Abstract
Lack of surface Fas expression is a main route for apoptotic resistance which is considered an important mechanism of tumorigenesis and tumor progression. Fas and FasL expressions in 110 non-small cell lung carcinomas (NSCLCs) were investigated to evaluate their roles in pulmonary carcinogenesis and to examine the clinicopathologic significance of Fas expression with its relationship with p53 and bcl-2 overexpressions. Immunohistochemical analysis using tissue microarray demonstrated that a large proportion of NSCLC patients (60%) showed lack of membranous Fas expression. The Fas-negative cases revealed the significantly lower survival rate than Fas-positive ones. Also, the loss of Fas receptor expression was found more frequently in advanced stage and higher nodal status. FasL protein was increased in most NSCLCs (89%) compared to normal lungs. p53 and bcl-2 overexpressions showed no association with Fas expression. Conclusively, reduced membranous Fas expression as a mechanism of apoptotic resistance is considered to play an important part of the pulmonary carcinogenesis, which may predict poor survival and have a bad prognostic influence. Increased FasL expression is thought to be a basis for the immune evasion in NSCLCs. The rare bcl-2 overexpression suggests that this anti-apoptotic protein is unlikely to play a role in the apoptotic resistance of NSCLCs.
Keywords: Antigens, CD95; FasL protein; Carcinoma, Non-Small-Cell Lung; Carcinogenesis; Tissue Array Analysis
INTRODUCTION
It is now clearly accepted that clonal expansion and tumor growth result not only from the acceleration of intrinsic proliferation, but also from escape from apoptotic cell death. The Fas receptor (APO-1 or CD95) and its ligand (FasL) play a key role in the initiation of one apoptotic pathway (1). Alterations in this pathway within tumor cells can result in escape from apoptosis and immune surveillance. In malignant cells, their reduced capability to undergo apoptosis in response to some physiological stimuli results in a significant survival advantage and may contribute to tumorigenesis (2, 3). A previous study reported that loss of Fas expression is an integral part of the process of lung tumorigenesis, by allowing lung tumor cells to escape attack by activated cytotoxic T cells which are frequently present in non-small cell lung carcinomas (NSCLCs) (4). Fas ligand (FasL) expression in lung carcinomas has also been proposed to play a role in allowing escape from immune surveillance via a mechanism of peripheral deletion of tumor-reactive T cell clones (5).
Therefore, the analysis of Fas and FasL protein expression is essential for the evaluation of potential pathologic alterations of apoptotic pathways in human NSCLCs, and in fact, the Fas pathway alterations have been reported in a variety of solid human tumors including lung carcinomas (6-11). The expression of Fas in tumor samples, however, does not necessarily predict susceptibility to death, because Fas-mediated apoptosis can be blocked by the lack of p53-mediated activation of Fas/FasL system (12). Recent evidence suggests an intriguing link between p53 and Fas pathway. Munsch et al. reported that intron 1 and promoter of the human Fas death receptor gene contain a p53-responsive element, which is specifically bound only by wild-type p53 (13). Also, mutated p53 has so far been identified as being involved in transcriptional down-regulation of Fas in malignant cells (14).
Initiation of apoptosis occurs principally by signals from two distinct but convergent pathways, the extrinsic or Fas receptor-mediated pathway and the intrinsic, or mitochondrial pathway (15). Bcl-2 normally resides in mitochondrial membranes and the cytoplasm, whose overexpression is another potential mechanism for apoptotic resistance. However, its role in the apoptotic resistance and the relationship with Fas expression has not yet been studied well (10).
Herein, I report results of the first study in which tissue microarrays (TMAs) were used for large-scale investigation of the biologic and prognostic value of Fas and FasL proteins in 110 NSCLC tumors. Also, I evaluate the relationships of p53 and bcl-2 overexpressions with the altered Fas expression in the respect of apoptotic resistance.
