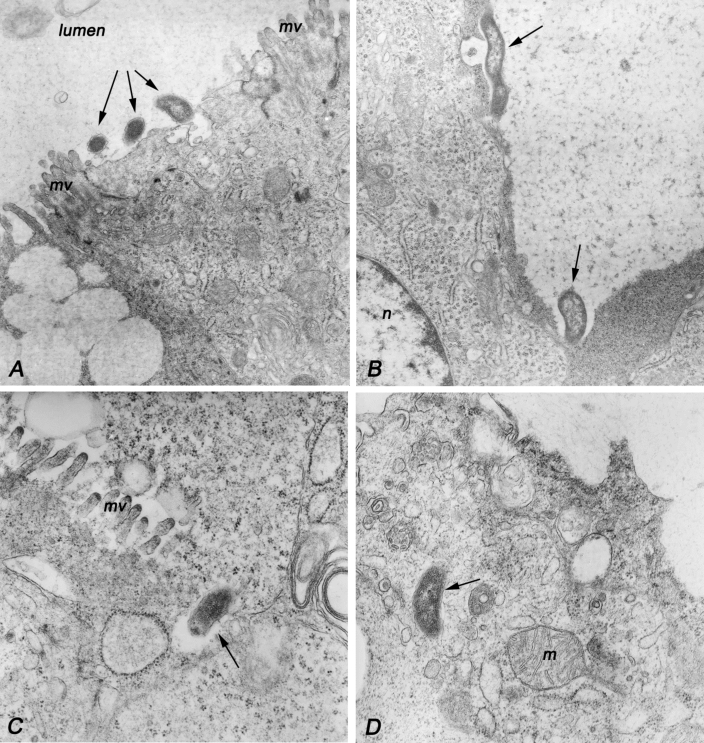
C. jejuni were most numerous in the lumen of the colon, in close association with colonic epithelial cells. At the sites of bacterial attachment, surface villi usually were absent (Figure 4 A). Occasionally, the cellular membrane next to bacteria was invaginated (Figure 4 A, B). However, intracellular bacteria did not appear to be contained in vacuoles (Figure 4 C, D).
Figure 4.
Transmission electron microscopy of Campylobacter jejuni in the colon of an infected ferret. (A) Electron micrograph showing attachment of 3 bacteria (arrows) to the epithelial cell surface. Notice the loss of microvilli (mv) at the site of attachment. Original magnification, ×35,000. (B) Loss of microvilli at the site of Campylobacter attachment to epithelial cells (arrows). A membranous vesicle is present next to the longer microorganism. Original magnification, × 27,000. (C) Epithelial cell invasion by C. jejuni. The clear space is being formed on one side of the microbe (arrow) is suggestive of direction of the invasion. No membrane is visible surrounding the internalized bacteria. Original magnification, ×54,000. (D) Intracellular Campylobacter apparently free in the cytoplasm (arrow). M, mitochondria. Original magnification, ×54,000.
Immune response to C. jejuni.
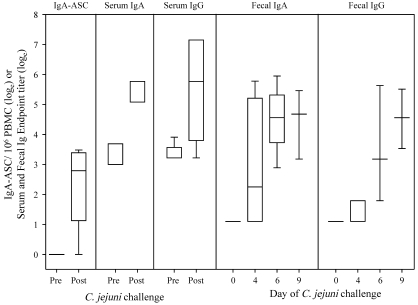
Blood samples collected at days 6 and 9 after infection and those from control animals were used to determine C. jejuni-specific IgA-ASC and IgA and IgG levels in serum (Figure 5). Control animals lacked ASC, whereas 5 of 6 animals infected with C. jejuni had about 20 ASC/106 mononuclear cells (mean loge, 2.92 ± 0.51) in their blood. Similarly, significantly (P < 0.05) higher levels of serum IgA and IgG specific for C. jejuni glycine-extracted protein were detected in infected animals. Infection was followed by induction of antigen-specific fecal IgA at day 4, which increased significantly (P < 0.05) at day 6 and remained at the same level on day 9. Significant (P < 0.05) fecal IgG was detected in 2 of 3 animals on day 6 and all 3 animals on day 9.
