Abstract
The characterization of porcine antithrombin III (ATIII)—a highly powerful anticoagulant—is essential for using porcine liver in xenotransplantation applications. The objective of this study was to clarify the functions of porcine ATIII through comparison with human ATIII. We cloned porcine ATIII and compared its important functional sites with those of human ATIII. The full-length cDNA of porcine ATIII was cloned by screening a porcine liver cDNA library, and the ATIII activities of 23 pigs were determined. The full-length cDNA of porcine ATIII spanned 1498 bp and encoded 463 amino acids. Porcine ATIII shared 87.67% nucleotide identity and 89.06% amino acid identity with human ATIII. Complete identity was found at active center Arg393–Ser394, and remarkably high similarities were found at 2 critical heparin-binding sites (residues 41 through 49 and 114 through 156) and in some key residues involved in heparin binding. An ATIII assay found no significant difference between porcine and human plasma. The high level of similarity between porcine ATIII and human ATIII suggests that porcine ATIII will function in a manner similar to human ATIII in xenotransplantation.
Abbreviation: ATIII, antithrombin III
Antithrombin III (ATIII) is a single-chain glycoprotein found in mammalian plasma that inhibits thrombin and other serine proteinases involved in the blood coagulation cascade, such as factor IX, factor X,20 and plasmin. ATIII is considered the most powerful serine proteinase inhibitor (serpin) and the most important contributor to the anticoagulation system.11 Previous studies have provided detailed knowledge of human ATIII1 and have identified 2 essential activities as prerequisites for its effective function: (1) recognizing and attacking target proteases and (2) interacting with its cofactor, heparin.2
The pig has played an important role in biomedical research,3,6,15,33 especially as a large animal model for surgical experiments and a promising candidate for xenotransplantation.9,12Although knowledge of the physiologic features of the porcine coagulation system is important for its successful application, differences (if any) between human and porcine coagulation factors have not been studied in detail.16 Considering the unknown properties of porcine ATIII when it is secreted into human blood and interacts with human thrombin and heparin after liver or hepatocyte xenotransplantation, there is a clear need for elucidating the properties of porcine ATIII.
Treatment with high doses of recombinant human ATIII prevents coagulopathy and protects renal xenografts from early injury in the pig-to-baboon model.8 Heparin-dependent inhibition of human factor Xa by porcine arterial endothelial cells is blocked completely by neutralizing ATIII but is unaffected by the antitissue factor pathway inhibitor antibody.18 These results suggest that human ATIII is an effective anticoagulant in the xenotransplantation model, but porcine tissue factor pathway inhibitor and human Xa are incompatible.18 However, porcine ATIII has not been evaluated with regard to xenotransplantation models. Consequently, in the present study, we cloned and characterized the full-length cDNA of porcine ATIII. We then compared sequence and function of porcine ATIII cDNA with those of human ATIII to gain insight into the molecular compatibility of porcine and human coagulation-related molecules.
Materials and Methods
Reagents.
TRIzol reagent was purchased from Invitrogen (Carlsbad, CA); Oligotex mRNA Mini Kit from Qiagen (Hilden, Germany); ZAP Express cDNA Synthesis Kit and ZAP Express cDNA Gigapack III Gold Cloning Kit from Stratagene (La Jolla, CA); RNA PCR Kit (AWV) version 3.0, from TaKaRa (Shiga, Japan); pGEM-T Easy Vector Systems from Promega (Madison, WI); and antithrombin III from Diagnostica Stago (Paris, France).
Animals.
