Abstract
To examine the immunohistochemical alterations associated with the histological dedifferentiation of thyroid carcinomas, we performed staining for HBME-1, high molecular weight cytokeratin (HCK), CK 19, thyroid transcription factor-1 (TTF-1) and E-cadherin (E-CD) on 125 various types of thyroid carcinomas. The HBME-1 staining was strong and diffuse in follicular carcinoma (FC), papillary carcinoma (PC), and poorly differentiated carcinoma (PDC), while it was rare in undifferentiated carcinoma (UC) as well as in benign lesions. Strong, diffuse staining for CK19 and HCK was predominantly found in PC, and these markers were not much found in other carcinomas. TTF-1 uniformly stained the tumor cells of all cases of PC, FC and Hurthle cell carcinoma (HC) and 42% of the PDC, while there was only focal staining in one case of the UC. Compared to the strong, diffuse reactivity in the benign lesions, E-CD staining was noted in 67% of PC, 80% of FC, 83% of HC, 58% of PDC and none of the UC. These results suggest that HBME-1 may be a marker for well-differentiated carcinomas while CK19 and HCK are phenotypic markers for papillary carcinoma. The loss or reduced expression of TTF-1 and E-CD may be markers for dedifferentiation.
Keywords: HBME-1 antigen, Keratin, thyroid nuclear factor 1, Cadherins, Thyroid Neoplasms
INTRODUCTION
According to the traditional scheme of thyroid neoplasia, malignant follicular cell tumors are subdivided into a well differentiated type composed of papillary and follicular carcinoma, a poorly differentiated or insular type and an undifferentiated type on the basis of both the morphologic appearance and the tumor behavior (1). Undifferentiated carcinoma (UC) is a highly aggressive neoplasm that is responsible for a large portion of the fatal thyroid cancers. The three major subtypes of UC are the spindle cell, giant cell and squamoid cell subtypes. Although its prognosis is not as abysmal as UC, poorly differentiated carcinomas (PDC) are another group of clinically aggressive thyroid carcinomas that include insular carcinoma and the other PDC, and these cancers can easily metastasize to regional lymph nodes and distant organs. PDC are morphologically characterized by the formation of solid nests and microfollicles, high mitotic activity and necrosis.
Monoclonal antibody HBME-1 was prepared from the epithelial malignant mesothelioma cell for use as an immunogen. This antibody recognizes an undetermined antigen that is abundant on the surface of normal and neoplastic mesothelial cells and it is also present in other epithelial cells. While this antibody has been found to be only relatively specific for mesothelium (2), it had demonstrated a diagnostic value for differentiating the papillary and follicular thyroid carcinomas from benign lesions on both the histologic sections and the fine-needle aspiration smears (3, 4).
There have been several studies that have attempted to elucidate the cytokeratin (CK) pattern of normal thyroid tissue and thyroid carcinomas. These studies have also tried to evaluate whether the process of neoplastic transformation of the thyroid, with a particular emphasis placed on papillary carcinoma, is associated with any specific change in the CK expression (5, 6). CK 19 has been found to be strongly and diffusely expressed in 80.9% papillary carcinoma (7), whereas it is usually absent or focally expressed in follicular carcinoma and in benign follicular nodules. Other CK subtypes, such as high molecular weight cytokeratin, CK17 and CK20, have been subsequently added to the differential laboratory panel that is used for diagnosis of tumors (8, 9).
The differentiated thyroid phenotype is characterized by the expression of a variety of thyroid-specific proteins, and the production of these proteins is regulated by thyroid-specific nuclear factors. Therefore, TTF-1 immunoreactivity, which is one of the thyroid-specific nuclear factors, can provide useful information on the functional activities and/or the differentiation of thyroid tumors.