MATERIALS AND METHODS
Patients and tissue samples
A total of 110 consecutive patients with completely resected stage I-III NSCLCs from 1994 to 2002 at Dankook University Hospital (DKUH) was retrospectively evaluated. Pathologic tumor staging was performed according to the tumor-node-metastasis (TNM) Classification of AJCC staging system. All patients had complete medical records and had a median follow-up period of 37 months (range, 15 days to 105 months). The demographic and clinical data such as age, sex, smoking history, and TNM status were collected from the hospital records. Death from lung cancer was the terminal event for survival calculations. Formalin-fixed and paraffin-embedded tumor specimens were obtained from the department of pathology at DKUH and all hematoxylin-eosin stained slides of the tumor samples were reviewed by a pathologist. They were histologically subtyped and graded according to the World Health Organization (WHO) diagnostic criteria for lung carcinomas. There were 58 squamous carcinomas, 32 adenocarcinomas, 3 adenosquamous carcinomas (ASCs), 3 bronchioloalveolar carcinomas (BACs), 6 large cell carcinomas, and 8 pleomorphic carcinomas.
Tissue microarray (TMA) construction
Briefly, the most representative tumor and normal areas were carefully selected in pairs and marked as a circle on the H&E slides in a diameter of 2 mm. The corresponding areas of the donor paraffin blocks were punched by the trephine needle with the hole size of 2 mm. These 2 mm-sized tissue cores were transferred and embedded into the recipient block with 60 empty 2 mm-sized holes. All study specimens were sampled with both tumor and normal regions from each donor block. Multiple 4 µm-thick sections were cut with a microtome and transferred to poly-L-lysine-coated slides.
Immunohistochemical staining and its semiquantitative analysis
The standard avidin-biotin-peroxidase complex method was used for the immunohistochemical examination with mouse monoclonal antibodies against Fas (GM30, Novocastra, Newcastle upon Tyne, U.K.), FasL (clone 33, Transduction Lab., Lexington, KY, U.S.A.), p53 (DO7, Novocastra), and bcl-2 (clone 124, DAKO, Goldstrup, Denmark).
The microarrayed tissue sections were deparaffinized with standard xylene and hydrated through graded ethyl alcohols into water. The sections were microwaved in 10 mM citrate buffer at 90℃ for 10 min, and treated with 3% H2O2-PBS solution to reduce endogenous peroxidase activity. Then, they were incubated with normal bovine serum to reduce nonspecific antibody binding and were subsequently subjected to the primary antibody reactions. The primary antibodies for Fas, FasL, p53, and bcl-2 were reacted with the sections at room temperature for one hour at the dilution of 1:40, 1:100, 1:50, and 1:50, respectively. Detection of the immunoreactive staining was carried out by avidin-biotin-peroxidase complex method using the LSAB kit (DAKO). The sections were subjected to a color reaction with 3,3'-diaminobenzidine tetrahydrochloride containing 3% H2O2 in Tris buffer and were lightly counterstained with Mayer's hematoxylin. A negative control was incubated without the primary antibodies.
Positive or negative (or reduced) immunoreactivity for Fas was defined by the following criteria; 1) the tumors were judged to be Fas-positive when at least 5% of the tumor cells were stained membranous only or mixed membranous and cytoplasmic; 2) they were Fas-negative (or reduced) when less than 5% of the tumor cells were stained membranous or when any proportions of the tumor cells were stained cytoplasmic only.
FasL expression was evaluated according to the criteria of Viard-Leveugle et al. (4), which was found in the cytoplasms along the cell borders. The tumors were defined as increased FasL expression, when the expression score is more than 100 for them. The FasL expression score is defined as percentage of stained cells (0-100%)×staining intensity (0-3), which is equal to 100 (area 100%×staining intensity 1) for normal bronchial epithelial cells. The cases were considered positive for p53 overexpression, when at least 50% of tumor cells showed distinct nuclear immunopositivity because high p53 expression would be expected if p53 mutations existed (16). The tumors were defined as bcl-2 overexpression when the cytoplasmic immunopositivity was found in more than 10% of tumor cells (17).
Statistical analysis
The chi-square test and the unpaired Student t-test were used to examine the association between Fas expression and various clinicopathologic parameters. The relationships of Fas expression with p53 and bcl-2 overexpressions were analyzed by chi-square and Fisher's exact tests, respectively (SPSS 12.0K for Windows, 2004). The survival period was calculated as the time from the date of surgery to the date of death or last follow-up. Postoperative survival curves were constructed using the Kaplan-Meier method and then compared by the log rank test. A p value less than 0.05 was defined as statistically significant.