For cDNA library construction, porcine liver tissue was excised from a euthanized healthy 6-y-old male pig of the Banna minipig inbred (BMI) line, JS151 substrain (a gift from Yangzhi Zeng, Yunnan Agriculture University). The pig was housed individually at the Animal Center of West China Hospital and was fed and watered ad libitum. When the pig was anesthetized (ketamine, 15 mg/kg IM), about 500 mL blood was exsanguinated from the thoracic aorta before its death, after which blood, tissues, and bones were collected for various studies. All procedures in this study were in accordance with the Guide for the Care and Use of Laboratory.17
For ATIII activity analysis, venous blood samples were collected from 23 (9 male, 14 female) Chinese Guizhou minipigs bred at the Agriculture Institute of Guizhou University. All pigs were 6 to 10 mo old, weighed 10 to 15 kg, and were free of common viruses, such as Salmonella spp., Pathogenic dermal fungi, Brucella spp., and Leptospira spp. They were housed in clean cages and had free access to water and pelleted food. The experiments were in accordance with the Guide for the Care and Use of Laboratory Animals.17
Screening of porcine liver cDNA library.
A porcine liver tissue cDNA library was constructed by the method described in our previous report.29 Briefly, total RNA from liver was isolated by using TRIzol reagent (Invitrogen), followed by poly(A)+ RNA purification by using Oligotex polystyrene–latex particles (Qiagen). Poly(A)+ RNA (5 µg) was used to synthesize cDNA with the ZAP Express cDNA Synthesis Kit (Stratagene). The in vitro lambda packaging reaction used to produce the primary cDNA library (ZAP Express cDNA Gigapack III Gold Cloning Kit, Stratagene) was set up according to the manufacturer's instructions.
Screening for the target gene from the cDNA library followed a PCR-based protocol described previously.7 Briefly, primers (forward ATIII A, 5′ GAT AGA GTC GGC AGT TCA GT 3′; reverse ATIII B, 5′ CCA GAC CTG TGG AAG ATT AG 3′) were designed based on the published partial sequence of porcine ATIII (GenBank accession number AF281653). Using total RNA from porcine liver tissue as a template, RT-PCR was performed, and the product was sequenced to verify the suitability of the primers, which in turn determined the specificity and efficiency of cDNA library screening. Then, using the cDNA library as the template and primers ATIII A and B, several rounds of PCR were conducted to eliminate unsuitable clones from the screening pool and isolate a single clone that bound to the target gene. The complete amplification cycle comprised the following steps: 94 °C for 2 min; 94 °C for 1 min, 60 °C for 1 min (annealing temperature reduced by 1 °C each cycle), and 72 °C for 1 min over 10 cycles (steps 2 through 4); 94 °C for 1 min, 50 °C for 1 min, and 72 °C for 1 min over 30 cycles (steps 5 through 7); 72 °C for 10 min. During the first round of PCR, the templates used were aliquots of the divided primary cDNA library supernatant; in the second round, the templates used were different dilutions of the positive aliquot from the first-round PCR. Then, the positive sample with the lowest concentration was dispensed into a 96-well plate in an 8 × 8 pattern. Aliquots of each row or each column were collected to serve as PCR templates. The double-positive well became the subpool for the next round of screening. After 2 rounds of PCR screening, a subpool with titer less than N × 103 pfu/mL (N presents any number between 1 and 9) was obtained; this subpool showed a small degree of complexity but still yielded positive clones on amplification. Next, 100 pfu of the last subpool was cultured on a plate, and several single clones were randomly selected for PCR to identify the positive recombinant; then, automated sequencing was performed (ABI 3700, Applied Biosystems, Carlsbad, CA).
Sequence analysis and alignments.
Identification of open reading frames, protein sequence translations, and multiple sequence alignments of the full-length cDNA was performed by using DNAMAN biological software (Lynnon, Pointe Claire, Quebec, Canada). The programs BLASTN and BLASTX (http://www.ncbi.nlm.nih.gov) were used to align the new sequence against various databases.23
For further analysis of the porcine ATIII protein sequence, several online ExPASy proteomics tools (http://au.expasy.org/tools/)14 such as Simple Modular Architecture Research Tool (SMART), SignalP, NetOGlyc, and NetNGlyc were used to predict functional domains, signal peptide cleavage sites, and glycosylation sites, respectively.
Antithrombin III activity assay.