E-cadherin (E-CD) is a cell adhesion molecule that has been directly implicated in the control of Ca2+-dependent interactions between epithelial cells. In vivo studies, E-CD, which is specifically expressed at the basolateral membrane of thyrocytes, mediated the initial cellular aggregation that was seen in follicle formation (10), and E-CD was under the control of the TSH-cAMP-dependent pathway. This suggested its important role on the action of this pathway for proliferation and differentiation (11). Brabant et al. (12) have revealed that the E-CD mRNA levels and/or its immunofluorescence intensity was reduced or altered in thyroid carcinomas, and particularly in those tumors displaying metastasis. The reduced and heterogeneous expression of E-CD in thyroid carcinomas appeared to reflect transcriptional regulation or post-transcriptional modulation in most cases (13), and the expression of E-CD seemed to be associated with the dedifferentiation, progression and metastatic spread of thyroid carcinomas, suggesting that E-CD immunoreactivity may be used as an independent prognostic indicator (14-16).
The aim of this study was to establish the CK19/HCK/HBME-1/TTF-1/E-CD immunoprofile of thyroid carcinomas in order to clarify the putative application of each immunostaining in diagnostic surgical pathology, and we also wanted to evaluate if the process of dedifferentiation in thyroid carcinoma might be associated with any particular changes in tumor immunoexpression.
MATERIALS AND METHODS
One hundred twenty-five consecutive cases of thyroid carcinomas were selected from the tissue files of the Department of Pathology, Samsung Medical Center, Seoul, Korea. All of these cases had sufficient materials available for immunohistochemical study. The total cases consisted of 84 cases of total thyroidectomy, 26 cases of near total or subtotal thyroidectomy, 11 cases of lobectomy and 4 cases of biopsy. Nine cases of benign lesions, including 6 nodular hyperplasias (NHs) and 3 follicular adenomas (FAs) were also retrieved. The relevant medical records and surgical pathology reports for all the cases were reviewed.
Hematoxylin and eosin-stained slides were reviewed in all cases, and the histologic patterns of the tumors were classified according to the already published criteria (17, 18). Immunohistochemical staining was performed on the formalin-fixed, paraffin-embedded tissue sections with the antibodies listed in Table 1 at the dilutions specified with when using the two-step procedure of the peroxidase-labelled EnVision+™ system (DAKO, Glostrup, Denmark). Antigen retrieval was done for all antibodies by heating the tissues for 5 min in an 800-W microwave oven to maintain the temperature of the buffer [0.01 mol citrate, pH 6.0] at about 100℃. Diaminobenzidine was used as a chromogen. Positive internal controls and negative controls (omission of primary antibody) were evaluated simultaneously. The results were scored as 0, 1+ and 2+ if the cell membrane (HBME-1), the cell membrane and cytoplasm (HCK, CK19), or the nucleus (TTF-1) was stained in less than 5%, 5% to 30% or and more than 30% of tumor cells, respectively. The results of E-CD staining, which was usually localized to the cell membranes, were scored as follows: 0, if E-CD-reactive tumor cells were not detected throughout the tumor; 1+, if the E-CD-reactive tumor cells were scattered; 2+, if the E-CD-reactive and E-CD-non-reactive cells were mixed; and 3+, if E-CD was expressed in all the tumor cells.
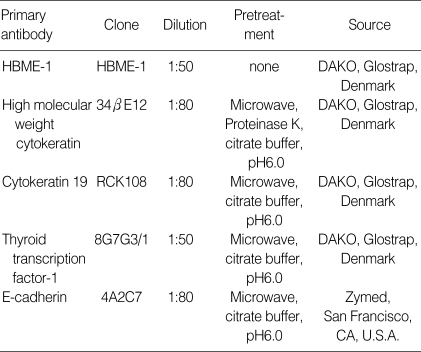
Table 1.
Antibodies and pretreatment conditions
RESULTS
Clinicopathological findings
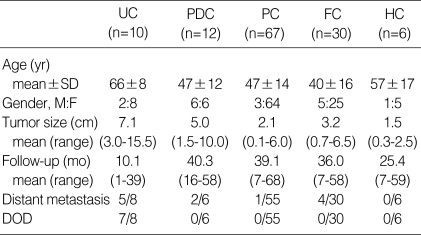
The case materials included for this study are shown in Table 2. At the time of diagnosis, the mean age of the patients with UC was the oldest among all the groups of cancer. The female predominance was evident in all groups of thyroid carcinomas, with the exception of the PDCs. The average UC tumor size was the largest among all the groups of cancer, and the PDCs had the next largest tumor size.
Table 2.
