Abstract
The heat shock proteins (HSPs) are ubiquitous molecules induced in cells exposed to various stress conditions, including carcinogenesis. The HSP70 and HSP27 among HSPs are of special relevance in human cancer inhibiting apoptosis. The aim of this study is to investigate the expressions of HSP70 and HSP27 in hepatocellular carcinoma (HCC) in association to tumor cell proliferation and apoptosis. We examined the expressions of HSP70 and HSP27 by immunohistochemical staining in 71 cases of HCC, and then related their expressions to clinicopathologic parameters and expressions of p53, Ki-67 and Apotag. HSP70 and HSP27 were frequently stained in the cytoplasm and nuclei of tumor cells, but not in the non-neoplastic hepatocytes. Immunoreactivities of HSP70 and HSP27 were observed in 56.3% and 61.9% of HCCs, respectively. HSP70 immunoreactivity correlated with high Ki-67 labeling indices (LIs) (p=0.0159), large tumor size (p=0.0129), presence of portal vein invasion (p=0.0231), and high tumor stage (p=0.0392). HSP27 immunoreactivity significantly related with the subgroup of HBV-associated HCCs (p=0.0003), but not with the others. Both HSP70 and HSP27 immunoreactivities showed no relation to Apotag LIs or p53 immunoreactivity. In conclusion, expressions of HSP70 and HSP27 may play an important role in hepatocarcinogenesis, and especially HSP70 showed a close relationship to the pathological parameters associated with tumor progression and high Ki-67 LIs. Our results could be additional evidence that HSP70 expressions can contribute to not only hepatocarcinogenesis but also tumor progression by promoting tumor cell proliferation.
Keywords: Heat-Shock Proteins; Heat-Shock Proteins 70; HSP27; Protein p53; Cell Proliferation; Apoptosis; Carcinoma, Hepatocellular
INTRODUCTION
The heat shock proteins (HSPs) are highly conserved and ubiquitous molecules with an essential defense mechanism for protecting cells from various environmental damages (1, 2). The HSPs are inducible in response to various physiologically or pathologically stressful conditions, including carcinogenesis (1-4). The functions of the HSPs in the tumorigenesis have been implicated in the regulation of cell cycle progression and apoptosis (5, 6), in multidrug resistance (7), and as a modulator of p53 function (8). HSPs have been classified into six major families and designated nomenclature according to their approximate molecular weight: HSP100, HSP90, HSP70, HSP60, HSP40, and small HSPs including HSP27 (9-12). Based on their regulatory roles in cell apoptosis, HSPs are divided into two groups: pro-apoptotic and anti-apoptotic HSPs (6). Both HSP70 and HSP27 are potent anti-apoptotic HSPs, and their overexpression allows cells to survive in the variable conditions (4, 6). Thus, they may participate in carcinogenesis and modulate tumor cell proliferation and survival through the regulation of apoptotic pathways (4-6, 13, 14). Until now, the exact mechanism of apoptosis-related HSP functions has not been clarified, however the chaperone function of HSP70 was clearly established in the regulation of pro-apoptotic p53 protein (15).
There were several reports about HSP70 expression in malignant tumors, such as breast cancer (7), lung cancer (16), oral squamous cell carcinoma (17), prostate cancer (18), and carcinoma of the uterine cervix (19). The majority of the published results demonstrated HSP70 overexpression correlated with poor prognosis and resistance to therapy (7, 17, 18). Increased levels of HSP27 were detected in a number of cancers, especially hormone-sensitive neoplasm (20, 21). Previous studies about the clinical implications of HSP27 showed different results according to tumor types (20-23).
Hepatocellular carcinoma (HCC) is one of the world's most common malignancies, especially in Asia and southern Africa. Most HCCs are associated with chronic liver diseases resulted from hepatitis B or C viral infection, and the processes of chronic inflammation and fibrosis act as a stressful condition. HSPs induced in response to this stress condition may contribute to hepatocarcinogenesis. Until now there have been a few comprehensive studies of the expression of HSP70 or HSP27 in HCC (22-24), however its prognostic relevance remains controversial. In addition, there have been few studies on HSP expressions in association with tumor cell proliferation or apoptosis in HCCs. The purpose of this study is to investigate the expressions of HSP70 and HSP27 in HCC in association to tumor cell proliferation and apoptosis. Therefore we examined the expressions of HSP70 and HSP27 by immunohistochemical staining in 71 cases of HCC, and then related their expressions to clinicopathologic parameters and expressions of p53, Ki-67 and Apotag.