Figure 5.
Immune response to C. jejuni after infection. ‘Pre’ indicates the samples collected from control animals (n = 4), and ‘Post’ are samples collected on study days 6 (n = 3) and 9 (n = 3) from infected animals. The levels of IgA-ASC and serum IgA and IgG were significantly (P < 0.05) higher after infection. Compared with those in controls, fecal IgA and IgG were significantly (P < 0.05) higher on days 6 and 9 after infection.
Discussion
The primary goal of this study was to determine the virulence of C. jejuni strain CG8421 and the antigen-specific immune responses in ferrets as part of the characterization of this strain for future use as a challenge strain in human vaccine efficacy studies. Ferrets infected orally with C. jejuni CG8421 developed diarrhea at rates and with illness patterns comparable to those reported for humans under experimental conditions11 or in natural settings in epidemiologic studies.2,8,15,39,40,43,44 C. jejuni CG8421-induced diarrhea in these animals contained mucus and gross blood. The inflammatory nature of the diarrhea was further confirmed by the presence of lactoferrin and blood in stools. The commercial kits we used had not been validated for use in ferrets; similar kits have been reported to detect occult blood in other animals.45 Although diarrhea resolved spontaneously within 3 d of infection, C. jejuni was excreted from feces throughout the study. The duration of diarrhea and infection rates associated with CG8421 were similar to those reported for strain 81-176 in ferrets.3,14,21 The pattern of diarrhea after C. jejuni CG8421 infection and colonization in ferrets was similar to those reported for humans.47 In humans, untreated C. jejuni cases may excrete bacteria asymptomatically for as long as 2 wk.11,12,47 In the present study, both mucosal (associated with production of ASC, fecal IgA and IgG) and systemic (serum IgA and IgG) immune responses were detected and appeared to be functional in controlling diarrhea, while fecal excretion of C. jejuni continued. In mice,4 ferrets,10 nonhuman primates,25,27 and humans,11,42 immunity to sickness is suggested to be controlled by an immune mechanisms distinct from that controlling immunity to infection.
C. jejuni CG8421 stimulated both mucosal and systemic antigen-specific IgA and IgG. However, the detection of IgG in feces is not a frequent finding, because IgG generally is not secreted across intestinal epithelial cells. It is unclear whether the IgG detected was synthesized locally or resulted from leakage due to intestinal epithelial disruption. However, a significant fecal IgG level was detected on day 9, without concurrent inflammation of the mucosa (gross blood, occult blood, lactoferrin). Because of the limited duration of the study, the kinetics and magnitude of the immune responses could not be determined.
C. jejuni generally is considered to cause an inflammatory diarrhea in humans, consistent with the observed pathology of C. jejuni CG8421 in ferrets. Numerous neutrophils were present within the epithelial layer and lamina propria of the colons of the infected animals (Figure 3 B). The neutropenia seen in infected animals may be the result of continual sequestering of the neutrophils to the intestinal epithelium. The significant rise in serum total protein during the recovery phase may be an indication of an active immune system. Significant pathology was observed in the colons of the infected animals. Damage of the brush border was noted in the colons of infected animals in areas adjacent to bacteria that appeared to be bound to the epithelial cells (Figure 3 B). Similar villus damage during C. jejuni infection of IL10 knockout mice35 and in a hamster model of C. jejuni infection23 have been reported recently. Studies of C. jejuni interactions with epithelial cells in vitro have indicated that the organism is invasive, although there is a considerable range in invasive level of individual strains19,31 Strain 81-176, one of the most highly invasive strains in vitro, invades by a microtubule-dependent mechanism and is internalized into vacuoles within the epithelial cells.32 After internalization, C. jejuni survives within the vacuoles by inhibition of endosomal acidification or prevention of fusion with lysosomes.48 The reported levels of in vitro invasion of CG8421 are significantly lower than those of 81-176.38 However, immunohistochemistry and electron microscopy showed intracellular, as well extracellular, CG8421 bacteria within the intestine (Figures 3 B and 4). Moreover, electron microscopy revealed no evidence of a membranous vacuole surrounding CG8421 (Figure 4). This observation will require additional studies to determine whether it is specific to the host (ferrets) or to this strain of C. jejuni.
