Abstract
TDCS - Transcranial Direct Current Stimulation - is an emerging technique of non-invasive brain stimulation that has been found useful in examining cortical function in normal subjects and in facilitating treatments of various neurological disorders. A better understanding of adaptive as well as maladaptive post-stroke neuroplasticity and its modulation through non-invasive brain stimulation has opened up experimental treatment options using TDCS for patients recovering from stroke. We will review TDCS’s role as a facilitator of stroke recovery, the different modes of transcranial direct current stimulation, and the potential mechanisms underlying the neural effects of TDCS.
Keywords: TDCS, Transcranial Direct Current Stimulation, Brain polarization, stroke, rehabilitation, recovery, adaptation, mal-adaptation, plasticity
Introduction and Historical Overview
The concept of therapeutic electricity on excitable tissues such as the brain is not new considering the attempts to cure epileptic disorders using electric catfish as early as the 11th century (for a historic perspective, see Priori, 2003).1 The initial experiments of Eduard Hitzig (1870; cited in Gross, 2007)2 on dog’s cortex subsequent to a serendipitous discovery of abnormal involuntary movements in patients treated with high voltage transcranial electric currents, led to an interest in using electric currents to identify the cortical representations of limb movements (for more historic details see Gross, 2007)2. Electro-sleep therapy which later came to be known as Cranial Electrical Stimulation (CES) was used to treat sleep disorders and depression since 1902 (Gilula, 2005).3
In the 1960s, Bindman’s4 experiments that reported long-lasting polarization effects following electric stimulation of the exposed motor cortex of animals led to a resurgence of studies exploring the clinical applications of electric stimulation, including the use of brain polarization in depressed patients. Although these studies showed some benefits, replicating these beneficial effects in controlled settings yielded mixed results which subsequently led to a diminished interest in transcranial electrical treatments. However, several years later, the effects of anodal direct currents on brain tissue in rats5 such as increased accumulation of calcium ions leading to increased cortical excitabilityand finding evidence for intra-cerebral currents during electro-sleep therapy studies in humans prompted Priori and colleagues1,6 to develop a novel approach of non-invasive brain stimulation with weak direct currents which came to be known as TDCS. Subsequent experiments by Nitsche and Paulus7,8 demonstrated the modulating effects of anodal (increases cortical excitability) and cathodal (decreases cortical excitability) TDCS on brain tissue whose effects surprisingly outlasted the duration of stimulation. Residual electrophysiological effects were detectable up to 90 minutes and sensorimotor/cognitive effects up to 30 minutes, after a 20–30 minute stimulation period.7,8 These early reports and others over the last 8–10 years have renewed the interest in the use of non-invasive regional brain polarization for various neurological disorders. Current research studies make use of the blocking/depressing effects of cathodal TDCS to create temporary cortical dysfunctions (“virtual lesions”) that allows investigators to causally examine functions of cortical regions. Similarly, several studies have examined whether anodal TDCS can be used to improve performance of certain sensorimotor or cognitive tasks (as an example of these two approaches see Vines et al., 2006a,b).9,10
Methods and Mechanisms of TDCS
The components required for TDCS include a Constant Current Stimulator and surface electrodes soaked in normal saline. A Constant Current Stimulator provides a steady flow of direct current (e.g., 0 – 4mA) while constantly monitoring the resistance in the system. Saline soaked electrodes applied and secured onto the scalp over desired areas such as the left or right precentral gyrus region (corresponding to C3 or C4 of the International 10–20 EEG system) form terminals relaying currents across the scalp and through the underlying brain tissue. The direction of the current flow determines the effects on the underlying tissue. With an active electrode over C3 or C4, a reference electrode over a control region (e.g., supra-orbital region) and current flowing from the active to the reference electrode, the excitability of the brain tissue under the anodal electrode is increased and when the current flow is reversed, the excitability of the brain tissue under this electrode is decreased (the electrode that was previously the anode now becomes the cathode; Fig. 1A). Once switched on, the constant current stimulator produces a transient tingling sensation under the electrode which fades off in 30sec to 1min thereby making it ideal for blinding subjects (in sham-controlled studies) by turning it off after the initial sensory experience. McCreery and colleagues11 found that current densities below 25 mA/cm2 did not cause brain tissue damage and the protocols that apply 1–2 mA as in present day studies fall well within these limits. Recent studies on brain modeling and current density distribution have suggested that in spite of a fraction of the direct current being shunted through the scalp, TDCS carries adequate currents to the underlying cortex, sufficient for neuronal excitability shifts.12 Our own studies have shown that measures of cerebral blood flow can change in brain regions that are targeted by transcranial anodal direct current providing further proof that transcranially applied direct currents can affect tissue excitability as well as regional blood flow as an indirect marker of change in regional tissue excitability (Fig. 1B).