MATERIALS AND METHODS
Clinicopathologic findings
Specimens used in this study consisted of 71 cases of surgically resected HCC. Seventy-one patients (65 male; 6 female, with a median age of 54.7 yr) who have HCC underwent liver resection between 1996 and 2003 in the Inje University Seoul Paik Hospital. The clinical data including the status of hepatitis markers were documented for all patients. There were 52 cases with HBV infection (positive serum HbsAg), 7 with HCV infection (positive anti-HCV), and 12 with no infection. All HCC specimens were comprised of both tumor and adjacent non-tumorous tissue. Sections from formalin-fixed, paraffin-embedded tissues were used for the immunohistochemical detection of HSP70, HSP27, p53, Ki-67, and Apotag. We compared their expressions with the following pathological characteristics: histologic grade, tumor size, portal vein invasion, intrahepatic metastasis, and tumor stage. HCC tissues were classified based on the degree of differentiation into well and poor according to the criteria of Edmondson and Steiner (25) with some modification; well (grades 1 & 2) (n=20) and poor (grades 3 & 4) (n=51). Tumor size was recorded as the greatest dimension of each specimen, and was classified based on the criteria of Yumoto et al. (26); small (tumor mass <3 cm) (n=24) and large (tumor mass ≥3 cm) (n=47). Tumor staging was determined in accordance with the TNM classification system of UICC (1997) (27), and cases were classified into two groups; low (T1 & T2) (n=21) and high (T3 & T4) (n=50).
Immunohistochemical staining
Immunostaining was performed by the conventional avidin-biotin complex (ABC) method. Commercially available monoclonal antibodies for HSP70 (1:50, Neomarker, Fremont, CA, U.S.A.), HSP27 (1:50, Neomarker), p53 (1:200, DAKO, Carpinteria, CA, U.S.A.), and Ki-67 (1:100, DAKO) were used. For antigen retrieval, the sections were immersed in a citrate buffer and processed in a microwave oven at 95℃ for 10 min. They were developed in a substrate solution of 0.01% 3,3'-diaminobenzidine tetrachloride (DAB) (DAKO) and counterstained with Meyer's hematoxylin. As positive controls for HSP70 and HSP27, p53, and Ki-67, breast carcinoma, colon carcinoma, and tonsilar tissue were used, respectively. Nonimmune mouse serum was used as a substitute for the primary negative controls. Apoptosis was measured by Apotag immunostaining in paraffin embedded sections using the Biogenex ISH detection kit (SH-1009-06; San Ramon, CA, U.S.A.) according to the manufacturer's instructions. In brief, sections were deparaffinized with xylene, pretreated with proteinase K to improve the exposure of DNA by digesting DNA binding protein, rinsed with PBS, and incubated in a reaction mixture containing terminal transferase and digoxigenin dUTP at 37℃ for 1 hr. The specimens were then washed, followed by the addition of antidigoxigenin peroxidase antibody coupled with horseradish peroxidase, and the tissues were incubated for 30 min at room temperature. Following rinsing with PBS, DAB was added for 10 min. Sections of lymph nodes were used as positive control for apoptosis.
Interpretation of immunohistochemical results
For HSP70 and HSP27, any staining either in the cytoplasms or nuclei was regarded as positive. The distribution of positive staining for HSP70 and HSP27 was semiquatitatively assessed by the percentage of positive cells and the cases were classified into three groups according to the proportion of positive tumor cells: 2+ (over 50%), 1+ (between 10% and 50%), and negative (less than 10%) (16). Given the reported correlation of p53 gene mutations with the accumulation of p53 protein in ≥10 percent tumor cell nuclei (28), the tumors were regarded as p53-positive when at least 10 percent of the tumor cells showed nuclear p53 protein accumulation. For the assessment of the expressions of Ki-67 and Apotag, the labeling index (LI) for these antigens (positive nuclei/total number of counted nuclei) was determined by a random evaluation of 1,000 tumor cells.