Clinical and pathological features of thyroid carcinomas
UC, undifferentiated carcinoma; PDC, poorly differentiated carcinoma; PC, papillary carcinoma; FC, follicular carcinoma; HC, Hurthle cell carcinoma; DOD, dead of diseased; SD, standard deviation.
After a variable duration of follow-up ranging from 1 to 68 months (mean: 35.1 months), 5 out of 8 patients with UC and 2 out of 6 patients with PDC developed distant metastasis. Seven out of 8 patients with UC died while none of the patients with other carcinomas died of disease. Six patients of UC died within one year after being diagnosed: two of them died within one month, and four of then developed distant metastasis to the lung, pleura, bone or peritoneum, respectively. One UC patient is still alive, but this patient has distant metastasis to the spine. Two out of 6 patients with PDC developed distant metastasis to the lung and bone. Fifty-four out of 55 cases of papillary carcinoma were alive at the time of this study with no evidence of disease; one patient has developed distant metastasis to the lung. Four out of the 30 cases of follicular carcinoma developed local recurrence and distant metastasis.
Of the 3 cases of the spindle cell type UC, one case showed angiosarcoma-like features. UC occasionally had the remaining well-differentiated carcinoma component in the background, such as the widely invasive follicular carcinoma (2 cases), the follicular variant of papillary carcinoma (one case), and encapsulated follicular neoplasm was observed in the background of 2 cases. One case of the mixed squamoid and spindle cell type and one case of the squamoid variant also showed occasional nuclear pseudoinclusions, and particularly in the squamoid cell component. All the PDCs shared such features as well-defined predominantly solid cell nests, high mitotic activity and necrosis. Of the 67 patients diagnosed as having PC, 4 patients had two separate papillary carcinomas. Three cases of classic PC and one case of the follicular variant of PC exhibited prominent Hurthle cell changes.
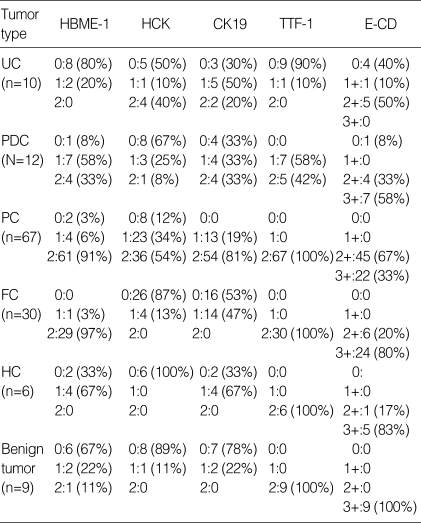
Immunohistochemical findings (Table 3)
Table 3.
Immunohistochemical findings of thyroid carcinomas
0, negative; 1, focally positive; 2, diffusely positive. UC, undifferentiated carcinoma; PDC, poorly differentiated carcinoma; PC, papillary carcinoma; FC, follicular carcinoma; HC, Hurthle cell carcinoma.
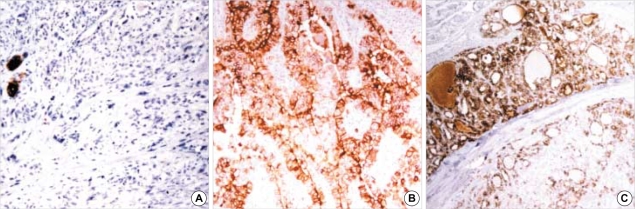
Of the 10 cases of UC, 8 cases showed non-reactivity for HBME-1 while 2 cases showed scattered reactivity (Fig. 1A). Of the 12 cases of PDC, 4 cases were diffusely reactive for HBME-1, 7 cases were focally reactive with heterogeneous staining and one case was non-reactive. Of the 7 cases with focal reactivity, one case exhibited a more diffuse and stronger staining in the coexisting well differentiated follicular carcinoma component than in the poorly differentiated carcinoma component, and one case exhibited a selective loss of reactivity in the pleomorphic tumor cells. Of the 67 cases of papillary carcinoma that were tested with HBME-1 staining, 61 cases showed diffuse reactivity (Fig. 1B), 4 cases showed focal reactivity and 2 cases, which were both the encapsulated follicular variant, showed no reactivity. HBME-1 staining tended to spare the areas of tumor cells having oxyphilic cytoplasm. Of the 30 cases of follicular carcinoma (FC), 29 cases showed strong reactivity (Fig. 1C), and one case of minimally invasive FC showed focal reactivity. Of the 6 cases of Hurthle cell carcinoma (HC), 4 cases showed focal reactivity and 2 cases of encapsulated HC showed no reactivity. Of the 9 cases of benign lesions, 6 cases showed no reactivity for HBME-1, one case of FA showed diffuse reactivity and two cases of FA showed focal reactivity.