Figure 1. TDCS setup and montage.
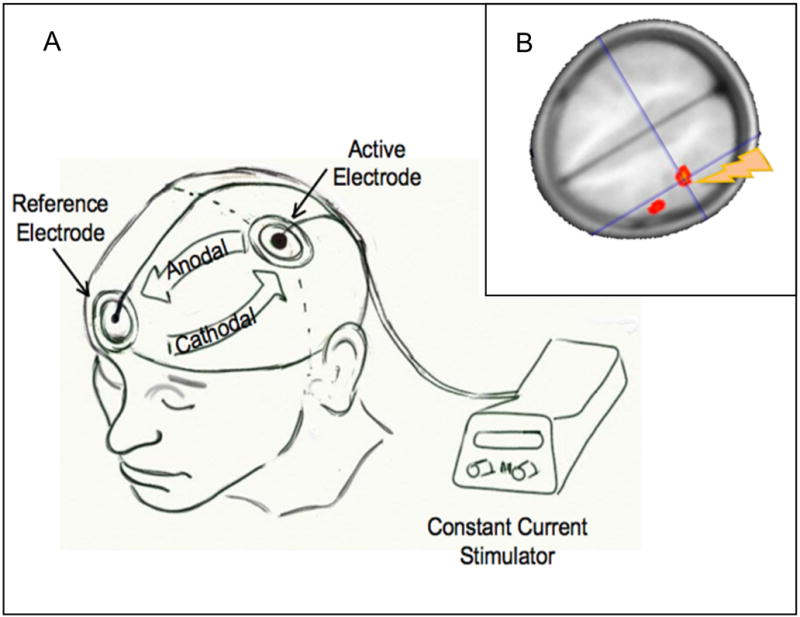
A) This drawing shows the TDCS setup using a mobile, battery-operated direct current stimulator connected with two electrodes. One electrode (active) is positioned over C3 (corresponding to the precentral gyrus) and the reference electrode is positioned over the contralateral supraorbital region. If current flows from C3 to the supraorbital region, then the tissue underlying C3 is subjected to anodal (increase in excitability) stimulation. If current is reversed, then the tissue underlying C3 is subjected to cathodal (decrease in excitability) stimulation; B) Regional cerebral blood increases in the motor region underlying the electrode positioned over C3 after anodal stimulation. Regional cerebral blood was determined using a non-invasive arterial spin-labeling technique30.
The advantages of TDCS over other non-invasive brain stimulation methods include its ease of use, its large electrode size allowing influence over a larger neural network, a sham mode allowing controlled experiments and randomized controlled clinical trials, and its portability making it possible to apply stimulation while the patient receives occupational/physical therapy. Nevertheless, TDCS is limited by its poor temporal resolution and anatomical localization. Furthermore, inter-individual variation in conductivity due to differences in hair, scalp, and bone composition can interfere with the current that is carried to the brain. Last but not least, although single sessions and multi-day sessions have been done and found to be safe, the safety of prolonged periods of stimulation requires further studies.