Statistical analysis
The immunoreactivities of HSP70 and HSP27 in relation to the pathological parameters including p53 immunoreactivity were examined with the chi-square test. To analyze the statistical differences of Ki-67 LI and Apotag LI according to the immunoreactivities of HSP70 and HSP27, the Kruskal-Wallis test was used. The correlation between Ki-67 LI and Apotag LI was analyzed by Spearman's correlation test. Significance was defined as p<0.05. All statistical analyses were performed using SPSS software (version 10.0, SPSS INC., Chicago, IL).
RESULTS
Immunoreactivities of HSP70 and HSP27
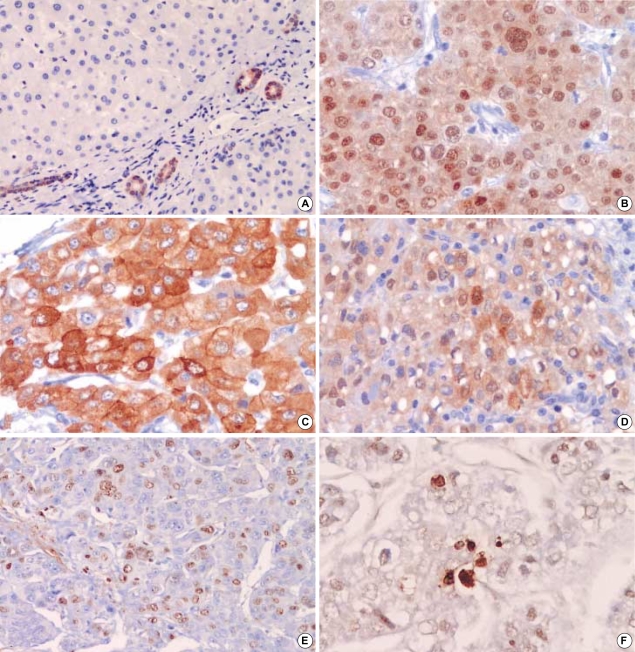
In non-neoplastic liver tissue, HSP70 and HSP27 were not significantly expressed in hepatocytes. We detected a few scattered HSP70-positive hepatocytes with a weak cytoplasmic staining. In contrast, the bile duct epithelium is constantly positive for HSP70 with a strong intensity (Fig. 1A). In HCCs, HSP70 immunoreactivity was seen in 40 cases (56.3%) (1+, 23 cases; 2+, 17 cases), and HSP27 was stained in 44 cases (61.9%) (1+, 27 cases; 2+, 17 cases). HSP70 immunoreactivity was observed in both the nucleus and cytoplasm with a finely granular pattern (Fig. 1B). HSP27 immunoreactivity was predominantly cytoplasmic (Fig. 1C), and in 20 cases, combined nuclear staining was also detected in variable numbers of neoplastic cells (Fig. 1D).
Fig. 1.
Representative photographs of immunohistochemical stainings of HSP70, HSP27, Ki-67, and Apotag. (A) Cytoplasmic staining of HSP70 in non-neoplastic bile ductules as internal control (×100), (B) Nucleocytoplasmic staining of HSP70 in HCCs with fine granular pattern (×200), (C) Strong cytoplasmic staining of HSP27 in HCC (×200), (D) Combined nuclear staining of HSP27 in HCC (×200), (E) High Ki-67 expression in HCC (×200), (F) Apoptotic cells by the Apotag staining in HCC (×400).
Correlation between HSP immunoreactivity and clinicopathologic findings
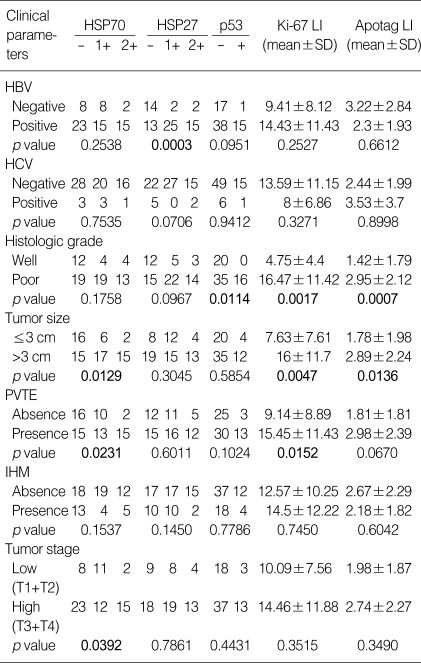
To understand the implications of the HSP expression in hepatocarcinogenesis, the immunoreactivities of HSP70 and HSP27 were analyzed for correlations with clinicopathologic features. As shown in Table 1, HSP70 immunoreactivity significantly correlated with large tumor size (p=0.0129), portal vein invasion (p=0.0231), and high tumor stage (p=0.0392). HSP27 immunoreactivity showed a high association with HBV-associated HCC (p=0.0003), however there was no correlation between any prognosis-related pathological parameters and HSP27 immunoreactivity regardless of the presence of nuclear expression.