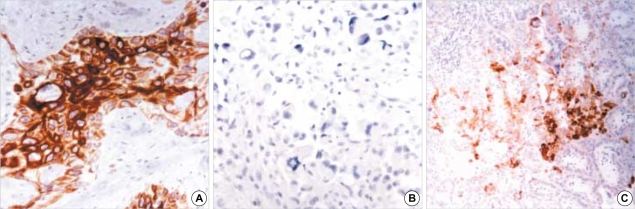
Fig. 1.
Expression of HBME-1 in thyroid carcinomas: (A) undifferentiated thyroid carcinoma showing scattered reactivity (×100), (B) papillary carcinoma (×200) and (C) follicular carcinoma with diffuse reactivity (×100).
Of the 10 cases of UC, all 4 cases of the squamoid cell type exhibited diffuse and strong reactivity for high molecular weight cytokeratin (HCK) (Fig. 2A), and one case of the mixed squamoid and spindle cell type showed scattered reactivity that was confined to the squamoid tumor cells. None of giant cell or spindle cell types showed reactivity for HCK (Fig. 2B). Of the 12 cases of PDC, only one case showed diffuse reactivity for HCK, and another three cases showed focal reactivity. Of the 67 cases of papillary carcinoma (PC), 36 cases showed diffuse reactivity, 23 cases showed focal reactivity and 8 cases, which included 7 out of 17 cases (41%) of follicular variant and one out of 46 cases (2%) of classic papillary carcinoma, showed no reactivity (Fig. 2C). Of the 30 cases of FC, 4 cases that consisted of 2 cases of minimally invasive FC and 2 cases of widely invasive FC showed focal reactivity. None of the remaining 26 cases were reactive for HCK. Of the 6 cases of HC, none showed reactivity, with the exception of focal reactivity in association with scarring or fibrosis, squamous metaplasia and non-specific staining of the vascular channels. Of the 9 benign lesions, one of the cases of follicular adenomas showed focal reactivity. Reactivity for HCK was occasionally noted in the areas having fibrous backgrounds or squamous metaplasia, and in the vascular channels.
Fig. 2.
Expression of HCK: (A) undifferentiated carcinoma of squamoid type showing strong reactivity (×200), (B) undifferentiated carcinoma of giant cell type showing non-reactivity (×200) and (C) papillary carcinoma with focal reactivity (×100).
With CK19, 2 of 10 cases of UC which were the squamoid type, exhibited diffuse cytoplasmic and membranous reactivity. Three cases of the spindle cell type, one case of the mixed type and one case of the squamoid type showed focal reactivity. Of the 12 cases of PDC, 4 cases showed diffuse reactivity for CK19, 4 cases showed focal or scattered reactivity that probably related with squamous metaplasia on the basis of the cytological features of the reactive cells, and 4 cases showed no reactivity. Of the 67 cases of papillary carcinoma, 54 cases showed diffuse reactivity and 13 cases, which included 4 cases of classic papillary carcinoma and 9 cases of follicular variant, showed focal reactivity. The follicular variant of papillary carcinoma showed diffuse reactivity for CK19 less frequently compared to the other subtypes. Of the 30 cases of follicular carcinoma, 16 cases showed no reactivity, 14 cases focal reactivity that was frequently associated with squamous metaplasia, scarring or a fibrous background. Of the 6 cases of HC, 4 cases showed focal reactivity. None of the FC or HC showed diffuse strong reactivity. Of the 9 benign lesions, 2 showed focal reactivity and the others showed no reactivity.
