Independently of their nucleation activity, γ-tubulin ring complex proteins localize along microtubules, limiting catastrophe events during interphase.
Abstract
γ-Tubulin is critical for the initiation and regulation of microtubule (MT) assembly. In Drosophila melanogaster, it acts within two main complexes: the γ-tubulin small complex (γ-TuSC) and the γ-tubulin ring complex (γ-TuRC). Proteins specific of the γ-TuRC, although nonessential for viability, are required for efficient mitotic progression. Until now, their role during interphase remained poorly understood. Using RNA interference in Drosophila S2 cells, we show that the γ-TuRC is not critical for overall MT organization. However, depletion of any component of this complex results in an increase of MT dynamics. Combined immunofluorescence and live imaging analysis allows us to reveal that the γ-TuRC localizes along interphase MTs and that distal γ-tubulin spots match with sites of pause or rescue events. We propose that, in addition to its role in nucleation, the γ-TuRC associated to MTs may regulate their dynamics by limiting catastrophes.
Introduction
The organization and dynamics of microtubules (MTs) are essential for different cellular processes such as migration or division. In animal cells, MT nucleation usually occurs at the centrosome, where γ-tubulin plays a key role. This protein is organized in multiprotein complexes (Moritz et al., 1995; Zheng et al., 1995; Raynaud-Messina and Merdes, 2007). In Drosophila melanogaster, two main complexes have been characterized (Oegema et al., 1999). The γ-tubulin small complex (γ-TuSC) is composed of γ-tubulin and two other proteins, Dgrip84 and -91. The γ-tubulin ring complex (γ-TuRC) is formed of γ-TuSCs associated with additional grip motif polypeptides, Dgrip75, -128, and -163, and a WD motif protein, Dgp71WD. Components of the γ-TuSC are highly conserved in eukaryotes. Deletion of any γ-TuSC subunit is lethal both in fungi and in Drosophila. This loss of function results in the accumulation of cells in mitosis, which is associated with defects such as monopolar spindles, impairment in centrosome maturation, and aneuploidy (Oakley and Oakley, 1989; Sunkel et al., 1995; Knop and Schiebel, 1997; Barbosa et al., 2000; Colombié et al., 2006). In contrast, γ-TuRC–specific grip motif proteins are nonessential for viability in yeast and in Drosophila (Fujita et al., 2002; Schnorrer et al., 2002; Anders et al., 2006; Vérollet et al., 2006; Vogt et al., 2006). Nevertheless, these grip proteins are necessary for the assembly of the large complex, for efficient mitotic progression (Vérollet et al., 2006; Izumi et al., 2008), and for specialized functions such as the organization of MT subarrays during oogenesis (Schnorrer et al., 2002; Vogt et al., 2006). Analysis of the nongrip component Dgp71WD reveals that this protein regulates the function and targeting of the γ-TuRC to the centrosome and along spindle MTs (Haren et al., 2006; Lüders et al., 2006).
The γ-tubulin complexes are involved in the nucleation of MTs from centrosomes but also from chromatin and spindle MTs (Joshi et al., 1992; Sunkel et al., 1995; Knop and Schiebel, 1997; Oegema et al., 1999; Wilde and Zheng, 1999; Wiese and Zheng, 2000; Goshima et al., 2008; Zhu et al., 2008). Additional observations in fungi suggest that γ-tubulin and its partners also affect the organization or dynamics of MTs (Oakley and Oakley, 1989; Paluh et al., 2000; Vardy and Toda, 2000; Fujita et al., 2002; Venkatram et al., 2004; Zimmerman and Chang, 2005; Masuda et al., 2006). To determine whether and how γ-TuRC proteins could influence MT dynamics, we determined dynamic parameters on individual MTs in Drosophila S2 cells during interphase. For the first time in metazoan cells, we show that γ-TuRCs contribute to the regulation of MT dynamics, independently of their nucleation activity. The γ-TuRCs localize along MTs, where they limit catastrophe events, thus enhancing MT stability.
Results and discussion
The γ-TuRC is not critical for MT organization during interphase in Drosophila cells
Down-regulation of any γ-TuSC component induces mitotic defects. Because these proteins act in complexes, we wondered whether the depletion of one of these proteins affected the levels of its partners, as seen for other multiprotein complexes (Goshima et al., 2008). RNAi treatment against any γ-TuSC protein (Dgrip84 or -91 or 23C γ-tubulin, the major γ-tubulin isotype in S2 cells) induced a decrease in the levels of the two others (Fig. S1 A, top). The levels of γ-TuRC–specific components were affected, but to a lower extent (Fig. S1 A, bottom). In contrast, when γ-TuRC–specific components were individually or concomitantly depleted, the levels of the three γ-TuSC proteins were not significantly altered (Fig. S1 B, top). These experiments support the idea that γ-TuSC components are coregulated independently of the assembly of the large complex. We confirmed this coregulation in vivo using Dgrip84 or -75 or Dgp71WD mutants (Fig. S1 C). Among the γ-TuRC–specific components, the levels of grip motif proteins also appeared to depend on each other (Fig. S1 B, bottom). These results are in agreement with a previous study in Drosophila ovaries (Vogt et al., 2006). To investigate the roles of γ-tubulin and its associated proteins, we depleted γ-tubulin as a marker of the γ-TuSC, and Dgrip75 or Dgp71WD as two representative proteins specific of γ-TuRCs.