Table 1.
Expressions of HSP70, HSP27, p53, Ki-67, and Apotag in association with clinicopathologic parameters in HCCs
LI, labeling index; -, no expression; 1+, positive cell proportion between 10% and 50%; 2+, positive cell proportion over 50%; SD, standard deviation; PVTE, portal vein tumor emboli; IHM, intrahepatic metastasis.
p53 immunoreactivity, Ki-67 LI, and apotag LI in HCCs
p53 immunoreactivity was noted in 16 cases (22.5%) and correlated with poor histologic grade (p=0.0114), high Ki-67 LIs (p=0.0308), and high Apotag LIs (p=0.0177). Ki-67 LIs were ranged from 0.1 to 40 (mean 13.2±10.9) (Fig. 1E), which significantly correlated with poor histologic grade (p=0.0017), large tumor size (p=0.0047), and portal vein invasion (p=0.0152). Tumor cells undergoing apoptosis displayed specific brown staining in fragmented chromatin within the nucleus, areas of chromatin condensation, as well as areas of pyknosis and karyorrhexis. Apotag LIs were ranged from 0 to 15 (mean 2.52±2.13) (Fig. 1F) and correlated with poor histologic grade (p=0.0007) and large tumor size (p=0.0136). Furthermore, the Apotag LIs showed close correlation with the Ki-67 LI (p=0.0001, Spearman's correlation test). There were significant relationships among the expressions of p53, Ki-67, and Apotag, all of which correlated with HCC histological grade (Table 1).
Correlation between HSP immunoreactivities and p53 immunoreactivity, Ki-67 LI, and apotag LI in HCCs
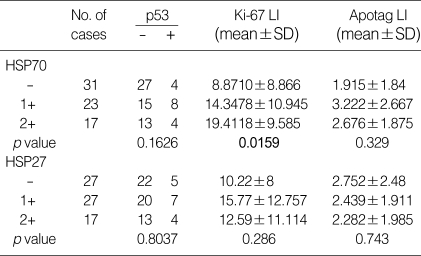
p53 immunoreactivity did not correlate with the immunoreactivities of HSP70 or HSP27 in the overall group of HCCs. We divided the group into two subgroups according to the type of hepatitis virus. A significant relationship was observed between immunoreactivities of HSP70 and p53 (p=0.0284) in the HBV-associated HCC group (n=53), but not in the HCV-associated HCC group (n=7). Ki-67 LIs were positively correlated with HSP70 immunoreactivity (p=0.0159). Apotag LIs showed no statistical significance with HSP immunoreactivities (Table 2).
Table 2.
Expressions of HSP70 and HSP27 in association to p53 expression, Ki-67 LI, and Apotag LI in HCCs (n=71)
LI, labeling index; -, no expression; 1+, positive cell proportion between 10% and 50%; 2+, positive cell proportion over 50%; SD, standard deviation.
DISCUSSION
Heat shock proteins have essential roles in protecting cells from the potentially lethal effects of stress (1-4). Heat-shock proteins have dual roles as a modifier to protein activities and as a central regulator in both cell proliferation and apoptosis (5, 6, 8, 15). Among them, HSP70 and HSP27 are often overexpressed in cells of various cancers and have been suggested to contribute to tumorigenesis (3, 4). According to the results of proteome analysis (29) or expression profiling using oligonucleotide array (24) studied in HCC, HSP70 was introduced as a useful HCC marker and may play an important role in stepwise progression of hepatocarcinogenesis. In our study, HSP70 immunoreactivity was specifically detected in tumor cells and showed a close relationship to the pathologic parameters related to tumor progression, such as large tumor size, portal vein invasion, and high tumor stage. In addition, a positive correlation between HSP70 immunoreactivity and Ki-67 LIs was confirmed, and Ki-67 LIs significantly correlated with adverse prognostic factors as well. The roles of HSP70 in promoting tumor cell proliferation were previously observed in the various types of carcinoma (16, 17, 19, 30), but not in HCC. Recently, it has been shown that HSP70 antisense oligomers can specifically inhibit tumor cell proliferation by inducing apoptosis (17, 31); these results suggest that anti-apoptotic functions of HSP70 may play an important role in tumor cell proliferation and tumor progression in HSP70 over-expressed tumors like HCC. Even though we were not able to demonstrate a relationship between apoptotic indices (Apotag LIs) and HSP70 immunoreactivity in this series, our results could be additional evidence that HSP70 expression can contribute to not only hepatocarcinogenesis but also tumor progression by promoting tumor cell proliferation.