Of the 10 cases of UC, only one case of them showed focal nuclear reactivity for TTF-1 in the several tumor cells, in contrast to the diffuse reactivity that was noted in the entrapped or adjacent follicular epithelial cells (Fig. 3A). Of the 12 cases of PDC, 7 cases showed mixed or heterogeneous reactivity and 5 cases showed a rather diffuse reactivity (Fig. 3B); 4 of these 5 cases exhibited a scattered loss of reactivity in the pleomorphic tumor cells such as the giant or rhabdoid tumor cells. All the cases of papillary carcinoma showed well-preserved reactivity for TTF-1, which was diffuse with or without focal mixed reactivity. The intensity of the nuclear staining tended to be lessened in the hypertrophic nuclei having empty appearing nucleoplasms or pseudoinclusions. All the cases of follicular carcinoma, HC and the benign lesion showed well-preserved nuclear reactivity for TTF-1 (Fig. 3C).
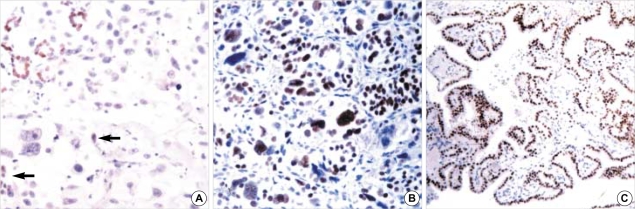
Fig. 3.
Expression of TTF-1 in thyroid lesions: (A) undifferentiated thyroid carcinoma showing focal reactivity in several tumor cells (arrows), in contrast to diffuse reactivity in entrapped follicular epithelial cells (×200); (B) diffuse and strong reactivity in poorly differentiated carcinoma (×200) and (C) follicular adenoma showing diffuse reactivity (×100).
Of the 10 cases of UC, 5 cases of the giant cell or spindle cell type showed no reactivity or scattered reactivity (1+) for E-CD (Fig. 4A), while all 4 cases of the squamoid type exhibited mixed reactivity with scattered aggregates of non-reactive tumor cells (2+). One case of the mixed squamoid and spindle cell type also showed mixed reactivity that was confined to the squamoid cell component (2+). Of the 12 cases of PDC, 4 cases showed diffuse reactivity for E-CD (3+), 3 cases showed mostly diffuse reactivity, but with scattered non-reactive tumor cells (3+), one case showed mixed reactivity (2+), 3 cases showed mixed reactivity with scattered aggregates of non-reactive tumor cells (2+), and one case showed no reactivity. Of the 67 papillary carcinomas, diffuse reactivity with or without scattered non-reactive tumor cells/focal mixed reactivity (3+) was noted in 22 cases, mixed reactivity (2+) was noted in 31 cases, and mixed reactivity with scattered aggregates of non-reactive tumor cells (2+) was seen in 14 cases, which included 13 cases of classic papillary carcinoma and one case of the follicular variant (Fig. 4B). Comparison among the 3 subtypes of papillary carcinoma revealed that the follicular variant exhibited diffuse reactivity more frequently than did the classic type and the microcarcinoma. Of the 30 cases of FC, 21 cases showed diffuse reactivity (3+), 3 cases showed diffuse reactivity with focally mixed reactivity (3+), and 6 cases, which consisted of one case of vascular invasive encapsulated FC, one case of minimally invasive FC and 4 cases of widely invasive FC, exhibited mixed reactivity (2+). Of the 6 cases of HC, 4 cases showed diffuse reactivity (3+), one case showed diffuse reactivity with focally mixed reactivity (3+), and one case showed mixed reactivity (2+). All the benign lesions showed diffuse reactivity (3+).
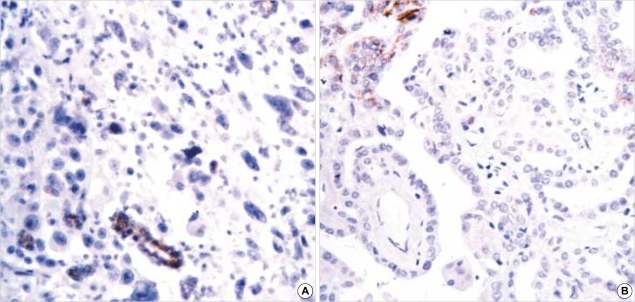
Fig. 4.