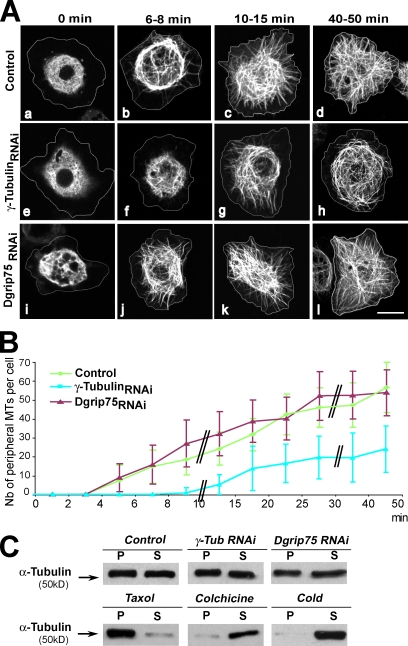
First, we clarified the role of γ-tubulin complexes in MT nucleation and organization in S2 cells. As in other Drosophila cells, the interphase MT array is not organized from a unique organizing center. Down-regulation of γ-tubulin induced a delay of ∼6–7 min in the regrowth of peripheral MTs compared with control cells (Fig. 1, A [a–h] and B). As expected from γ-TuSC protein coregulation, we obtained comparable results after treatment with Dgrip84 RNAi (unpublished data). In contrast, after depletion of the γ-TuRC–specific protein Dgrip75 (Fig. 1, A [i–l] and B) or Dgp71WD (not depicted), the growth kinetics of newly assembled MTs were indistinguishable from controls. Moreover, depletion of γ-tubulin, Dgrip75, or Dgp71WD did not induce any significant modification in the mass of polymerized MTs or in the organization of the interphase MT cytoskeleton (Fig. 1 C; Raynaud-Messina et al., 2004; Colombié et al., 2006; Vérollet et al., 2006; Rogers et al., 2008). Thus, in Drosophila cells, it appears that the γ-TuSC promotes initial phases of MT nucleation or polymerization, whereas the γ-TuRC does not play a major role in these processes. However, none of these complexes seem critical for the steady-state organization of the interphase MT array, suggesting that additional mechanisms function redundantly with γ-tubulin complexes (Rogers et al., 2008).
Figure 1.
γ-TuSC promotes MT regrowth in interphase. (A and B) MT regrowth after cold-induced depolymerization. (A) Control (a–d), γ-tubulin RNAi–treated (e–h), and Dgrip75 RNAi–treated (i–l) GFP–α-tubulin cells were incubated for 40 min at 4°C (t = 0), and MT regrowth at 24°C was followed over 50 min. The white lines indicate the cell outlines obtained from differential interference contrast images. (B) Graph showing the number (Nb) of newly assembled MTs per cell that reached the subcortical area. The double black bars in the graph indicate a change in the time scale. The graph is representative of at least three independent experiments. Error bars indicate ±SD (n = 15–40 cells). (C) Solubility characteristics of α-tubulin after γ-tubulin (γ-Tub) and Dgrip75 depletion. (top) 40-µg protein extracts of control and RNAi-treated cells were centrifuged for 15 min at 10,000 g. The pellet (P) and the supernatant (S) were analyzed by Western blotting using antibodies against α-tubulin. For the three conditions (control, γ-tubulin RNAi, or Dgrip75 RNAi), the ratio P/S was evaluated as ∼1 ± 0.1 by densitometry quantification after ECL. (bottom) Taxol-, colchicine-, and cold-treated cells were used as solubility controls with ratios of 7.7, 0.2, and 0.15, respectively. Data are representative of three independent experiments. Bar, 5 µm.
The γ-TuRC contributes to the regulation of MT plus end dynamics
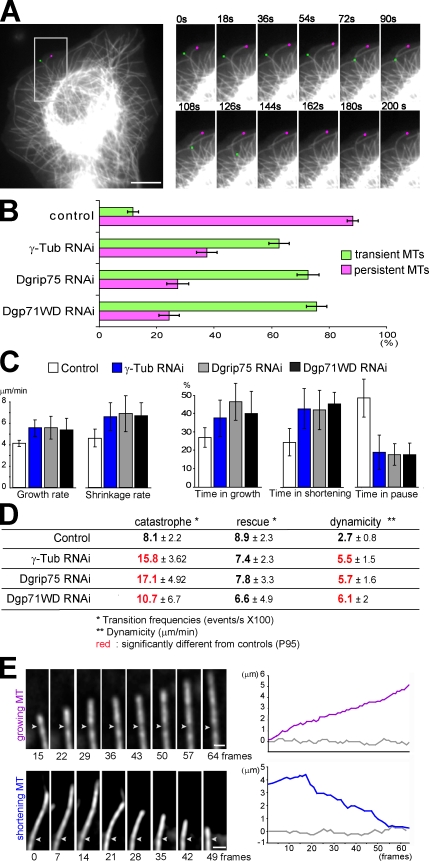
We then wondered whether the γ-TuRCs influenced MT dynamics. Live analysis on GFP–α-tubulin S2 cells allowed us to classify MTs into two arbitrary classes (Fig. 2 A). MTs that could be tracked during the whole video (200 s) in the subcortical area (6 µm) were defined as persistent, whereas MTs that appeared or disappeared from this area during the recording period were defined as transient. In control cells, most of the MTs were persistent (∼90%). Dgrip75 depletion resulted in a decrease in this class (25%; Fig. 2 B and Video 1), suggesting that γ-tubulin complexes act as MT-stabilizing factors. This effect on MT dynamics was even more noticeable on free MTs, probably because of severing activity. In control cells, the MT length was maintained or even elongated, whereas in γ-TuRC–depleted cells, they rapidly disappeared (Video 2).
Figure 2.