RESULTS
Clinicopathologic data and its relationship with Fas expression
The data were summarized in Table 1. The 110 NSCLC patients consisted of 85 men and 25 women, with median ages of 61 and 60 yr, respectively. Overall, the median age at diagnosis was 60.4 yr. The pathological staging revealed 41 cases of stage 1, 30 of stage 2, and 39 of stage 3. Pathological T stages were pT1 in 21 cases, pT2 in 60, pT3 in 24, and pT4 in 5. Pathological N stages were pN0 in 51 cases, pN1 in 28, pN2 in 31. Of 110 NSCLCs, only 90 cases of squamous carcinoma and adenocarcinoma were histologically graded as 14 cases of grade 1 (well differentiated), 54 of grade 2 (moderately differentiated), and 22 of grade 3 (poorly differentiated). Overall, 66 (60%) of 110 cases showed negative immunostaining for membranous Fas protein expression. The negative immunoreactivity for Fas protein was found more frequently in the advanced stage (stage 3) than in earlier ones (stage 1-2) (p=0.023). Also, the membranous Fas expression tended to be reduced more significantly in higher (pN2) than in lower nodal status (pN0-1) (p=0.057). Other clinocopathologic variables such as age, sex, smoking history, tumor size (pT), and histologic grade and subtypes were not significantly associated with the Fas expression. Bronchioloalveolar carcinoma (BACs), which are thought to be a well-differentiated form of adenocarcinoma, showed the highest rate of membranous Fas expression (100%) among all histologic types, while ordinary adenocarcinomas expressed membranous Fas positivity in only 37% of the cases.
Table 1.
Correlation between Fas expression and clinicopathologic features in 110 non-small cell lung carcinomas
S.D., standard deviation; ca., carcinoma; ASC, adenosquamous carcinoma; BAC, bronchioloalveolar carcinoma; p value *between squamous carcinoma and adenocarcinoma.
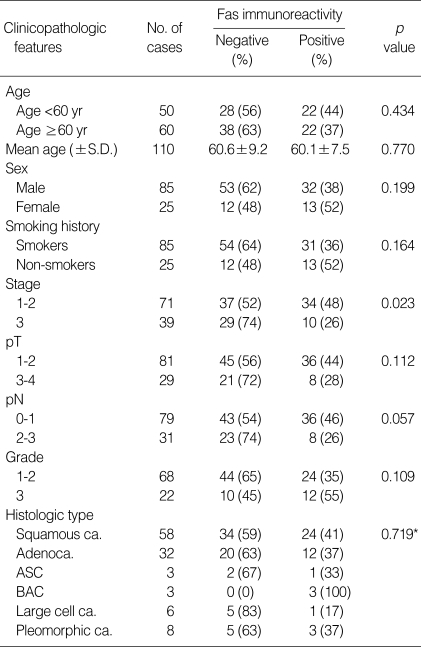
Relationship of Fas expression with survival
Patients with Fas-negative NSCLCs exhibit significantly shorter survival times than did patients with Fas-positive carcinomas, when their survival data were analyzed by Kaplan-Meier method and then log-rank test (Fig. 1). The median survival time of patients with Fas-negative tumors was 32 months, while that of patients with Fas-positive tumors was 67 months. The difference was statistically significant (p=0.019). Additionally, the survival curves according to the pathologic staging and lymph nodal status showed the statistically significant difference in survival rate between the lower (stage 1-2) and higher (stage 3) stages (p=0.019), but not between pN (0-1) and pN (2-3) (p=0.131) (data not shown).
Fig. 1.
The Kaplan-Meier survival curves according to Fas immunostaining for 110 NSCLC patients. Significant difference in survival times was observed between Fas-positive and Fas-negative cases (log rank test p=0.019).