Loss of expression of E-CD in (A) undifferentiated carcinoma (×200) and (B) papillary carcinoma (×200).
DISCUSSION
Although HBME-1 had been developed as a positive marker for mesothelial cells, it has proved its utility as a positive marker for thyroid carcinomas (3, 4). This study reveals that HBME-1 may be useful as a positive marker for differentiated carcinomas such as PC, FC, and HC, and it may be an equivocal marker for PDC, but not for UC.
The utility of HCK and CK19 in distinguishing papillary carcinoma from follicular carcinoma has become controversial (5). The present study showed that both HCK and CK19 might be positive markers for the follicular variant of PC and they are not so useful for classic PC. The reasons why there have been some differences among several studies, including ours, might be related to the usage of different biotin blocking methods from study to study: this is particularly true when considering the frequently encountered various degrees of oxyphilic cell changes in PC and FC. In addition, the considerable staining that was found in PDC and UC seemed to be related with the squamous metaplasia of the tumor cells. When considering the role of cytokeratin, which is an intermediate filament playing important roles for the morphologic changes that occur within cells, CK 19 and HCK both turned out to be morphologic markers in the papillary configuration or in the squamoid features, and these are not important changes in oncogenesis of the papillary or poorly-differentiated carcinomas.
Compared to the result of the previous studies (Table 4) (16, 19), follicular carcinomas showed a lower percentage of E-CD loss in our study. Although the follicular carcinoma group in the study by Cerrato et al. (19) included 3 cases of the poorly differentiated, insular type of carcinoma, the inclusion of PDC does not seem to be the only reason for this difference because the study by von Wasielewski et al. (16), which included only differentiated carcinomas, also showed a higher percentage of E-CD loss than the present study. We used Envision for blocking the endogenous biotin activity, which is known to be high in Hurthle cells, and we did not observe cytoplasmic staining, but rather, we noted lateral intercellular staining in the Hurthle cells. That might explain our findings of the higher E-CD reactivity for the follicular carcinomas. It has been reported that for thyroid epithelial malignancies, E-CD down-regulation is associated with dedifferentiation and a poor prognosis. In particular, distant metastases were observed for papillary carcinomas showing E-CD positivity in less than 30 percent of the tissue (14). The loss of E-CD expression in primary human thyroid carcinoma has not been associated with irreversible genetic E-CD alterations, but with methylation of the E-Cd 5'CpG island. This has been particularly true in the case of papillary carcinoma. Poorly differentiated or undifferentiated carcinomas have been observed to have only infrequent involvement of promoter region methylation compared to papillary carcinoma. The altered subcellular distribution observed for E-CD, such as diffuse cytoplasmic staining, has suggested aberrant cell signaling as the alternative plausible mechanism for the reduced E-CD expression in poorly differentiated or undifferentiated carcinomas (20). Frequent mutation and the nuclear localization of β-catenin, which has a crucial role in E-cadherin-mediated cell-to-cell adhesion and as a downstream signaling molecule in the wingless pathway, has been detected in anaplastic thyroid carcinoma, suggesting that β-catenin acts as an oncogene and it contributes to the highly aggressive behavior of this tumor (21). The greatly reduced immunohistochemical expression of E-CD correlated with the progression to primary tumor stage 4 tumors and the higher rates of locoregional tumor recurrence and distant metastasis. Patients with positive E-CD staining of more than 30% of their thyroid carcinoma cells rarely showed regional recurrence or new distant metastasis. E-cadherin reactivity in papillary carcinomas has been reported as an important prognostic marker, but our present study did not show any statistical difference about prognosis because all of our papillary carcinoma cases displayed a good prognosis.
Table 4.
Immunoreactivity of E-cadherin reported for thyroid carcinomas
PC, papillary carcinoma; FC, follicular carcinoma; UC, undifferentiated carcinoma.