Venous blood samples were collected from 23 Chinese Guizhou Minipig. Venous blood was collected into plastic tubes with 0.11 M trisodium citrate and the plasma separated by centrifugation at 2500 × g for 15 min. The ATIII assay was performed in an autoanalyzer (STA, Diagnostica Stago) at 405 nm in a clinical laboratory of West China Hospital according to the universal protocol for clinical samples.
Results
Full-length cDNA cloning of porcine ATIII.
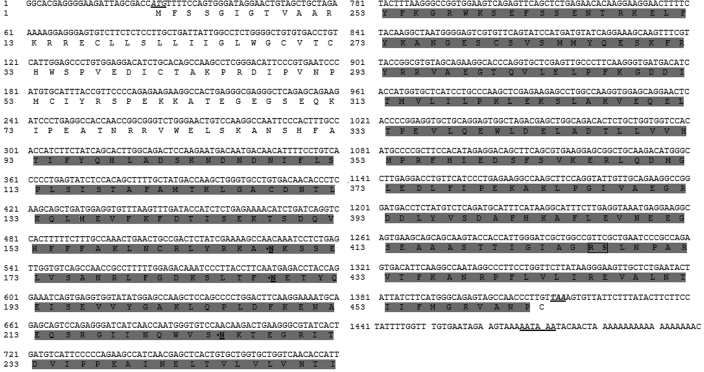
The partial sequence of porcine ATIII cloned by RT-PCR completely matched the published sequence in GenBank (AF281653). Sequencing of the positive recombinant obtained from the porcine liver cDNA library revealed that the full-length cDNA of porcine ATIII (Figure 1) contained 1498 bp with a single complete open reading frame of 1389 bp, a 24-bp 5′ untranslated flanking region, and a 82-bp 3′ untranslated flanking region. The deduced protein sequence had 463 amino acids with a predicted molecular weight of 52,376.9 Da. The sequence of the full-length porcine ATIII cDNA was submitted to GenBank (GenBank accession number, DQ530373).
Figure 1.
Full-length cDNA sequence and deduced protein sequence of porcine ATIII. The full-length cDNA sequence and corresponding amino acid sequence are shown (GenBank accession number DQ530373). The initiation codon (ATG) and termination codon (T) are italicized and underlined. The polyadenylation site in the 3′ untranslated region (AATAAA) is underlined. The trypin domain is shaded. The 3 potential N-glycosylation sites (N) are bolded, underlined, and indicated with asterisks. The active center (Arg–Ser) is bolded and boxed.
Sequence analysis and comparison with factor X from human and other species.
The nucleotide and amino acid sequences of porcine ATIII were aligned with those of human, bovine, and murine ATIII. The results indicated that porcine ATIII was highly homologous to human ATIII, with 87.67% nucleotide identity and 89.06% amino acid identity. Porcine and bovine ATIII showed nucleotide and amino acid identities of 91% and 90.20%, respectively; for porcine and mouse ATIII, the corresponding identities were 84% and 87.23%.
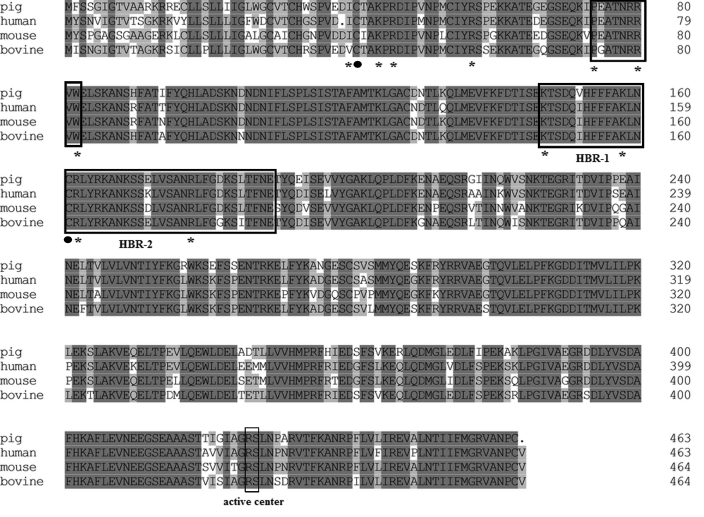
Analysis of the deduced protein sequence of porcine ATIII identified a unique catalytic domain, called the serpin domain (amino acids 234 through 462). This pattern is conserved within ATIII derived from several animal species. Comparison of the serpin domain of porcine ATIII with those of bovine, human, and murine ATIII revealed a higher degree of similarity than that for alignment of the entire sequence. Amino acid identities between porcine ATIII and bovine, human, and murine ATIII were 91.03%, 90.22%, and 87.23%, respectively (Figure 2). As in other previously studied coagulation factors, all of the cysteine residues were conserved between species, suggesting that ATIII from different species may share a similar folding conformation due to the conserved disulfide bonds.