TDCS by itself provides a sub-threshold stimulus that modulates the likelihood that neurons will fire by hyperpolarizing or depolarizing the brain tissue, without direct neuronal depolarization. The prolonged sensory, motor, and cognitive effects of TDCS have been attributed to a persistent bidirectional modification of post-synaptic connections similar to long-term potentiation (LTP) and long-term depression (LTD) effects.5,7 Dextromethorphan, an N-methyl-D-aspartate (NMDA) antagonist suppressed both anodal and cathodal TDCS effects, strongly suggesting the involvement of NMDA receptors in both types of DC-induced neuroplasticity.13 In contrast, Carbamazepine selectively eliminated anodal effects.14 Since Carbamazepine stabilizes the membrane potential through voltage-gated sodium channels (stabilizing the inactivated state of sodium channels), the results reveal that after-effects of anodal TDCS require a depolarization of membrane potentials.14,15 More studies are needed, particularly in humans to verify TDCS’s actions on brain tissue, its underlying mechanism, and the associated behavioral and cognitive effects.
Stroke Recovery, Neuroplasticity, and Effects of Brain Polarization
Stroke is the major cause of severe disability in the US population with about half of the stroke victims left with residual disabilities.16 Spontaneous recovery has been primarily attributed to neuroplasticity which occurs predominantly by means of regeneration (e.g., axonal and dendritic sprouting) and reorganization (e.g., remapping of lesional area representations onto non-lesional cortex, either in the peri-lesional region or in the contralesional hemisphere). Functional magnetic resonance imaging (fMRI) studies have shown that early reorganization of the brain is associated with increased bi-hemispheric activation when the affected hand/arm is moved which in chronic stroke stages becomes more lateralized.17,18 The significance of the contralesional (ipsi-lateral to the moving hand) activation during motor tasks involving the recovering hand/arm still remains uncertain. Explanations range from an epiphenomenon of recovery or an adaptive neuroplastic process, to a sign of mal-adaptation which might possibly interfere with the recovery process.
Early re-activation or an over-activation of the remnant ipsilesional sensori-motor and premotor cortex generally correlates with good recovery.17,18 Whether or not the contralesional activation pattern (ipsilateral to the recovering hand/arm) is an epiphenomenon or a maladaptive phenomenon in the recovery process could be examined by blocking or depressing this activation using non-invasive brain stimulation methods such as TDCS. The electrophysiological correlate of an apparent mal-adaptive activation pattern is an imbalance of interhemispheric inhibition due to inhibition from the contralesional unaffected hemisphere onto the lesional hemisphere that is not balanced by a similar level of inhibition from the lesional hemisphere onto the contralesional, normal hemisphere. This abnormal and imbalanced inter-hemispheric inhibition is the hypothetical model that underlies the experimental therapy of applying anodal TDCS to the lesional hemisphere or cathodal TDCS to the non-lesional, unaffected hemisphere.
Experimental Animal Studies
Spontaneous, training induced, and post-polarization neuroplasticity with or without physical rehabilitation have been studied in primates and rodent brain models. Factors such as delay between the stroke and the time of initiation of therapy, as well as the type (monopolar and bipolar), frequency, and duration of the stimulation have different outcomes on motor improvement, remapping of cortical representation and on the overall functional outcomes.19–21 For example, there was a significant difference in sensorimotor improvement in recovering rats receiving a 50Hz direct cortical stimulation compared to those receiving either 250Hz stimulation or no stimulation at all.19–21 Histological analysis of brains of these animals that received 50Hz stimulation revealed a significantly higher surface density of microtubule-associated protein2 (MAP 2) in the peri-lesional cortex which is typically associated with high dendritic activity.21 Most experimental animal studies have shown that rehabilitation dependent improvement in motor performance is associated with remapping of movement representations towards the peri-lesional motor cortices which seems to be significantly enhanced when cortical stimulation is combined with rehabilitative motor training in the recovery phase.22,20 Combining peripheral and central stimulation might lead to an increase in synaptic plasticity modulated by depolarization induced intracortical connectivity. Both monopolar and bipolar currents showed significant benefits in increasing peri-lesional movement representations.19 It was also seen that in comparison to the non-stimulated groups, the cortically stimulated rats maintained their performance improvements for days without any intervening decline.20
Human Studies
Studies in humans can be divided into invasive and non-invasive brain-stimulation studies and further into those that are or not coupled with simultaneous physical/occupational therapy. Epidural electric stimulation around an FMRI ‘hotspot’ in the perilesional area coupled with simultaneous occupational therapy has shown benefits in pilot studies.23 However the early benefits seen in the uncontrolled and un-blinded phase I and phase II studies, were not replicated in a recently concluded randomized controlled clinical trial24 comparing the effects of combined epidural stimulation and occupational therapy against occupational therapy alone.