Down-regulation of γ-TuRC components enhances MT plus end dynamics. (A and B) MT behavior. (A, left) Low magnification of a control GFP–α-tubulin cell showing a persistent (pink dot) and a transient MT (green dot). The boxed area is magnified in the right panels. (right) High magnification of the two marked MTs over time. (B) Proportion of persistent and transient MTs (n > 150). Data are representative of four experiments. See Video 1. (C and D) Dynamic parameters. The life history of at least 50 individual MTs (extracted from 15 independent cells) was analyzed to determine the dynamic parameters in control and γ-tubulin–, Dgrip75-, and Dgp71WD-depleted cells. Bars indicate ±SD (four independent experiments), and numbers in red are significantly different from controls (P95). (E) MT plus end dynamics. (left) High magnification after deconvolution of a growing (purple) and a shortening (blue) MT in Dgrip75 RNAi–treated cells. White arrowheads indicate sites of uneven GFP–α-tubulin incorporation into the MT walls (speckles). (right) Graphs indicate the position of the speckle (gray) and of the MT plus end (colored) in micrometers. For the bottom graph, frame 0 corresponds with the beginning of the video. For the top graph, frame 0 corresponds with the appearance of the speckle (1 frame = 2 s). These examples are representative of 20 individually monitored MTs. See Video 3 for the growing MT. Bars: (A) 5 µm; (E) 1 µm.
We randomly tracked MTs localized in the peripheral area, which contains internal MTs and MTs reaching the cell cortex (Brittle and Ohkura, 2005). These MTs alternated between growth and shrinkage states with dynamic parameters consistent with published data (Fig. 2, C and D; Rogers et al., 2002; Brittle and Ohkura, 2005; Sousa et al., 2007). In Dgrip75 RNAi–treated cells, the MT shrinkage rate was slightly higher (Fig. 2 C, left). We also noticed a significant increase in the frequencies of catastrophe, whereas rescues were not clearly affected (Fig. 2 D). The most striking difference was observed for the time spent in pause. Although pauses represented ∼50% of the time in control cells, they dropped to 18% in Dgrip75-depleted cells (Fig. 2 C, right). Corroborating these data, the dynamicity, a parameter which reflects the overall exchange of tubulin with the MT ends (Rusan et al., 2001), was more than twofold increased in Dgrip75-depleted cells. Comparable results were obtained after RNAi treatment against Dgp71WD or a γ-TuSC component (γ-tubulin and Dgrip84; Fig. 2, B–D; and not depicted).
We treated with latrunculin A, a drug which depolymerizes actin, to test which MT population (cortical or internal) was affected by γ-TuRC depletion. This treatment had no effect on the phenotype induced by γ-TuRC depletion, whereas it increased the proportion of transient MTs in control cells (Fig. S2 A). A simple explanation could be that the increase of MT dynamics after γ-TuRC depletion induces a relaxation of the interactions between cortical MTs and lamellae. Nevertheless, we observed a differential effect between latrunculin A–treated control cells and γ-TuRC–depleted cells, suggesting that the γ-TuRC also acts on the dynamics of internal MTs (Fig. S2 A). To support the idea that the γ-TuRC might contribute to MT stabilization, we showed that the depletion of EB1, a +TIP (plus end tracking protein) described to be an MT destabilization factor during interphase (Rogers et al., 2002), rescued the defects in MT dynamics induced by γ-tubulin depletion (Fig. S2, B–D).
To determine whether the increase in MT dynamics was caused by altered plus end dynamics rather than resulting from translocation of entire MTs, we took advantage of the uneven incorporation of GFP–α-tubulin in the form of speckles. In cells depleted of Dgrip75 or γ-tubulin, the MT plus ends were growing or shortening, whereas speckle positions oscillated around a constant value (Fig. 2 E, Video 3, and not depicted), showing that γ-TuRCs play a role in the dynamics of MT plus ends. Our data indicate that, in addition to their well-characterized role in MT nucleation, γ-TuRCs also influence MT plus end dynamics, acting as a pause factor.
The γ-TuRC is localized along MTs during interphase
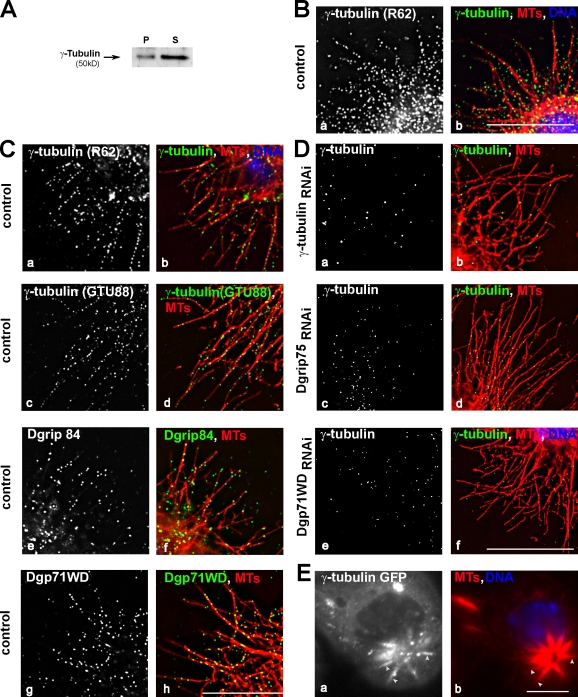
This observation led us to further investigate the localization of γ-tubulin and associated proteins during interphase. Despite the large pool of γ-tubulin in the cytoplasm (Fig. 3 A), immunofluorescence analyses after methanol fixation (Fig. 3 B, a and b) or permeabilization before fixation (Fig. 3 C, a–d) allowed the detection of γ-tubulin along peripheral MTs and, in some cases, near the plus ends. This staining was reduced after γ-tubulin RNAi treatment (Fig. 3 D, b) or cold-induced MT depolymerization (not depicted). However, it was resistant to treatment by latrunculin A (unpublished data). Antibodies directed against Dgrip84 or Dgp71WD also decorated MTs (Fig. 3 C, e–h). These results strongly suggest that γ-tubulin localizes along MTs in the form of γ-TuRCs. Accordingly, γ-tubulin staining on cytoplasmic MTs was reduced after depletion of Dgrip75 or Dgp71WD (Fig. 3 D, c–f). After down-regulation of Dgp71WD, neither the levels of γ-TuRC proteins nor the amount or the size of the γ-tubulin complexes were modified (Vérollet et al., 2006). Although γ-TuRCs lacking Dgp71WD were indistinguishable from regular γ-TuRCs in sedimentation gradient analysis, they were no longer able to bind to MTs. Therefore, it is unlikely that γ-TuRCs along MTs were trapped unspecifically during fixation simply because of their large size. Moreover, the γ-tubulin localization on MTs was observed by live analysis using γ-tubulin–GFP-expressing cells. Treatment with low concentrations of taxol induced MT bundles, along which a clear accumulation of γ-tubulin was detected (Fig. 3 E). Unfortunately, because of the large pool of soluble γ-TuRC, the detection at the level of an individual MT was not possible.