Figure 2.
Multiple alignment of amino acid sequences of ATIII from swine, human, bovine, and mouse. Identical residues are shaded in gray. The sequences of active center and heparin-binding regions 1 (HBR-1; residues 41 through 49, as numbered from the initial of mature protein without the signal peptide, including residues 1 through 32) and 2 (HRB-2; residues 114 through 156) are boxed and indicated. The key amino acids involved in heparin binding (that is, Ile7, Lys11, Arg13, Arg24, Pro41, Arg47, Trp49, Lys114, Lys125, Arg129, and Arg145) are indicated by asterisks. Cys8 and Cys128, which form a disulphide linkage involved in heparin binding, are indicated with circles. The alignment shows that all of these important residues are highly conserved among the 4 species.
Sequence analysis of the glycosylation sites revealed some variations in the 4 species. Three potential N-glycosylation sites (Asn136, Asn156, and Asn193) were present in both the porcine and bovine ATIII sequences. However, an additional site was revealed in both human ATIII (Asn96, Asn135, Asn155, and Asn192), and mouse ATIII (Asn97, Asn136, Asn156, and Asn193).
Functional sites analysis and comparison with human ATIII.
Alignment of the deduced protein sequence of porcine ATIII with human ATIII demonstrated the presence of an identical reactive center (Arg393–Ser394) in porcine ATIII (Figure 2), indicating a similar inhibitory action to α-thrombin. In addition, alignment results revealed 100% identity between porcine ATIII and human ATIII at the first heparin-binding region, whereas 2 variations in Val119Ile and Glu139Lys were identified at the second heparin-binding region. It is worth noting that all of the additional key residues (that is, Ile7, Lys11, Arg13, Arg24, Pro41, Arg47, Trp49, Lys114, Lys125, Arg129, and Arg145) were identical between the primary sequences of the porcine and human proteins (Figure 2). Finally, Cys8 and Cys128, residues involved in a disulfide linkage important for heparin binding, were conserved in porcine ATIII (Figure 2). Therefore, our data provide genetic evidence to support the idea that the heparin-binding ability of porcine ATIII is similar to that of human ATIII.
Comparison of antithrombin III activity in pigs and humans.
ATIII assays of porcine plasma showed that the average level of ATIII activity in the 23 pigs tested was 117.39% ± 11.87%. In comparison, the reference value for plasma ATIII activity in healthy humans is 80% to 120%;35 therefore, ATIII activity did not differ significantly between the 2 species.