The immunohistochemical expressions of Fas, FasL, p53, and bcl-2 proteins in normal lung and tumor tissues
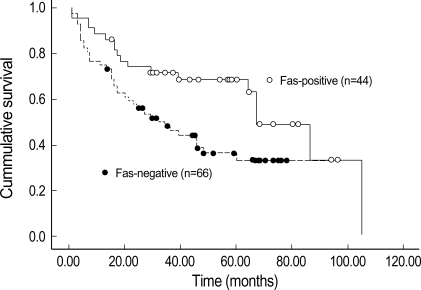
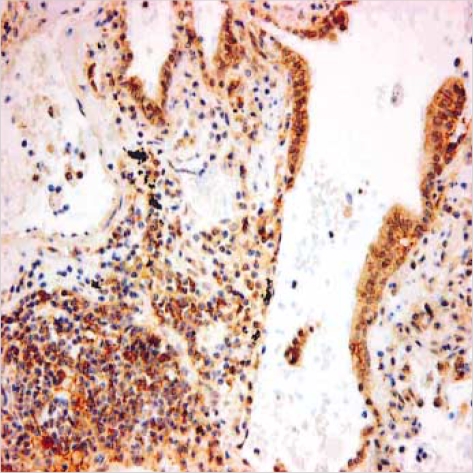
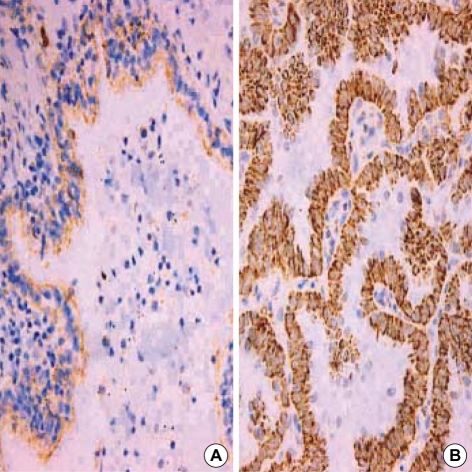
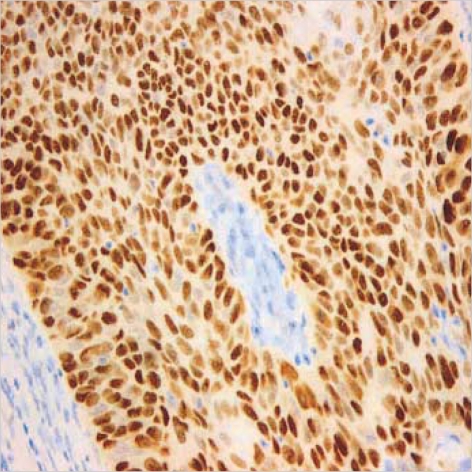
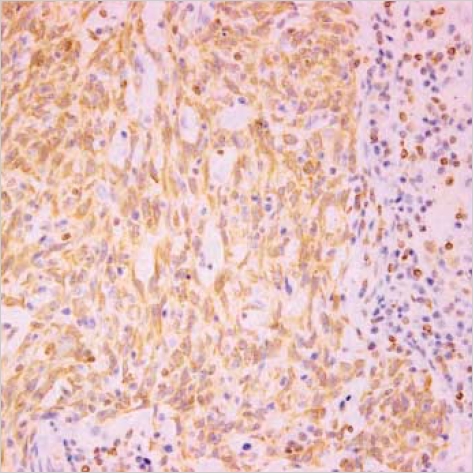
110 microarrayed normal lung tissues revealed the distinct membranous staining for Fas in bronchial and bronchiolar epithelia, reactive lymphocytes, and histiocytes (Fig. 2), whereas the alveolar lining cells were not stained. Most of the paired NSCLC tumor samples (60%) showed a remarkable reduction of the membranous expression for Fas protein (Fig. 3A), while there were some tumors showing strong immunopositivity for surface Fas protein (Fig. 3B). Of 66 Fas-negative cases, 38 tumors (58%) showed complete loss of surface Fas expression. On the other hand, expression of FasL protein was found to be increased in the cytoplasms along the cell borders in most NSCLCs (98 cases, 89%), when the threshold value was chosen as the score 100 for normal bronchial epithelial cells (Fig. 4A). The percentage of FasL-positive cells ranged from 0% to 100% in each tumor, with the average rate of about 92%, and 79 tumors (80%) showed immunoreactivity of 100% with at least moderate intensity (Fig. 4B). In normal and neoplastic lung tissues, expression of FasL protein was mainly found in the cytoplasms along the cell membrane borders of bronchial epithelia. In addition, reactive lymphocytes and histiocytes were also stained for FasL. p53 immunostaining revealed the distinct nuclear expression in tumors, in comparison to no immunoreactivity in normal lung tissues (Fig. 5). The cytoplasmic bcl-2 protein was expressed around the nuclei of the tumor cells as well as normal lymphoid cells (Fig. 6).