Figure 3.
γ-Tubulin localizes along interphase MTs. (A) Solubility property of γ-tubulin. Same legend as in Fig. 1 C, except Western blot analysis was performed using γ-tubulin antibodies. P, pellet; S, supernatant. (B–D) γ-TuRC components localize along interphase MTs. S2R+ cells fixed in methanol (control; B) or permeabilized before formaldehyde fixation (control [C] or depleted cells [D]). Cells were stained using antibodies against γ-tubulin (rabbit R62: B [a], C [a], and D [a, c, and e]; or monoclonal GTU88: C [c]), against Dgrip84 (C, e), or against Dgp71WD (C, g). (right) MTs are shown in red, γ-TuRC components are shown in green, and chromosomes are shown in blue. (E) Live γ-tubulin–GFP S2 cells. A cell treated with taxol was examined live by recording GFP (a), and then fixed and stained for α-tubulin (b; n = 20). The arrowheads indicate MT bundles stained by γ-tubulin. Bars, 5 µm.
This is the first study of γ-TuRC localization on interphase MTs in animal cells. γ-Tubulin along MTs has been previously reported in fission yeast and higher plants during interphase and in animal and plant cells during mitosis. γ-Tubulin complexes associated with MTs have been proposed to act as secondary MT organization centers in mitosis (Lüders et al., 2006; Mahoney et al., 2006; Goshima et al., 2008; Zhu et al., 2008) or in systems in which interphase MTs are arranged in noncentrosomal arrays such as fission yeast and higher plants (Sawin et al., 2004; Venkatram et al., 2004; Janson et al., 2005; Murata et al., 2005). Similar principles may govern nonradial MT organization in cultured Drosophila cells. However, nascent MTs or free MT minus ends were rarely visible at the proximity of MT-bound γ-tubulin (Fig. 3). Another nonexclusive possibility is that γ-tubulin linked to MTs contributes to their stabilization. Consistently, γ-tubulin localizes on spindle MTs with a preferential distribution on stable kinetochore fibers (Lajoie-Mazenc et al., 1994). Moreover, FAM29A, a human protein of the augmin complex which targets γ-TuRCs to spindle MTs, is described to have a stabilizing effect on spindle MTs (Zhu et al., 2008).
The γ-TuRC along MTs acts as an anticatastrophe factor
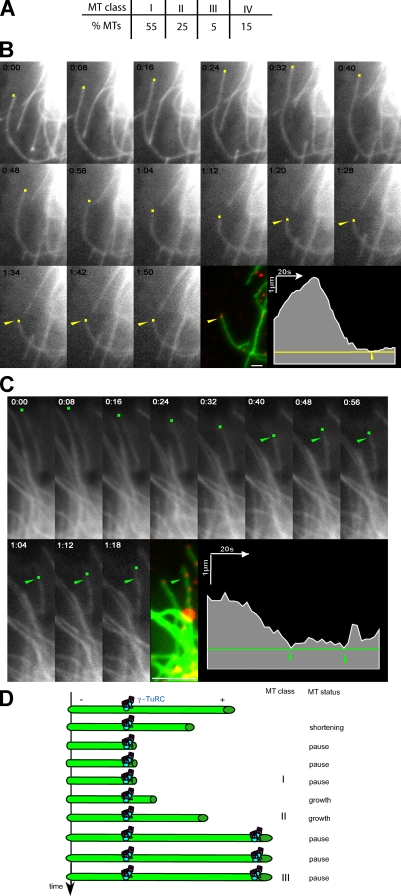
To test whether γ-tubulin bound to MTs might control MT stability, we performed immunofluorescence staining of γ-tubulin just after live analysis of the GFP-MT dynamics (Dimitrov et al., 2008). We verified by live microscopy that upon permeabilization, MTs no longer exhibited dynamic behavior (unpublished data). We defined four classes of MTs according to their dynamic behavior and the location of the distal γ-tubulin spot (Fig. 4, A–C; and Videos 4 and 5). In 55% of MTs, γ-tubulin was localized to the MT tip, and this endpoint corresponded with a pause status independently of the previous dynamic behavior (class I). As illustrated in Fig. 4 B and Video 4, the tracked MT started growing and then depolymerized and remained in pause for the last 45 s. In class II (25%), exemplified in Fig. 4 C and Video 5, MTs oscillated between growth and shortening phases, but in all cases, γ-tubulin spots coincided with a point beyond which the MT no longer depolymerized. MTs of class III (5%) were at least marked by two γ-tubulin points and satisfied both characteristics of MTs of classes I and II. In 85% of the MTs studied (classes I, II, and III), the distal γ-tubulin dot was identified at a domain where a pause or a rescue event was recorded. The remaining 15% of MTs (class IV) showed a γ-tubulin dot located further toward the cell center, relative to the zone of dynamic growth and shrinkage. In these cases, γ-tubulin did not appear to affect MT dynamics for the duration of the video.
Figure 4.