The presence of C. jejuni (by microbiologic isolation and immunohistochemical reactivity) in liver within 24 h after inoculation did not elicit a detectable inflammatory response, as verified by lack of C-reactive protein in the blood. The failure to detect this protein may be due at least in part to the use of reagents for testing humans. However no histopathologic lesions were seen in any of the ferret liver samples, although the isolation of C. jejuni from liver in other animal species has been reported previously.16,34 The mechanism of dissemination of Campylobacter has not been defined, but potentially C. jejuni enter the portal system and are filtered by the liver, travel retrograde through the bile duct, or are carried by monocytes or macrophages.22,29,37
We designed the present study to increase understanding of the pathogenesis, histopathology, and acquired immunity of ferrets infected with C. jejuni CG8421. The results confirm the virulence of this strain, which lacks a siaylated lipooligosaccharide core, and underscore the usefulness of the ferret model for studies of C. jejuni pathogenesis.
Acknowledgments
This work was made possible with the assistance of Rodney Sturdivant (Center for Data Analysis and Statistics, Department of Mathematical Sciences, United States Military Academy, West Point, NY), and the Divisions of Veterinary Medicine and Pathology at Walter Reed Army Institute of Research, Silver Spring, MD. We thank Ms Lanfong Lee for preparing Campylobacter glycine-extract antigens for immunologic assays.
This material has been reviewed by the Walter Reed Army Institute of Research and Naval Medical Research Center. There is no objection to its presentation or publication. The opinions or assertions contained herein are private views of the authors and are not to be construed as official or as reflecting views of the Walter Reed Institute of Research, Department of the Army, the Naval Medical Research Center, Department of the Navy, the Department of Defense, nor the US Government.
These studies were supported by US Navy Research and Development Command Work Units 6000.RAD1.DA3.A0308. The authors have no conflicting financial interests. KWN, AWB, SMW, LVA, PG, and SB are employees of the US Government. This work was prepared as part of their official duties. Title 17 USC 101 defines a US Government work as a work prepared by a military service member or employee of the US Government as part of that person's official duties.
References
- 1.Al-Banna NA, Junaid TA, Mathew TC, Raghupathy R, Albert MJ. 2008. Histopathological and ultrastructural studies of a mouse lung model of Campylobacter jejuni infection. J Med Microbiol 57:210–217 [DOI] [PubMed] [Google Scholar]
- 2.Allos BM. 2001. Campylobacter jejuni infections: update on emerging issues and trends. Clin Infect Dis 32:1201–1206 [DOI] [PubMed] [Google Scholar]
- 3.Bacon DJ, Szymanski CM, Burr DH, Silver RP, Alm RA, Guerry P. 2001. A phase-variable capsule is involved in virulence of Campylobacter jejuni 81-176. Mol Microbiol 40:769–777 [DOI] [PubMed] [Google Scholar]