Non-invasive brain stimulation in humans has been done with TMS and recently also TDCS. In this review we will focus on TDCS studies. TDCS with its filtered current may have some advantages over direct cortical stimulation by influencing a wider region of brain involving not only the primary motor, but also premotor, supplementary motor, and somatosensory cortex, all of which have been shown to have a role in the recovery process in various studies. Moreover non-invasive transcranial stimulation is less risky than direct cortical or epidural stimulation, it is portable, and can be performed on an outpatient basis, with optimal montage of electrodes suited to individual subjects. Two modes of TDCS have been used in human stroke rehabilitation studies: anodal stimulation (increase in excitability) of the lesional hemisphere (Fig. 2) or cathodal stimulation (decrease in excitability) of the contralesional hemisphere. Proof-of-principle studies have been done for both of these approaches with TMS as well as TDCS. These studies mostly applied a single session of either TMS or TDCS and evaluated the effects comparing performance in pre and post intervention batteries of motor assessments. Effects of multiple sessions are currently being studied. Preliminary analyses of an ongoing trial in our own institution involving five days of combined TDCS with occupational therapy in a crossover, sham-controlled study suggested a significant improvement in motor outcomes that lasted for at least one week.25 Results of this cathodal TDCS study (stimulation applied to the contralesional hemisphere) however contrasts with an anodal TDCS study by Hesse et al.26 who subjected sub-acute stroke patients to multiple sessions of anodal TDCS (applied to the lesional hemisphere) in combination with an robot-assisted arm training protocol, but failed to find significant motor improvements. These differences between cathodal stimulation to the unaffected hemisphere and anodal stimulation to the lesional hemisphere may be due to factors such as the extent of the lesion, the amount of cortical involvement, or the involvement of the pyramidal tract on the lesional hemisphere. Further studies and possibly direct contrasts between cathodal and anodal stimulation approaches are needed to explore these issues. Previous studies in chronic stroke patients using behavioral parameters and TMS as a diagnostic tool have shown that anodal TDCS applied to the lesional motor region is associated with significant improvements in motor tasks and the improvements correlated with the increase in excitability of the lesional hemisphere as indicated by a rise in the slope of the recruitment curve and a reduction in the short interval intra-cortical inhibition (SICI) as evidenced by TMS.27 Similar findings have recently been made in our group applying cathodal stimulation to the contralesional, unaffected hemisphere in chronic stroke patients. Improvements in motor tasks correlated with a rise in the slope of the recruitment curve in the affected hemisphere and a decrease in the activation of the contralesional hemisphere as revealed by analysis of functional imaging data25. Future studies might be able to use pre-therapy assessments (e.g., lesion size and location, integrity of pyramidal tract, presence of abnormal interhemispheric inhibition etc.) to tailor stimulation parameters to individual stroke patients. Such parameters include the mode of stimulation (e.g., anodal versus cathodal), the strength of the stimulation, the region of the brain to which stimulation should be delivered and the extent of this region that is being stimulated. TDCS over the non-affected hemisphere may have some inherent advantages over the stimulation of the affected hemisphere by relying on a model of the current density distribution that is not disturbed by a lesion, a normal topography, intact intracortical connections, and a lesser risk of triggering a seizure (“scar epilepsy”). Apart from the site of stimulation and lesion size and location many other factors can contribute to the variability in natural and facilitated stroke recovery studies. Those factors include the hemisphere affected (right versus left, dominant versus non-dominant), lesion site (e.g., cortical/subcortical versus deep white matter lesions), the relation between lesion location and retained pyramidal tract, severity of the initial impairment, age, gender etc. The integrity of the pyramidal tract as examined with Diffusion Tensor Imaging (DTI) or as indicated by the presence of Motor Evoked Potentials (MEPs) in the affected hand is an important determinant of recovery as well as a predictor of stroke recovery potential. Fig. 3 shows two patients with incomplete recovery. Both patients underwent cathodal TDCS to their non-affected hemisphere in combination with simultaneous occupational therapy. One of the patients had a pronounced improvement while the other had only minimal improvement. While the patient with prominent improvement had maintained an intact pyramidal tract (although a reduced number of fibers) in the lesional hemisphere, the patient showing only a minor improvement had a disrupted pyramidal tract (see Fig. 3). This highlights the importance of pyramidal tract integrity and appropriate selection of candidates for experimental interventions.