γ-TuRCs along interphase MTs act as anticatastrophe factors. (A) Table showing the distribution of MTs in the different classes as defined in the Results and discussion section. The γ-TuRC along MTs acts as an anticatastrophe factor (53 MTs, 12 cells, and three independent experiments). (B and C) Analysis of MTs of classes I (B) and II (C). S2 cells expressing GFP–α-tubulin were imaged at the indicated times (colored dots). After cytosol extraction, cells were stained with γ-tubulin (red) and imaged again (arrowheads). The appearance of a γ-tubulin spot coincided with MT pause (class I; B), or marked a point beyond which the MT no longer depolymerized (class II; C). The graphs represent the tracking of the MT plus end extremity over time; the arrowheads indicate the position of the distal γ-tubulin spots. See Videos 4 and 5. (D) Model for the role of the γ-TuRCs bound to cytoplasmic MTs. The γ-TuRC localized along MTs induces a pause signal that could be transient, followed by MT regrowth, or long-lasting, corresponding to an arrest. Bars, 1 µm.
As recent data show that GTP-bound tubulin in MTs may be responsible for rescue events (Dimitrov et al., 2008), we explored whether γ-tubulin colocalized with GTP-tubulin. Although the majority of the MTs were labeled by both γ- and GTP-tubulin antibodies, the patterns of the two epitopes did not overlap (Fig. S3). These findings were not surprising, as γ-tubulin acts mainly as a pause factor, whereas GTP-tubulin conformation promotes MT rescue.
Altogether, our data indicate that γ-TuRCs associated to MTs influence their dynamics, acting as a stabilizing factor. This effect appears to be independent of nucleation activity. Even if a slight decrease in nucleation was induced by the depletion of a γ-TuRC–specific component, it should result in an increase of the free α/β-tubulin pool, promoting tubulin polymerization and thus minimizing any increase of MT dynamics. A role of γ-tubulin and its associated proteins in MT dynamics fits with previous data obtained in fungi. For example, γ-tubulin was first characterized in Aspergillus nidulans as an extragenic suppressor of a β-tubulin mutation, inducing MT hyperstabilization (Oakley and Oakley, 1989). In Schizosaccharomyces pombe, mutants in γ-TuRC–specific genes are hypersensitive to MT-destabilizing agents (Fujita et al., 2002), and mutants in the Dgrip84 orthologue or strains overexpressing the C-terminal domain of this protein exhibit altered MT dynamics (Zimmerman and Chang, 2005; Masuda et al., 2006).
So far, the mechanisms through which γ-TuRCs control MT dynamics remain unclear. An explanation would be that MTs formed in the absence of the γ-TuRCs have an abnormal protofilament number, resulting in different dynamic properties. This seems unlikely as we have previously shown that in Dgrip75-depleted cells, most of the MTs exhibit 13 protofilaments (Vérollet et al., 2006). It could also be argued that γ-tubulin complexes control the assembly or the loading of factors that regulate MT dynamics, as previously suggested (Zimmerman and Chang, 2005; Cuschieri et al., 2006). γ-TuRCs associated to MTs could counteract the function of MT-destabilizing factors either directly by occluding binding sites on the MTs or indirectly by altering the MT tip structure.
We propose a model in which the γ-TuRC localized along MTs may represent a pause signal, switching from a shortening or a growing phase to a pause (Fig. 4 D). This pause status could be either transient, followed by a growth event (rescue), or could be long lasting and stabilize MTs, especially at the cell periphery. In conclusion, we suggest that γ-TuRCs are essential to mediate noncentrosome functions such as organization of cell type–specific MT networks by regulating MT dynamics in addition to nucleation.
Materials and methods
Cell culture and RNA-mediated interference
Drosophila Schneider S2 cells were stably transfected with a plasmid encoding GFP–α-tubulin or γ-tubulin–GFP under the control of a constitutive active promoter (gifts from S. Rogers [University of North Carolina at Chapel Hill, Chapel Hill, NC] and R. Vale [University of California, San Francisco, San Francisco, CA]). RNAi treatment was performed according to Vérollet et al. (2006). Cells were treated with double-stranded RNAs (dsRNAs) at day 1 and harvested on day 5. For cosilencing, cells were incubated with the four dsRNAs together (20 mM each). The dsRNAs against EB1 (CG3265) were generated from the library of Drosophila cDNA (Thermo Fisher Scientific).
Fly strains
Strains y1w118 or w118 were used as control strains, and mutant strains Dgrip75175 (Schnorrer et al., 2002), Dgrip84 R20 (R20/FM7; Colombié et al., 2006), and Dgp71WDGE30807 (GenExel-Sein, Inc.; Vérollet et al., 2006) were used for the study. Each mutant chromosome was balanced over CyO, P[Kr:Gal4], P[UAS:GFP] to select the homozygous mutant larvae (Vérollet et al., 2006).
Western blotting and cytological analysis
Protein extracts from S2 cells (Raynaud-Messina et al., 2004) and from total larval brains were prepared and subjected to Western blot analyses (7.5% polyacrylamide gel and SDS). Total brain extracts were prepared from ∼10 brains of each genotype, dissected in saline (0.7% NaCl), boiled for 3 min in 2× Laemmli sample buffer, and diluted twice (Colombié et al., 2006). To determine the solubility characteristics of tubulins, extracts were centrifuged for 15 min at 10,000 g at room temperature, and then the supernatant and the pellet were analyzed by Western blotting. For controls, cells were treated with taxol (24 h at 5.10−5 M) or colchicine (24 h at 25.10−3 M) or incubated at 4°C for 2 h before harvesting. S2R+ cells, cultured on 0.5 mg/ml concanavalin A–coated coverslips for at least 3 h, were fixed with precooled −20°C methanol for 10 min and then immunostained (Vérollet et al., 2006). To detect γ-tubulin, Dgrip84, and Dgp71WD along MTs, S2R+ cells were permeabilized for 1 min in PEM (100 mM Pipes, 1 mM EGTA, and 2 mM MgCl2, pH 6.8) containing 0.5% vol/vol Triton X-100, fixed for 1 h in PEM containing 1% dimethyl sulfoxide and 3.7% vol/vol paraformaldehyde, and then immunolabeled (Raynaud-Messina et al., 2004). Taxol treatments were performed for 24 h at 5.10−5 M. Fluorescence microscopy was performed with Deltavision RT equipment (Applied Precision) on a microscope (IX71; Olympus), using a 100× 1.4 NA objective. Images were obtained with a camera (CoolSNAP; Roper Industries) and subsequently deconvolved (ratio conservative method), and optical sections were projected. Images and Western blot quantification were created using Photoshop (Adobe).