Fig. 2.
Normal lung tissue reveal distinct membranous immunoreactivity for Fas protein in bronchiolar epithelia and reactive lymphocytes (×400).
Fig. 3.
Immunohistochemical finding of Fas-negative (A) and Faspositive (B) squamous carcinomas (×400). The Fas-negative carcinoma (A) shows complete loss of surface Fas expression compared to Fas-expressing reactive lymphocytes. The Fas-positive carcinoma (B) reveals diffuse membranous immunopositivity similar to that of adjacent lymphocytes.
Fig. 4.
A case of well-differentiated adenocarcinoma (B) showing increased FasL expression compared to the normal bronchial epithelia (A). FasL protein is immunohistochemically expressed in the cytoplasms along the cell borders (×400).
Fig. 5.
Diffuse and strong immunopositivity for nuclear p53 protein in a case of squamous carcinoma (×400).
Fig. 6.
A rare case of large cell carcinoma with bcl-2 overexpression reveals diffuse perinuclear immunoreactivity for the cytoplasmic bcl-2 protein (×400).
Association of Fas expression with p53 and bcl-2 overexpressions
The statistical analysis for the correlations between Fas expression and p53 or bcl-2 overexpression was shown in Table 2, 3. Of 109 NSCLCs, 59 cases (54%) showed p53 overexpression which is defined as distinct nuclear immunoreactivity for p53 protein in the tumor cells over than 50%, but there was no significant correlation between Fas expression and overexpression of p53 protein which is known for a regulator of Fas expression. The cytoplasmic bcl-2 protein was overexpressed in 8 cases only (7.3%), and the bcl-2 overexpression, which is considered as a mechanism of apoptotic resistance, was not correlated with the Fas expression, either. The immunostaining results for p53 and bcl-2 overexpressions are shown representatively in Fig. 5, 6.
Table 2.
Correlation between Fas expression and p53 overexpression in non-small cell lung carcinomas
*, A case was lost in this analysis due to the failure in p53 immunostaining.
Table 3.
Correlation between Fas expression and bcl-2 overexpression in non-small cell lung carcinomas
*, Fisher exact test.
DISCUSSION
The surface expression of Fas on a malignant target cell is a prerequisite for a tumoricidal attack by immune effector cells through Fas/FasL interactions (6). There is now broad evidence demonstrating that malignant cells draw advantage from aberrant loss of Fas and increased Fas-ligand expression, as compared to their normal counterparts (18). In order to eventually determine a pathogenetic and prognostic role of the Fas-FasL pathway in lung cancer, their expressions in a well-defined group of 110 NSCLCs were investigated by a high-throughput study using TMA, with a reference to the relationship with overexpression of p53 and bcl-2 proteins.
It is generally believed that the tumor cells must achieve resistance toward FasL-induced killing in order to escape a cytotoxic attack by FasL-positive T cells (6). This selective pressure has led them to either down-regulate or even lose receptor expression. Resistance to Fas-mediated apoptosis has been reported previously from several malignant tumors including carcinomas of the colon (7), liver (19), and lung (4, 9). The strong membranous expression of Fas receptor could be readily demonstrated by immunoperoxidase staining using anti-Fas monoclonal antibody. In this immunohistochemical study using TMA, the membranous Fas was markedly decreased in 66 cases (60%) of 110 NSCLCs. This study, therefore, demonstrates that the loss of Fas expression might be an important pathway of pulmonary carcinogenesis by allowing lung tumors to escape from Fas-mediated apoptosis, that is, apoptotic resistance. The reduced Fas expression was found more frequently in the advanced stage than in the earlier stages. It also appeared to be significantly associated with higher nodal status. The clinicopathologic significance of Fas expression in NSCLCs has not been fully understood yet. A few previous studies reported that a Fas/FasL ratio less than 1 was more frequent in advanced stages III-IV than in limited stages I-II (7) and the Fas expression was correlated with the nodal status (11). Although this study showed no association between Fas expression and the clinicopathologic parameters such as age, tumor extent (pT), histologic type and grade, two well-known prognostic factors such as stage and nodal status were significantly associated with Fas expression. The associations between the loss of Fas expression and the advanced NSCLCs suggest that defects in the apoptotic pathway represent a critical element in the progression of the NSCLCs. Together, these data support a role for the loss of Fas-mediated apoptosis during tumorigenesis and tumor progression in NSCLCs.