Another plausible role for HSPs in tumorigenesis is as a modifier of protein activities (3, 4). The tumor suppressor p53 protein, involved in the control of cell proliferation, represses transcription by direct protein-protein interaction with the promoter region of the human HSP70 gene (15, 32). Indeed, HSP70-p53 complexes can be detected in extracts from human cancer tissues (33). Cui et al. (34) has reported that coimmunoprecipitation of anti-HSP70 mAb and anti-p53 mAb was shown in 3 out of 12 HCC tissues (25%). In the current study, a statistically significant association between the immunoreactivities of HSP70 and p53 was not shown. However, there was a tendency that the majority of cases showing p53 immunoreactivity (75%, 12 of 16 cases) also demonstrated HSP70 immunoreactivity, and a significant association between the immunoreactivities of HSP70 and p53 was found in the HBV-associated HCC group. Besides HSP70, multiple chaperone complexes including HSP90, HSP40, and p23 mediate the stabilization and cytoplasmic sequestration of conformational mutant p53 or wild-type p53 (15). Therefore studying the coordinated expression of HSPs with p53 protein may be helpful to understand the roles of HSPs in the regulation of p53 function.
King et al. (22) reported that HSP27 expression was related to histologic grade and the survival of patients with HCC. According to the results reported by Harimoto et al. (23), HSP27 expression did not correlated with clinicopathologic factor in the overall group of HCCs; however, it is noticeable that HSP27 expression correlated significantly with the prognosis, disease-free survival and overall survival in HBV-associated group. In the present study, HSP27 immunoreactivity was also detected specifically in tumor cells, and showed a high relevance to HBV-associated HCCs, while we were not able to find any correlation between HSP27 immunoreactivities and the other parameters examined. HSP27 has been reported as one of the most up-regulated genes (over 4-folds) performed cDNA microarray analysis on 588 cellular DNA in HepG2 cell line stably expressing Hepatitis B viral X protein (HBX) (35). HBX is known as a transcription factor and potential oncogene, and it is strongly implicated in the development of hepatocellular carcinoma in chronic HBV-infected patients (36). In this respect, the induction of HSP27 genes by HBX could suggest an additional role for viral protein in HBV-associated carcinogenesis. More comprehensive research will be required to clarify the association between HBV-associated HCC and HSP27 up-regulation.
In the current study, we found expressions of HSP70 and HSP27 may play an important role in hepatocarcinogenesis, and especially HSP70 can contribute tumor progression by promoting tumor cell proliferation in HCC. HSP27 might contribute to the hepatocarcinogenesis of the HBV-associated subgroup.
Footnotes
This work was supported by Grant from Inje University, 2003.