Figure 2. Brain model of imbalanced interhemispheric inhibition and the therapeutic options to ameliorate this imbalance.
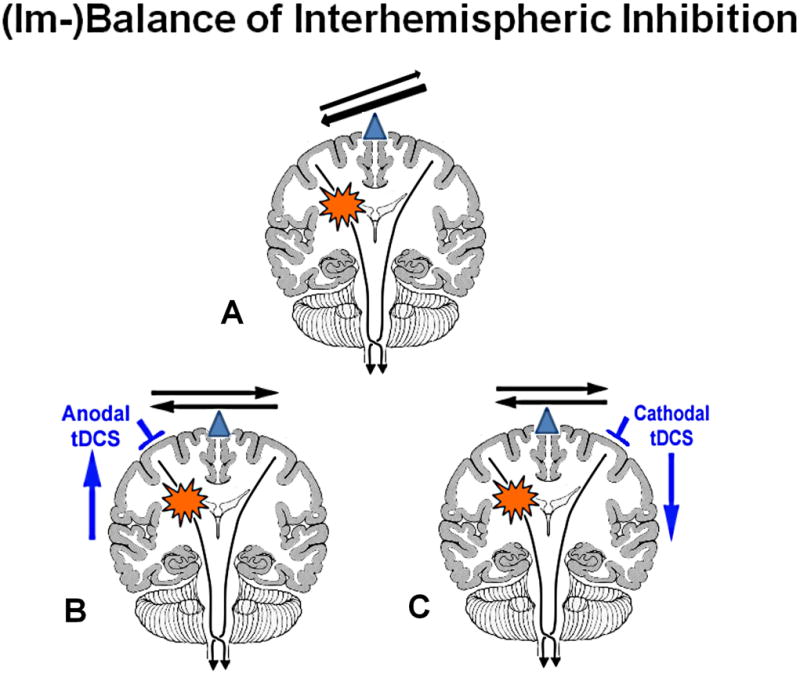
The balance of interhemispheric inhibition becomes disrupted after a stroke (A). This leaves the healthy hemisphere in a position that it could exert too much of an unopposed/imbalanced inhibitory influence onto the lesional hemisphere and possibly interfere in the recovery process of the affected hemisphere. There are two possible ways to ameliorate this imbalance: either one upregulates the excitability in the affected (lesional) hemisphere (B) or one downregulates the excitability in the unaffected (normal) hemisphere (C).
Figure 3. Diffusion Tensor Imaging (DTI) and Stroke Recovery Potential.