TTF-1 is a 38 kDa nuclear protein that activates the translation of the thyroglobulin and thyroperoxidase genes (22). TTF-1 showed a complete loss of immunoreactivity in the cases with undifferentiated carcinomas. All of the well-differentiated thyroid cancers such as the follicular and papillary carcinomas showed strong diffuse positivity, but in the poorly-differentiated carcinomas, the positivity reactivity was diminished and the most part of undifferentiated carcinomas showed a complete loss of immunoreactivity. These findings share similar characteristics to the studies with thyroglobulin, and this molecule can be interpreted as a marker of differentiation for thyroid carcinomas (23).
In conclusion, these results suggest that HBME-1 may be a marker for well-differentiated carcinomas while CK19 and HCK are phenotypic markers for papillary carcinoma. The loss or reduced expression of TTF-1 and E-CD may be markers for dedifferentiation, which is known to lead to the transcriptional inhibition of thyroid specific proteins such as thyroglobulin, and to the acquisition of invasiveness and the loss of the epithelial phenotype, and all of these features are characteristics of UC.
Footnotes
This work was supported by a Samsung Medical Center Clinical Research Development Program grant.
References
- 1.Rosai J. Thyroid gland. In: Rosai J, editor. Rosai and Ackermann's Surgical Pathology. 9 ed. Phildelphia: Elsevier; 2004. pp. 515–594. [Google Scholar]
- 2.van Hoeven KH, Kovatich AJ, Miettinen M. Immunocytochemical evaluation of HBME-1, CA 19-9, and CD-15 (Leu-M1) in fine-needle aspirates of thyroid nodules. Diagn Cytopathol. 1998;18:93–97. doi: 10.1002/(sici)1097-0339(199802)18:2<93::aid-dc3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 3.Casey MB, Lohse CM, Lloyd RV. Distinction between papillary thyroid hyperplasia and papillary thyroid carcinoma by immunohistochemical staining for cytokeratin 19, galectin-3, and HBME-1. Endocr Pathol. 2003;14:55–60. doi: 10.1385/ep:14:1:55. [DOI] [PubMed] [Google Scholar]
- 4.Sack MJ, Astengo-Osuna C, Lin BT, Battifora H, LiVolsi VA. HBME-1 immunostaining in thyroid fine-needle aspirations: a useful marker in the diagnosis of carcinoma. Mod Pathol. 1997;10:668–674. [PubMed] [Google Scholar]
- 5.Cameron BR, Berean KW. Cytokeratin subtypes in thyroid tumors: immunohistochemical study with emphasis on the follicular variant of papillary carcinoma. J Otolaryngol. 2003;32:319–322. doi: 10.2310/7070.2003.11429. [DOI] [PubMed] [Google Scholar]
- 6.Kragsterman B, Grimelius L, Wallin G, Werga P, Johansson H. Cytokeratin 19 expression in papillary thyroid carcinoma. Appl Immunohistochem Mol Morphol. 1999;7:181–185. [Google Scholar]
- 7.Shin E, Chung WY, Yang WI, Park CS, Hong SW. RET/PTC and CK19 expression in papillary thyroid carcinoma and its clinicopathologic correlation. J Korean Med Sci. 2005;20:98–104. doi: 10.3346/jkms.2005.20.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baloch ZW, Abraham S, Roberts S, LiVolsi VA. Differential expression of cytokeratins in follicular variant of papillary carcinoma: an immunohistochemical study and its diagnostic utility. Hum Pathol. 1999;30:1166–1171. doi: 10.1016/s0046-8177(99)90033-3. [DOI] [PubMed] [Google Scholar]
- 9.Liberman E, Weidner N. Papillary and follicular neoplasms of the thyroid gland. Differential immunohistochemical staining with high-molecular-weight keratin and involucrin. Appl Immunohistochem Mol Morphol. 2000;8:42–48. doi: 10.1097/00129039-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Yap AS, Stevenson BR, Keast JR, Manley SW. Cadherin-mediated adhesion and apical membrane assembly define distinct steps during thyroid epithelial polarization and lumen formation. Endocrinology. 1995;136:4672–4680. doi: 10.1210/endo.136.10.7664688. [DOI] [PubMed] [Google Scholar]