Discussion
Knowledge about the porcine coagulation system is required for the successful application of pigs as large animal models in a variety of biomedical settings.15Investigating the differences between the coagulation factors in pigs and humans can facilitate a better understanding of pig-to-human liver or hepatocyte transplantation, because the porcine liver-synthesized coagulation factors will be secreted into the human circulation. In tests of factor-deficient human plasma, human and porcine plasma were similar in terms of their prothrombin and activated partial thromboplastin times, whereas porcine coagulation factors (including factors II, VII, and X) showed higher activities than their human homologs.35 Issues related to anticoagulation in porcine tissues have attracted considerable attention from researchers studying xenotransplantation because specific molecular incompatibilities are well demonstrated, such as the incompatibility between porcine thrombomodulin and human thrombin and that between porcine tissue factor pathway inhibitor and human Xa.10 However, the possible function of porcine ATIII in the human body has not been elucidated to date. In our study, we cloned the full-length cDNA of porcine ATIII from a liver tissue cDNA library, and we compared the nucleotide and amino acid sequences and protease activity of porcine ATIII with those of human ATIII. The considerably high conservation of primary sequences and the similar protease activities between the 2 species suggest that porcine ATIII has a similar function to human ATII in the coagulation system, which is to maintain the balance between coagulation and anticoagulation.
The ATIII protein belongs to the clade C subgroup of the serine protease inhibitor superfamily.5 Our results showed that porcine ATIII showed remarkably high values of nucleotide identity (87.67%) and amino acid sequence identity (89.06%) with human ATIII; these values are markedly higher than the identity values between other members of the porcine and human serpin superfamilies. In particular, alignment studies indicated that the similarity between the human and porcine α1-antitrypsin proteins is 73% and those between the porcine and human homologs of α1-antichymotrypsin, heparin cofactor, and protein C inhibitor are 60%, 75%, and 75%, respectively (data not shown). Moreover, the identity between porcine ATIII and human ATIII is the highest among other coagulation and anticoagulation factors. For example, the identity between various porcine and human coagulation and anticoagulation factors is: factor II, 83%;7 factor VII, 74%; factor X, 73%; anticoagulant protein C, 74%; tissue factor pathway inhibitor, 73%; and thrombomodulin, 69% (data not shown). Despite the considerable evolutionary divergence between humans and pigs, the remarkably high homology between porcine ATIII and human ATIII implies that the 2 proteins have a significantly similar primary role in the anticoagulation systems of the respective species. Whereas the function of porcine ATIII has not been reported previously, considerable research has been directed at elucidating the functional domains of human ATIII.34 The human data provide us with sufficient theoretical evidence to predict the characteristics of porcine ATIII from comparisons between the nucleotide and amino acid sequences of the 2 species.
Protease inactivation, the major function of ATIII, is based primarily on 2 prerequisites: the formation of complexes with (1) serine proteases as substrates and (2) heparin as an accelerating cofactor. As far as the biologic application of porcine tissues is concerned, affinity with heparin is relatively more important for porcine ATIII, because exogenous heparin is a regular treatment when surgery is performed. The difference between human and porcine ATIII is significant when determining the postsurgery heparin dose to ensure that abnormal bleeding or thrombosis do not occur in experimental animals. Some clinical evidence supports the hypothesis of similar functions of human ATIII and porcine ATIII based on the fact that commercial heparin reagents prepared from porcine tissue, such as intestine, have been used widely in patients for years and have been proved safe and efficient. However, no supporting molecular or genetic evidence has been presented previously.