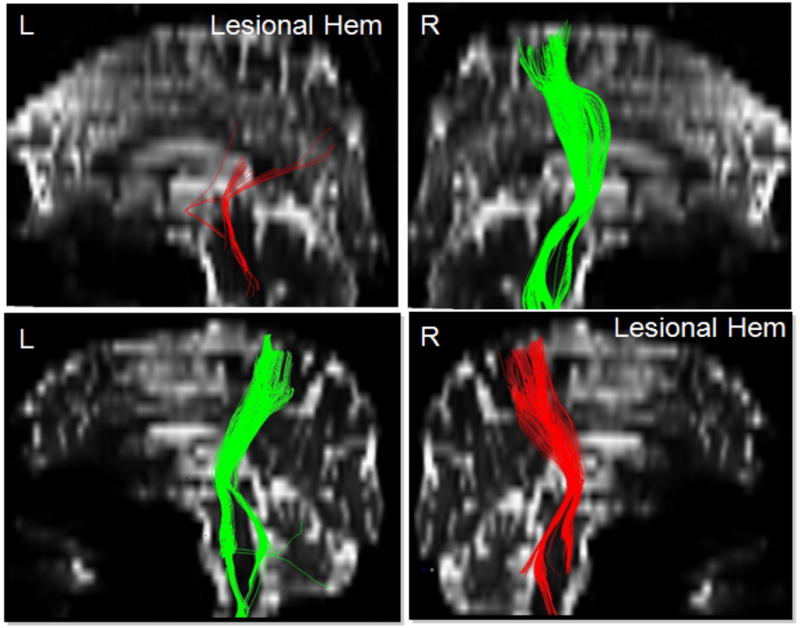
This picture shows two patients with their representative corticospinal tract (CST) fibers that originate from the white matter underlying the precentral gyrus and travel through the internal capsule into the brainstem. The CST of the lesional hemispheres differs between both patients. Patient #1 shows a severely reduced number of fibers that don’t seem to originate from the dorsal part of the motor region (hand/arm region of the precentral gyrus), but still show a path through the posterior limb of the internal capsule into the brainstem while patient #2 has a very mild reduction in the number of CST fibers on the lesional hemisphere, but otherwise shows a similar origin and descent of the CST between the lesional and normal hemisphere. The improvement after TDCS stimulation was pronounced in the patient with intact pyramidal tract, but only minimal in the patient with the disrupted pyramidal tract.
The magnitude of improvement that can be seen after a combined peripheral and central stimulation has varied from study to study and is also dependent on the number of combined peripheral and central brain stimulation sessions a patient undergoes. In our experience, a 5 day treatment trial of central and peripheral stimulation might lead to at least 20% change in the Upper Extremity-Fugl-Meyer score in those patients with incomplete recovery, but who still have intact pyramidal tract fibers.
TDCS in Combination with Rehabilitative Therapy
The effects of non-invasive brain stimulation on stroke recovery might be enhanced by combining it with peripheral stimulation using neuromuscular facilitation techniques as applied in routine rehabilitative therapy or other sensorimotor activities. Initial pilot and proof-of-principle single session studies using TDCS alone have shown significant short lasting excitability shifts and motor improvements. More recent studies have combined brain stimulation with simultaneous peripheral stimulation to further enhance the facilitating effect of non-invasive brain stimulation, the idea being that combined peripheral and central input can enhance synaptic plasticity and skill re-learning. Motor skill learning has shown to produce LTP- and LTD-like changes in the primary motor cortex in animal studies.28 Similar changes were seen following TDCS applied to the motor cortex in animal experiments.5 It seems possible that combining the effects of these two interventions can potentiate relearning of motor skills to a level unattained by either of these alone. This is supported by the fact that paired associative brain stimulation and repetitive peripheral nerve stimulation generated MEPs and improved motor performance which was of greater magnitude than the ones obtained by cortical stimulation alone.29
Summary
Transcranial Direct Current Stimulation (TDCS), a portable, safe, non-invasive, brain stimulation technique, is capable of modulating the excitability of targeted brain regions by altering neuronal membrane potentials based on the polarity of the current transmitted through the scalp via sponge electrodes. Anodal stimulation increases cortical excitability in the stimulated brain tissue while cathodal stimulation decreases it. Corresponding behavioral effects have been seen if the behavior tested draws on the region that is stimulated. TDCS has enormous clinical potential for use in stroke recovery because of its ease of use, its non-invasiveness, its safety (does not provoke seizures), its sham mode (important for controlled clinical trials), and the possibility to combine it with other stimulation/stroke recovery enhancing methods (e.g., simultaneous occupational/physical therapy). If results of pilot and proof-of-principle studies showing long lasting benefits and can be replicated, TDCS might become a very important adjuvant therapy in routine rehabilitative procedures, both in acute and chronic stroke settings.