The mouse monoclonal antibodies T-5168, GTU88 (Sigma-Aldrich), and 1501 (Millipore) were used to stain α-tubulin, γ-tubulin, and actin (used as an internal loading control), respectively. The following rabbit antibodies were used: R62 against Drosophila 23C γ-tubulin (antibody used in all cases except when specified in the text for GTU88; Raynaud-Messina et al., 2004), R367 against Dgrip84, R403 against Dgrip91, R300 against Dgrip75, R360 against Dgrip163, and R267 against Dgp71WD (Vérollet et al., 2006). We also used rabbit antibody Rb276 against Mast (Sousa et al., 2007), rabbit antibodies against Dgrip128 (gift from Y. Zheng, Carnegie Institution of Washington, Baltimore, MD; Gunawardane et al., 2000), rabbit antibodies against EB1 (gift from S. Rogers; Rogers et al., 2002), and human antibody hMB11 against GTP-MTs (gift from F. Perez, Institut Curie, Paris, France; Dimitrov et al., 2008).
Live imaging of MTs
Drosophila S2 GFP–α-tubulin and γ-tubulin–GFP cells were grown in Schneider medium in an aluminum culture chamber with concanavalin A–coated coverslips. Images were acquired at 2-s intervals for 200 s with a wild-field microscope (Axiovert; Carl Zeiss, Inc.) using a 100× 1.4 NA objective (22°C) and a camera (AxioCam MRm; Carl Zeiss, Inc.)/Axiovision software (Carl Zeiss, Inc.), except for speckle experiments (Deltavision RT and deconvolution as in immunofluorescence analysis). MT plus ends (or speckles) were tracked using ImageJ software (National Institutes of Health). Catastrophes were defined as transition from growth or pause to shrinkage, and rescues were defined as transition from shortening or pause to growth (Sousa et al., 2007). Dynamicity was calculated by dividing the sum of the total length grown and shortened by the life span of the MT (Rusan et al., 2001). At least 150 MTs were analyzed for the quantification of the two arbitrary classes (transient and persistent), at least 50 MTs (from 15 independent cells) were analyzed for the quantification of dynamic parameters, and 20 individual MTs were tracked for MT plus end analysis. Statistical independent two-sample t test analysis of dynamic parameters was performed using SPSS version 14.0 software (SPSS, Inc.; Sousa et al., 2007). Means are significantly different for P < 0.05, meaning a 95% confidence level (P95).
For γ-tubulin immunostaining immediately after time-lapse recording, cells were permeabilized with PEM/Triton X-100 0.3% (1 min) and subjected to γ-tubulin labeling as described in the previous section for S2R+ cells. The MT plus end of the image was fitted with the MT end of the last frame of the video. 53 MTs taken from 12 cells were analyzed. Only MTs clearly resolved near the cell periphery and labeled with γ-tubulin were selected for the analysis.
Regrowth assays
Cells were placed for 50 min on ice with ethanol inside a Petri dish and then transferred directly to a controlled 25°C chamber. Images were obtained at regular intervals over 50 min using a confocal microscope (SP2; Leica) with a 63× 1.4 NA objective (Leica). The MTs were counted in a region of interest of 6 µm from the cell cortex (Sousa et al., 2007).
Online supplemental material
Fig. S1 shows that γ-TuSC proteins are coregulated. Fig. S2 shows the effects of latrunculin A treatment and EB1 depletion on the increase of MT dynamics induced by γ-TuRC depletion. Fig. S3 shows that γ- and GTP-tubulin do not colocalize on the MTs. Video 1 shows that Dgrip75 down-regulation enhances MT dynamics. Video 2 shows that γ-tubulin down-regulation destabilizes short MTs. Video 3 shows that MT plus end dynamics are affected after depletion of a γ-TuRC component. Videos 4 and 5 show that γ-tubulin along interphase MTs acts as an anticatastrophe factor. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200905060/DC1.
Acknowledgments
We thank Drs. S. Rogers, R. Vale, H. Ohkura (University of Edinburgh, Edinburgh, Scotland, UK), F. Perez, G. Goshima (Nagoya University, Chikusa-ku, Nagoya, Japan), H. Maiato (Instituto de Biologia Molecular e Celular, Porto, Portugal), and Y. Zheng for the donation of cell lines and antibodies. We also thank J. Coppin and D. Rebérioux for help with computer analysis and Drs. S. Carréno and J.E. Gairin for critical comments.
This work was funded by grants from the Association pour la Recherche sur le Cancer (4720XP0230F), Centre National de la Recherche Scientifique, and Pierre Fabre Laboratories, and support for equipment was provided by the Fonds Européen de Développement Economique Régional and Action Concertée Incitative (grant 04 5 566).