- 4.Baqar S, Applebee LA, Gilliland TC, Jr, Lee LH, Porter CK, Guerry P. 2008. Immunogenicity and protective efficacy of recombinant Campylobacter jejuni flagellum-secreted proteins in mice. Infect Immun 76:3170–3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baqar S, Bourgeois AL, Applebee LA, Mourad AS, Kleinosky MT, Mohran Z, Murphy JR. 1996. Murine intranasal challenge model for the study of Campylobacter pathogenesis and immunity. Infect Immun 64:4933–4939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baqar S, Bourgeois AL, Schultheiss PJ, Walker RI, Rollins DM, Haberberger RL, Pavlovskis OR. 1995. Safety and immunogenicity of a prototype oral whole-cell killed Campylobacter vaccine administered with a mucosal adjuvant in nonhuman primates. Vaccine 13:22–28 [DOI] [PubMed] [Google Scholar]
- 7.Baqar S, Nour El Din AA, Scott DA, Bourgeois AL, Mourad AS, Kleinosky MT, Oplinger MJ, Murphy JR. 1997. Standardization of measurement of immunoglobulin-secreting cells in human peripheral circulation. Clin Diagn Lab Immunol 4:375–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baqar S, Rice B, Lee L, Bourgeois AL, El Din AN, Tribble DR, Heresi GP, Mourad AS, Murphy JR. 2001. Campylobacter jejuni enteritis. Clin Infect Dis 33:901–905 [DOI] [PubMed] [Google Scholar]
- 9.Bell JA, Manning DD. 1990. A domestic ferret model of immunity to Campylobacter jejuni-induced enteric disease. Infect Immun 58:1848–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bell JA, Manning DD. 1991. Evaluation of Campylobacter jejuni colonization of the domestic ferret intestine as a model of proliferative colitis. Am J Vet Res 52:826–832 [PubMed] [Google Scholar]
- 11.Black RE, Levine MM, Clements ML, Hughes TP, Blaser MJ. 1988. Experimental Campylobacter jejuni infection in humans. J Infect Dis 157:472–479 [DOI] [PubMed] [Google Scholar]
- 12.Blaser MJ, Duncan DJ, Osterholm MT, Istre GR, Wang WL. 1983. Serologic study of 2 clusters of infection due to Campylobacter jejuni. J Infect Dis 147:820–823 [DOI] [PubMed] [Google Scholar]
- 13.Burr DH, Caldwell MB, Bourgeois AL, Morgan HR, Wistar R, Jr, Walker RI. 1988. Mucosal and systemic immunity to Campylobacter jejuni in rabbits after gastric inoculation. Infect Immun 56:99–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burr DH, Rollins D, Lee LH, Pattarini DL, Walz SS, Tian JH, Pace JL, Bourgeois AL, Walker RI. 2005. Prevention of disease in ferrets fed an inactivated whole-cell Campylobacter jejuni vaccine. Vaccine 23:4315–4321 [DOI] [PubMed] [Google Scholar]
- 15.Coker AO, Isokpehi RD, Thomas BN, Amisu KO, Obi CL. 2002. Human campylobacteriosis in developing countries. Emerg Infect Dis 8:237–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enokimoto M, Kubo M, Bozono Y, Mieno Y, Misawa N. 2007. Enumeration and identification of Campylobacter species in the liver and bile of slaughtered cattle. Int J Food Microbiol 118:259–263 [DOI] [PubMed] [Google Scholar]
- 17.Epoke J, Obi CL, Coker AO. 1992. In vivo effect of cadmium chloride on intestinal colonization of rats by Campylobacter jejuni. East Afr Med J 69:609–610 [PubMed] [Google Scholar]
- 18.Fox JG, Ackerman JI, Taylor N, Claps M, Murphy JC. 1987. Campylobacter jejuni infection in the ferret: an animal model of human campylobacteriosis. Am J Vet Res 48:85–90 [PubMed] [Google Scholar]
- 19.Friis LM, Pin C, Pearson BM, Wells JM. 2005. In vitro cell culture methods for investigating Campylobacter invasion mechanisms. J Microbiol Methods 61:145–160 [DOI] [PubMed] [Google Scholar]
- 20.Frost JA. 2001. Current epidemiological issues in human campylobacteriosis. Symp Ser Soc Appl Microbiol 30:85S–95S [DOI] [PubMed] [Google Scholar]