The data for the influences of the Fas expression on the survival of NSCLC patients have been limited and controversial. Uramoto et al. and Koomagi and Volm showed the patients with positive Fas staining survived for longer period than those with negative staining (10, 20). However, Esposito et al. reported no prognostic value of Fas expression on overall survival in NSCLC patients (11). Our result for the survival data showed that the survival times of Fas-negative carcinomas were significantly shorter than those of Fas-positive tumors. This fact might indicate that the decreased Fas expression in NSCLCs has a bad prognostic implication.
FasL, the ligand of Fas, has been reported to transmit the apoptotic signal via Fas receptor and to be produced by tumors of diverse histogenetic origins including the lung (4, 5, 7, 19, 21, 22). It has also been advocated that FasL-expressing tumor cells might induce the killing of Fas-expressing normal tissue cells including immune cells such as T lymphocytes (7, 23). In lung carcinomas, especially NSCLCs, there are somewhat contradictory opinions for the pattern of FasL expression. Niehans et al. reported that virtually all tumors including NSCLCs express functional FasL protein (5), whereas Viard-Leveugle et al. demonstrated decreased FasL expression in most NSCLCs (72%), compared with normal lung tissue (4). In this study using the criteria of Viard-Leveugle et al., however, most NSCLCs (89%) showed increased FasL expression in comparison to their normal counterparts. These contradictory results could be explained by the use of different technological methodologies or interpretation criteria. Also, our data for FasL protein suggest that NSCLC cells showing increased FasL expression could counteract the immune system by killing activated T cells that have been shown to express Fas strongly.
p53 tumor suppressor gene is the most frequently identified genetic change in human neoplasms including lung carcinomas (24, 25) and known to regulate the Fas gene expression as its promoter gene (13). A few precedent works showed that accumulation of mutations with p53 gene and within Fas promoter region containing putative p53-binding site, could progressively silence Fas expression in the development of NSCLCs (18, 26). In this study, 59 (54%) of the 109 NSCLCs were positive for p53 overexpression which is thought to be equivalent to p53 gene mutation (16), but there was no correlation between negative Fas expression and p53 overexpression. However, their functions are not simple in vivo and thus results of further genetic studies must be combined with the functional data.
The bcl-2 oncogene is a suppressor of apoptosis and overexpression of bcl-2 oncogene has been reported to be an important mechanism of apoptotic resistance in Fas-expressing malignant cells (27). The only published data for bcl-2 in NSCLCs (10) showed no correlation between the Fas and bcl-2 expressions, although there had been a suggestion that the balance between Fas and bcl-2 may play an important role in the induction of apoptosis (28). In this study, only 8 (7%) of the 110 NSCLCs were positive for bcl-2 overexpression, and which showed no relationship with the Fas expression. Taken together, bcl-2 overexpression does not seem to play a role as a main mechanism of apoptotic resistance in NSCLCs.
The large-scale immunohistochemical study was performed using a high-throughput TMA, for which will facilitate the screening of a large number of tumors with minimal outlay in reagents. Concerns about tumor representation in the microarray need to be addressed, but Hoos et al. validated the use of TMAs for immunophenotyping of malignant tumors and showed an excellent concordance for staining of three antibodies between TMAs with triplicate cores per tumor and the full sections (29).
In conclusion, the reduced membranous Fas expression as a mechanism of apoptotic resistance seems to serve a large part of the pulmonary carcinogenesis, which might predict poor survival and have a bad prognostic implication. Also, the rare bcl-2 overexpression suggests that it is unlikely to be involved as a mechanism for the apoptotic resistance in NSCLCs, whereas the increased FasL expression is considered an important basis for the immune evasion of the lung tumor cells.
Footnotes
The present research was conducted by the research fund of Dankook University in 2004.
