Abstract
We present here a case of acrodermatitis enteropathica-like eruption associated with essential free fatty acid and protein deficiencies as well as borderline zinc deficiency that occurred after Whipple's operation in a 31-yr-old woman. Her eruptions were improved not by zinc supplements alone, but her condition was improved by total parenteral nutrition including amino acids, albumin, lipid and zinc. Although we could not exactly decide which of the nutrients contributed the most to her manifestations, we inferred that all three elements in concert caused her dermatoses. This case shows that even though the patient's skin manifestations and laboratory results are suggestive of acrodermatitis enteropathica, the physicians should keep in mind the possibility that this disease can be associated with other nutritional deficiencies such as free fatty acid or protein deficiency.
Keywords: Acrodermatitis; Acrodermatitis Enteropathica; Malnutrition; Zinc; Fatty Acids, Essential; Protein Deficiency
INTRODUCTION
Nutritional deficiency states result in a diverse group of diseases with many distinctive cutaneous manifestations. These nutritional diseases are seen in a wide variety of clinical settings, and they result from inadequate diet, impaired absorption or the defective metabolism of specific nutrients. Until now, however, the exact pathophysiologic mechanisms for many of the skin changes associated with nutritional deficiency are unclear.
Acrodermatitis enteropathica (AE) is the clinical phenotype of zinc (Zn) deficiency characterized by pustular and bullous dermatitis with an acral and periorificial distribution (1). Pustular paronychia, angular stomatitis, glossitis and generalized alopecia are also usually present (2). It can be associated with the acquired form of zinc deficiency resulting from Crohn's disease (3), chronic pancreatitis (4), and chemotherapy (5).
Cutaneous eruption is a rare complication of Whipple's operation. From the literature review, a few cases have been reported including necrolytic migratory erythema (6), protein energy malnutrition (7) and pseudoglucagonoma (8).
We herein present a case of AE-like eruptions that were associated with essential free fatty acid (EFA) and protein deficiencies as well as a borderline Zn deficiency following Whipple's operation in a 31-yr-old woman. We have also reviewed the medical literatures about the interconnection of these nutritional deficiencies.
CASE REPORT
A 31-yr-old woman was referred to our hospital for the evaluation of a refractory dermatitis and a diffuse alopecia for 1 yr. She had had a Whipple's operation 6 yr ago due to periampullary carcinoma. After that, her stool became loose and her weight had decreased by 10 kg over the last 6 yr. Diffuse alopecia began 1 yr ago and skin manifestation developed 6 months ago. Her medical history also revealed the onset of amenorrhea for 4 months.
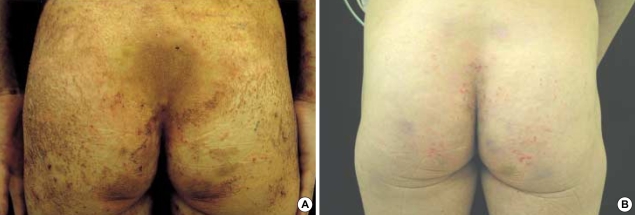
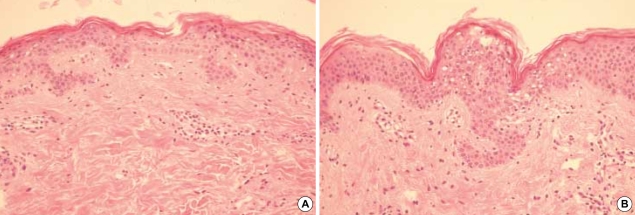
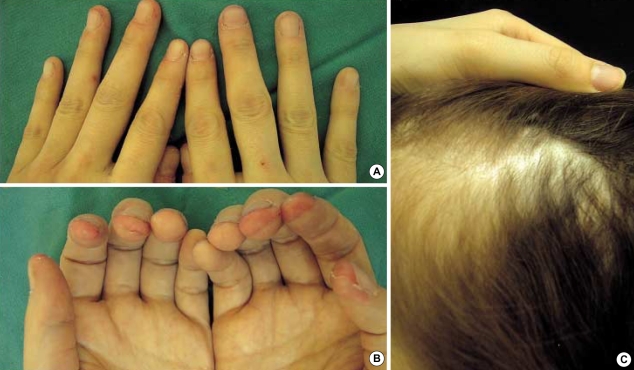
On physical examination, her height and weight were 161 cm and 41 kg, respectively, and body mass index was 15.1, and this was well below the normal average value 22. The cutaneous manifestations were characterized by interconnected, erythematous, scaly, erosive patches, papules and tiny vesicles on her arms, abdomen, lower back and buttocks, and these lesions were especially severe in the flexural areas (Fig. 1A). Microscopic examination of the skin lesions showed scattered parakeratosis and upper epidermal clear cell changes with a mild dermal infiltration of lymphocytes. Irregular acanthosis, mild parakeratosis and dermal perivascular infiltration were also observed on another skin section (Fig. 2). The transepidermal water loss (TEWL) measurement in the lesions was shown to be 7.4 times higher, on average, than in normal skin. There were mild angular cheilitis and oral mucosal erosions as well as her having a beefy red tongue accompanied by papillary atropy. Her fingers showed the erosion of the finger tips, but there was no paronychia involvement (Fig. 3). The scalp hair was diffusely sparse and light brown but it was not brittle or otherwise abnormal (Fig. 3). Pitting edema was noted over both lower legs and feet. There was no symptom and sign suggesting photophobia or hepatosplenomegaly.