- 21.Guerry P, Ewing CP, Schirm M, Lorenzo M, Kelly J, Pattarini D, Majam G, Thibault P, Logan S. 2006. Changes in flagellin glycosylation affect Campylobacter autoagglutination and virulence. Mol Microbiol 60:299–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hickey TE, Majam G, Guerry P. 2005. Intracellular survival of Campylobacter jejuni in human monocytic cells and induction of apoptotic death by cytholethal distending toxin. Infect Immun 73:5194–5197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Humphrey CD, Montag DM, Pittman FE. 1985. Experimental infection of hamsters with Campylobacter jejuni. J Infect Dis 151:485–493 [DOI] [PubMed] [Google Scholar]
- 24.Institute of Laboratory Animal Resources. 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academy Press [Google Scholar]
- 25.Islam D, Lewis MD, Srijan A, Bodhidatta L, Aksomboon A, Gettayacamin M, Baqar S, Scott D, Mason CJ. 2006. Establishment of a nonhuman primate Campylobacter disease model for the preclinical evaluation of Campylobacter vaccine formulations. Vaccine 24:3762–3771 [DOI] [PubMed] [Google Scholar]
- 26.Jesudason MV, Hentges DJ, Pongpech P. 1989. Colonization of mice by Campylobacter jejuni. Infect Immun 57:2279–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones FR, Baqar S, Gozalo A, Nunez G, Espinoza N, Reyes SM, Salazar M, Meza R, Porter CK, Walz SE. 2006. New World monkey Aotus nancymae as a model for Campylobacter jejuni infection and immunity. Infect Immun 74:790–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones TF, Scallan E, Angulo FJ. 2007. FoodNet: overview of a decade of achievement. Foodborne Pathog Dis 4:60–66 [DOI] [PubMed] [Google Scholar]
- 29.Kiehlbauch JA, Albach RA, Baum LL, Chang KP. 1985. Phagocytosis of Campylobacter jejuni and its intracellular survival in mononuclear phagocytes. Infect Immun 48:446–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kita E, Katsui N, Nishi K, Emoto M, Yanagase Y, Kashiba S. 1986. Hepatic lesions in experimental Campylobacter jejuni infection of mice. J Gen Microbiol 132:3095–3103 [DOI] [PubMed] [Google Scholar]
- 31.Konkel ME, Joens LA. 1989. Adhesion to and invasion of HEp2 cells by Campylobacter spp. Infect Immun 57:2984–2990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kopecko DJ, Hu L, Zaal KJ. 2001. Campylobacter jejuni microtubule-dependent invasion. Trends Microbiol 9:389–396 [DOI] [PubMed] [Google Scholar]
- 33.Korlath JA, Osterholm MT, Judy LA, Forfang JC, Robinson RA. 1985. A point-source outbreak of campylobacteriosis associated with consumption of raw milk. J Infect Dis 152:592–596 [DOI] [PubMed] [Google Scholar]
- 34.Lamb-Rosteski JM, Kalischuk LD, Inglis GD, Buret AG. 2008. Epidermal growth factor inhibits Campylobacter jejuni-induced claudin-4 disruption, loss of epithelial barrier function, and Escherichia coli translocation. Infect Immun 76:3390–3398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mansfield LS, Bell JA, Wilson DL, Murphy AJ, Elsheikha HM, Rathinam VA, Fierro BR, Linz JE, Young VB. 2007. C57BL/6 and congenic interleukin10-deficient mice can serve as models of Campylobacter jejuni colonization and enteritis. Infect Immun 75:1099–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mansfield LS, Patterson JS, Fierro BR, Murphy AJ, Rathinam VA, Kopper JJ, Barbu NI, Onifade TJ, Bell JA. 2008. Genetic background of IL10(−/−) mice alters host–pathogen interactions with Campylobacter jejuni and influences disease phenotype. Microb Pathog 45:241–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poly F, Guerry P. 2008. Pathogenesis of Campylobacter. Curr Opin Gastroenterol 24:27–31 [DOI] [PubMed] [Google Scholar]