References
- 1.Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- 2.Morimoto RI. Cells in stress: transcriptional activation of heat shock genes. Science. 1993;259:1409–1410. doi: 10.1126/science.8451637. [DOI] [PubMed] [Google Scholar]
- 3.Mosser DD, Morimoto RI. Molecular chaperones and the stress of oncogenesis. Oncogene. 2004;23:2907–2918. doi: 10.1038/sj.onc.1207529. [DOI] [PubMed] [Google Scholar]
- 4.Jolly C, Morimoto RI. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst. 2000;92:1564–1572. doi: 10.1093/jnci/92.19.1564. [DOI] [PubMed] [Google Scholar]
- 5.Helmbrecht K, Zeise E, Rensing L. Chaperones in cell cycle regulation and mitogenic signal transduction: a review. Cell Prolif. 2000;33:341–365. doi: 10.1046/j.1365-2184.2000.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garrido C, Gurbuxani S, Ravagnan L, Kroemer G. Heat shock proteins: endogenous modulators of apoptotic cell death. Biochem Biophys Res Commun. 2001;286:433–442. doi: 10.1006/bbrc.2001.5427. [DOI] [PubMed] [Google Scholar]
- 7.Ciocca DR, Clark GM, Tandon AK, Fuqua SA, Welch WJ, McGuire WL. Heat shock protein hsp70 in patients with axillary lymph node-negative breast cancer: prognostic implications. J Natl Cancer Inst. 1993;85:570–574. doi: 10.1093/jnci/85.7.570. [DOI] [PubMed] [Google Scholar]
- 8.Levine AJ, Momand J, Finlay CA. The p53 tumour suppressor gene. Nature. 1991;351:453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- 9.Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 10.Craig EA, Weissman JS, Horwich AL. Heat shock proteins and molecular chaperones: mediators of protein conformation and turnover in the cell. Cell. 1994;78:365–372. doi: 10.1016/0092-8674(94)90416-2. [DOI] [PubMed] [Google Scholar]
- 11.Morimoto RI, Tisseres A, Georgopoulos C. Heat shock proteins in biology and medicine. Cold Spring Harbor, New York: Cold Spring Harbor Press; 1994. [Google Scholar]
- 12.Scharf KD, Höhfeld I, Nover L. 1998. Heat stress response and heat stress transcription factors. J Biosci. 1998;23:313–329. [Google Scholar]
- 13.Nylandsted J, Brand K, Jaattela M. Heat shock protein 70 is required for the survival of cancer cells. Ann N Y Acad Sci. 2000;926:122–125. doi: 10.1111/j.1749-6632.2000.tb05605.x. [DOI] [PubMed] [Google Scholar]
- 14.Mosser DD, Caron AW, Bourget L, Denis-Larose C, Massie B. Role of human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol Cell Biol. 1997;17:5317–5327. doi: 10.1128/mcb.17.9.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zylicz M, King FW, Wawrzynow A. Hsp70 interactions with the p53 tumour suppressor protein. EMBO J. 2001;20:4634–4638. doi: 10.1093/emboj/20.17.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malusecka E, Zborek A, Krzyzowska-Gruca S, Krawczyk Z. Expression of heat shock proteins HSP70 and HSP27 in primary non-small cell lung carcinomas. An immunohistochemical study. Anticancer Res. 2001;21:1015–1021. [PubMed] [Google Scholar]
- 17.Kaur J, Ralhan R. Differential expression of 70-kDa heat shock-protein in human oral tumorigenesis. Int J Cancer. 1995;63:774–779. doi: 10.1002/ijc.2910630604. [DOI] [PubMed] [Google Scholar]
- 18.Cornford PA, Dodson AR, Parsons KF, Desmond AD, Woolfenden A, Fordham M, Neoptolemos JP, Ke Y, Foster CS. Heat shock protein expression independently predicts clinical outcome in prostate cancer. Cancer Res. 2000;60:7099–7105. [PubMed] [Google Scholar]
- 19.Park CS, Joo IS, Song SY, Kim DS, Bae DS, Lee JH. An immunohistochemical analysis of heat shock protein 70, p53, and estrogen receptor status in carcinoma of the uterine cervix. Gynecol Oncol. 1999;74:53–60. doi: 10.1006/gyno.1999.5429. [DOI] [PubMed] [Google Scholar]
- 20.Elpek GO, Karaveli S, Simsek T, Keles N, Aksoy NH. Expression of heat-shock proteins hsp27, hsp70 and hsp90 in malignant epithelial tumour of the ovaries. APMIS. 2003;111:523–530. doi: 10.1034/j.1600-0463.2003.1110411.x. [DOI] [PubMed] [Google Scholar]