Footnotes
Abbreviations used in this paper:
- dsRNA
- double-stranded RNA
- γ-TuRC
- γ-tubulin ring complex
- γ-TuSC
- γ-tubulin small complex
- MT
- microtubule
References
- Anders A., Lourenço P.C., Sawin K.E. 2006. Noncore components of the fission yeast gamma-tubulin complex. Mol. Biol. Cell. 17:5075–5093 10.1091/mbc.E05-11-1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa V., Yamamoto R.R., Henderson D.S., Glover D.M. 2000. Mutation of a Drosophila gamma tubulin ring complex subunit encoded by discs degenerate-4 differentially disrupts centrosomal protein localization. Genes Dev. 14:3126–3139 10.1101/gad.182800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittle A.L., Ohkura H. 2005. Mini spindles, the XMAP215 homologue, suppresses pausing of interphase microtubules in Drosophila. EMBO J. 24:1387–1396 10.1038/sj.emboj.7600629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombié N., Vérollet C., Sampaio P., Moisand A., Sunkel C., Bourbon H.M., Wright M., Raynaud-Messina B. 2006. The Drosophila gamma-tubulin small complex subunit Dgrip84 is required for structural and functional integrity of the spindle apparatus. Mol. Biol. Cell. 17:272–282 10.1091/mbc.E05-08-0722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuschieri L., Miller R., Vogel J. 2006. Gamma-tubulin is required for proper recruitment and assembly of Kar9-Bim1 complexes in budding yeast. Mol. Biol. Cell. 17:4420–4434 10.1091/mbc.E06-03-0245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov A., Quesnoit M., Moutel S., Cantaloube I., Poüs C., Perez F. 2008. Detection of GTP-tubulin conformation in vivo reveals a role for GTP remnants in microtubule rescues. Science. 322:1353–1356 10.1126/science.1165401 [DOI] [PubMed] [Google Scholar]
- Fujita A., Vardy L., Garcia M.A., Toda T. 2002. A fourth component of the fission yeast γ-tubulin complex, Alp16, is required for cytoplasmic microtubule integrity and becomes indispensable when γ-tubulin function is compromised. Mol. Biol. Cell. 13:2360–2373 10.1091/mbc.02-01-0603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima G., Mayer M., Zhang N., Stuurman N., Vale R.D. 2008. Augmin: a protein complex required for centrosome-independent microtubule generation within the spindle. J. Cell Biol. 181:421–429 10.1083/jcb.200711053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardane R.N., Martin O.C., Cao K., Zhang L., Dej K., Iwamatsu A., Zheng Y. 2000. Characterization and reconstitution of Drosophila γ-tubulin ring complex subunits. J. Cell Biol. 151:1513–1524 10.1083/jcb.151.7.1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haren L., Remy M.-H., Bazin I., Callebaut I., Wright M., Merdes A. 2006. NEDD1-dependent recruitment of the γ-tubulin ring complex to the centrosome is necessary for centriole duplication and spindle assembly. J. Cell Biol. 172:505–515 10.1083/jcb.200510028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi N., Fumoto K., Izumi S., Kikuchi A. 2008. GSK-3beta regulates proper mitotic spindle formation in cooperation with a component of the gamma-tubulin ring complex, GCP5. J. Biol. Chem. 283:12981–12991 10.1074/jbc.M710282200 [DOI] [PubMed] [Google Scholar]
- Janson M.E., Setty T.G., Paoletti A., Tran P.T. 2005. Efficient formation of bipolar microtubule bundles requires microtubule-bound γ-tubulin complexes. J. Cell Biol. 169:297–308 10.1083/jcb.200410119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi H.C., Palacios M.J., McNamara L., Cleveland D.W. 1992. Gamma-tubulin is a centrosomal protein required for cell cycle-dependent microtubule nucleation. Nature. 356:80–83 10.1038/356080a0 [DOI] [PubMed] [Google Scholar]
- Knop M., Schiebel E. 1997. Spc98p and Spc97p of the yeast gamma-tubulin complex mediate binding to the spindle pole body via their interaction with Spc110p. EMBO J. 16:6985–6995 10.1093/emboj/16.23.6985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajoie-Mazenc I., Tollon Y., Détraves C., Julian M., Moisand A., Gueth-Hallonet C., Debec A., Salles-Passador I., Puget A., Mazarguil H., et al. 1994. Recruitment of antigenic gamma-tubulin during mitosis in animal cells: presence of gamma-tubulin in the mitotic spindle. J. Cell Sci. 107:2825–2837 [DOI] [PubMed] [Google Scholar]
- Lüders J., Patel U.K., Stearns T. 2006. GCP-WD is a gamma-tubulin targeting factor required for centrosomal and chromatin-mediated microtubule nucleation. Nat. Cell Biol. 8:137–147 10.1038/ncb1349 [DOI] [PubMed] [Google Scholar]
- Mahoney N.M., Goshima G., Douglass A.D., Vale R.D. 2006. Making microtubules and mitotic spindles in cells without functional centrosomes. Curr. Biol. 16:564–569 10.1016/j.cub.2006.01.053 [DOI] [PubMed] [Google Scholar]
- Masuda H., Miyamoto R., Haraguchi T., Hiraoka Y. 2006. The carboxy-terminus of Alp4 alters microtubule dynamics to induce oscillatory nuclear movement led by the spindle pole body in Schizosaccharomyces pombe. Genes Cells. 11:337–352 10.1111/j.1365-2443.2006.00947.x [DOI] [PubMed] [Google Scholar]
- Moritz M., Braunfeld M.B., Sedat J.W., Alberts B., Agard D.A. 1995. Microtubule nucleation by γ-tubulin-containing rings in the centrosome. Nature. 378:638–640 10.1038/378638a0 [DOI] [PubMed] [Google Scholar]
- Murata T., Sonobe S., Baskin T.I., Hyodo S., Hasezawa S., Nagata T., Horio T., Hasebe M. 2005. Microtubule-dependent microtubule nucleation based on recruitment of gamma-tubulin in higher plants. Nat. Cell Biol. 7:961–968 10.1038/ncb1306 [DOI] [PubMed] [Google Scholar]