- 38.Poly F, Read TD, Chen YH, Monteiro MA, Serichantalergs O, Pootong P, Bodhidatta L, Mason CJ, Rockabrand D, Baqar S, Porter CK, Tribble D, Darsley M, Guerry P. 2008. Characterization of 2 Campylobacter jejuni strains for use in volunteer experimental-infection studies. Infect Immun 76:5655–5667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanders JW, Isenbarger DW, Walz SE, Pang LW, Scott DA, Tamminga C, Oyofo BA, Hewitson WC, Sanchez JL, Pitarangsi C, Echeverria P, Tribble DR. 2002. An observational clinic-based study of diarrheal illness in deployed United States military personnel in Thailand: presentation and outcome of Campylobacter infection. Am J Trop Med Hyg 67:533–538 [DOI] [PubMed] [Google Scholar]
- 40.Sanders JW, Putnam SD, Gould P, Kolisnyk J, Merced N, Barthel V, Rozmajzl PJ, Shaheen H, Fouad S, Frenck RW. 2005. Diarrheal illness among deployed US military personnel during Operation Bright Star 2001–Egypt. Diagn Microbiol Infect Dis 52:85–90 [DOI] [PubMed] [Google Scholar]
- 41.Scott DA. 1997. Vaccines against Campylobacter jejuni. J Infect Dis 176 Suppl 2:S183–S188 [DOI] [PubMed] [Google Scholar]
- 42.Taylor DN, Perlman DM, Echeverria PD, Lexomboon U, Blaser MJ. 1993. Campylobacter immunity and quantitative excretion rates in Thai children. J Infect Dis 168:754–758 [DOI] [PubMed] [Google Scholar]
- 43.Tribble DR, Baqar S, Pang LW, Mason C, Houng HS, Pitarangsi C, Lebron C, Armstrong A, Sethabutr O, Sanders JW. 2008. Diagnostic approach to acute diarrheal illness in a military population on training exercises in Thailand, a region of Campylobacter hyperendemicity. J Clin Microbiol 46:1418–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tribble DR, Sanders JW, Pang LW, Mason C, Pitarangsi C, Baqar S, Armstrong A, Hshieh P, Fox A, Maley EA, Lebron C, Faix DJ, Lawler JV, Nayak G, Lewis M, Bodhidatta L, Scott DA. 2007. Traveler's diarrhea in Thailand: randomized, double-blind trial comparing single-dose and 3-day azithromycin-based regimens with a 3-day levofloxacin regimen. Clin Infect Dis 44:338–346 [DOI] [PubMed] [Google Scholar]
- 45.Tuffli SP, Gaschen F, Neiger R. 2001. Effect of dietary factors on the detection of fecal occult blood in cats. J Vet Diagn Invest 13:177–179 [DOI] [PubMed] [Google Scholar]
- 46.Walker RI, Schmauder-Chock EA, Parker JL, Burr D. 1988. Selective association and transport of Campylobacter jejuni through M cells of rabbit Peyer's patches. Can J Microbiol 34:1142–1147 [DOI] [PubMed] [Google Scholar]
- 47.Wassenaar TM, Blaser MJ. 1999. Pathophysiology of Campylobacter jejuni infections of humans. Microbes Infect 1:1023–1033 [DOI] [PubMed] [Google Scholar]
- 48.Watson RO, Galan JE. 2008. Campylobacter jejuni survives within epithelial cells by avoiding delivery to lysosomes. PLoS Pathog 4:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watson RO, Novik V, Hofreuter D, Lara-Tejero M, Galan JE. 2007. A MyD88-deficient mouse model reveals a role for Nramp1 in Campylobacter jejuni infection. Infect Immun 75:1994–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yao R, Burr DH, Guerry P. 1997. CheY-mediated modulation of Campylobacter jejuni virulence. Mol Microbiol 23:1021–1031 [DOI] [PubMed] [Google Scholar]
- 51.Yuki N, Koga M. 2006. Bacterial infections in Guillain–Barre and Fisher syndromes. Curr Opin Neurol 19:451–457 [DOI] [PubMed] [Google Scholar]
- 52.Yuki N, Odaka M. 2005. Ganglioside mimicry as a cause of Guillain–Barre syndrome. Curr Opin Neurol 18:557–561 [DOI] [PubMed] [Google Scholar]