Fig. 1.
(A) Interconnected, erythematous, scaly, erosive patches, papules and tiny vesicles on the lower back and buttocks. (B) After 1 month of treatment with total parenteral nutrition, oral zinc supplement and topical linoleic acid.
Fig. 2.
(A) Irregular acanthosis, mild parakeratosis and dermal perivascular infiltration. (B) Upper epidermal clear cell changes (H&E, (A) ×100, (B) ×200).
Fig. 3.
(A, B) Erosion of the finger tips. (C) Diffuse alopecia and yellowish change of hair.
Abnormal laboratory results were as follows; hemoglobin, 9.4 g/dL (12-16 g/dL); post prandial 2 hr glucose test, 255 mg/dL (<200 mg/dL); alkaline serine transaminase, 60 IU/L (5-38 IU/L); serum protein and albumin, 3.7 g/dL (6.4-8.3 g/dL) and 1.6 g/dL (3.3-5.2 g/dL), respectively; stool fat, positive (negative); beta carotene, 3.41 µg/dL (50-250 µg/dL); total cholesterol, 80 mg/dL (128-250 mg/dL); transferrin, 76.5 mg/dL (200-360 mg/dL); retinal binding protein, 1.05 mg/dL (3-6 mg/dL); calcium and magnesium, 6.4 mg/dL (8.2-10.2 mg/dL) and 1.8 mg/dL (1.9-3.1 mg/dL) respectively; and serum zinc, 67 µg/dL (70-121 µg/dL). Normal or negative values were obtained for the followings: urinalysis, serum glucagons and blood gas analysis.
Based on the clinical, histophathological and initial laboratory findings, we diagnosed this woman as a case of zinc deficiency. However, in spite of a 1-week treatment with an oral zinc sulfate supplement, 50 mg a day, her skin lesions did not improve and her serum zinc level did not increase either. With the aid of additional laboratory results, we inferred that her dermatitis came from a combined malnutrition disorder resulting from protein deficiency, EFA deficiency and a borderline Zn deficiency due to Whipple's operation. The patient was given total parenteral nutrition containing amino acids, albumin, lipids and electrolytes and oral Zn supplements, 50 mg a day, and topical linolenic acid. The patient's dermatitis improved markedly after 1 month of treatment, except for her postinflammatory hyperpigmentation (Fig. 1B). The abnormal results of her laboratory examination became normalized. The pitting edema also cleared up earlier than the dermatitis, and the TEWL returned to that of normal skin. Thereafter, she has been maintained on the regular infusion of albumin and lipids together with an oral zinc supplement, and she is without recurrence of her disease.
DISCUSSION
Our patient clearly showed a nutritionally responsive dermatosis. We inferred that it was because of exocrine pancreatic insufficiency resulting from the Whipple's operation. It is likely that the borderline Zn, low EFA and protein deficiency or perhaps all three in concert, was responsible for this patient's clinical manifestations, although we could not precisely decide which nutrient was the most important. Therefore, we will discuss here the possible contributions and relationship of each of three nutritional deficiencies.
AE is the clinical phenotype of Zn deficiency. It is a congenital autosomal recessive condition characterized by pustular and bullous dermatitis with an acral and periorificial distribution (1). Pustular paronychia, angular stomatitis, glossitis and generalized alopecia are also usually present (2). Although the term AE was initially used to describe the congenital form, it is also associated nowadays with the acquired form of zinc deficiency resulting from Crohn's disease (3), chronic pancreatitis (4) and chemotherapy (5).
The clinical presentations of erythematous, scaly, crusting and erosive patches with alopecia and mucosal involvement and the histological findings in our patient were consistent with AE. However, it is unusual that the lesions were distributed not only on the periorificial and acral area, but also on the buttocks, thigh and both arms, and that there was no paronychia.