Acknowledgments
Financial Disclosure: G.S. acknowledges grant support from NIH/NINDS (NS045049). The authors have no conflicts of interest related to this manuscript, including employment, consultancies, honoraria, ownership or options, expert testimony, grants or patents receiving or pending, or royalties.
Footnotes
Author Contributions: Study concept and design: Schlaug, Renga, Nair. Acquisiton of data: Schlaug, Renga, Nair. Analysis and interpretation of data: Schlaug, Renga, Nair. Drafting of the manuscript: Schlaug, Renga, Nair. Critical revision of the manuscript for important intellectual content: Schlaug, Renga, Nair. Administrative, technical and material support: Schlaug, Renga, Nair. Study supervision: Schlaug;
References
- 1.Priori A. Brain polarization in humans: a reappraisal of an old tool for prolonged non-invasive modulation of brain excitability. Clin Neurophysiol. 2003;114(4):589–95. doi: 10.1016/s1388-2457(02)00437-6. [DOI] [PubMed] [Google Scholar]
- 2.Gross CG. The discovery of motor cortex and its background. J Hist Neurosci. 2007;16(3):320–31. doi: 10.1080/09647040600630160. [DOI] [PubMed] [Google Scholar]
- 3.Gilula MF, Kirsch DL. Cranial Electrotherapy Stimulation review: A Safer Alternative to Pyschopharmaceuticals in the Treatment of Depression. J Neurotherapy. 2005;9(2):7–26. [Google Scholar]
- 4.Bindman LJ, Lippold OC, Redfearn JW. The action of brief polarization on the cerebral cortex of rat (1) during the current flow and (2) in the production of long lasting after effects. J Physiol. 1964;172:369–82. doi: 10.1113/jphysiol.1964.sp007425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Islam N, Aftabuddin M, Moriwaki A, Hattori Y, et al. Increase in the calcium level following anodal polarization in the rat brain. Brain Res. 1995;684(2):206–8. doi: 10.1016/0006-8993(95)00434-r. [DOI] [PubMed] [Google Scholar]
- 6.Priori A, Berardelli A, Rona S, Accornero N, et al. Polarization of the human motor cortex through the scalp. Neuroreport. 1998;9(10):2257–60. doi: 10.1097/00001756-199807130-00020. [DOI] [PubMed] [Google Scholar]
- 7.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527(3):633–9. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57(10):1899–901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- 9.Vines BW, Nair DG, Schlaug G. Contralateral and ipsilateral motor effects after transcranial direct current stimulation. Neuroreport. 2006a;17:671–674. doi: 10.1097/00001756-200604240-00023. [DOI] [PubMed] [Google Scholar]
- 10.Vines BW, Schnider NM, Schlaug G. Testing for causality with tDCS: Pitch memory and the left supramarginal gyrus. Neuroreport. 2006b;17:1047–1050. doi: 10.1097/01.wnr.0000223396.05070.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCreery DB, Agnew WF, Yuen TG, Bullara L. Charge density and charge per phase as cofactors in neural injury induced by electrical stimulation. IEEE Trans Biomed Eng. 1990;37(10):996–1001. doi: 10.1109/10.102812. [DOI] [PubMed] [Google Scholar]
- 12.Wagner T, Fregni F, Fecteau S, Grodzinsky A, et al. Transcranial direct current stimulation: a computer-based human model study. Neuroimage. 2007;35(3):1113–24. doi: 10.1016/j.neuroimage.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 13.Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain. 2002;125(Pt 10):2238–47. doi: 10.1093/brain/awf238. [DOI] [PubMed] [Google Scholar]
- 14.Nitsche MA, Liebetanz D, Schlitterlau A, Henschke U, et al. GABAergic modulation of DC stimulation-induced motor cortex excitability shifts in humans. Eur J Neurosci. 2004;19(10):2720–6. doi: 10.1111/j.0953-816X.2004.03398.x. [DOI] [PubMed] [Google Scholar]