- 11.Brabant G, Hoang-Vu C, Behrends J, Cetin Y, Potter E, Dumont JE, Maenhaut C. Regulation of the cell-cell adhesion protein, E-cadherin, in dog and human thyrocytes in vitro. Endocrinology. 1995;136:3113–3119. doi: 10.1210/endo.136.7.7789339. [DOI] [PubMed] [Google Scholar]
- 12.Brabant G, Hoang-Vu C, Cetin Y, Dralle H, Scheumann G, Molne J, Hansson G, Jansson S, Ericson LE, Nilsson M. E-cadherin: a differentiation marker in thyroid malignancies. Cancer Res. 1993;53:4987–4993. [PubMed] [Google Scholar]
- 13.Soares P, Berx G, van Roy F, Sobrinho-Simoes M. E-cadherin gene alterations are rare events in thyroid tumors. Int J Cancer. 1997;70:32–38. doi: 10.1002/(sici)1097-0215(19970106)70:1<32::aid-ijc5>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 14.Scheumman GF, Hoang-Vu C, Cetin Y, Gimm O, Behrends J, von Wasielewski R, Georgii A, Birchmeier W, von Zur Muhlen A, Dralle H. Clinical significance of E-cadherin as a prognostic marker in thyroid carcinomas. J Clin Endocrinol Metab. 1995;80:2168–2172. doi: 10.1210/jcem.80.7.7608273. [DOI] [PubMed] [Google Scholar]
- 15.Rocha AS, Soares P, Fonseca E, Cameselle-Teijeiro J, Oliveira MC, Sobrinho-Simoes M. E-cadherin loss rather than beta-catenin alterations is a common feature of poorly differentiated thyroid carcinomas. Histopathology. 2003;42:580–587. doi: 10.1046/j.1365-2559.2003.01642.x. [DOI] [PubMed] [Google Scholar]
- 16.von Wasielewski R, Rhein A, Werner M, Scheumann GF, Dralle H, Potter E, Brabant G, Georgii A. Immunohistochemical detection of E-cadherin in differentiated thyroid carcinomas correlates with clinical outcome. Cancer Res. 1997;57:2501–2507. [PubMed] [Google Scholar]
- 17.Rosia J, Carcangiu ML, Delellis RA. AFIP third series, fascicle 5. 1992. Atlas of Tumor Pathology: Tumors of the thyroid gland. [Google Scholar]
- 18.LiVolsi VA. In: Surgical pathology of the thyroid. Saunders, editor. Philadelphia, PA: 1990. [Google Scholar]
- 19.Cerrato A, Fulciniti F, Avallone A, Benincasa G, Palombini L, Grieco M. Beta- and gamma-catenin expression in thyroid carcinomas. J Pathol. 1998;185:267–272. doi: 10.1002/(SICI)1096-9896(199807)185:3<267::AID-PATH113>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 20.Graff JR, Greenberg VE, Herman JG, Westra WH, Boghaert ER, Ain KB, Saji M, Zeiger MA, Zimmer SG, Baylin SB. Distinct patterns of E-cadherin CpG island methylation in papillary, follicular, Hurthle's cell, and poorly differentiated human thyroid carcinoma. Cancer Res. 1998;58:2063–2066. [PubMed] [Google Scholar]
- 21.Garcia-Rostan G, Tallini G, Herrero A, D'Aquila TG, Carcangiu ML, Rimm DL. Frequent mutation and nuclear localization of beta-catenin in anaplastic thyroid carcinoma. Cancer Res. 1999;59:1811–1815. [PubMed] [Google Scholar]
- 22.Lonigro R, De Felice M, Biffali E, Macchia PE, Damante G, Asteria C, Di Lauro R. Expression of thyroid transcription factor 1 gene can be regulated at the transcriptional and posttranscriptional levels. Cell Growth Differ. 1996;7:251–261. [PubMed] [Google Scholar]
- 23.Katoh R, Kawaoi A, Miyagi E, Li X, Suzuki K, Nakamura Y, Kakudo K. Thyroid transcription factor-1 in normal, hyperplastic, and neoplastic follicular thyroid cells examined by immunohistochemistry and nonradioactive in situ hybridization. Mod Pathol. 2000;13:570–576. doi: 10.1038/modpathol.3880098. [DOI] [PubMed] [Google Scholar]