- Oakley C.E., Oakley B.R. 1989. Identification of γ-tubulin, a new member of the tubulin superfamily encoded by mipA gene of Aspergillus nidulans. Nature. 338:662–664 10.1038/338662a0 [DOI] [PubMed] [Google Scholar]
- Oegema K., Wiese C., Martin O.C., Milligan R.A., Iwamatsu A., Mitchison T.J., Zheng Y. 1999. Characterization of two related Drosophila γ-tubulin complexes that differ in their ability to nucleate microtubules. J. Cell Biol. 144:721–733 10.1083/jcb.144.4.721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluh J.L., Nogales E., Oakley B.R., McDonald K., Pidoux A.L., Cande W.Z. 2000. A mutation in gamma-tubulin alters microtubule dynamics and organization and is synthetically lethal with the kinesin-like protein pkl1p. Mol. Biol. Cell. 11:1225–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynaud-Messina B., Merdes A. 2007. Gamma-tubulin complexes and microtubule organization. Curr. Opin. Cell Biol. 19:24–30 10.1016/j.ceb.2006.12.008 [DOI] [PubMed] [Google Scholar]
- Raynaud-Messina B., Mazzolini L., Moisand A., Cirinesi A.M., Wright M. 2004. Elongation of centriolar microtubule triplets contributes to the formation of the mitotic spindle in gamma-tubulin-depleted cells. J. Cell Sci. 117:5497–5507 10.1242/jcs.01401 [DOI] [PubMed] [Google Scholar]
- Rogers S.L., Rogers G.C., Sharp D.J., Vale R.D. 2002. Drosophila EB1 is important for proper assembly, dynamics, and positioning of the mitotic spindle. J. Cell Biol. 158:873–884 10.1083/jcb.200202032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers G.C., Rusan N.M., Peifer M., Rogers S.L. 2008. A multicomponent assembly pathway contributes to the formation of acentrosomal microtubule arrays in interphase Drosophila cells. Mol. Biol. Cell. 19:3163–3178 10.1091/mbc.E07-10-1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusan N.M., Fagerstrom C.J., Yvon A.M., Wadsworth P. 2001. Cell cycle-dependent changes in microtubule dynamics in living cells expressing green fluorescent protein-alpha tubulin. Mol. Biol. Cell. 12:971–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin K.E., Lourenco P.C., Snaith H.A. 2004. Microtubule nucleation at non-spindle pole body microtubule-organizing centers requires fission yeast centrosomin-related protein mod20p. Curr. Biol. 14:763–775 10.1016/j.cub.2004.03.042 [DOI] [PubMed] [Google Scholar]
- Schnorrer F., Luschnig S., Koch I., Nüsslein-Volhard C. 2002. γ-tubulin37C and γ-tubulin ring complex protein 75 are essential for bicoid RNA localization during Drosophila oogenesis. Dev. Cell. 3:685–696 10.1016/S1534-5807(02)00301-5 [DOI] [PubMed] [Google Scholar]
- Sousa A., Reis R., Sampaio P., Sunkel C.E. 2007. The Drosophila CLASP homologue, Mast/Orbit regulates the dynamic behaviour of interphase microtubules by promoting the pause state. Cell Motil. Cytoskeleton. 64:605–620 10.1002/cm.20208 [DOI] [PubMed] [Google Scholar]
- Sunkel C.E., Gomes R., Sampaio P., Perdigão J., González C. 1995. Gamma-tubulin is required for the structure and function of the microtubule organizing centre in Drosophila neuroblasts. EMBO J. 14:28–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardy L., Toda T. 2000. The fission yeast γ-tubulin complex is required in G(1) phase and is a component of the spindle assembly checkpoint. EMBO J. 19:6098–6111 10.1093/emboj/19.22.6098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatram S., Tasto J.J., Feoktistova A., Jennings J.L., Link A.J., Gould K.L. 2004. Identification and characterization of two novel proteins affecting fission yeast gamma-tubulin complex function. Mol. Biol. Cell. 15:2287–2301 10.1091/mbc.E03-10-0728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vérollet C., Colombié N., Daubon T., Bourbon H.M., Wright M., Raynaud-Messina B. 2006. Drosophila melanogaster γ-TuRC is dispensable for targeting γ-tubulin to the centrosome and microtubule nucleation. J. Cell Biol. 172:517–528 10.1083/jcb.200511071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt N., Koch I., Schwarz H., Schnorrer F., Nüsslein-Volhard C. 2006. The gammaTuRC components Grip75 and Grip128 have an essential microtubule-anchoring function in the Drosophila germline. Development. 133:3963–3972 10.1242/dev.02570 [DOI] [PubMed] [Google Scholar]
- Wiese C., Zheng Y. 2000. A new function for the gamma-tubulin ring complex as a microtubule minus-end cap. Nat. Cell Biol. 2:358–364 10.1038/35014051 [DOI] [PubMed] [Google Scholar]
- Wilde A., Zheng Y. 1999. Stimulation of microtubule aster formation and spindle assembly by the small GTPase Ran. Science. 284:1359–1362 10.1126/science.284.5418.1359 [DOI] [PubMed] [Google Scholar]
- Zheng Y., Wong M.L., Alberts B., Mitchison T. 1995. Nucleation of microtubule assembly by a γ-tubulin-containing ring complex. Nature. 378:578–583 10.1038/378578a0 [DOI] [PubMed] [Google Scholar]
- Zhu H., Coppinger J.A., Jang C.Y., Yates J.R., III, Fang G. 2008. FAM29A promotes microtubule amplification via recruitment of the NEDD1–γ-tubulin complex to the mitotic spindle. J. Cell Biol. 183:835–848 10.1083/jcb.200807046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman S., Chang F. 2005. Effects of gamma-tubulin complex proteins on microtubule nucleation and catastrophe in fission yeast. Mol. Biol. Cell. 16:2719–2733 10.1091/mbc.E04-08-0676 [DOI] [PMC free article] [PubMed] [Google Scholar]