- 15.Nitsche MA, Fricke K, Henschke U, Schlitterlau A, et al. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. 2003;553(1):293–301. doi: 10.1113/jphysiol.2003.049916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarke PJ. Handicap in stroke survivors. Disability and Rehabilitation. 1996;21(3):116–23. doi: 10.1080/096382899297855. [DOI] [PubMed] [Google Scholar]
- 17.Loubinoux I, Carel C, Pariente J, Dechaumont S, et al. Correlation between cerebral reorganization and motor recovery after subcortical infarcts. Neuroimage. 2003;20(4):2166–80. doi: 10.1016/j.neuroimage.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 18.Nair DG, Hutchinson S, Fregni F, Alexander M, et al. Imaging correlates of motor recovery from cerebral infarction and their physiological significance in well-recovered patients. Neuroimage. 2007;34(1):253–63. doi: 10.1016/j.neuroimage.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleim JA, Bruneau R, VandenBerg P, MacDonald E, et al. Motor cortex stimulation enhances motor recovery and reduces peri-infarct dysfunction following ischemic insult. Neurol Res. 2003;25(8):789–93. doi: 10.1179/016164103771953862. [DOI] [PubMed] [Google Scholar]
- 20.Teskey GC, Flynn C, Goertzen CD, Monfils MH, et al. Cortical stimulation improves skilled forelimb use following a focal ischemic infarct in the rat. Neurol Res. 2003;25(8):794–800. doi: 10.1179/016164103771953871. [DOI] [PubMed] [Google Scholar]
- 21.Adkins-Muir DL, Jones TA. Cortical electrical stimulation combined with rehabilitative training: enhanced functional recovery and dendritic plasticity following focal cortical ischemia in rats. Neurol Res. 2003;25(8):780–8. doi: 10.1179/016164103771953853. [DOI] [PubMed] [Google Scholar]
- 22.Plautz E, Nudo R. Post-infarct cortical plasticity and behavioral recovery using concurrent cortical stimulation and rehabilitative training: a feasibility study in primates. Neurol Res. 2003;25(8):801–10. doi: 10.1179/016164103771953880. [DOI] [PubMed] [Google Scholar]
- 23.Brown JA, Lutsep H, Cramer SC, Weinand M. Motor cortex stimulation for enhancement of recovery after stroke: case report. Neurol Res. 2003;25(8):815–8. doi: 10.1179/016164103771953907. [DOI] [PubMed] [Google Scholar]
- 24.Levy RM, Benson RR, Winstein CJ. Cortical Stimulation for Upper-Extremity Hemiparesis from Ischemic Stroke. Stroke. 2008;39(2):568. [Google Scholar]
- 25.Nair DN, Renga V, Hamelin S, Pascual-Leone A, Schlaug G. Improving motor function in chronic stroke patients using simultaneous occupational therapy and tDCS. Stroke. 2008;39(2):542. [Google Scholar]
- 26.Hesse S, Werner C, Schonhardt EM, Bardeleben A, et al. Combined transcranial direct current stimulation and robot-assisted arm training in subacute stroke patients: a pilot study. Restor Neurol Neurosci. 2007;25(1):9–15. [PubMed] [Google Scholar]
- 27.Hummel F, Cohen LG. Improvement of motor function with noninvasive cortical stimulation in a patient with chronic stroke. Neurorehabil Neural Repair. 2005;19(1):14–9. doi: 10.1177/1545968304272698. [DOI] [PubMed] [Google Scholar]
- 28.Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290(5491):533–6. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- 29.Uy J, Ridding MC. Increased cortical excitability induced by transcranial DC and peripheral nerve stimulation. J Neurosci Methods. 2003;127(2):193–7. doi: 10.1016/s0165-0270(03)00142-0. [DOI] [PubMed] [Google Scholar]
- 30.Alsop DC, Detre JA. Multisection cerebral blood flow MR imaging with continuous arterial spin labeling. Radiology. 1998;208(2):410–6. doi: 10.1148/radiology.208.2.9680569. [DOI] [PubMed] [Google Scholar]