References
- 1.Nagata S, Goldstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 2.Wyllie AH, Kerr JF, Currie AR. Cell death: significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 3.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 4.Viard-Leveugle I, Veyrenc S, French LE, Brambilla C, Brambilla E. Frequent loss of Fas expression and function in human lung tumors with overexpression of FasL in small cell lung carcinoma. J Pathol. 2003;201:268–277. doi: 10.1002/path.1428. [DOI] [PubMed] [Google Scholar]
- 5.Niehans GA, Brunner T, Frizelle SP, Liston JC, Salerno CT, Knapp DJ, Green DR, Kratzke RA. Human lung carcinomas express Fas ligand. Cancer Res. 1997;57:1007–1012. [PubMed] [Google Scholar]
- 6.Ungefroren H, Voss M, Jansen M, Roeder C, Henne-Bruns D, Kremer B, Kalthoff H. Human pancreatic adenocarcinomas express Fas and Fas ligand yet are resistant to Fas-mediated apoptosis. Cancer Res. 1998;58:1741–1749. [PubMed] [Google Scholar]
- 7.O'Connell J, Bennett MW, Nally K, Houston A, O'Sullivan GC, Shanahan F. Altered mechanisms of apoptosis in colon cancer: Fas resistance and couterattack in the tumor-immune conflict. Ann NY Acad Sci. 2000;910:178–192. doi: 10.1111/j.1749-6632.2000.tb06708.x. [DOI] [PubMed] [Google Scholar]
- 8.Mottolese M, Buglioni S, Bracalenti C, Cardarelli MA, Ciabocco L, Giannarelli D, Botti C, Natali PG, Concetti A, Venanzi FM. Prognostic relevance of altered Fas (CD95)-system in human breast cancer. Int J Cancer. 2000;89:127–132. doi: 10.1002/(sici)1097-0215(20000320)89:2<127::aid-ijc5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 9.Nambu Y, Hughes SJ, Rehemtulla A, Hamstra D, Orringer MB, Beer DG. Lack of cell surface Fas/APO-1 expression in pulmonary adenocarcinomas. J Clin Invest. 1998;101:1102–1110. doi: 10.1172/JCI1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uramoto H, Osaki T, Inoue M, Taga S, Takenoyama M, Hanagiri T, Yoshino I, Nakanishi R, Ichiyoshi Y, Yasumoto K. Fas expression in non-small cell lung cancer: its prognostic effect in completely resected stage III patients. Eur J Cancer. 1999;35:1462–1465. doi: 10.1016/s0959-8049(99)00157-4. [DOI] [PubMed] [Google Scholar]
- 11.Esposito V, Baldi A, Liuzzi G, Tonini G, Vincenzi B, Persichetti P, Santini M, Ambrogi V, Mineo TC, Montesarchio V, Wolner E, Baldi F, Groeger AM. Analysis of Fas (Apo-1/CD95) expression in non-small cell lung cancer. Anticancer Res. 2003;23:4901–4906. [PubMed] [Google Scholar]
- 12.Bennett M, Macdonald K, Chan SW, Luzio JP, Simari R, Weissberg P. Cell surface trafficking of fas: a rapid mechanism of p53-mediated apoptosis. Science. 1998;282:290–293. doi: 10.1126/science.282.5387.290. [DOI] [PubMed] [Google Scholar]
- 13.Munsch D, Watanabe-Fukunaga R, Bourdon JC, Nagata S, May E, Yonish-Rouach E, Reisdorf P. Human and mouse Fas (APO-1/CD95) death receptor genes each contain a p53-responsive element that is activated by p53 mutants unable to induce apoptosis. J Biol Chem. 2000;275:3867–3872. doi: 10.1074/jbc.275.6.3867. [DOI] [PubMed] [Google Scholar]
- 14.Fukazawa T, Fujiwara T, Morimoto Y, Shao J, Nishizaki M, Kadowaki Y, Hizuta A, Owen-Schaub LB, Roth JA, Tanaka N. Differential involvement of the CD95 (Fas/APO-1) receptor/ligand system on apoptosis induced by the wild-type p53 gene transfer in human cancer cells. Oncogene. 1999;18:2189–2199. doi: 10.1038/sj.onc.1202561. [DOI] [PubMed] [Google Scholar]
- 15.Kumar V, Abbas AK, Fausto N. Pathologic basis of disease. 7th ed. Philadelphia: Elsevier Saunders; 2005. Cellular adaptations, cell injury, and cell death; pp. 3–46. [Google Scholar]