- 21.Ciocca DR, Oesterreich S, Chamness GC, McGuire WL, Fuqua SA. Biological and clinical implications of heat shock protein 27,000 (Hsp27) : a review. J Natl Cancer Inst. 1993;85:1558–1570. doi: 10.1093/jnci/85.19.1558. [DOI] [PubMed] [Google Scholar]
- 22.King KL, Li AF, Chau GY, Chi CW, Wu CW, Huang CL, Lui WY. Prognostic significance of heat shock protein-27 expression in hepatocellular carcinoma and its relation to histologic grading and survival. Cancer. 2000;88:2464–2470. doi: 10.1002/1097-0142(20000601)88:11<2464::aid-cncr6>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 23.Harimoto N, Shimada M, Aishima S, Kitagawa D, Itoh S, Tsujita E, Maehara S, Taketomi A, Tanaka S, Shirabe K, Maehara Y. The role of heat shock protein 27 expression in hepatocellular carcinoma in Japan: special reference to the difference between hepatitis B and C. Liver Int. 2004;24:316–321. doi: 10.1111/j.1478-3231.2004.0927.x. [DOI] [PubMed] [Google Scholar]
- 24.Chuma M, Sakamoto M, Yamazaki K, Ohta T, Ohki M, Asaka M, Hirohashi S. Expression profiling in multistage hepatocarcinogenesis: identification of HSP70 as a molecular marker of early hepatocellular carcinoma. Hepatology. 2003;37:198–207. doi: 10.1053/jhep.2003.50022. [DOI] [PubMed] [Google Scholar]
- 25.Edmondson HA, Steiner PE. Primary carcinoma of the liver: A study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462–503. doi: 10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 26.Yumoto Y, Hanafusa T, Hada H, Morita T, Ooguchi S, Shinji N, Mitani T, Hamaya K, Koide N, Tsuji T. Loss of heterozygosity and analysis of mutation of p53 in hepatocellular carcinoma. J Gastroenterol Hepatol. 1995;10:179–185. doi: 10.1111/j.1440-1746.1995.tb01075.x. [DOI] [PubMed] [Google Scholar]
- 27.Sobin LH, Wittekind C, editors. UICC: TNM classification of malignant tumors. 5th eds. New York: Wiley-Liss; 1997. pp. 74–77. [Google Scholar]
- 28.Esrig D, Elmajian D, Groshen S, Freeman JA, Stein JP, Chen SC, Nichols PW, Skinner DG, Jones PA, Cote RJ. Accumulation of nuclear p53 and tumor progression in bladder cancer. N Engl J Med. 1994;331:1259–1264. doi: 10.1056/NEJM199411103311903. [DOI] [PubMed] [Google Scholar]
- 29.Lim SO, Park SJ, Kim W, Park SG, Kim HJ, Kim YI, Sohn TS, Noh JH, Jung G. Proteome analysis of hepatocellular carcinoma. Biochem Biophys Res Commun. 2002;291:1031–1037. doi: 10.1006/bbrc.2002.6547. [DOI] [PubMed] [Google Scholar]
- 30.Vargas-Roig LM, Fanelli MA, Lopez LA, Gago FE, Tello O, Aznar JC, Ciocca DR. Heat shock proteins and cell proliferation in human breast cancer biopsy samples. Cancer Detect Prev. 1997;21:441–451. [PubMed] [Google Scholar]
- 31.Zhao ZG, Shen WL. Heat shock protein 70 antisense oligonucleotide inhibits cell growth and induces apoptosis in human gastric cancer cell line SGC-7901. World J Gastroenterol. 2005;11:73–78. doi: 10.3748/wjg.v11.i1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinhasi-Kimhi O, Michalovitz D, Ben-Zeev A, Oren M. Specific interaction between the p53 cellular tumour antigen and major heat shock proteins. Nature. 1986;320:182–184. doi: 10.1038/320182a0. [DOI] [PubMed] [Google Scholar]
- 33.Davidoff AM, Iglehart JD, Marks JR. Immune response to p53 is dependent upon p53/HSP70 complexes in breast cancers. Proc Natl Acad Sci USA. 1992;89:3439–3442. doi: 10.1073/pnas.89.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cui CW, Yang SJ, Liu YP, Liu YF. Interaction between p53 and HSP70 in human hepatocellular carcinoma tissues. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2003;19:195–196. [PubMed] [Google Scholar]
- 35.Han J, Yoo HY, Choi BH, Rho HM. Selective transcriptional regulations in the human liver cell by hepatitis B viral X protein. Biochem Biophys Res Commun. 2000;272:525–530. doi: 10.1006/bbrc.2000.2801. [DOI] [PubMed] [Google Scholar]
- 36.Andrisani OM, Barnabas S. The transcriptional function of the hepatitis B virus X protein and its role in hepatocarcinogenesis. Int J Oncol. 1999;15:373–379. doi: 10.3892/ijo.15.2.373. [DOI] [PubMed] [Google Scholar]