Serpins contain an exposed reactive site loop that is recognized and attacked by the target protease. Once attacked, the serpin and protease become part of a 1:1 covalent complex, which is then cleared via receptor-mediated endocytosis.31 With the addition of heparin, the inhibitory activity of ATIII is accelerated by as much as 10,000 times its initial activity.24 The locations of heparin-binding sites in human ATIII were discovered during the last century.25 It has been elucidated that the C-terminal domain of ATIII contains the reactive center Arg393–Ser394, which is involved in protease inactivation, whereas the N-terminal domain of ATIII is essential for both heparin binding and complex-formation with α-thrombin.2,25 Considerable research has addressed the accurate heparin-binding sites in ATIII.13,21,30 Blackburn4 and Smith27 suggested that amino acid residues 41 to 49 were involved in heparin binding and that amino acid residues 114 to 156 constituted a second heparin-binding region. In particular, some key residues are involved in the binding site. Taken together, Ile7, Lys11, Arg13, Arg24, Pro41, Arg47, Trp49, Lys114, Lys125, Arg129, and Arg145 are identified as the most important residues for binding with the pentasaccharide sequence from heparin.13,26 In addition, disulfide linkages contribute to heparin binding.28,32 The reduction of Cys8–Cys128 correlates quantitatively with the loss of the heparin cofactor activity of ATIII.28
In light of genetic variants, chemical modifications, glycosylation isoforms, and structural data, the location of core binding site has been narrowed down to the basic residues on the D-helix, the N-terminal end of the A-helix, and other surrounding structural features, particularly residues from the amino terminus.13,26 A cluster of positively charged residues in the D-helix region has been proposed to interact with essential negatively charged sulfate and carboxylate groups on heparin. Alanine scanning mutagenesis of basic residues in the D-helix region found that Arg47Ala, Lys125Ala, and Arg129Ala mutant proteins exhibited severely reduced affinity for immobilized heparin, eluting in the 0.5- to 0.7-M NaCl range, and the affinities of the Lys11Ala, Arg13Ala, Arg24Ala, and Arg145Ala variants for immobilized heparin were reduced also.13 Recombinant studies have suggested the additional involvement of Lys114 in the broader heparin-binding site.19 In addition, Lys114 is crucial for the antiangiogenic activity of ATIII.36 The alignments of porcine and human ATIII protein sequences in our study focused on these key sites and indicated high identities. Therefore, our results provide us with valuable evidence in favor of our assumption that porcine and human ATIII have similar functions and affinities in heparin binding.
Glycosylation of ATIII plays a critical role in heparin binding and ATIII activity. Two isoforms exist in the circulation—α-antithrombin and β-antithrombin—which differ in the amount of glycosylation present on the polypeptide chain; β-antithrombin lacks the carbohydrate present at Asn135 in α-antithrombin. Of the 2 forms, β-antithrombin has higher affinity for heparin and thus functions as the major inhibitor in vivo even though it is the less-abundant form. Differences between the heparin affinities of the α and β forms arise because the additional carbohydrate changes the rate of conformational change.22 According to our findings, in comparison with human ATIII, porcine and bovine ATIII lack the N-linked glycosylation site at Asn96, whereas all 3 species have a common Asn135 glycosylation site. Although there is no evidence to suggest that differences between the activities of porcine and human ATIII are caused by different compositions of α- and β-antithrombin, the variant glycosylation at the additional site should be further studied to elucidate its influence on ATIII activity.
In summary, we have reported the full-length cDNA of porcine ATIII for the first time. The genetic comparison of porcine ATIII with human ATIII revealed high conservation between them, and ATIII assays of porcine and human plasma showed no remarkable difference with regard to ATIII activity. Together, these results provide valuable genetic and functional evidence for the potential replacement of human ATIII by porcine ATIII after liver xenotransplantation. The availability of this gene sequence will facilitate research on the porcine coagulation system.
Acknowledgments
This study was supported by the National Basic Research Program of China (grant 2009CB522401), National Natural Science Foundation of China (grant 30772037), China Postdoctoral Science Foundation (grant 20080430195), National Program for High Technology Research and Development of China (grant 2006AA02A117), and Program for Changjiang Scholars and Innovative Research Team in University, Ministry of Education.