The diagnostic value of Zn in AE is usually below 70 µg/dL in comparison with 67 µg/dL in our case. Some authors have reported AE without hypozincemia (9), explaining that accumulation of zinc bound in an immobile chemical form in the tissue could make the element unavailable for metabolic processes. However, the patient with classic AE often has lower Zn levels and Neldner and Hambidge (10) have reported a patient showing the recurrence of symptoms even at a plasma Zn level of 36 µg/dL. Moreover, AE usually responds to Zn supplement therapy within a few days (11), as compared with 1 month in our case. From these atypical findings, we have to consider another possible cause other than the Zn deficiency in our patient.
Our patient showed markedly decreased serum protein and albumin with peripheral edema. Kwashiorkor, chronic protein deficiency, is characterized by hypopigmentation with fine, branlike desquamation in the circumoral and edematous locations (2, 12). There may be a clinical resemblance between kwashiorkor and AE. Animal-derived proteins from our diet are the major source of zinc in humans. Conversely, poor protein absorption resulting from exocrine pancreatic insufficiency, as was shown in our patient, may cause a zinc deficiency. In addition, because albumin is the major carrier protein for zinc in human, hypoalbuminemia is associated with a low plasma zinc level. Hansen et al. (13) have shown that pancreatic proteolytic enzyme deficiency leaded to AE without zinc deficiency, and Golden et al. (14) have reported a patient with kwashiorkor who responded to zinc sulfate ointment: they concluded that the skin lesions of kwashiorkor seem to be caused by a deficiency of zinc. These facts suggest a close relationship between protein deficiency and zinc deficiency.
Clinical findings in our patient were also suggestive of EFA deficiency. AE and EFA deficiency share in common a large portion of clinical manifestations and histological features. EFAs in the skin have both synthetic and structural functions. They are precursors of the prostaglandins, hydroxyeicosatetraenoic acids and leukotrienes. Structurally, EFAs elevate the degree of cell membrane lipoprotein unsaturation and influence membrane fluidity (2, 15). EFA deficiency can lead to chronic epidermal hyperproliferation causing the thickening, scaling and loss of elasticity in the skin, and to impaired eisocsanoid production resulting in eczematous changes in the skin (16, 17). In addition, TEWL measurement in the skin lesions of EFA deficiency, as shown in our patient, reveals a marked increase as compared with normal skin (2).
The association of AE and EFA deficiency has been previously noted (18), although this link has not been consistently seen. Zinc is thought to be the element required in more than 24 metalloenzymes including δ-6-desaturase and δ-5-desaturase, and zinc is also required in the mobilization of dihomo-δ-linolenic acid (19). From the therapeutic points of view, the clinical manifestations of zinc deficient patients were greatly improved by EFA supplement (9), pancreatic enzyme preparation (20) and diiodohydroxyquinoline (17), and so some authors have recommended that both EFA and zinc determinations be made for the patient with AE-like eruption.
Pseudoglucagonoma syndrome can also show similar clinical features as is seen in the AE. Liver cirrhosis, Crohn's disease, malabsorption and chronic pancreatitis can cause necrolytic migratory erythema, the prototype of pseudoglucagonoma (21). Although the pathogenesis is unclear, deficiencies of zinc, amino acids, or essential fatty acids can be associated with this disease (22).
Nutritional deficiency states result in many distinctive cutaneous manifestations depending upon the deficient elements. However, in some cases, there can be an intermingling of features from more than two nutritional dermatoses. In those cases, the diagnosis may be carried by author's propensity. Our patient clearly showed protein, essential fatty acid and borderline zinc deficiency on the physical and laboratory examinations. We inferred that her malnutrition states and symptoms were attributable to pancreatic insufficiency caused by Whipple's operation and, perhaps, all three elements in concert, caused our patient's clinical manifestations.