- 16.Rodrigues NR, Rowan A, Smith MEF, Kerr IB, Bodmer WF, Gannon JV, Lane DP. p53 mutations in colorectal cancer. Proc Natl Acad Sci USA. 1990;87:7555–7559. doi: 10.1073/pnas.87.19.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishida H, Irie K, Itoh T, Furukawa T, Tokunaga O. The prognostic significance of p53 and bcl-2 expression in lung adenocarcinoma and its correlation with Ki-67 growth fraction. Cancer. 1997;80:1034–1045. [PubMed] [Google Scholar]
- 18.Boldrini L, Faviana P, Pistolesi F, Gisfredi S, Quirico DD, Lucchi M, Mussi A, Angeletti CA, Baldinotti F, Fogli A, Simi P, Basolo F, Fontanini G. Alterations of Fas (APO-1/CD95) gene and its relationship with p53 in non-small cell lung cancer. Oncogene. 2001;20:6632–6637. doi: 10.1038/sj.onc.1204727. [DOI] [PubMed] [Google Scholar]
- 19.Strand S, Hofmann WJ, Hug H, Muller M, Otto G, Strand D, Mariani SM, Stremmel W, Krammer PH, Galle PR. Lymphocyte apoptosis induced by CD95 (APO-1/Fas) ligand-expressing tumor cells-A mechanism of immune evasion? Nat Med. 1996;2:1361–1366. doi: 10.1038/nm1296-1361. [DOI] [PubMed] [Google Scholar]
- 20.Koomagi R, Volm M. Expression of Fas (CD95/APO-1) and Fas ligand in lung cancer, its prognostic and predictive relevance. Int J Cancer. 1999;84:239–243. doi: 10.1002/(sici)1097-0215(19990621)84:3<239::aid-ijc7>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 21.Shiraki K, Tsuji N, Shioda T, Isselbacher KJ, Takahashi H. Expression of Fas ligand in liver metastases of human colonic adenocarcinomas. Proc Natl Acad Sci USA. 1997;94:6420–6425. doi: 10.1073/pnas.94.12.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hahne M, Rimoldi D, Schroter M, Romero P, Schreier M, French LE, Schneider P, Bornand T, Fontana A, Lienard D, Cerottini J, Tschopp J. Melanoma cell expression of Fas (Apo-1/CD95) ligand: implications for tumor immune escape. Science. 1996;274:1363–1366. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- 23.Zeytun A, Nagarkatti M, Nagarkatti PS. Growth of FasL-bearing tumor cells in syngeneic murine host induces apoptosis and toxicity in Fas+ organs. Blood. 2000;95:2111–2117. [PubMed] [Google Scholar]
- 24.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi T, Nau MM, Chiba I, Birrer MJ, Rosenberg RK, Vinocour M, Levitt M, Pass H, Gazdar AF, Minna JD. P53: a frequent target for genetic abnormalities in lung cancer. Science. 1989;246:491–494. doi: 10.1126/science.2554494. [DOI] [PubMed] [Google Scholar]
- 26.Boldrini L, Faviana P, Gisfredi S, Di Quirico D, Lucchi M, Mussi A, Angeletti CA, Baldinotti F, Fogli A, Simi P, Basolo F, Pingitore F, Fontanini G. Identification of Fas (APO-1/CD95) and p53 gene mutations in non-small cell lung cancer. Int J Oncol. 2002;20:155–159. doi: 10.3892/ijo.20.1.155. [DOI] [PubMed] [Google Scholar]
- 27.Owen-Schaub LB, Radinsky R, Kruzel E, Berry K, Yonehara S. Anti-Fas on nonhematopoietic tumors: levels of Fas/APO-1 and bcl-2 are not predictive of biological resonsiveness. Cancer Res. 1994;54:1580–1586. [PubMed] [Google Scholar]
- 28.Itoh N, Tsuimoto Y, Nagata S. Effect of bcl-2 on Fas antigen-mediated cell death. J Immunol. 1993;151:621–627. [PubMed] [Google Scholar]
- 29.Hoos A, Urist MJ, Stojadinovic A, Mastorides S, Dudas ME, Leung DH, Kuo D, Brennan MF, Lewis JJ, Cordon-Cardo C. Validation of tissue microarrays for immunohistochemical profiling of cancer specimens using the example of human fibroblastic tumors. Am J Pathol. 2001;158:1245–1251. doi: 10.1016/S0002-9440(10)64075-8. [DOI] [PMC free article] [PubMed] [Google Scholar]