References
- 1.Abildgaard U. 2007. Antithrombin–early prophecies and present challenges. Thromb Haemost 98:97–104 [PubMed] [Google Scholar]
- 2.Austin RC, Sheffield WP, Rachubinski RA, Blajchman MA. 1992. The N-terminal domain of antithrombin-III is essential for heparin binding and complex-formation with, but not cleavage by, alpha-thrombin. Biochem J 282:345–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bermejo A, Gonzalez O, Gonzalez JM. 1993. The pig as an animal model for experimentation on the temporomandibular articular complex. Oral Surg Oral Med Oral Pathol 75:18–23 [DOI] [PubMed] [Google Scholar]
- 4.Blackburn MN, Smith RL, Carson J, Sibley CC. 1984. The heparin-binding site of antithrombin III. Identification of a critical tryptophan in the amino acid sequence. J Biol Chem 259:939–941 [PubMed] [Google Scholar]
- 5.Bock SC, Wion KL, Vehar GA, Lawn RM. 1982. Cloning and expression of the cDNA for human antithrombin III. Nucleic Acids Res. 10:8113–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cameron JS, Simmonds HA, Hatfield PJ, Jones AS, Cadenhead A. 1974. The pig as an animal model for purine metabolic studies. Adv Exp Med Biol 41:691–692 [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Tan W, Lu X, Lu Y, Qin S, Li S, Zeng Y, Bu H, Li Y, Cheng J. 2007. Full-length cDNA cloning and protein three-dimensional structure modeling of porcine prothrombin. Blood Cells Mol Dis 38:93–99 [DOI] [PubMed] [Google Scholar]
- 8.Cowan PJ, Aminian A, Barlow H, Brown AA, Dwyer K, Filshie RJ, Fisicaro N, Francis DM, Gock H, Goodman DJ, Katsoulis J, Robson SC, Salvaris E, Shinkel TA, Stewart AB, d'Apice AJ. 2002. Protective effects of recombinant human antithrombin III in pig-to-primate renal xenotransplantation. Am J Transplant 2:520–525 [DOI] [PubMed] [Google Scholar]
- 9.Cozzi E, Bosio E, Seveso M, Vadori M, Ancona E. 2006. Xenotransplantation: current status and future perspectives. Br Med Bull 75-76:99–114 [DOI] [PubMed] [Google Scholar]
- 10.Crikis S, Cowan PJ, d'Apice AJ. 2006. Intravascular thrombosis in discordant xenotransplantation. Transplantation 82:1119–1123 [DOI] [PubMed] [Google Scholar]
- 11.Davie EW, Fujikawa K, Kisiel W. 1991. The coagulation cascade: initiation, maintenance, and regulation. Biochemistry 30:10363–10370 [DOI] [PubMed] [Google Scholar]
- 12.Deschamps JY, Roux FA, Sai P, Gouin E. 2005. History of xenotransplantation. Xenotransplantation 12:91–109 [DOI] [PubMed] [Google Scholar]
- 13.Ersdal-Badju E, Lu A, Zuo Y, Picard V, Bock SC. 1997. Identification of the antithrombin III heparin-binding site. J Biol Chem 272:19393–19400 [DOI] [PubMed] [Google Scholar]
- 14.Expasy ExPASy Proteomics tools. [Cited 12 Sep 2007]. Available from http://au.expasy.org/tools/
- 15.Fourtanier A, Berrebi C. 1989. Miniature pig as an animal model to study photoaging. Photochem Photobiol 50:771–784 [DOI] [PubMed] [Google Scholar]
- 16.Ibrahim Z, Busch J, Awwad M, Wagner R, Wells K, Cooper DK. 2006. Selected physiologic compatibilities and incompatibilities between human and porcine organ systems. Xenotransplantation 13:488–499 [DOI] [PubMed] [Google Scholar]
- 17.Institute for Laboratory Animal Resources 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academy Press [Google Scholar]
- 18.Kopp CW, Siegel JB, Hancock WW, Anrather J, Winkler H, Geczy CL, Kaczmarek E, Bach FH, Robson SC. 1997. Effect of porcine endothelial tissue factor pathway inhibitor on human coagulation factors. Transplantation 63:749–758 [DOI] [PubMed] [Google Scholar]