References
- 1.Perafan-Riveros C, Franca LF, Alves AC, Sanches JA., Jr Acrodermatitis enteropathica: case report and review of the literature. Pediatr Dermatol. 2002;19:426–431. doi: 10.1046/j.1525-1470.2002.00200.x. [DOI] [PubMed] [Google Scholar]
- 2.Miller SJ. Nutritional deficiency and the skin. J Am Acad Dermatol. 1989;21:1–30. doi: 10.1016/s0190-9622(89)70144-4. [DOI] [PubMed] [Google Scholar]
- 3.Krasovec M, Frenk E. Acrodermatitis enteropathica secondary to Crohn's disease. Dermatology. 1996;193:361–363. doi: 10.1159/000246296. [DOI] [PubMed] [Google Scholar]
- 4.Kim BC, Joo KR, Lee HS, Jeong YK, Suh HS, Kim DH, Park NM, Park JH. A case of chronic pancreatitis associated with liver infarction and acrodermatitis enteropathica. Korean J Intern Med. 2002;17:263–265. doi: 10.3904/kjim.2002.17.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawai T, Sugiura H, Danno K, Uchiyama M, Ohta S. Acquired acrodermatitis enteropathica during chemotherapy for acute lymphocytic leukemia in a child with Down syndrome. Br J Dermatol. 1996;135:659–660. doi: 10.1111/j.1365-2133.1996.tb03862.x. [DOI] [PubMed] [Google Scholar]
- 6.Jang MS, Kim YJ, Chae YS, Suh KS, Kim ST. A case of necrolytic migratory erythema induced by a pancreatic insufficiency. Korean J Dermatol. 1996;34:166–170. [Google Scholar]
- 7.Park MA, Ha SG, Won YH, Chun IK. A case of protein energy malnutrition after Whipple's operation. Korean J Dermatol. 1994;32:130–133. [Google Scholar]
- 8.Seo KH, Park JH, Jang HS, Kwon KS, Chung TA. A case of pseudoglucagonoma syndrome treated with medium-chain triglyceride. Korean J Dermatol. 1997;35:593–599. [Google Scholar]
- 9.Krieger J, Evans GW. Acrodermatitis enteropathica without hypozincemia: therapeutic effect of a pancreatic enzyme preparation due to zinc binding ligand. J Pediatr. 1980;96:32–35. doi: 10.1016/s0022-3476(80)80319-2. [DOI] [PubMed] [Google Scholar]
- 10.Neldner KN, Hambidge KM. Zinc therapy of acrodermatitis enteropathica. N Eng J Med. 1975;292:879–882. doi: 10.1056/NEJM197504242921702. [DOI] [PubMed] [Google Scholar]
- 11.Kang JD, You DO, Park SD. A case of transient acrodermatitis enteropathica. Korean J Dermatol. 2003;41:786–789. [Google Scholar]
- 12.McLaren DS. Skin in protein energy malnutrition. Arch Dermatol. 1987;123:1674–1676. [PubMed] [Google Scholar]
- 13.Hansen RC, Lynch PJ, Morrow GM. Proteolytic deficiency causing acrodermatitis enteropathica. Clin Res. 1976;24:168. [Google Scholar]
- 14.Golden MH, Golden BE, Jackson AA. Skin breakdown in kwashiorkor responds to zinc. Lancet. 1980;1:1256. doi: 10.1016/s0140-6736(80)91722-5. [DOI] [PubMed] [Google Scholar]
- 15.Schreiner V, Gooris GS, Pfeiffer S, Lanzendorfer G, Wenck H, Diembeck W, Proksch E, Bouwstra J. Barrier characteristics of different human skin types investigated with X-ray diffraction, lipid analysis, and electron microscopy imaging. J Invest Dermatol. 2000;114:654–660. doi: 10.1046/j.1523-1747.2000.00941.x. [DOI] [PubMed] [Google Scholar]
- 16.Lowe NJ, Stoughton RB. Essential fatty acid deficient hairless mouse: a model of chronic epidermal hyperproliferation. Br J Dermatol. 1977;96:155–162. doi: 10.1111/j.1365-2133.1977.tb12537.x. [DOI] [PubMed] [Google Scholar]
- 17.Ziboh VA, Cho Y, Mani I, Xi S. Biological significance of essential fatty acids/prostanoids/lipoxygenase-derived monohydroxy fatty acids in the skin. Arch Pharm Res. 2002;25:747–758. doi: 10.1007/BF02976988. [DOI] [PubMed] [Google Scholar]
- 18.Ginsburg R, Robertson A, Jr, Michel B. Acrodermatitis enteropathico: abnormalities of fat metabolism and integumental ultrastructures in infants. Arch Dermatol. 1976;112:653–660. doi: 10.1001/archderm.112.5.653. [DOI] [PubMed] [Google Scholar]
- 19.Horrobin DF, Cunnane SC. Interactions between zinc, essential fatty acids and prostaglandins: relevance to acrodermatitis enteropathica, total parenteral nutrition, the glucagonoma syndrome, diabetes, anorexia nervosa and sickle cell anemia. Med Hypotheses. 1980;6:277–296. doi: 10.1016/0306-9877(80)90125-5. [DOI] [PubMed] [Google Scholar]
- 20.Schroeter AL, Tucker SB, Minn R. Essential fatty acid deficiency. Arch Dermatol. 1978;114:800–801. [PubMed] [Google Scholar]
- 21.Schwartz RA. Glucagonoma and pseudoglucagonoma syndromes. Int J Dermatol. 1997;36:81–89. doi: 10.1046/j.1365-4362.1997.00042.x. [DOI] [PubMed] [Google Scholar]
- 22.Blackford S, Wright S, Robert DL. Necrolytic migratory erythema without glucagonoma: the role of dietary essential fatty acids. Br J Dermatol. 1991;125:460–462. doi: 10.1111/j.1365-2133.1991.tb14773.x. [DOI] [PubMed] [Google Scholar]