- 19.Kridel SJ, Chan WW, Knauer DJ. 1996. Requirement of lysine residues outside of the proposed pentasaccharide binding region for high-affinity heparin binding and activation of human antithrombin III. J Biol Chem 271:20935–20941 [DOI] [PubMed] [Google Scholar]
- 20.Kurachi K, Fujikawa K, Schmer G, Davie EW. 1976. Inhibition of bovine factor IXa and factor Xaβ by antithrombin III. Biochemistry 15:373–377 [DOI] [PubMed] [Google Scholar]
- 21.Lellouch AC, Lansbury PT., Jr 1992. A peptide model for the heparin-binding site of antithrombin III. Biochemistry 31:2279–2285 [DOI] [PubMed] [Google Scholar]
- 22.McCoy AJ, Pei XY, Skinner R, Abrahams JP, Carrell RW. 2003. Structure of beta-antithrombin and the effect of glycosylation on antithrombin's heparin affinity and activity. J Mol Biol. 326:823–33 [DOI] [PubMed] [Google Scholar]
- 23.NCBI National Center for Biotechnology Information. BLASTN and BLASTX programs. [Cited 20 Sep 2007]. Available from http://www.ncbi.nlm.nih.gov/
- 24.Olson ST, Srinivasan KR, Bjork I, Shore JD. 1981. Binding of high affinity heparin to antithrombin III. Stopped flow kinetic studies of the binding interaction. J Biol Chem 256:11073–11079 [PubMed] [Google Scholar]
- 25.Petitou M, Casu B, Lindahl U. 2003. 1976–1983, a critical period in the history of heparin: the discovery of the antithrombin binding site. Biochimie 85:83–89 [DOI] [PubMed] [Google Scholar]
- 26.Skinner R, Abrahams JP, Whisstock JC, Lesk AM, Carrell RW, Wardell MR. 1997. The 2.6-Å structure of antithrombin indicates a conformational change at the heparin-binding site. J Mol Biol 266:601–609 [DOI] [PubMed] [Google Scholar]
- 27.Smith JM, Daum HA., 3rd 1987. Identification and nucleotide sequence of a gene encoding 5′-phosphoribosylglycinamide transformylase in Escherichia coli K12. J Biol Chem 262:10565–10569 [PubMed] [Google Scholar]
- 28.Sun XJ, Chang JY. 1990. Re-formation of disulphide bonds in reduced antithrombin III. Biochem J 269:665–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan W, Chen Y, Zhang L, Lu Y, Li S, Zeng R, Zeng Y, Li Y, Cheng J. 2006. Construction and characterization of a cDNA library from liver tissue of Chinese Banna minipig inbred line. Transplant Proc 38:2264–2266 [DOI] [PubMed] [Google Scholar]
- 30.Villanueva GB. 1984. Predictions of the secondary structure of antithrombin III and the location of the heparin-binding site. J Biol Chem 259:2531–2536 [PubMed] [Google Scholar]
- 31.Villanueva GB, Allen N. 1983. Demonstration of a two-domain structure of antithrombin III during its denaturation in guanidinium chloride. J Biol Chem 258:11010–11013 [PubMed] [Google Scholar]
- 32.Villanueva GB, Allen N. 1983. Refolding properties of antithrombin III. Mechanism of binding to heparin. J Biol Chem 258:14048–14053 [PubMed] [Google Scholar]
- 33.Wang S, Liu Y, Fang D, Shi S. 2007. The miniature pig: a useful large animal model for dental and orofacial research. Oral Dis 13:530–537 [DOI] [PubMed] [Google Scholar]
- 34.Watton J, Longstaff C, Lane DA, Barrowcliffe TW. 1993. Heparin binding affinity of normal and genetically modified antithrombin III measured using a monoclonal antibody to the heparin-binding site of antithrombin III. Biochemistry 32:7286–7293 [DOI] [PubMed] [Google Scholar]
- 35.Zhang L, Li Y, Jiang H, Liu J, Zeng Y, Cheng J. 2005. Comparison of hepatic coagulant, fibrinolytic, and anticoagulant functions between Banna Minipig Inbred line and humans. Transplantation 79:1128–1131 [DOI] [PubMed] [Google Scholar]
- 36.Zhang W, Swanson R, Izaguirre G, Xiong Y, Lau LF, Olson ST. 2005. The heparin-binding site of antithrombin is crucial for antiangiogenic activity. Blood 106:1621–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]